Abstract
Background: Protease inhibitors (PIs) are believed to affect insulin sensitivity. We aimed to analyze the effect of PIs on insulin sensitivity and the onset of diabetes mellitus (DM) in patients with HIV.
Methodology: We searched PubMed, Google Scholar, ClinicalTrals.gov, and the WHO International Clinical Trials Registry Platform till November 2020 for randomized controlled trials (RCTs) that studied the effects of PIs on insulin sensitivity and DM in patients with HIV. We followed the PRISMA and PICOS frameworks to develop the search strategy. We used the random-effects meta-analysis model to estimate the mean difference (MD), standardized mean difference (SMD), and risk ratios for our outcomes, using Stata 14 software.
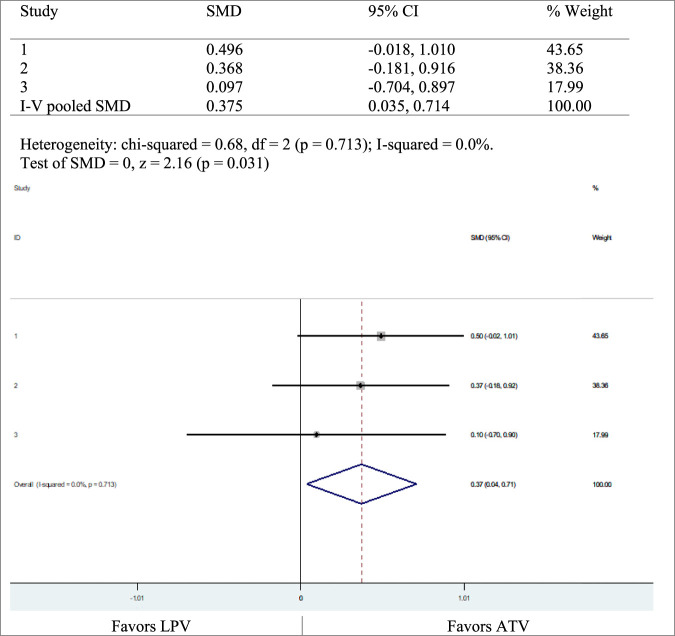
Results: We included nine RCTs that enrolled 1,000 participants, with their ages ranging from 18 to 69 years. The parameters and investigations used in the studies to determine insulin sensitivity were glucose disposal rates, hyperglycemia, and mean glucose uptake. The majority of results showed an association between PIs and insulin sensitivity. The pooled analysis showed no statistically significant difference in insulin sensitivity with atazanavir, whether the study was performed on healthy individuals for a short term or long term in combination with other drugs like tenofovir or emtricitabine [SMD = 0.375, 95% CI (0.035, 0.714)]. The analysis showed reduced glucose disposal rates and hence reduced insulin sensitivity with lopinavir (heterogeneity chi-squared = 0.68, I-squared [variation in SMD attributable to heterogeneity] = 0.0%, p = 0.031). The heterogeneity with chi-squared was substantial (61–80%), while with I-squared was not significant (0–40%), p = 0.031). Less adverse events were observed with atazanavir than with lopinavir [RR = 0.987, 95% CI (0.849, 1.124)]. Darunavir and indinavir did not demonstrate any significant changes in insulin sensitivity. Most of the studies were found to have a low risk of bias.
Conclusions: There are significant variations in the effects of PIs on insulin sensitivity and onsets of DM. Atazanavir, fosamprenavir, and darunavir did not demonstrate any significant changes in insulin sensitivity, compared to the rest of the group. There is a need to assess the benefits of PIs against the long-term risk of impaired insulin sensitivity. All patients newly diagnosed with HIV should have DM investigations before the start of ARVs and routinely. RCTs should focus on sub-Saharan Africa as the region is worst affected by HIV, but limited studies have been documented.
Keywords: HIV, protease inhibitors, insulin resistance, diabetes, antiretrovirals
Introduction
The human immunodeficiency virus (HIV) is a retrovirus that carries a single-stranded RNA as its genetic material, with high mutation rates that often lead to viral escape from multiple drugs (Fidler et al., 2020; Saag et al., 2020). A variety of antiretrovirals (ARVs) have been developed that highly suppress HIV replication, while there has been no effective cure. According to the World Health Organization 2021 global progress report on HIV (World Health Organization, 2021), an estimated 3.7 million people were living with HIV at the end of 2020, of whom two-thirds were in Africa. In the same year, 68,000 people died due to HIV and 1.5 million people acquired new HIV infection (World Health Organization, 2021). Since 2016, the WHO has recommended a lifelong antiretroviral treatment for all people living with HIV, including children and pregnant women, regardless of the CD4 count or clinical status (World Health Organization (WHO), 2016). This eventually improved the survival of patients (Chang et al., 2020) but lifted chronic complications in clinical practice (Bygrave et al., 2020).
