Abstract
Background: Thyroid cancer is a common malignancy whose detection has increased significantly in past decades. Most of the increased incidence is due to detection of early well-differentiated thyroid cancer, but the incidence of more advanced thyroid cancers has increased as well. Recent methodological advancements have allowed for a deep understanding of the molecular underpinnings of the various types of thyroid cancer.
Summary: Thyroid cancers harbor a high frequency of potential druggable molecular alterations, including the highest frequency of oncogenic driver kinase fusions seen across all solid tumors. Analyses of poorly differentiated and anaplastic thyroid carcinoma confirmed that these tumors develop from more well-differentiated follicular-derived thyroid cancers through acquired additional mutations. The recognition of driver genomic alterations in thyroid cancers not only predicts tumor phenotype but also now can inform treatment approaches.
Conclusions: Major progress in understanding the oncogenic molecular underpinnings across the array of thyroid cancers has led to considerable gains in gene-specific systemic therapies for many cancers. This article focuses on the molecular characteristics of aggressive follicular-derived thyroid cancers and medullary thyroid cancer and highlights advancements in treating thyroid cancer in the era of targeted therapy.
Keywords: BRAF, targeted therapy, thyroid cancer
Introduction
Thyroid cancer is a relatively common malignancy whose detection has increased more than 400% over the past four decades. Given the long-term survival of patients with thyroid cancer, the prevalence of thyroid cancer in the United States now exceeds 800,000 cases (0.2% of the U.S. population) (1,2). The increased incidence of thyroid cancer is thought to be due to an increase in the diagnosis of small, indolent papillary thyroid carcinomas (PTCs) that do not impact survival and have led to concerns for overtreatment (3–7). However, recent evidence shows that the incidence of more advanced thyroid cancers is also increasing, along with a small but significant increase in thyroid cancer mortality (8).
Follicular cell-derived thyroid cancers (FDTC) include PTC, follicular thyroid carcinoma (FTC), Hürthle cell carcinoma (HCC), poorly differentiated thyroid carcinoma (PDTC), and undifferentiated (anaplastic) thyroid carcinoma (ATC), whereas medullary thyroid carcinoma (MTC) is derived from the thyroid parafollicular C cell. FDTCs range from very indolent tumors (papillary thyroid microcarcinomas with a <1% disease-specific mortality) to some of the most lethal of all human cancers (ATCs with 98% disease-specific mortality) (9). This article focuses on the molecular characteristics of aggressive follicular-derived thyroid cancers and medullary thyroid cancer, particularly related to advancements in targeted therapies.
Follicular-Derived Thyroid Cancer
Recent methodological advancements have allowed for a deep understanding of the molecular underpinnings of thyroid cancer. Landmark work by The Cancer Genome Atlas (TCGA) program identified genotype–phenotype correlations in 496 PTCs, characterizing the majority of PTCs into BRAF- or RAS-like tumors based on transcriptional output (10). BRAF-like tumors include classical and infiltrative follicular variant of PTC (FVPTC), whereas RAS-like tumors include benign follicular adenomas, noninvasive follicular tumors with papillary-like nuclear features (NIFTP), FTC, and the encapsulated, invasive FVPTC (11). The findings by TCGA set the stage for reclassifying thyroid cancers into molecular subtypes that predict their natural history. With follicular histologies, Yoo et al. found that expression profiles of minimally invasive FTC, benign follicular adenoma, and encapsulated FVPTC were similar, suggesting that reclassification of tumors based on genotype may be more useful clinically (12). Subsequent comprehensive analyses of PDTCs and ATCs confirmed that these tumors develop from more well-differentiated FDTCs through the accumulation of additional mutations, even when no well-differentiated precursor is noted on pathology (13,14).
HCC is now considered distinct from FTC based on two landmark studies of genetic drivers of HCC (15–17). Specifically, many HCCs exhibit widespread chromosomal loss leading to near-haploid state, recurrent mitochondrial DNA mutations affecting complex I of the mitochondrial electron transport chain, and recurrent nuclear DNA mutations and kinase fusions. The recognition of driver genomic alterations in FDTC not only predicts tumor phenotype but also may inform treatment approaches. In fact, recent next-generation sequencing (NGS) of >10,000 cancers revealed that 61% of thyroid cancers harbored potentially druggable genomic alterations, which was the second highest rate of actionable alterations seen across all tumor types studied (18).
Radioiodine-Resistant Differentiated Thyroid Cancer
Radioactive iodine (RAI, 131I) is the most common adjuvant therapy for FDTC after thyroidectomy. Unfortunately, many patients with advanced thyroid cancer have de novo or develop resistance to 131I. Although the term radioiodine-resistant (RAIR) differentiated thyroid cancer (DTC) is variably defined, the overall prognosis of patients with unresectable and/or metastatic RAIR-DTC is much worse compared with those with metastatic FDTC that is RAI-sensitive (10-year survival of <20% vs. 92%) (19–21).
Two recent phase III trials, DECISION and SELECT, established nonselective multikinase inhibitors (MKIs) as the standard of care for patients with progressive RAIR-DTC (Table 1). In DECISION, 417 patients with progressive RAIR-DTC were randomized to receive sorafenib versus placebo (22,23). In SELECT, 392 patients with progressive RAIR-DTC were randomized to receive lenvatinib versus placebo. Both sorafenib and lenvatinib are oral small-molecule MKIs that inhibit several shared kinases, including VEGFR1–3, RET, KIT, and PDGFR. The primary endpoint of both trials was progression-free survival (PFS). With sorafenib, the median PFS was 10.8 months versus 5.8 months with placebo, and the objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST) was 12.2%. With lenvatinib, the median PFS was 18.7 months versus 3.7 months with placebo, and the ORR was 64.8%. Although neither trial demonstrated an overall survival (OS) advantage, this may have been due to the trial designs incorporating crossover from placebo to active treatment in patients randomized to placebo at the time of disease progression. Adverse events (AEs) related to sorafenib and lenvatinib are primarily attributed to VEGFR2 inhibition and include hand–foot skin reaction, hypertension, diarrhea, anorexia, weight loss, and fatigue. Treatment-related AEs led to dose reductions in 64.3% and discontinuation in 18.8% of participants in DECISION, and 67.8% and 14.2% in SELECT, respectively. While significant improvements in PFS were seen in both trials, toxicity and impact on quality of life (QoL) are a concern, creating challenges for patients and clinicians, particularly with regard to deciding when to initiate MKI therapy. In clinical practice, the need to initiate MKI therapy in patients who are symptomatic, or who have a significant burden of rapidly progressive disease is clear. However, in patients with less aggressive disease, it can be difficult to decide in favor of ongoing active surveillance versus starting treatment despite impact of treatment-related AEs on QoL (24,25).
