Abstract
Objectives
Acute respiratory distress syndrome (ARDS) in patients with traumatic brain injury (TBI) is associated with increased mortality. Information on the prevalence of ARDS and its neurological outcome after TBI is sparse. We aimed to systematically review the prevalence, risk factors, and outcome of ARDS in TBI population.
Data Sources
PubMed and four other databases (Embase, Cochrane Library, Web of Science Core Collection, and Scopus) from inception to July 6, 2020.
Study Selection
Randomized controlled trials (RCTs) and observational studies in patients older than 18 years old.
Data Extraction
Two independent reviewers extracted the data. Study quality was assessed by the Cochrane Risk of Bias tool for RCTs, the Newcastle–Ottawa Scale for cohort and case–control studies. Good neurological outcome was defined as Glasgow Outcome Scale ≥ 4. Random-effects meta-analyses were conducted to estimate pooled outcome prevalence and their 95% confidence intervals (CI).
Data Synthesis
We included 20 studies (n = 2830) with median age of 44 years (interquartile range [IQR] = 35–47, 64% male) and 79% (n = 2237) suffered severe TBI. In meta-analysis, 19% patients (95% CI = 0.13–0.27, I2 = 93%) had ARDS after TBI. The median time from TBI to ARDS was 3 days (IQR = 2–5). Overall survival at discharge for the TBI cohort was 70% (95% CI = 0.64–0.75; I2 = 85%) and good neurological outcome at any time was achieved in 31% of TBI patients (95% CI = 0.23–0.40; I2 = 88%). TBI cohort without ARDS had higher survival (67% vs. 57%, p = 0.01) and good neurological outcomes (34% vs. 23%, p = 0.02) compared to those with ARDS. We did not find any specific risk factors for developing ARDS.
Conclusion
In this meta-analysis, approximately one in five patients had ARDS shortly after TBI with the median time of 3 days. The presence of ARDS was associated with worse neurological outcome and mortality in TBI. Further research on prevention and intervention strategy of TBI-associated ARDS is warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00408-021-00491-1.
Keywords: Acute respiratory distress syndrome, Traumatic brain injury, Acute brain injury, Neurogenic pulmonary edema, Acute lung injury
Introduction
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide and one of the most common causes of death in individuals under the age 40 [1]. One of the main interventions in TBI to prevent secondary hypoxic brain insults is through the maintenance of an adequate cerebral oxygen delivery, which may improve the outcome in this population [2]. An important risk factor for mortality in TBI is the development of pulmonary complication such as acute respiratory distress syndrome (ARDS), which increase the risk of in-hospital death by threefold [3]. ARDS is characterized by diffuse lung parenchymal inflammation, noncardiogenic pulmonary edema due to increased alveolar-capillary vascular permeability, leading to impaired gas exchange resulting in hypoxemia and abnormal lung physiology [4]. The mechanism of ARDS associated with TBI is related to catecholamine surge, and systemic inflammation response causing abnormally elevated pulmonary hydrostatic pressure and vascular permeability, pulmonary edema coupled with systemic arterial hypertension to maintain the cerebral perfusion pressure (CPP) in the presence of intracranial pressure (ICP) crisis [5–7].
Despite its impact on mortality, the prevalence, timing, and risk factors of ARDS is not well characterized in TBI population. The presence of ARDS is independently associated with brain hypoxia in TBI patients when monitored with LICOX (Integra Neuroscience) [8]. In addition, prior studies have reported a wide range of frequency of TBI-associated ARDS ranging between 1 and 50% [9, 10]. Herein, we aimed to determine the prevalence, risk factors, and outcome of TBI-associated ARDS by performing a systematic review and meta-analysis.
Materials and Methods
Search Strategy
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. An information specialist (C.P) with experience in systematic reviews and expert searching designed the search after a preliminary consultation that identified key concepts and terminology. The designed search was translated and applied to PubMed, both legacy (results = 4991) and new (results = 4998) via NCBI, Embase via Elsevier (results = 7527), the Cochrane Library via Wiley (results = 644, the Web of Science Core Collection via Clarivate (results = 4449), and Scopus via Elsevier (results = 8768). The search included subject headings and controlled vocabulary as well as keywords and natural language related to acute lung injury (ALI), ARDS, and brain injury. The search was applied from database inception through July 6, 2020. There were no limits for language. An effort was made to account for plurals, acronyms, and synonyms. In total, there were 31,327 total results, 17,414 duplicates removed, and 13,913 remaining results. Deduplication was performed with EndNote X9 (Endnote, Clarivate, available at www.endnote.com). The results were uploaded to Covidence (Covidence, Veritas Health Innovation, Melbourne, Australia; available at www.covidence.org) for title and abstract screening and further reviewed for eligibility. All articles meeting the inclusion criteria were retrieved and the full text was reviewed. References from the included studies were manually reviewed for additional relevant reports. The detailed search strategy is available in Appendix A.