Metabolic disorders such as diabetes mellitus (DM) have been associated with HIV and ARVs (Echecopar-Sabogal et al., 2018; Sarfo et al., 2021), and carbohydrate metabolism abnormalities like insulin resistance, hyperglycemia, and DM have been associated with ARVs of the class protease inhibitors (PIs) (Noor et al., 2006). Some earlier PIs such as indinavir and lopinavir/ritonavir exasperate lipid profiles and increase the risk of developing insulin resistance, hyperglycemia, and DM (Overton et al., 2016). PIs also mediate the blockage of glucose transport. In non-HIV metabolic syndrome, insulin resistance is further integrated with endoplasmic reticulum and oxidative stresses, lipotoxicity and lipid metabolism disruption, and altered adipocytokine secretion (Hruz, 2011; Zha et al., 2013).
In preclinical studies, PIs altered glucose transporter 4 (GLUT4)—one of the most important glucose transporters—decreased insulin secretion by affecting β-cells, thereby increasing insulin resistance (Hertel et al., 2004; Vyas et al., 2010; Monroe et al., 2015). The clinical outcomes and the overall impacts of ARV initiations on glucose metabolism remain ambiguous (Erlandson et al., 2014; Monroe et al., 2015). Several studies have evaluated the development of DM when PIs are administered in combination or with other classes of ARVs. In this particular review, we aimed to compare PIs towards their effects on insulin sensitivity and the onset of diabetes mellitus (DM) in patients with HIV.
Methods
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Shamseer et al., 2015). We compared PIs separately and looked at their effects on insulin sensitivity and determined if the findings can be used for the present and future in the creation of a proper combination of ARVs for patients with HIV and comorbidity of DM.
Search Methods
We searched PubMed, Google Scholar, ClinicalTrals.gov, and the WHO International Clinical Trials Registry Platform for completed randomized controlled trials (RCTs) up to November 1, 2020. Medical Subject Headings (MeSH) (Baumann, 2016) was used to develop search terms, using combined key terms that we drove from our research questions. For PubMed, Boolean operations were used in between search terms like protease inhibitors and hyperglycemia or protease inhibitors and insulin sensitivity or protease inhibitors and cholesterol. References of the retrieved articles were also searched to identify other similar articles.
Search Strategy
PubMed MeSH terms used were “insulin sensitivity” [Text Word] OR “insulin”[MeSH Terms] OR (“insulin resistance, Type 2”[Mesh]) AND “Protease inhibitors”[MeSH Terms] OR “HIV protease inhibitors” [Text Word] OR “Lopinavir” [Text Word] OR “Atazanavir” [Text Word] OR “Darunavir” [Text Word] AND “Antiretroviral” [Text Word] OR “Antiretroviral Agents”[Mesh] OR “Antiretroviral Therapy,” OR “Highly Active”[Mesh] AND “Incidence”[Text Word] OR “Incidence”[Mesh] AND “Epidemiology”[Mesh] OR “epidemiology” [Subheading] AND “Incidence”[Mesh].
Eligibility Criteria
PICOS (participants, interventions, comparison, outcomes, and study design) (Gebrie et al., 2020) was used to formulate the eligibility criteria.
- Population: individuals with HIV.
- Intervention: PIs.
- Comparison: PIs of different classes.
- Outcome:
- Primary outcome: effects on insulin sensitivity.
- Secondary outcomes: adverse events.
- Study design: RCTs published from 2000 to November 1, 2020.
Inclusion Criteria
We used the Jadad scale with three main characteristics of description (RCT, blinding, withdrawal, and dropouts). We considered RCTs that studied any drug in the PI group and conducted in any part of the world. We considered studies with HIV-seronegative participants (phase 1) and with seropositive participants but not diagnosed with DM that evaluated one or more of the outcomes listed before and of English language.
Exclusion Criteria
We excluded studies that had patients with DM at baseline, those that had combined therapies of other ARTs, and those not published in peer-reviewed publisher/journals or not yet completed.