Table 1.
Registrational Trials in Nonselective Inhibitors for Advanced Thyroid Cancers
| Drug | Target(s) | Trial | Patient population | Trial design | Primary outcome |
|---|---|---|---|---|---|
| RAIR-DTC | |||||
| Sorafenib | VEGFR1–3, RET, RAF, PDGFRβ | DECISION (22) | 417 Patients with progressive RAIR-DTC | Placebo-controlled phase III | Median PFS = 10.8 months (sorafenib) vs. 5.8 months (placebo) |
| Lenvatinib | VEGFR1–3, FGFR1–4, PDGFRα, RET, KIT | SELECT (23) | 392 Patients with progressive RAIR-DTC | Placebo-controlled phase III | Median PFS = 18.3 months (lenvatinib) vs. 3.6 months (placebo) |
| MTC | |||||
| Vandetanib | VEGFR1–3, RET, EGFR, BRK, TIE2, EPHR, SRC | ZETA (63) | 331 Patients with RECIST measurable MTC | Placebo-controlled phase III | Median PFS = 30.5 months (est.) (vandetanib) vs. 19.3 months (placebo) |
| Cabozantinib | VEGFR1–3, RET, MET, KIT, TRKB, FLT-3 | EXAM (65) | 330 Patients with progressive MTC | Placebo-controlled phase III | Median PFS = 11.2 months (cabozantinib) vs. 4.0 months (placebo) |
MTC, medullary thyroid carcinoma; PFS, progression-free survival; RAIR-DTC, radioiodine-resistant differentiated thyroid cancer; RECIST, Response Evaluation Criteria in Solid Tumors.
Fusion-Driven Thyroid Cancer
TCGA analysis showed that thyroid cancers harbor the highest frequency of oncogenic driver kinase fusions of all solid tumors, at a rate of 12% (26). Recent evaluation of fusion-driven thyroid cancers identified typical histological features, including multinodular growth, prominent fibrosis, and lymphovascular spread (27). This triad of features in combination with the absence of BRAFV600E positivity suggests the presence of a driver kinase fusion, many of which are now druggable. Two unique aspects of oncogenic fusions have particular clinical relevance in thyroid cancer: (i) Oncogene fusions are especially common in pediatric thyroid cancers, seen in 56% thyroid cancers diagnosed in patients up to 20 years of age; and (ii) Most kinase genes involved in rearrangements have multiple different 5′ fusion partners, and thus, NGS assays used to interrogate thyroid cancers for actionable alterations in treatment decision-making should have the capability of detecting all the many known and as yet unidentified fusion partners (28).
The neurotrophic tyrosine kinase receptor genes (NTRK1–3) encode for TRK kinases that play a role in neuronal development. NTRK genes are not expressed in normal thyroid follicular epithelium. However, gene rearrangements with 5′ fusion partners and NTRK lead to expression of the fusion protein and constitutive activation of TRK kinases (29). Larotrectinib, a TRK-specific oral small-molecule inhibitor, was the first small-molecule inhibitor that gained tumor-agnostic Food and Drug Administration (FDA) approval in oncology (Table 2). In a pooled analysis of three phase I and II trials enrolling a total of 159 patients with various NTRK fusion-positive cancers, thyroid cancer was the second most common adult cancer type enrolled (30). Of the 24 thyroid cancer patients enrolled, 19 (79%) experienced an objective response by RECIST v1.1, and the median duration of response (DoR) was not reached at a median follow-up of 25.9 months. Larotrectinib demonstrated a favorable toxicity profile with grade 3 or higher treatment-related AEs being uncommon. On-target AEs attributed to TRK inhibition, including paresthesias and dizziness, were generally manageable (30). Entrectinib, designed to inhibit TRK, ROS1, and ALK kinases, soon followed in approval (31,32). As seen in other oncology settings in which potent gene-specific therapies have been developed, acquired resistance to TRK inhibition has emerged, a common theme of which is acquired mutation in the kinase domain blocking drug activity. Second-generation therapies, including selitrectinib and repotrectinib, developed to maintain potency against multiple acquired TRK kinase resistance mutations, are now in clinic trials.
Table 2.
Registrational Trials in Gene-Specific Inhibitors for Advanced Thyroid Cancers
| Drug | Target(s) | Trial | Patient population | Trial design | Primary outcome |
|---|---|---|---|---|---|
| RAIR-DTC | |||||
| Larotrectinib | TRK A, B, C | SCOUT and NAVIGATE (30) | 19 Patients with NTRK fusion-positive RECIST measurable diseasea | Pooled results from three phase I/II trials | ORR = 79% |
| Entrectinib | TRK A, B, C, ROS1, ALK | ALKA, STARTRK 1&2 (31) | 5 Patients with NTRK fusion-positive RECIST measurable disease | Pooled results from three phase I/II trials | ORR = 20% |
| Selpercatinib | RET | LIBRETTO-001 (36) | 19 Patients with RET fusion-positive RECIST measurable diseaseb | Phase I/II | ORR = 79% |
| Pralsetinib | RET | ARROWc (37) | |||
| MTC | |||||
| Selpercatinib | RET | LIBRETTO-001 (36) | 55 Patients with prior vandetanib and/or cabozantinib and 88 patients with no prior vandetanib or cabozantinib, RECIST measurable | Phase I/II | ORR = 69% (prior vandetanib and/or cabozantinib); ORR = 73% (no prior vandetanib or cabozantinib) |
| Pralsetinib | RET | ARROWc (37) | |||
Included seven patients with ATC.
Included two patients with ATC.
Results not published by the time of this writing.
ATC, anaplastic thyroid carcinoma; NTRK, neurotrophic tyrosine kinase receptor; ORR, objective response rate; RET, REarranged in Transfection.
REarranged in Transfection (RET) is the most commonly rearranged oncogene in thyroid cancer. Less than 10% of PTCs in the TCGA analysis harbored RET fusions, while RET fusions are seen in ∼30% of PTCs occurring in children and young adults and are even more common in radiation-induced PTCs. CCDC6 (also known as RET/PTC1) and NCOA4 (also known as RET/PTC3) are the two most common 5′ RET fusion partners (10,33,34). However, in thyroid cancer, more than 20 different 5′ fusion partners with RET have now been identified. The first two RET-specific inhibitors, selpercatinib and pralsetinib, have now completed first-in-human clinical studies. Selpercatinib and pralsetinib were designed to potently and specifically inhibit RET while minimizing off target toxicity from inhibition of other kinases, including VEGFR2. Both drugs inhibit the wild-type RET kinase activated in RET fusion-driven cancers, as well as the array of RET mutations seen in MTC, including the multiple endocrine neoplasia type 2A (MEN2A)-related RET V804M/T mutations that have also arisen as acquired resistance mutations in RET-driven cancers treated with MKIs (35).