Eligibility Criteria
Eligibility criteria were applied following the PICOS (population, intervention, comparator, outcome, and study design) approach [11].
Inclusion Criteria
We included (1) all randomized controlled trials (RCTs) and observational studies with adult patients (> 18 years old); (2) the studies with ALI or ARDS (defined by either the American-European Consensus Conference [AECC] or Berlin criteria) occurring after TBI [12, 13]. Studies that reported ALI patients based on AECC criteria (PaO2/FiO2 [P/F] ratio ≤ 300) are classified as mild ARDS in this study, patients with a P/F ratio ≤ 200 are classified as moderate/severe ARDS [12, 13].
Exclusion Criteria
We excluded (1) editorials, commentaries, research protocols, reviews (including systemic review and meta-analysis articles), case series/reports, and abstracts; (2) articles with pediatric population (age < 18); (3) the studies without description or definition of ALI or ARDS; (4) animal and in-vitro studies; and (5) ARDS was diagnosed prior to the occurrence of brain injury.
Study Selection and Data Extraction
The literature results were independently assessed by two reviewers (T.H.F., and M.H.) for eligibility. Any disagreements on the inclusion or exclusion of literature were resolved by a third reviewer (S.-M.C). Data were extracted from eligible studies and recorded in an excel spreadsheet (Microsoft, Redmond, WA). Full text and charts were reviewed in detail for data on study design, study population, TBI relevant information (head abbreviated injury score[head-AIS], injury severity score [ISS], Marshall computed tomography [CT] classification of TBI severity, admission Glasgow coma score [GCS]) [14], patient characteristics (age, sex, ethnicity, and baseline comorbidities), complications during hospital admission, ARDS related information (etiology, severity, P/F ratio), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, neurological outcomes, and survival.
Definition of Outcomes
Primary outcome was prevalence of ARDS occurred after TBI. Secondary outcomes included survival at hospital discharge and prevalence of good neurologic outcome at any time after TBI (defined as Glasgow Outcome Scale [GOS] ≥ 4) of discharged patients [15].
Quality Assessment/Risk of Bias
The Cochrane Risk of Bias assessment tool was used to assess risk of bias in RCT in eight domains [16]. The trial was considered at high risk of bias if at least one domain was rated as a high risk. The trial was considered low risk if all domains were judged as low. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies was used to evaluate the risk of bias of cohort studies. The NOS scores were based on three domains: patient selection, comparability, and assessment of outcome or exposure [17]. Studies scoring 6 or more points were considered to have a low risk for bias. Publication quality was assessed independently by two investigators (T.H.F, M.H.). Any discrepancies were resolved in consensus with a third investigator (S.-M.C.).