Study Selection
Published studies were needed to include information on the interaction between PIs and insulin sensitivity (Béïque et al., 2007) based on the pre-identified criteria. We imported articles from the electronic databases into STATA software 14, screened for titles, followed by the abstracts and full text by two reviewers. Any discrepancies in findings between the two reviewers were discussed and resolved. All studies were RCTs written in English. Before the screening, we identified and removed redundant papers.
Data Extraction, Synthesis, and Analysis
Data were extracted by two independent reviewers using a data extraction tool adopted from the Cochrane Library (Noyes et al., 2018). This included the author’s name, article publication year, study country, study design, number of participants, length of follow-up, intervention PIs, outcomes, and adverse events. Data from eligible studies were transferred to a spreadsheet on Microsoft Excel. Studies that had designs and interventions that were alike, with assessments of the same outcomes and sufficient enough data, were used to perform the meta-analysis. Articles were summarized using a clinical-based approach (Siwek et al., 2002).
Assessment of Risk of Bias
The risk of bias was assessed using the revised Cochrane risk of bias tool for randomized trials (RoB 2) (Sterne et al., 2019). Two authors independently judged these risks as low, unclear, or high based on critical domains including random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
Statistical Analysis
STATA was used for statistical analysis. The random-effects model was used in performing the meta-analysis to estimate the mean differences and standardized mean difference for continuous outcomes and risk ratio for dichotomous outcomes of adverse events, with 95% CI. Data were analyzed according to the intention-to-treat principle (Sedgwick, 2015), and heterogeneity was measured using the chi-squared and I2 statistics.
Operational Definitions
ARV: antiretroviral drugs used to treat HIV.
ART: a combination of antiretroviral drugs for the treatment of HIV.
Seronegative patients: patients who have tested negative for HIV.
Patients with highly active ART failure: patients on ARTs having at least 1,000 and more copies of HIV viral load.
Phase 1 trial: the earliest trial carried out to test HIV treatment for the first time in a small group of people.
Biomarker: the characteristics that is measured as an indicator of responses to an HIV treatment.
Results
Search Results
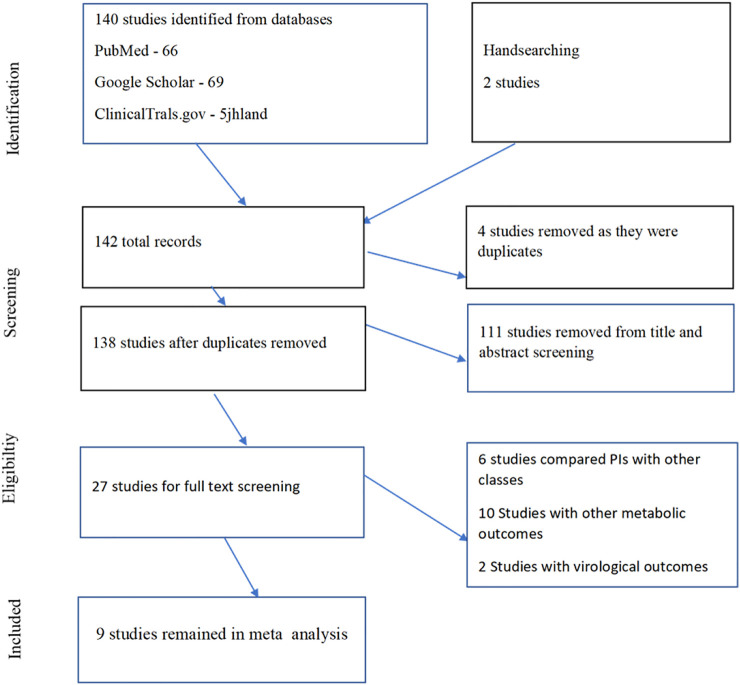
We identified 142 studies, with four articles removed as they were duplicates and 111 studies removed because they did not meet the inclusion criteria (Figure 1). We reviewed 27 full-text studies and finally left with nine studies. The selected studies were mostly from the United States, and one multinational study included Europe and Latin America, with people of different ethnicities (Black, White, Latinas, and Caucasians).
FIGURE 1.
Study flow diagram.
Study Characteristics
Table 1 presents a summary of the included nine RCTs. The studies included 1,000 adults with ages ranging from 18 to 69 years.
TABLE 1.