Selpercatinib was studied in LIBRETTO-001, an open-label phase I/II trial in patients with RET-driven nonsmall cell lung and thyroid cancers (36). A total of 531 patients were enrolled across all cohorts. Nineteen patients with previously treated RET fusion-positive advanced thyroid cancer were enrolled, including two patients with ATC. The ORR was 79%, with a median DoR and PFS of 18.4 and 20.1 months, respectively. Activity in MTC is detailed below. The safety profile was consistent with the RET-specific drug design, with most AEs being grade 1 or 2 and reversible. The most common treatment-related grade 3/4 AEs included hypertension, transaminitis, and diarrhea. Thirty percent of patients had dose reduction, and only 2% of patients had discontinuation of therapy due to treatment-related AEs. Based on LIBRETTO-001 outcomes, selpercatinib was the first RET-specific inhibitor to gain a line-agnostic approval by the FDA for RET-driven nonsmall cell lung and thyroid cancers.
Although only preliminary results from the pralsetinib phase I/II ARROW trial are available at the time of this writing, these promising data led to recent FDA approval for both RET-driven nonsmall cell lung and thyroid cancers (37). As seen with NTRK-specific therapy, acquired resistance to selpercatinib and pralsetinib has emerged, as is described below.
ALK fusions are less commonly seen in FDTC. To the best of our knowledge, ALK-driven thyroid cancer patients have not been enrolled in any ALK inhibitor clinical trials; however, case reports detail efficacy of ALK inhibitor therapy in patients with ALK fusion-positive RAIR-DTC. Remarkable efficacy has even been described in a patient with ALK fusion-positive ATC (38–40).
PAX8-PPARG gene fusions have been identified in approximately one-third of FTCs and are thought to be oncogenic (41). While identification of this gene fusion may aid in the cytological diagnosis of FTC, targeted therapy against the PAX8-PPAR-γ fusion protein has not yet emerged as a viable treatment approach in patients with FTC.
Mitogen-Activated Protein Kinase-Driven DTCs
Activating BRAFV600E and N/H/K RAS mutations are the most common potentially druggable alterations in PTCs and FTCs, respectively. In TCGA analysis, 60% of PTCs were found to harbor BRAFV600E mutations (10). These tumors exhibit robust mitogen-activated protein kinase (MAPK) activation with high ERK transcriptional output promoting cell cycle progression, proliferation, and survival. Vemurafenib and other MAPK pathway inhibitors were initially developed to treat BRAFV600E-mutant melanoma (42). Success in melanoma prompted phase II study of vemurafenib in progressive BRAFV600E-mutant RAIR PTC (43). Twenty-six treatment-naive patients were enrolled in cohort 1, and 25 patients previously treated with a VEGFR MKI were enrolled in cohort 2. ORRs were 38.5% and 27.3%, and the median PFS was 18.2 and 8.9 months, respectively. BRAF inhibition without concomitant MEK inhibition can lead to the development of cutaneous squamous cell carcinomas, which emerged on treatment in 27% and 20% of patients in cohorts 1 and 2, respectively. Other grade 3 or higher AEs were uncommon, including lymphopenia and transaminitis. The BRAF inhibitor, dabrafenib, alone or in combination with the MEK inhibitor, trametinib, has also been studied in a similar patient population [NCT01723202]. To date, no large-scale definitive study evaluating the role of BRAF inhibition in RAIR BRAFV600E-mutant PTC has been reported. As a result, this approach has not gained regulatory approval, nor become an established first-line gene-specific treatment in advanced BRAFV600E-mutant PTC.
RAS mutations are found in ∼30% of all human cancers. Yoo et al. found activating H/N/K RAS mutations in FVPTC, minimally invasive FTC, and follicular adenomas at rates of 48%, 50%, and 24%, respectively (12). RAS genes encode for small GTPase proteins that act as molecular switches conducting MAPK signaling and thus are attractive targets for therapeutic intervention. However, the development of conventional small-molecule inhibitors of RAS isoforms has been challenged by their lack of large pockets for drug binding and intracellular protein–protein interactions that are difficult to overcome (44). Novel RAS inhibitory compounds are under active investigation, but have yet to make headway in thyroid cancer (45).
Blockade of the MAPK signaling cascade is of interest for the direct treatment of BRAF- and RAS-driven RAIR-DTCs. Upregulation of the pathway is one mechanism underlying thyroid cancer dedifferentiation, correlating with loss of expression of the sodium/iodine symporter and other thyroid hormone biosynthesis genes (46). Therefore, treatment with MAPK pathway inhibitors may reverse the loss of 131I uptake in RAIR disease and re-sensitize these tumors to treatment with 131I. This redifferentiation strategy is particularly appealing in low volume, slow growing metastatic disease for which MKI treatment seems overly aggressive. MAPK inhibition need only be prescribed for weeks at a time before 131I therapy and, if successful, could be repeated at a future time.
A proof-of-principle study by Ho et al. established the potential for MAPK pathway blockade to reverse loss of 131I uptake by pretreating patients with RAIR-DTC with the MEK inhibitor, selumetinib (47). Of 20 evaluable patients enrolled, 8 met a prespecified dosimetry threshold by 124I positron emission tomography (PET) indicating redifferentation. All five patients harboring NRAS mutations met the dosimetry threshold for treatment compared with only one of nine patients with BRAFV600E mutations. These results led to ASTRA, a randomized phase III trial of selumetinib versus placebo followed by 131I treatment in 233 patients at high risk of treatment failure based on surgical outcomes, with a primary endpoint of complete remission at 18 months [NCT02393690] (48). Patients were enrolled regardless of mutation status, which may be one reason why the trial did not meet its primary endpoint. A third trial of pretreatment with selumetinib versus placebo plus 131I treatment in patients with RAI-avid recurrent/metastatic thyroid cancers sponsored by the International Thyroid Oncology Group has completed enrollment [NCT02393690].
Direct BRAFV600E blockade with dabrafenib or vemurafenib for redifferentiation in BRAF-mutant RAIR PTC has also been evaluated in two small studies (49,50). Pretreatment with both drugs reversed 131I insensitivity in a subset of patients enrolled. These patients were then treated with 131I and some experienced regression of measurable disease. Taken together, these redifferentiation studies show promise, but definitive evidence of clinical benefit is lacking.
Medullary Thyroid Carcinoma
Medullary thyroid carcinoma (MTC) is a primary neoplasm of the thyroid gland arising from parafollicular or C cells, which are developmentally, phenotypically, and functionally distinct from thyrocytes. MTCs account for <5% of thyroid cancer diagnoses, but ∼13% of thyroid cancer deaths (51). Patients presenting with early-stage disease without nodal metastasis have a >80% chance of cure with surgery alone, whereas lymph node metastasis at presentation significantly reduces the rate of cure (52). For patients with recurrent/metastatic MTC, the median 10-year disease-specific survival is 44%.