Statistical Analysis
We reported this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Appendix C). The prevalence of each outcome was calculated in each study based on the number of patients with the specific outcome divided by the total number of TBI patients, which was then pooled in meta-analysis across studies. For all meta-analyses of prevalence, we used random-effects models with the inverse variance method. The Sidik–Jonkman estimator was used for tau [18] and Hartung–Knapp adjustment was used to calculate Confidence intervals (CI) [19]. The Freeman–Tukey double arcsine transformation was used to calculate prevalence for all outcomes. Heterogeneity was assessed using the Cochrane Q statistic (Chi-square test) and the magnitude of the heterogeneity was evaluated with the I2 statistic [16]. I2 quantified the degree of heterogeneity that ranges between 0 and 100%. Meta-regression analyses were performed to evaluate the association between prespecified patient variables (age, sex, GCS score on admission, Marshall CT score on admission, presence of pneumonia, sepsis and systemic inflammatory response syndrome [SIRS]), and the outcome of ARDS. We chose relevant variables a priori based on prior literature. However, certain relevant variables such as patient's baseline comorbidities were unable to be assessed due to insufficient data and reporting. Effects were described as odds ratios (OR) and their 95% CI. A p value < 0.05 means there is association between potential predictor and outcome. We conducted a subgroup analyses by presence or not of ARDS for good neurologic outcome, and survival rate. Differences of prevalence between subgroups (ARDS vs. no-ARDS) were tested with the p for interaction (a p < 0.1 means that proportions are different between them). Analysis were performed with R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
Results
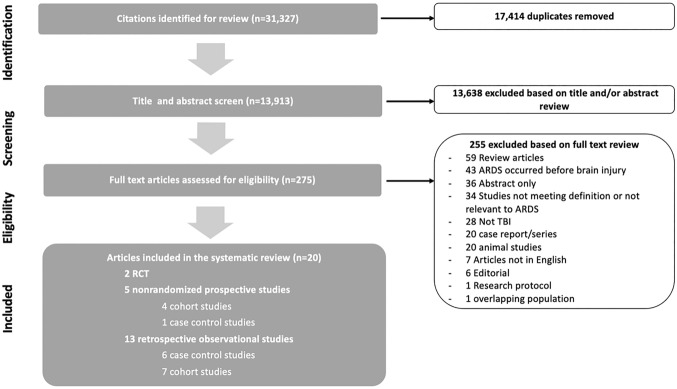
Our search yielded 13,550 citations after duplicates were removed. Following title and abstract screening, 275 articles were eligible for full text review. Of these studies, 255 were excluded based on inclusion/exclusion criteria leaving the final 21 studies (n = 3203). Figure 1 demonstrates the flowchart of the selection process. The total studies included 2 RCTs (n = 254), five prospective observational studies (4 cohort studies, 1 case control studies; n = 638), and 13 retrospective observational studies (7 cohort studies, 6 case control studies; n = 1938). A reference of the final studies was included in Appendix B.
Fig. 1.
Study flowchart for literature search and selection of studies. ARDS acute respiratory distress stress; RCT randomized controlled trials; TBI traumatic brain injury
Risk of Bias Assessment
The Cochrane tool showed overall high risk of bias for the 2 RCTs (Supplemental Table 1). The NOS was conducted on studies and did not indicate high risk of bias for any study with the median NOS score of 7 (Supplemental Table 2).
Prevalence of ARDS in TBI
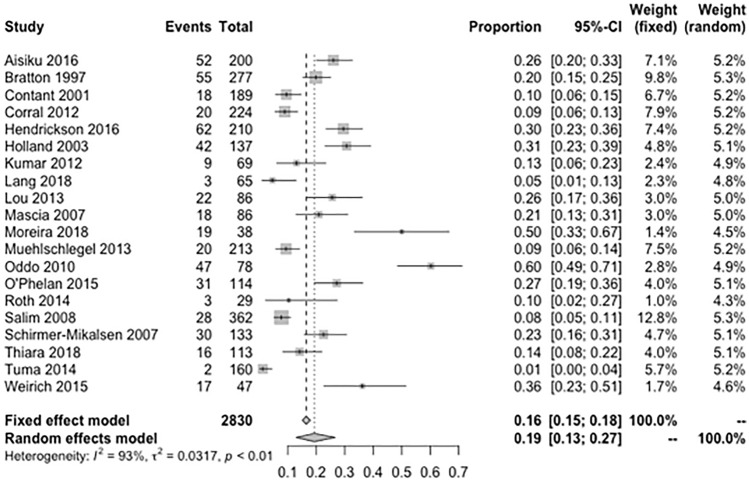
Of the total 2830 patients with TBI, the median age was 44 years (interquartile range [IQR] = 35–47) and 64% were male (n = 1805) (Table 1). Majority (79%) had severe TBI (head-AIS score > 3); six studies (n = 1070) included only patients with isolated TBI and the remaining 14 studies (n = 1760) included multi-trauma with a median ISS of 27 (IQR = 26–32). Of the TBI patients with multi-trauma, 20% had evidence of chest injuries. The median admission GCS of all studies was 5 (IQR = 4–6) and 49% of TBI patients had severe diffuse injury or mass lesion on brain CT based on Marshall classification (Marshall CT score > 3). Of the reported complications, 18% of TBI patients had pneumonia, 23% developed sepsis, and 31% had SIRS during their hospital stay. The pooled prevalence of ARDS in patients after TBI was 19% (95% CI = 0.13–0.27; I2 = 93%; Fig. 2) and the median time of developing ARDS from initial TBI diagnosis was 3 days (IQR = 2–5). Etiology of ARDS was not reported in the included studies. The proportion of ARDS in patients with isolated TBI was 22% vs. 21% in patients with multi-trauma. Six studies (n = 1047) reported number of patients with mild ARDS, and the pooled prevalence of mild ARDS was 8% (95% CI = 0.03–0.15; I2 = 88%; Supplemental Fig. 1). Fourteen studies (n = 2317) reported number of patients with moderate/severe ARDS and the pooled prevalence of moderate/severe ARDS was 14% (95% CI = 0.08–0.22; I2 = 91%; Supplemental Fig. 2), of which 25% had severe ARDS with the median P/F ratio < 100.