Characteristics of included RCTs.
| Author, year | Country | Study design | Participants number | Follow-up duration | Intervention drugs | Treatment outcomes | Adverse events |
| Overton et al. (2016) | The United States | Phase 4 randomized trial | 22 | 48 weeks | DRV/r | 9.9 | 2/12 |
| ATV/r | 9.1 | 1/10 | |||||
| Glucose disposal rate | |||||||
| Noor et al. (2004) | The United States | Randomized, double-blind, crossover study | 30 | 5 days | Placebo | 9.88 | None |
| ATV | 9.80 | ||||||
| LPV/r | 7.52 | ||||||
| Glucose disposal rate | |||||||
| Noor et al. (2006) | The United States | Randomized, crossover study | 26 | 10 days | ATV/r | 10.4 | None |
| LPV/r | 8.6 | ||||||
| Glucose disposal rate | |||||||
| Lee et al. (2007) | The United States | Randomized, double-blind, crossover study | 14 | 28 days | RTV | 8.0 | 1/8 |
| Amprenavir | 8.4 glucose disposal rate | 0/6 | |||||
| Molina et al. (2010) | Multicenter | Multicenter, open-label, non-inferiority randomized trial | 883 | 96 weeks | ATV/RTV | 3/434 | 283 |
| LPV/RTV | 2/428 | 282 | |||||
| Hyperglycemia | |||||||
| Stanley et al. (2009) | The United States | Randomized non-blinded trial | 12 | 6 months | ATV/RTV | 26.7 | 3/5 |
| LPV/RTV | 24.4 | 1/7 | |||||
| Glucose disposal rate | |||||||
| Dubé et al. (2008) | The United States | Placebo-controlled trial | 30 | 4 weeks | ATV/RTV | 6.73 | |
| LPV/RTV | 8.88 | ||||||
| Placebo | 7.53 | ||||||
| Mean glucose uptake | |||||||
| Noor et al. (2002) | The United States | Randomized placebo-controlled trial | 06 | 7–10 days | IDV | 13.5 | |
| Placebo | 14.1 | ||||||
| Glucose disposal rate | |||||||
| Randell et al. (2010) | The United Kingdom | Randomized trial | 27 | 2 weeks | FPV | 4.48 | |
| LPV | 0.28 | ||||||
| M/I (ratio of glucose disposal rate) |
DRV, darunavir; ATV, atazanavir; IDV, indinavir; RTV, ritsonavir; LPV, lopinavir; FPV, fosamprenavir.
The Risk of Bias and Methodological Quality
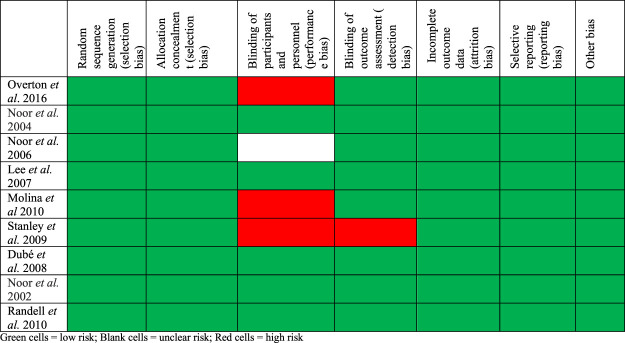
Figure 2 summarizes what the authors reviewed and their judgments about each risk of bias for included trials. Most of the studies were found to have a low risk of bias. Four of the studies did not have any bias at all. The studies by Overton et al. (2016) and Molina et al. (2010) were found with bias due to the blinding of participants as they were single-blinded trials. The study by Stanley et al. (2009) was an open-labeled trial, hence increasing its level of bias.
FIGURE 2.
Risk of bias summary: review authors’ judgments about each’ risk of bias’ item for included trials.
Glucose Disposal Rates Post-Intervention
Table 2 summarizes the glucose disposal rates post-intervention. A euglycemic clamp was used for all the aforementioned studies. The gold standard for assessing insulin resistance in humans is the hyperinsulinemic–euglycemic clamp. Overton et al. (2016) and Stanley et al. (2009) were open-labeled studies, while the rest were double-blinded studies.
TABLE 2.