The majority of MTCs are driven by a mutation in RET. Approximately 7% of MTCs diagnosed as an isolated nodule occur in patients with hereditary MEN2A or MEN2B due to a germline RET mutation. However, screening of family members of probands with germline RET mutation uncovers many other cases. Therefore, 25% of all MTCs are due to germline RET mutations. Approximately 60% of patients with sporadic MTC harbor somatic RET mutations (53). In RET wild-type MTC, somatic mutations in RAS family genes are the next most common potentially actionable genomic alterations, occurring in 10% to 15% of sporadic MTCs (53–56).
Genotype–phenotype correlations in MTC are well characterized. In MEN2A, more than 95% of patients harbor germline RET mutations in the extracellular cysteine-rich domain, especially at codon 634. In MEN2B, the germline RET M918T mutation predominates. Specific germline RET mutations correlate with both the age of onset of MTC in carriers, as well as the aggressiveness of the disease. For example, RET M918T carries the highest risk of aggressive MTC, according to the American Thyroid Association (ATA) guidelines (57). Children harboring this mutation are at risk for developing MTC before one year of age and should undergo surgery as soon as the diagnosis is made. RET C634 mutations fall into the ATA higher risk category. Children with mutations at this codon can develop MTC in the first few years of life, and thus should undergo prophylactic thyroidectomy before five years of age. Specific germline RET mutations influence of prevalence of other components of MEN2, including pheochromocytoma, primary hyperparathyroidism, and cutaneous lichen amyloidosis. Primary hyperparathyroidism does not occur in MEN2B, but carriers do have unique somatic characteristics, including typical facies, marfanoid body habitus, mucosal neuromas of the tongue, lips, and eyelids, and ganglioneuromatosis of the gastrointestinal tract (57).
Genotype–phenotype correlation is also seen in sporadic MTC. For example, RET M918T is the most frequent somatic mutation in sporadic MTC and correlates with more advanced stage at diagnosis, distant metastatic disease, and death, compared to patients without this somatic mutation (58,59). In contrast, RAS mutations are generally associated with a less aggressive MTC phenotype and better overall prognosis (56,60,61).
Vandetanib, an MKI originally developed to inhibit VEGFR2 and VEGFR3, and EGFR, was also found to inhibit the RET tyrosine kinase, leading to the first clinical trials of an MKI in MTC (62). Promising activity seen in the trial of vandetanib in hereditary MTC subsequently led to the international double-blind phase III ZETA trial (63,64). ZETA enrolled 331 patients with unresectable or metastatic MTC. Participants were randomized to receive vandetanib or placebo until disease progression. Upon disease progression, participants were unblinded, and those on placebo were offered crossover to open-label vandetanib. The primary endpoint was PFS, the median of which not been reached at a median follow-up of 24 months in the vandetanib group, with modeling projecting the median would approximate 30.5 months, compared with 19.3 months with placebo. Of note, disease progression was not required for enrollment, likely explaining the long median PFS in the placebo group. ORR with vandetanib was 45%. AEs, including diarrhea, rash, nausea, and hypertension, were common and led to dose reduction and discontinuation in 35% and 12% of participants, respectively. A less common but potentially serious AE was QTc prolongation, and five deaths on the vandetanib arm were attributed to AEs.
Cabozantinib is a similar MKI targeting VEGFR2, MET, and RET. The EXAM trial randomized to cabozantinib or placebo (65). Three hundred thirty patients with unresectable or metastatic MTC with disease progression per RECIST at study entry were enrolled. This study did not incorporate crossover from placebo to active drug upon progression. In EXAM, the PFS was prolonged from just 4.0 months with placebo to 11.2 months with cabozantinib, while the ORR was 28%. Of note, the median PFS times observed in both the placebo and cabozantinib arms are shorter than those observed in either arm of the ZETA trial, likely explained by differences in patient eligibility. While no significant difference in OS was observed in EXAM, exploratory analysis did reveal an OS benefit with cabozantinib in the subset of participants with RET M918T mutation-positive tumors (median OS = 44.3 months vs. 18.9 months, respectively) (66). Additional subset analyses in both trials demonstrated benefit regardless of the presence or absence of RET mutations, suggesting that efficacy of either drug cannot be attributed to RET inhibition alone. Typical of MKIs, AEs were common with cabozantinib, with grade 3 or higher AEs reported in 69% of participants, the most common of which were diarrhea, hand–foot syndrome, and fatigue. AEs attributed to VEGFR inhibition included hypertension and hemorrhage, observed in 25% of participants or more. Less common side effects included fistula formation and gastrointestinal perforation. Dose reduction for toxicity was required in 79% of cabozantinib-treated patients, and 16% had discontinuation of treatment due to toxicity.
Activating RET mutations and fusions emerged as attractive targets for the development of selective RET inhibitors given the toxicity and disappointing clinical activity of less selective MKIs (35,67). As noted above, two new small-molecule inhibitors, selpercatinib and pralsetinib, were developed as highly selective and potent RET inhibitors with minimal off target activity (67).
LIBRETTO-001 enrolled patients with RET-driven cancers into a phase I trial of selpercatinib, followed by expansion phase II cohorts in NSCLC, thyroid cancers, and other cancers harboring activating RET alterations (36,68). The three thyroid cancer cohorts were: (i) patients with RET-mutant MTC previously treated with vandetanib and/or cabozantinib (n = 55); (ii) patients with RET-mutant MTC not previously treated with vandetanib or cabozantinib (n = 88); and (iii) patients with RET fusion-positive previously treated advanced FDTC (n = 19), described above. In the two MTC thyroid cohorts, ORRs were 69% and 73%, respectively. Responses were durable, with PFS rates at one year of 82% and 92%, respectively. Responses were seen across all RET mutations. Consistent with the RET specificity of the drug, fewer participants experienced AEs than would be expected with MKIs. AEs were primarily grade 1 and 2, with the most common grade 3 or higher being hypertension, transaminitis, hyponatremia, and diarrhea. Of all participants in the trial, 30% required dose reduction and only 2% had discontinuation of selpercatinib due to toxicity. Results of patient-reported outcomes in the MTC cohorts paralleled efficacy and safety outcomes (69). Based on LIBRETTO-001 outcomes, selpercatinib is now FDA approved for adult and pediatric patients aged ≥12 years with advanced or metastatic RET-mutant MTC and RET fusion-positive thyroid cancer, and for adults with RET fusion-positive NSCLC, all of which are agnostic to the line of therapy. Importantly, an international randomized trial, LIBRETTO-531, comparing selpercatinib with physician's choice of vandetanib or cabozantinib in patients with advanced progressive RET-mutant MTC is underway [NCT04211337].