Table 1.
Baseline characteristics of patients with TBI
| Characteristics | All patients with traumatic brain injuries (n = 2830) |
|---|---|
| Demographics | |
| Age, year, median (IQR) | 44 (35–47) |
| Male, n (%) | 1805 (64%) |
| Pts with isolated TBI | 1070 (33%) |
| Severity of injury on admission | |
| APACHE II score, median (IQR) | 24 (21–43) |
| Pts with head-AIS > 3, n (%) | 2237 (80%) |
| Pts with chest injury, n (%) | 426 (13%) |
| GCS score on admission, median (IQR) | 5 (4–6) |
| Pts with GCS score 3–5 on admission, n (%) | 645 (46%) |
| Marshall CT score > 3, n (%) | 630 (49%) |
| Median ICP*, mmHg (IQR) | 17 (15–20) |
| ARDS | |
| All ARDS, n (%) | 657 (21%) |
| Mild ARDS, n (%) | 105 (13%) |
| Moderate/Severe ARDS, n (%) | 281 (14%) |
| P/F ratio, median (IQR) | 276 (204–291) |
| Hospital complications | |
| Pneumonia, n (%) | 304 (18%) |
| Sepsis, n (%) | 207 (23%) |
| SIRS, n (%) | 131 (32%) |
| Outcomes | |
| Length of hospital stay, days, median, IQR | 14 (13–25) |
| Good neurological outcome at any time after TBI, n (%) | 443 (28%) |
| Survivors at discharge, n (%) | 1627 (57%) |
ARDS acute respiratory distress syndrome; APACHE acute physiology and chronic health evaluation; CT computed tomography; GCS Glasgow coma scale; ICP intracranial pressure; IQR interquartile range; ISS injury severity score; SIRS systemic inflammatory response syndrome; P/F PaO2/FiO2
*Reported as median ICP throughout hospital stay
Fig. 2.
Meta-analysis of the prevalence of ARDS among patients with traumatic brain injury. ARDS acute respiratory distress stress. τ2 is the variance of the effect size parameters across the population of studies and it reflects the variance of the true effect sizes. I2 quantified the degree of heterogeneity across the studies that ranges between 0 and 100%
Survival and Neurological Outcome
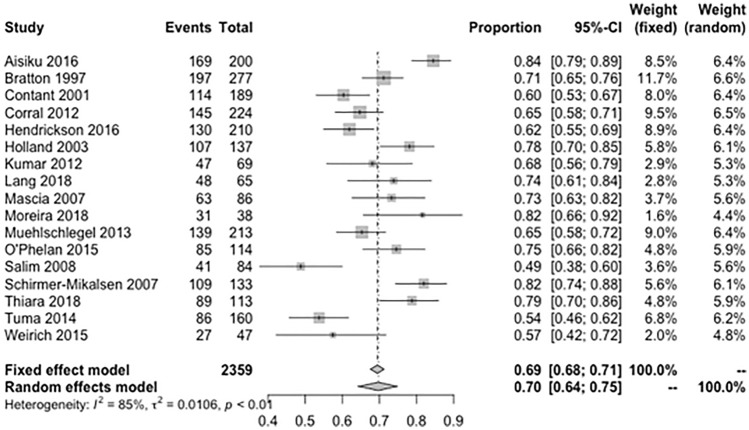
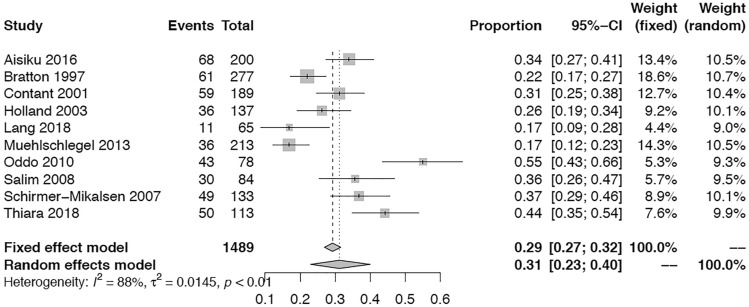
Seventeen studies (n = 2359, 83%) reported number of in-hospital survivors after TBI. The pooled overall survival proportion at discharge after TBI was 70% (95% CI = 0.64–0.75; I2 = 85%; Fig. 3). The survival proportion was significantly higher in TBI cohort without ARDS vs. those with ARDS (67% vs. 57%, p = 0.01, Supplemental Fig. 3). Ten studies (n = 1767, 55%) reported neurological outcome after TBI. Good neurological outcome at any time was achieved in 31% of TBI patients (95% CI = 0.23–0.40; I2 = 88%, Fig. 4). When patients with and without ARDS were compared, the ARDS group had significantly fewer patients with good neurological outcome at any time after TBI (23% vs. 34%, p = 0.02, Supplemental Fig. 4).