Glucose disposal rate post-intervention.
| Trial | Atazanavir | Lopinavir | Darunavir | Ritonavir | Amprenavir | Placebo | Indinavir |
| Overton et al. (2016) | 9.1 | — | 9.9 | ||||
| Noor et al. (2004) | 9.80 | 7.52 | |||||
| Noor et al. (2006) | 10.4 | 8.6 | |||||
| Lee et al. (2007) | — | — | — | 8.0 | 8.4 | ||
| Stanley et al. (2009) | 26.7 | 24.4 | |||||
| Dubé et al. (2008) | 6.73 | 8.88 | — | — | — | 7.53 | |
| Noor et al. (2002) | — | — | — | — | — | 14.1 | 13.5 |
Seven studies reported glucose disposal rates with different PIs (Table 2). From the observations, we notice the IDV placebo study has the highest glucose disposal. There were no statistically significant differences in baseline fasting body weight, plasma glucose, insulin, lipid, and lipoprotein levels between placebo- and indinavir-treated subjects (Noor et al., 2002). The euglycemic–hyperinsulinemic clamp duration was 3-h, which did not show a difference from other studies (most were between 3 and 3.5 h). DRV as compared to ATV had a higher glucose disposal rate. Lee et al. (2007) compared RTV and amprenavir, which had a slight statistical difference in glucose disposal rates. One study, Randell et al. (2010), calculated the insulin sensitivities between FPV and LPV by M/I (glucose disposal rates over the mean insulin concentrations). There was decreased insulin sensitivity with patients on LPV.
Three studies, Noor et al. (2004), Noor et al. (2006), and Stanley et al. (2009), compared ATV and LPV with variable results. We carried out a meta-analysis, showing that the glucose disposal rates were in favor of ATV (Figure 3).
FIGURE 3.
Glucose disposal rate, LPV versus ATV.
Adverse Events
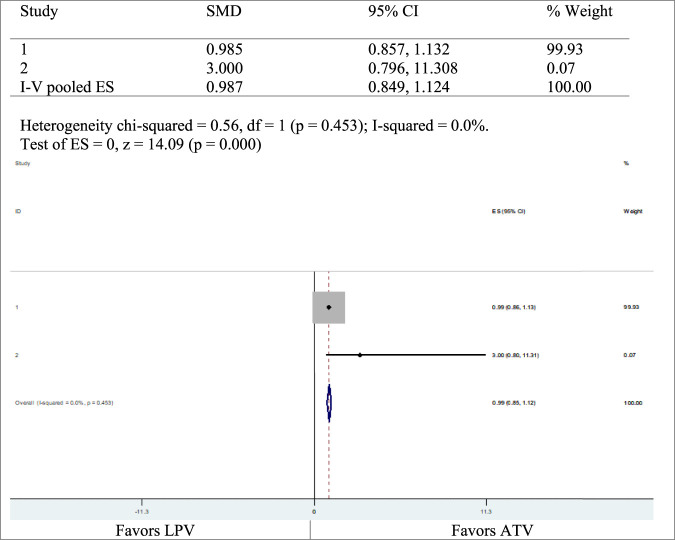
Table 3 summarizes the adverse events observed. Two studies reported AEs of ATV and LPV. The first study had a risk ratio of 0.9 with 95% CI (0.8574586–1.131936), and the second study had a risk ratio of 3 with 95% CI (0.7958937–11.30804) (Figure 4).
TABLE 3.
Adverse events.
| Author, year | Country | Risk ratio | Lower confidence interval | Upper confidence interval |
| Molina et al. (2010) | Multicenter | 0.9851843 | 0.8574586 | 1.131936 |
| Stanley et al. (2009) | The United States | 3 | 0.7958937 | 11.30804 |
FIGURE 4.
Adverse events of ATV and LPV.
Discussion
Nine of the included RCTs studied the effects of PIs on insulin sensitivity. Insulin sensitivity did not show significant changes with ATV, whether the study is performed on healthy individuals for short term like 5, 10, or 28 days (Noor et al., 2004; Overton et al., 2016) or on HIV-infected individuals for a long term in combination with other drugs like tenofovir and emtricitabine parallelly. LPV reduced glucose disposal rates and hence reduced insulin sensitivity. This was supported by a meta‐analysis of the three studies [heterogeneity chi-squared = 0.68, I-squared (variation in SMD attributable to heterogeneity) = 0.0%, p = 0.031]. The heterogeneity with chi-squared was substantial (61–80%), while that with I-squared was not significant (0–40%), p = 0.031). Darunavir does not demonstrate any significant changes in insulin resistance in HIV-infected individuals, on long-term use in combination with other drugs (Overton et al., 2016). The plasma glucose levels are within normal ranges when using amprenavir as demonstrated by healthy individuals for a short term of 28 days (Lee et al., 2007). RTV and LPV increase plasma glucose levels in healthy individuals on acute use and increase insulin sensitivity (Noor et al., 2004). A meta-analysis on two studies, Molina et al. (2010) and Stanley et al. (2009), showed that there was a higher risk of developing adverse events on the LPV arm than on the ATV arm, with heterogeneity chi-squared = 0.56, p = 0.453 and I-squared (variation in ES attributable to heterogeneity) = 0.0%, p = 0.000; chi-squared heterogeneity was moderate (41–60%) while I-squared was not significant (0–40%). For treatment of adults with HIV, the current WHO guidelines for ART recommend two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase (NNRTI) or an integrase inhibitor (INSTI) consisting of the first-line drugs (World Health Organization (WHO), 2016). In special situations and circumstances, abacavir (ABC) or boosted PIs (ATV/r, DRV/r, LPV/r) are an option (World Health Organization (WHO), 2016).