The ARROW trial similarly investigated pralsetinib in RET-altered cancers. Preliminary results presented for two MTC cohorts enrolled in the phase II portion of the trial: (i) RET-mutant MTC with prior vandetanib and/or cabozantinib (n = 53); and (ii) RET-mutant MTC with no prior systemic therapy (n = 19) were promising enough to lead to recent FDA approval (37).
While these first-generation RET-specific inhibitors are practice-changing, acquired resistance has now emerged in selpercatinib- and pralsetinib-treated NSCLC and MTC (70–72). The first report detailed two patients with RET fusion-positive NSCLC and one patient with germline RET M918T MTC, who initially responded to RET-specific inhibition, only to subsequently progress on therapy. At the time of progression, acquired RET G810 mutations in the kinase solvent front were found. Based on structural modeling, alteration of this glycine residue appears to sterically hinder drug binding at the kinase domain. Further study of acquired resistance to RET-specific inhibition has revealed acquired MET and KRAS amplification as additional mechanisms of resistance. These findings are already leading to new clinical trials for patients with RET-driven malignancy, including a first-in-human trial of TPX-0046, a small-molecule inhibitor designed to inhibit the SRC kinase as well as RET, including RET G810 mutations [NCT04161391].
Our Approach
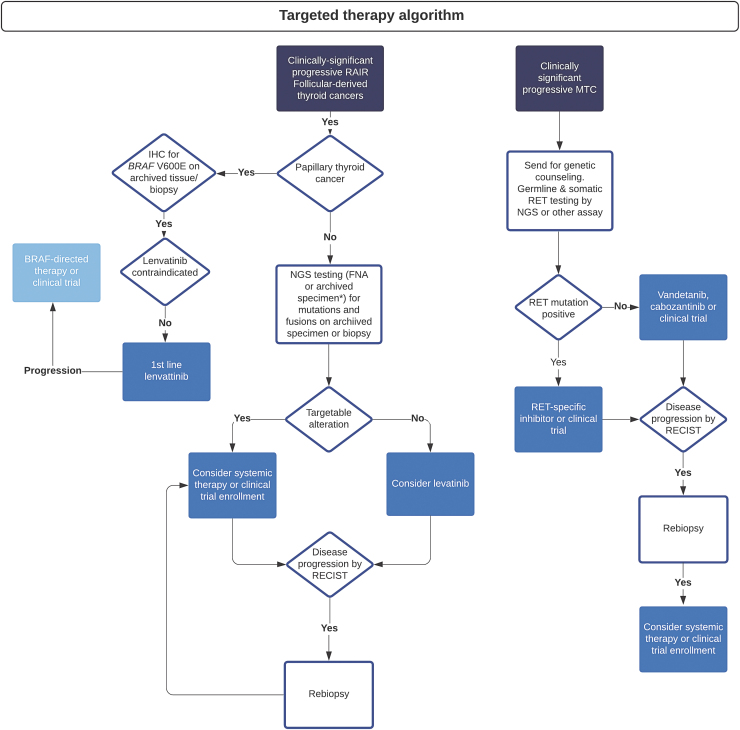
Molecular profiling optimizes clinical management with systemic therapy in patients with advanced thyroid cancers and is consistent with current National Comprehensive Cancer Network guidelines (73). We advocate for an algorithmic approach to testing in all patients with advanced thyroid cancer in need of systemic treatment (Fig. 1). In FDTC, BRAFV600E can first be excluded by immunohistochemistry, followed by more extensive testing in BRAF wild-type tumors (27). Like other institutions, we have developed a comprehensive NGS platform for identifying germline and somatic alterations relevant to prognosis and treatment in multiple tumor types, and we find this to be an efficient upfront test for most cases of advanced thyroid cancer. In addition, several validated commercial platforms are available, predominantly for fine-needle aspiration specimens (74), but also validated on biopsy tissues (75).
FIG. 1.
Suggested algorithm for approach to patients with clinically significant advanced thyroid cancers. IHC, immunohistochemistry; NGS, next-generation sequencing; RAIR, radioiodine resistant. Color images are available online.
Given the prominent role oncogenic fusions play in FDTC, and the numerous different 5′ fusion partners found with RET, NTRK 1/3, and ALK, our NGS testing incorporates a robust method for detecting fusions. This involves Anchored Multiplex PCR (AMP™) that permits amplification of both known and unknown genomic sequences of interest, allowing for detection of both known and novel gene fusions (76). Because most targetable molecular alterations are oncogenic drivers present in the primary tumor and metastatic disease, archival specimens are often adequate for initial testing. If inadequate, biopsy of locoregionally recurrent or metastatic disease specifically for testing is indicated given the potential impact on treatment decisions. Re-biopsy should also be considered upon disease progression on targeted therapy, to evaluate for potentially targetable mechanisms of acquired resistance (77). Liquid biopsy by assaying circulating tumor (ct) DNA is of interest in advanced thyroid cancer, although challenging given the lower levels of circulating substrates. The utility of ctDNA is under investigation and may be particularly useful in detecting the emergence of acquired resistance in patients on gene-specific therapy (78,79).
In addition to testing for oncogenic point mutations, insertions and deletions, gene amplifications, and fusions, biomarkers related to immunotherapy, such as tumor mutational burden (TMB) and programmed death-ligand 1 (PD-L1) expression, are becoming increasingly relevant and are incorporated into many molecular platforms in use or available as adjuvant testing. We have recently incorporated TMB as a subheading in our standard NGS reports, with PD-L1 testing in thyroid still under investigation, particularly in regard to ATC (80). Identifying the best NGS platform for a given patient can be challenging and any specific NGS platform recommendation is outside the scope of this review. However, best practice guidelines are available (81).
We also believe that offering all thyroid cancer patients treatment within clinical trials is a priority whenever possible. When clinical trial participation is not possible, our multidisciplinary team personalizes recommendations based on multiple factors, including histological diagnosis, tumor burden and pace of disease progression, tumor genotype, and patient preference. In RAI-refractory DTC, systemic therapy is not recommended for patients with increasing thyroglobulin alone but is considered for patients with disease progression by RECIST. Given the frequency of brain metastases in patients with advanced RAI-refractory DTC, brain magnetic resonance imaging (MRI) is usually incorporated into baseline imaging. RET- or NTRK-directed therapies are considered as possible first-line options in these fusion-driven cancers based on the clinical efficacy of these agents, favorable toxicity profiles, and line agnostic FDA approvals. In the absence of a targetable fusion, we favor lenvatinib as the standard first-line MKI given the high ORR and durable PFS benefit seen in SELECT.