Fig. 3.
Meta-analysis of overall survival at discharge in patients with traumatic brain injury. τ2 is the variance of the effect size parameters across the population of studies and it reflects the variance of the true effect sizes. I2 quantified the degree of heterogeneity across the studies that ranges between 0 and 100%
Fig. 4.
Meta-analysis of overall good neurological outcomes at any time in patients with traumatic brain injury. τ2 is the variance of the effect size parameters across the population of studies and it reflects the variance of the true effect sizes. I2 quantified the degree of heterogeneity across the studies that ranges between 0 and 100%
Meta-Regression for Pre-specified Risk Factors of ARDS
A meta-regression analysis of 11 studies (n = 1462) showed that age, male gender, white race, admission APACHE II score, head-AIS > 3, Marshall CT score > 3, GCS score on admission, SIRS during hospitalization, and median ICP during hospitalization were not significant risk factors for ARDS in TBI (Supplemental Table 3).
Discussion
To date, there has not been a systematical effort to review the prevalence of ARDS in patients with TBI in a meta-analysis. To our knowledge, this is the largest systematic review and meta-analysis to assess the prevalence, risk factors, survival and neurological outcome in patients with ARDS after acute TBI.
Our study involving 2830 adult TBI patients demonstrated high prevalence of ARDS (19%). Previously, the occurrence of ARDS was reported to have bimodal distribution, highest at 2–3 days following initial TBI and 7–8 days later in association with development of pneumonia [20]. In our study, the occurrence of ARDS was observed shortly after TBI with the median of 3 days. This highlights the important interplay between the brain and lung, suggesting catecholamine surge and systemic inflammation response after acute TBI may be responsible in causing ARDS shortly after the initial insult. Not surprisingly, the presence of ARDS was associated with worse neurological outcome and mortality compared to those without ARDS. Therefore, early identification and early prevention of ARDS as well as pulmonary intervention such as lung protective ventilation is important in improving the outcome of these critically ill patients [21]. In addition, a better understanding and research on brain–lung interaction is necessary in TBI-associated with ARDS, especially with the presence of high ICP and its interaction with lung protective ventilation [22, 23].
We have not found any specific risk factors for developing ARDS. Prior studies reported several risk factors of ARDS in TBI including younger age [24], Hispanic and White race [25], male sex [3], and severity of TBI such as lower GCS at admission [8, 26], high ICP [27], and extent of brain injury in CT scan [8]. We chose relevant variables a priori to investigate if admission GCS < 5, Marshall CT score > 3, Head-AIS > 3, median ICP, and SIRS were independent risk factors for ARDS. None of these risk factors was significant in our analysis. However, caution needs to be taken in interpreting the data as there were many missing data and significant heterogeneity across the studies. Previously, multi-trauma patients with lung injury were a concern for increased risk of ARDS, however, studies have shown that ARDS may not develop as the result of a chest trauma or any kind of direct injury to the lungs [28, 29]. The overall prevalence of ARDS in multi-trauma population is unknown, current data varies from 6% to 51.7% [30–32]. In our cohort, the frequency of ARDS (22% vs. 20%) in patients with isolated TBI and those with multi-trauma was similar. We were unable to accurately assess other important risk factors such as baseline comorbidities [3], presence of acute pneumonia [33], sepsis [33], midline shift on CT [27], blood/platelet transfusion [34], certain ventilator settings [35] due to insufficient data.