Evaluating the quality of included studies, we noticed that standard biomarkers were used in all the trials. RCTs were mainly used and emphasized as they are most likely to be representative of the true difference in the comparison arms. In rare events, it is statistically limited to detect the power by use of RCTs. Some studies were conducted on persons with no ART experience, while other studies considered ART-experienced patients, with a history of treatment failure and HIV-seronegative; these could have led to recruitment bias. The sample sizes of most studies were small, with less than 300 participants, and short study duration (48 weeks and less), which could have led to selection bias.
Randell et al. (2010) demonstrated effects using both euglycemic clamp and HOMA IR. The results of both analyses correlate with each other, showing no significant changes. In a cross-sectional study in Angola (Francisco et al., 2020), insulin resistance based on the homeostatic model (HOMA-R) was 20% and glucose intolerance was 40%. Two other cross-sectional analyses of longitudinal cohort and cohort studies conducted in Italy (Rosso et al., 2007) and Rwanda (Dusingize et al., 2013) compared median HOMA IR and fasting glucose. The first study (Rosso et al., 2007) demonstrates that FPG was in normal ranges (78.5), while HOMA IR was significantly high (2.18). The second study (Dusingize et al., 2013) reports that the median for both is in normal ranges (FPG = 77.5, HOMA IR = 0.66). A cross-sectional study conducted in the United States (Tiozzo et al., 2021) compared results of HOMA IR and HbA1c for patients with a shorter duration of fewer than 15 years or a longer duration of greater than 16 years. For the shorter duration, the HOMA IR was above normal ranges, while HbA1c was normal (2.8 and 5.8, respectively). For a longer duration, the HOMA IR was above normal ranges, while HbA1c was normal (4.0 and 6.1, respectively). From the aforementioned analysis, we can conclude that more RCTs need to be done with a comparison of HOMA IR with the other methods (OsGTT, HbA1c). Treating patients with DM is complex due to the progressive nature of the disease (Gebrie et al., 2021a; Gebrie et al., 2021b), and the burden could be higher in HIV comorbidity (Godman et al., 2020; Ye et al., 2020; Lopez-Alvarenga et al., 2021; Phoswa, 2021) that calls for more robust studies in the area.
Conclusion
In this review, there were significant variations in the effects of PIs on insulin sensitivity and onsets of DM. Atazanavir, fosamprenavir, and darunavir do not demonstrate any significant changes in insulin sensitivity, compared to the rest of the group. There is a need to assess the benefits of the PIs against the long-term risk of impaired insulin sensitivity. We recommend that all patients newly diagnosed with HIV should be investigated for DM and hyperglycemic factors, with the tests available in a particular setting before the start of ARTs and routinely. Close monitoring of the patients should be done in every visit for any signs and symptoms. From the aforementioned review, it is evident that just one class of drugs has different variations on the onset of DM in PLHIV. More studies should be conducted on the evaluation of not only PIs but also all ARV classes and the effects or onsets of DM. RCTs should focus on sub-Saharan Africa as the region is worse affected by HIV, but limited studies have been documented.
Acknowledgments
Thanks to the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, for supporting this work.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
VK, MG, YM, JA, and TM design the study. VK, DA, HT, and TM preformed the search. VK, MG, HT, WO, and TM analyzed data. VK wrote the first draft of the manuscript. TM critically reviewed the manuscript. All authors have read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AE, adverse events; AMP, amprenavir; ART, antiretroviral therapy; ARV, antiretrovirals; ATV, atazanavir; DM, diabetes mellitus; FBG/FPG, fasting blood glucose/plasma glucose; FPV, fosamprenavir; HAART, highly active antiretroviral therapy; HbA1c, glycated hemoglobin; HIV, human immunodeficiency virus; HOMA IR, homeostatic model; IDV, indinavir; INSTIs, integrase strand transfer inhibitors; LPV, lopinavir; MS, metabolic syndrome; NCD, non-communicable diseases; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase; OGTT, oral glucose tolerance test; PI, protease inhibitors; PLHIV, people living with HIV; RBG, resting blood glucose; and RTV, ritonavir.