The decision of when to start kinase inhibitor therapy can be challenging, especially with the potential for MKI toxicities impacting patient QoL. Some practitioners may wish to hold off on starting an MKI until symptoms from disease progression are impending; however, recent post hoc data from SELECT suggest that clinical benefit may be compromised when therapy is initiated late in the course of disease (82,83). Thus, we generally favor initiating lenvatinib in patients with progressive RAI-refractory DTC earlier in the disease process, before the imminent development of symptoms. We use the FDA-approved starting dose in most patients, and when dose holds are needed for toxicity, we keep the holding time as short as possible. In patients with BRAFV600E-mutant RAI-refractory DTC, we do not favor adopting BRAF-directed therapy as first-line treatment, as the phase II data suggest that BRAF-directed activity is not as robust as lenvatinib activity in these patients. Exceptions include cases in which lenvatinib is contraindicated due to comorbid conditions. BRAF-directed therapy in a clinical trial or off label is a reasonable second-line approach for BRAFV600E-mutant patients who discontinue lenvatinib due to disease progression or treatment-related AEs. An additional second-line option is now available based on the COSMIC-311 phase III trial demonstrating a significant decrease risk of disease progression and death with cabozantinib compared with placebo in patients with previously treated RAI-refractory DTC [NCT03690388].
Many patients with RAI-refractory DTC have low volume, slow-growing disease. It can be difficult to justify committing such patients to ongoing systemic therapy for months to years, even if gene-specific therapy is available. When alterations are present in the MAPK pathway, our multidisciplinary team considers redifferentiation strategies, preferably in a clinical trial, with the hope to elicit tumor response as well as to delay the need to initiate more intensive systemic therapy.
In patients with advanced MTC, if not already done, patients are referred for genetic counseling and germline RET testing. In patients with sporadic disease, we use our in-house NGS platform for somatic RET testing, which also can detect RAS-mutant MTC in RET-wild-type tumors. Monitoring calcitonin and carcinoembryonic antigen (CEA) doubling times is useful in estimating prognosis. Our standard imaging includes computed tomography plus MRI protocoled for optimal imaging of the liver. Brain MRI is also frequently performed. Treatment in the case of rising tumor markers alone is not indicated at present. However, when patients have RECIST-measurable disease that is progressive within one year or less, or when patients have bulky and/or symptomatic disease at presentation, systemic therapy is generally indicated. In RET-driven MTC, two new phase III trials are underway comparing, respectively, selpercatinib and pralsetinib, to physician's choice vandetanib or cabozantinib [NCT04211337; NCT04760288]. Both studies ask not only the question of which approach, gene-specific or MKI therapy, offers the best clinical outcomes for patients with RET-mutant MTC but also should help establish the optimal sequence of treatment.
Conclusions
With improved multidisciplinary approaches to the treatment of advanced thyroid cancers, patient outcomes are improving. Major progress in understanding the oncogenic molecular underpinnings across the array of thyroid cancers has led to considerable gains in gene-specific systemic therapies for many cancers. It is now clear that there is a compelling need for NGS testing in patients with advanced thyroid cancer in need of systemic therapy to identify those patients for whom gene-specific therapy may be available.
The presence of distant metastatic disease is often sufficient to dictate the initiation of systemic treatment. However, many patients with metastatic FDTC and MTC are asymptomatic with relatively slowly progressive disease. Decisions regarding when to start treatment in these patients are difficult. If a highly effective treatment with minimal toxicity is available, then earlier treatment, such as when distant disease is first recognized, may offer the greatest clinical benefit. Conversely for treatments with high toxicity, striking a balance between benefit and risk is more challenging.
We remain in our infancy in terms of deciding who needs treatment for advanced disease and what variables will best inform that decision. For example, patients with DTC whose distant metastases are FDG-PET positive have a worse outcome than those whose tumors are FDG-PET negative (2). The presence of TERT promoter mutations in addition to BRAF or RAS predicts a worse outcome (84). Moreover, rapid calcitonin and/or CEA doubling times in MTC are associated with a worse prognosis (85). How these additional variables should influence the decision to treat based on clinical findings remains uncertain.
Further future directions will hopefully include definitive studies of redifferentiation, neoadjuvant therapy in patient with bulky neck disease, immunotherapy particularly in ATC, and the optimal sequencing of systemic therapy in all patients with advanced thyroid cancer now that multiple treatment options are available for most. More study is needed, and the principle of primum non nocere remains an important principle in the field.
Authors' Contributions
All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
C.C.L.: Drafting, review, revision, and submission of the article.
P.M.S. and G.H.D.: Drafting, review, and revision of the article.
L.J.W.: Drafting, review, and revision of the article, and concept.
Author Disclosure Statement
C.C.L., P.M.S., G.H.D.: No competing financial interests exist. L.J.W.: Consulting fees from Ayala Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Blueprint Medicines, Cue Biopharma, Eli Lilly, Eisai, Genentech USA, Loxo Oncology, Merck.
Funding Information
This study was supported by NIH/NCI R37 CA231957 (C.C.L.) and NIH/NCI 1P01 CA240239-01 (L.J.W. and P.M.S.).
References
- 1. Surveillance Epidemiology and End Results. Available at http://seer.cancer.gov/ (accessed January 26, 2016).
- 2. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, Tuttle RM, Drucker W, Larson SM. 2006. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 91:498–505. [DOI] [PubMed] [Google Scholar]
- 3. Brito JP, Ito Y, Miyauchi A, Tuttle RM. 2016. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 26:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. 2016. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg 40:523–528. [DOI] [PubMed] [Google Scholar]
- 5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahn HS, Kim HJ, Welch HG. 2014. Korea's thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 371:1765–1767. [DOI] [PubMed] [Google Scholar]
- 7. Welch HG, Black WC. 2010. Overdiagnosis in cancer. J Natl Cancer Inst 102:605–613. [DOI] [PubMed] [Google Scholar]
- 8. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. 2017. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, Williams MD, Gunn GB, Hofmann MC, Cote G, Sperling J, Gross ND, Sturgis EM, Goepfert RP, Lai SY, Cabanillas ME, Zafereo M. 2020. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000–2019. JAMA Oncol 6:1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cancer Genome Atlas Research N 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asa SL, Giordano TJ, LiVolsi VA. 2015. Implications of the TCGA genomic characterization of papillary thyroid carcinoma for thyroid pathology: does follicular variant papillary thyroid carcinoma exist? Thyroid 25:1–2. [DOI] [PubMed] [Google Scholar]
- 12. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, Song YS, Cho SW, Won JK, Shin JY, Park do J, Kim JI, Lee KE, Park YJ, Seo JS. 2016. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet 12:e1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. 2016. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. 2018. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gopal RK, Kubler K, Calvo SE, Polak P, Livitz D, Rosebrock D, Sadow PM, Campbell B, Donovan SE, Amin S, Gigliotti BJ, Grabarek Z, Hess JM, Stewart C, Braunstein LZ, Arndt PF, Mordecai S, Shih AR, Chaves F, Zhan T, Lubitz CC, Kim J, Iafrate AJ, Wirth L, Parangi S, Leshchiner I, Daniels GH, Mootha VK, Dias-Santagata D, Getz G, McFadden DG. 2018. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in Hurthle cell carcinoma. Cancer Cell 34:242–255.e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bychkov A 2020. WHO thyroid tumor classification. Available at http://www.pathologyoutlines.com/topic/thyroidwho.html (accessed on February 14, 2020).