The mechanism of TBI-associated ARDS is multifactorial. Primary mechanisms includes brain injury causing an activation of sympathoadrenal axis, and systemic inflammation response, especially during ICP crisis [7, 36]. The sympathetic surge in response to TBI and elevated ICP, driven by hypothalamic pituitary pathway, causes vasoconstriction of peripheral vessels, leading to elevated systemic arterial pressure and pulmonary hydrostatic pressure resulting in pulmonary edema [37]. The inflammatory response following TBI leads to increased inflammatory mediators causing end-organ damage, including ultrastructural changes in the type II pneumocytes in the lung and increased vascular permeability, which worsens pulmonary edema and ARDS [7, 36]. Furthermore, although controversial, lung protective ventilation strategy may have an adverse effect on cerebral perfusion causing potential secondary brain damage [22]. In a RCT comparing different CPP target for severe TBI, patients with a higher CPP goal (70 vs. 50 mmHg) were five times more likely to develop ARDS [35]. Although we were not able to investigate this important question regarding the interplay between the severity of TBI (such as ICP crisis) and ARDS, our study serves as a foundational work in reporting common occurrence of ARDS and the limitation of current literatures.
A strength of this study is the large number of patients included in the analysis, assessing the prevalence and neurological outcomes of ARDS in TBI patients. Our study has several limitations. First, our study showed substantial heterogeneity (I2 > 90%) in estimating the prevalence owing to variability in the included studies, representing the current state of literature on this topic. Similarly, we were unable to account for observer's bias between clinicians’ interpretation of imaging and ventilation data while making the diagnosis of ARDS. Included studies also have wide range of timeframe, we were not able to account for differences in incidence of ARDS due to changes in clinical practice and lung protective ventilation strategies overtime. In addition, some studies only included patients with isolated TBI while others included patients with multi-trauma, which introduces some degree of heterogeneity. Although we demonstrated there is no difference in prevalence of ARDS in patients with isolated TBI and multi-trauma with TBI, similarly to previous studies. Our study did not account for the variation in patient’s baseline comorbidities, ventilation strategies, and extent of chest injuries and their potential impact on mortality and development of ARDS. In addition, heterogeneity exists across studies reporting the timing of neurologic outcome, although majority of the neurologic outcomes were evaluated between 3 and 6 months post-TBI. Lastly, the assessment for risk factors for ARDS was limited by missing data with only few studies reporting the pre-specified risk factor variables.
Conclusion
In this meta-analysis, approximately one in five patients had ARDS shortly after TBI with the median time 3 days. The presence of ARDS was associated with worse neurological outcome and mortality in TBI. Further research on prevention and intervention strategy of TBI-associated ARDS is warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Funding
No funding was received for this study.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sosin DM, Sacks JJ, Smith SM. Head injury-associated deaths in the United States from 1979 to 1986. JAMA J Am Med Assoc. 1989;262(16):2251–2255. doi: 10.1001/jama.1989.03430160073033. [DOI] [PubMed] [Google Scholar]
- 2.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 3.Rincon F, Ghosh S, Dey S, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012;71(4):795–803. doi: 10.1227/NEU.0b013e3182672ae5. [DOI] [PubMed] [Google Scholar]
- 4.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest. 1999;116(1 Suppl):74S–82S. doi: 10.1378/chest.116.suppl_1.74S-a. [DOI] [PubMed] [Google Scholar]
- 5.Thiara S, Griesdale DE, Henderson WR, Sekhon MS. Effect of cerebral perfusion pressure on acute respiratory distress syndrome. Can J Neurol Sci. 2018;45(3):313–319. doi: 10.1017/cjn.2017.292. [DOI] [PubMed] [Google Scholar]
- 6.Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95(4):560–568. doi: 10.3171/jns.2001.95.4.0560. [DOI] [PubMed] [Google Scholar]
- 7.Rogers FB, Shackford SR, Trevisani GT, et al. Neurogenic pulmonary edema in fatal and nonfatal head injuries. J Trauma - Injury Infection Crit Care. 1995;39(5):860–868. doi: 10.1097/00005373-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Oddo M, Nduom E, Frangos S, et al. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery. 2010;67(2):338–344. doi: 10.1227/01.NEU.0000371979.48809.D9. [DOI] [PubMed] [Google Scholar]