References
- Baumann N. (2016). How to Use the Medical Subject Headings (MeSH). Int. J. Clin. Pract. 70 (2), 171–174. 10.1111/ijcp.12767 [DOI] [PubMed] [Google Scholar]
- Béïque L., Giguère P., la Porte C., Angel J. (2007). Interactions between Protease Inhibitors and Acid-Reducing Agents: a Systematic Review. HIV Med. 8 (6), 335–345. 10.1111/j.1468-1293.2007.00482.x [DOI] [PubMed] [Google Scholar]
- Bygrave H., Golob L., Wilkinson L., Roberts T., Grimsrud A. (2020). Let’s Talk Chronic Disease: Can Differentiated Service Delivery Address the Syndemics of HIV, Hypertension and Diabetes?. Curr. Opin. HIV AIDS 15 (4), 256–260. 10.1097/COH.0000000000000629 [DOI] [PubMed] [Google Scholar]
- Chang H., Sewda A., Marquez-Luna C., White S. R., Whitney B. M., Williams-Nguyen J., et al. (2020). Genetic Architecture of Cardiometabolic Risks in People Living with HIV. BMC Med. 18, 288–314. 10.1186/s12916-020-01762-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M. P., Shen C., Greenwald M., Mather K. J. (2008). No Impairment of Endothelial Function or Insulin Sensitivity with 4 Weeks of the HIV Protease Inhibitors Atazanavir or Lopinavir-Ritonavir in Healthy Subjects without HIV Infection: a Placebo-Controlled Trial. Clin. Infect. Dis. 47 (4), 567–574. 10.1086/590154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusingize J. C., Hoover D. R., Shi Q., Mutimura E., Kiefer E., Anastos K. (2013). Associations of HIV Infection with Insulin and Glucose Levels in Antiretroviral-Naive Rwandan Women: A Cross-Sectional Analysis. BMJ Open 3 (12), e003879. 10.1136/bmjopen-2013-003879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echecopar-Sabogal J., D'Angelo-Piaggio L., Chanamé-Baca D. M., Ugarte-Gil C. (2018). Association between the Use of Protease Inhibitors in Highly Active Antiretroviral Therapy and Incidence of Diabetes Mellitus And/or Metabolic Syndrome in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Int. J. STD AIDS 29 (5), 443–452. 10.1177/0956462417732226 [DOI] [PubMed] [Google Scholar]
- Erlandson K. M., Kitch D., Tierney C., Sax P. E., Daar E. S., Melbourne K. M., et al. (2014). Impact of Randomized Antiretroviral Therapy Initiation on Glucose Metabolism. AIDS 28 (10), 1451–1461. 10.1097/QAD.0000000000000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler S., Stöhr W., Pace M., Dorrell L., Lever A., Pett S., et al. (2020). Antiretroviral Therapy Alone Versus Antiretroviral Therapy with a Kick and Kill Approach, on Measures of the HIV Reservoir in Participants with Recent HIV Infection (The RIVER Trial): a Phase 2, Randomised Trial. Lancet 395 (10227), 888–898. 10.1016/S0140-6736(19)32990-3 [DOI] [PubMed] [Google Scholar]
- Francisco K., Pole B., Tchiva S., Ngwiri T., Nduati R., Mungai L., et al. (2020). Insulin Resistance and Glucose Intolerance in HIV Infected Children on Antiretroviral Therapy at Lubango Pediatric Hospital - Angola. Int. J. Virol. AIDS 7, 071. 10.23937/2469-567X/1510071 [DOI] [Google Scholar]
- Gebrie D., Getnet D., Manyazewal T. (2021). Cardiovascular Safety and Efficacy of Metformin-SGLT2i versus Metformin-Sulfonylureas in Type 2 Diabetes: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 11 (1), 137. 10.1038/s41598-020-80603-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrie D., Getnet D., Manyazewal T. (2020). Efficacy of Remdesivir in Patients with COVID-19: A Protocol for Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open 10 (6), e039159. 10.1136/bmjopen-2020-039159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrie D., Manyazewal T., Ejigu D. A., Makonnen E. (2021). Metformin-Insulin versus Metformin-Sulfonylurea Combination Therapies in Type 2 Diabetes: A Comparative Study of Glycemic Control and Risk of Cardiovascular Diseases in Addis Ababa, Ethiopia. Diabetes Metab. Syndr. Obes. 14, 3345–3359. 