- 17. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA. 2018. Integrated genomic analysis of Hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 34:256–270.e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. 2017. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, Dillehay G, Draganescu C, Flux G, Fuhrer D, Giovanella L, Greenspan B, Luster M, Muylle K, Smit JWA, Van Nostrand D, Verburg FA, Hegedus L. 2019. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 29:461–470. [DOI] [PubMed] [Google Scholar]
- 20. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, Pacini F. 2014. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol 2:356–358. [DOI] [PubMed] [Google Scholar]
- 21. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899. [DOI] [PubMed] [Google Scholar]
- 22. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ; DECISION investigators. 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630. [DOI] [PubMed] [Google Scholar]
- 24. Brose MS, Frenette CT, Keefe SM, Stein SM. 2014. Management of sorafenib-related adverse events: a clinician's perspective. Semin Oncol 41(Suppl 2):S1–S16. [DOI] [PubMed] [Google Scholar]
- 25. Cabanillas ME, Terris DJ, Sabra MM. 2017. Information for clinicians: approach to the patient with progressive radioiodine-refractory thyroid cancer-when to use systemic therapy. Thyroid 27:987–993. [DOI] [PubMed] [Google Scholar]
- 26. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. 2014. The landscape of kinase fusions in cancer. Nat Commun 5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu YH, Wirth LJ, Farahani AA, Nose V, Faquin WC, Dias-Santagata D, Sadow PM. 2020. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol 33:2458–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, Astl J, Vlcek P, Kodetova D, Vcelak J, Bendlova B. 2020. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid 30:1771–1780. [DOI] [PubMed] [Google Scholar]
- 29. Cocco E, Scaltriti M, Drilon A. 2018. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15:731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. 2020. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr., Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. 2020. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 21:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. 2018. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vanden Borre P, Schrock AB, Anderson PM, Morris JC, 3rd, Heilmann AM, Holmes O, Wang K, Johnson A, Waguespack SG, Ou SI, Khan S, Fung KM, Stephens PJ, Erlich RL, Miller VA, Ross JS, Ali SM. 2017. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations, including kinase fusions. Oncologist 22:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova T, Thomas GA, Mason CE, Fagin JA. 2013. Identification of kinase fusion oncogenes in post-chernobyl radiation-induced thyroid cancers. J Clin Invest 123:4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault V, Corsi-Travali S, Henry D, Burkard M, Hamor R, Bouhana K, Winski S, Wallace RD, Hartley D, Rhodes S, Reddy M, Brandhuber BJ, Andrews S, Rothenberg SM, Drilon A. 2018. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 29:1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardiere C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH, Cabanillas ME. 2020. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 383:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu M S, V, Wirth LJ, Schuler M, Mansfield AS, Brose MS. 2020. Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients with advanced RET mutation-positive medullary thyroid cancer. Ann Oncol 31:S1026–S1033. [Google Scholar]
- 38. Demeure MJ, Aziz M, Rosenberg R, Gurley SD, Bussey KJ, Carpten JD. 2014. Whole-genome sequencing of an aggressive BRAF wild-type papillary thyroid cancer identified EML4-ALK translocation as a therapeutic target. World J Surg 38:1296–1305. [DOI] [PubMed] [Google Scholar]
- 39. de Salins V, Loganadane G, Joly C, Abulizi M, Nourieh M, Boussion H, Belkacemi Y, Tournigand C, Kempf E. 2020. Complete response in anaplastic lymphoma kinase-rearranged oncocytic thyroid cancer: a case report and review of literature. World J Clin Oncol 11:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Godbert Y, Henriques de Figueiredo B, Bonichon F, Chibon F, Hostein I, Perot G, Dupin C, Daubech A, Belleannee G, Gros A, Italiano A, Soubeyran I. 2015. Remarkable response to crizotinib in woman with anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma. J Clin Oncol 33:e84–e87. [DOI] [PubMed] [Google Scholar]
- 41. Raman P, Koenig RJ. 2014. Pax-8-PPAR-gamma fusion protein in thyroid carcinoma. Nat Rev Endocrinol 10:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S. 2011. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. 2016. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 17:1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pei D, Chen K, Liao H. 2018. Targeting Ras with macromolecules. Cold Spring Harb Perspect Med 8:a031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Bryan JP 2019. Pharmacological targeting of RAS: recent success with direct inhibitors. Pharmacol Res 139:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagarajah J, Le M, Knauf JA, Ferrandino G, Montero-Conde C, Pillarsetty N, Bolaender A, Irwin C, Krishnamoorthy GP, Saqcena M, Larson SM, Ho AL, Seshan V, Ishii N, Carrasco N, Rosen N, Weber WA, Fagin JA. 2016. Sustained ERK inhibition maximizes responses of BrafV600E thyroid cancers to radioiodine. J Clin Invest 126:4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Dominguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. 2013. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho A DM, Wirth LJ 2018 ASTRA: A Phase III, Randomized, Placebo-Controlled Study Evaluating Complete Remission Rate (CRR) with Short-Course Selumetinib Plus Adjuvant Radioactive Iodine (RAI) in Patients (pts) with Differentiated Thyroid Cancer (DTC). American Thyroid Association, Washington, DC. [Google Scholar]
- 49. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, Pentlow KS, Haque S, Tuttle RM, Sabra MM, Fish S, Boucai L, Walters J, Ghossein RA, Seshan VE, Ni A, Li D, Knauf JA, Pfister DG, Fagin JA, Ho AL. 2019. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab 104:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. 2015. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 21:1028–1035. [DOI] [PubMed] [Google Scholar]
- 51. Randle RW, Balentine CJ, Leverson GE, Havlena JA, Sippel RS, Schneider DF, Pitt SC. 2017. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 161:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torresan F, Cavedon E, Mian C, Iacobone M. 2018. Long-term outcome after surgery for medullary thyroid carcinoma: a single-center experience. World J Surg 42:367–375. [DOI] [PubMed] [Google Scholar]