- 9.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, Mañez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care. 2012;16(2):1–7. doi: 10.1186/cc11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuma M, El-Menyar A, Abdelrahman H, et al. Prehospital intubation in patients with isolated severe traumatic brain injury: a 4-Year. Crit Care Res Pract. 2014;2014:1–6. doi: 10.1155/2014/135986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 13.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA - J Am Med Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Marshall LF, Marshall SB, Klauber MR, Van Berkum CM, Eisenberg H, Jane JA, Luerssen TG, Marmarou AFM. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1981;9:287–292. [PubMed] [Google Scholar]
- 15.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow outcome scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Sidik K, Jonkman JN. A comparison of heterogeneity variance estimators in combining results of studies. Stat Med. 2007;26(9):1964–1981. doi: 10.1002/sim.2688. [DOI] [PubMed] [Google Scholar]
- 19.Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014 doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piek J, Chesnut RM, Marshall LF, et al. Extracranial complications of severe head injury. J Neurosurg. 1992;77(6):901–907. doi: 10.3171/jns.1992.77.6.0901. [DOI] [PubMed] [Google Scholar]
- 21.De Haro C, Martin-Loeches I, Torrents E, Artigas A. Acute respiratory distress syndrome: prevention and early recognition. Annal Intensive Care. 2013 doi: 10.1186/2110-5820-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Torre V, Badenes R, Corradi F, et al. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis. 2017;9(12):5368–5381. doi: 10.21037/jtd.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robba C, Poole DMM, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European society of intensive care medicine consensus. Intensive Care Med. 2020;46(12):2397–2410. doi: 10.1007/s00134-020-06283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(SUPPL. 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryb GE, Cooper C. Race/ethnicity and acute respiratory distress syndrome: a national trauma data bank study. J Natl Med Assoc. 2010;102(10):865–869. doi: 10.1016/S0027-9684(15)30700-8. [DOI] [PubMed] [Google Scholar]
- 26.Bratton SL, Davis RL. Acute lung injury in isolated traumatic brain injury. Neurosurgery. 1997;40(4):707–712. doi: 10.1097/00006123-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Lou M, Chen X, Wang K, Xue Y, Cui D, Xue F. Increased intracranial pressure is associated with the development of acute lung injury following severe traumatic brain injury. Clin Neurol Neurosurg. 2013;115(7):904–908. doi: 10.1016/j.clineuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55(1):106–111. doi: 10.1097/01.TA.0000071620.27375.BE. [DOI] [PubMed] [Google Scholar]
- 29.Salim A, Martin M, Brown C, et al. The presence of the adult respiratory distress syndrome does not worsen mortality or discharge disability in blunt trauma patients with severe traumatic brain injury. Injury. 2008;39(1):30–35. doi: 10.1016/j.injury.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Mp O, Ja K, A Y,, et al. Clinical predictors of early acute respiratory distress syndrome in trauma patients. Am J Surg. 2016;212(6):1096–1100. doi: 10.1016/j.amjsurg.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Haider T, Halat G, Heinz T, Hajdu S, Negrin LL. Thoracic trauma and acute respiratory distress syndrome in polytraumatized patients: a retrospective analysis. Minerva Anestesiol. 2017;83(10):1026–1033. doi: 10.23736/S0375-9393.17.11728-1. [DOI] [PubMed] [Google Scholar]
- 32.KJP van W, LPH L (2018) Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg Acute Care Open. 3(1) [DOI] [PMC free article] [PubMed]
- 33.Bronchard R, Albaladejo P, Brezac G, et al. Early onset pneumonia: risk factors and consequences in head trauma patients. Anesthesiology. 2004;100(2):234–239. doi: 10.1097/00000542-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Hendrickson CM, Howard BM, Kornblith LZ, et al. The acute respiratory distress syndrome following isolated severe traumatic brain injury. J Trauma Acute Care Surg. 2016;80:989–997. doi: 10.1097/TA.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27(10):2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Yildirim E, Kaptanoglu E, Ozisik K, et al. Ultrastructural changes in pneumocyte type II cells following traumatic brain injury in rats. Eur J Cardiothorac Surg. 2004;25:523–529. doi: 10.1016/j.ejcts.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Sarnoff SJ, Sarnoff LC. Neurohemodynamics of pulmonary edema. II. The role of sympathetic pathways in the elevation of pulmonary and stemic vascular pressures following the intracisternal injection of fibrin. Circulation. 1952;6(1):51–62. doi: 10.1161/01.CIR.6.1.51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Not applicable.