- 53. Ciampi R, Romei C, Ramone T, Prete A, Tacito A, Cappagli V, Bottici V, Viola D, Torregrossa L, Ugolini C, Basolo F, Elisei R. 2019. Genetic landscape of somatic mutations in a large cohort of sporadic medullary thyroid carcinomas studied by next-generation targeted sequencing. iScience 20:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simbolo M, Mian C, Barollo S, Fassan M, Mafficini A, Neves D, Scardoni M, Pennelli G, Rugge M, Pelizzo MR, Cavedon E, Fugazzola L, Scarpa A. 2014. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch 465:73–78. [DOI] [PubMed] [Google Scholar]
- 55. Ciampi R, Mian C, Fugazzola L, Cosci B, Romei C, Barollo S, Cirello V, Bottici V, Marconcini G, Rosa PM, Borrello MG, Basolo F, Ugolini C, Materazzi G, Pinchera A, Elisei R. 2013. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid 23:50–57. [DOI] [PubMed] [Google Scholar]
- 56. Moura MM, Cavaco BM, Pinto AE, Leite V. 2011. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 96:E863–E868. [DOI] [PubMed] [Google Scholar]
- 57. Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. 2015. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schilling T, Burck J, Sinn HP, Clemens A, Otto HF, Hoppner W, Herfarth C, Ziegler R, Schwab M, Raue F. 2001. Prognostic value of codon 918 (ATG—>ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. Int J Cancer 95:62–66. [DOI] [PubMed] [Google Scholar]
- 59. Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A. 2008. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93:682–687. [DOI] [PubMed] [Google Scholar]
- 60. Boichard A, Croux L, Al Ghuzlan A, Broutin S, Dupuy C, Leboulleux S, Schlumberger M, Bidart JM, Lacroix L. 2012. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab 97:E2031–E2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moura MM, Cavaco BM, Leite V. 2015. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer 22:R235–R252. [DOI] [PubMed] [Google Scholar]
- 62. Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. 2002. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62:7284–7290. [PubMed] [Google Scholar]
- 63. Wells SA Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. 2012. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wells SA Jr., Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M. 2010. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 28:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. 2013. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schlumberger M, Elisei R, Muller S, Schoffski P, Brose M, Shah M, Licitra L, Krajewska J, Kreissl MC, Niederle B, Cohen EEW, Wirth L, Ali H, Clary DO, Yaron Y, Mangeshkar M, Ball D, Nelkin B, Sherman S. 2017. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol 28:2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Drilon A, Hu ZI, Lai GGY, Tan DSW. 2018. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15:151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, Peled N, Weiss J, Kim YJ, Ohe Y, Nishio M, Park K, Patel J, Seto T, Sakamoto T, Rosen E, Shah MH, Barlesi F, Cassier PA, Bazhenova L, De Braud F, Garralda E, Velcheti V, Satouchi M, Ohashi K, Pennell NA, Reckamp KL, Dy GK, Wolf J, Solomon B, Falchook G, Ebata K, Nguyen M, Nair B, Zhu EY, Yang L, Huang X, Olek E, Rothenberg SM, Goto K, Subbiah V. 2020. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med 383:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wirth LJ RB, Boni V, Tan DSW, McCoach CE, Massarelli E. 2020. Exploratory patient-reported outcomes among patients with RET-mutant medullary thyroid cancer in LIBRETTO-001: a phase I/II trial of selpercatinib (LOXO-292). Ann Oncol 31:S1026–S1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Solomon BJ, Zhou CC, Drilon A, Park K, Wolf J, Elamin Y, Davis HM, Soldatenkova V, Sashegyi A, Lin AB, Lin BK, HH FL, Novello S, Arriola E, Perol M, Goto K, Santini FC. 2020. Phase III study of selpercatinib vs chemotherapy +/− pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol 17:763–773. [DOI] [PubMed] [Google Scholar]
- 71. Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, Hu M, Cabanillas M, Behrang A, Meric-Bernstam F, Vo PTT, Mooers BHM, Wu J. 2020. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann Oncol 32:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lin JJ, Liu SV, McCoach CE, Zhu VW, Tan AC, Yoda S, Peterson J, Do A, Prutisto-Chang K, Dagogo-Jack I, Sequist LV, Wirth LJ, Lennerz JK, Hata AN, Mino-Kenudson M, Nardi V, Ou SI, Tan DS, Gainor JF. 2020. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol 31:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Network NCC. NCCN Guidelines for Thyroid Carcinoma. Available at www.nccn.org (accessed December 1, 2020).
- 74. Ohori NP 2020. Molecular testing and thyroid nodule management in North America. Gland Surg 9:1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. 2013. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 98:E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, Chen J, Robinson HE, Shim HS, Chmielecki J, Pao W, Engelman JA, Iafrate AJ, Le LP. 2014. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 77. Naoum GE, Morkos M, Kim B, Arafat W. 2018. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer 17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Allin DM, Shaikh R, Carter P, Thway K, Sharabiani MTA, Gonzales-de-Castro D, O'Leary B, Garcia-Murillas I, Bhide S, Hubank M, Harrington K, Kim D, Newbold K. 2018. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur J Cancer 103:165–175. [DOI] [PubMed] [Google Scholar]
- 79. Pogliaghi G 2021. Liquid biopsy in thyroid cancer: from circulating biomarkers to a new prospective of tumor monitoring and therapy. Minerva Endocrinol (Torino) 46:45–61. [DOI] [PubMed] [Google Scholar]
- 80. Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA, Fasolo A, Fuhrer D, Hutter-Kronke ML, Forde PM, Wrona A, Santoro A, Sadow PM, Szpakowski S, Wu H, Bostel G, Faris J, Cameron S, Varga A, Taylor M. 2020. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol 38:2620–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jennings LJ, Arcila ME, Corless C, Kamel-Reid S, Lubin IM, Pfeifer J, Temple-Smolkin RL, Voelkerding KV, Nikiforova MN. 2017. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn 19:341–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Sasaki T, Suzuki T, Fujino K, Dutcus CE, Takahashi S. 2017. Lenvatinib for anaplastic thyroid cancer. Front Oncol 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taylor MH, Takahashi S, Capdevila J, Tahara M, Leboulleux S, Kiyota N, Dutcus CE, Xie R, Robinson B, Sherman SI, Habra MA, Elisei R, Wirth LJ. 2021. Correlation of performance status and neutrophil-lymphocyte ratio with efficacy in radioiodine-refractory differentiated thyroid cancer treated with lenvatinib. Thyroid 31:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. 2014. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 32:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meijer JA, le Cessie S, van den Hout WB, Kievit J, Schoones JW, Romijn JA, Smit JW. 2010. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol (Oxf) 72:534–542. [DOI] [PubMed] [Google Scholar]