10.2147/DMSO.S312997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godman B., Basu D., Pillay Y., Mwita J. C., Rwegerera G. M., Anand Paramadhas B. D., et al. (2020). Review of Ongoing Activities and Challenges to Improve the Care of Patients with Type 2 Diabetes across Africa and the Implications for the Future. Front. Pharmacol. 11, 108. 10.3389/fphar.2020.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel J., Struthers H., Horj C. B., Hruz P. W. (2004). A Structural Basis for the Acute Effects of HIV Protease Inhibitors on GLUT4 Intrinsic Activity. J. Biol. Chem. 279 (53), 55147–55152. 10.1074/jbc.M410826200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz P. W. (2011). Molecular Mechanisms for Insulin Resistance in Treated HIV-Infection. Best Pract. Res. Clin. Endocrinol. Metab. 25, 459–468. 10.1016/j.beem.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. A., Rao M., Mulligan K., Lo J. C., Aweeka F., Schwarz J. M., et al. (2007). Effects of Ritonavir and Amprenavir on Insulin Sensitivity in Healthy Volunteers. AIDS 21 (16), 2183–2190. 10.1097/QAD.0b013e32826fbc54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alvarenga J. C., Martinez D. A., Diaz-Badillo A., Morales L. D., Arya R., Jenkinson C. P., et al. (2021). Association of HIV-1 Infection and Antiretroviral Therapy With Type 2 Diabetes in the Hispanic Population of the Rio Grande Valley, Texas, USA. Front. Med. 8, 676979. 10.3389/fmed.2021.676979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J. M., Andrade-Villanueva J., Echevarria J., Chetchotisakd P., Corral J., David N., et al. (2010). Once-daily Atazanavir/ritonavir Compared with Twice-Daily Lopinavir/Ritonavir, Each in Combination with Tenofovir and Emtricitabine, for Management of Antiretroviral-Naive HIV-1-Infected Patients: 96-week Efficacy and Safety Results of the CASTLE Study. J. Acquir. Immune Defic. Syndr. 53 (3), 323–332. 10.1097/QAI.0b013e3181c990bf [DOI] [PubMed] [Google Scholar]
- Monroe A. K., Glesby M. J., Brown T. T. (2015). Diagnosing and Managing Diabetes in HIV-Infected Patients: Current Concepts. Clin. Infect. Dis. 60 (3), 453–462. 10.1093/cid/ciu779 [DOI] [PubMed] [Google Scholar]
- Noor M. A., Flint O. P., Maa J. F., Parker R. A. (2006). Effects of Atazanavir/ritonavir and Lopinavir/ritonavir on Glucose Uptake and Insulin Sensitivity: Demonstrable Differences In Vitro and Clinically. AIDS 20 (14), 1813–1821. 10.1097/01.aids.0000244200.11006.55 [DOI] [PubMed] [Google Scholar]
- Noor M. A., Parker R. A., O'Mara E., Grasela D. M., Currie A., Hodder S. L., et al. (2004). The Effects of HIV Protease Inhibitors Atazanavir and Lopinavir/Ritonavir on Insulin Sensitivity in HIV-Seronegative Healthy Adults. AIDS 18 (16), 2137–2144. 10.1097/00002030-200411050-00005 [DOI] [PubMed] [Google Scholar]
- Noor M. A., Seneviratne T., Aweeka F. T., Lo J. C., Schwarz J. M., Mulligan K., et al. (2002). Indinavir Acutely Inhibits Insulin-Stimulated Glucose Disposal in Humans: A Randomized, Placebo-Controlled Study. AIDS 16 (5), F1–F8. 10.1097/00002030-200203290-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes J., Booth A., Flemming K., Garside R., Harden A., Lewin S., et al. (2018). Cochrane Qualitative and Implementation Methods Group Guidance Series-Paper 3: Methods for Assessing Methodological Limitations, Data Extraction and Synthesis, and Confidence in Synthesized Qualitative Findings. J. Clin. Epidemiol. 97, 49–58. 10.1016/j.jclinepi.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Overton E. T., Tebas P., Coate B., Ryan R., Perniciaro A., Dayaram Y. K., et al. (2016). Effects of Once-Daily Darunavir/Ritonavir Versus Atazanavir/Ritonavir on Insulin Sensitivity in HIV-Infected Persons over 48 Weeks: Results of an Exploratory Substudy of METABOLIK, a Phase 4, Randomized Trial. HIV Clin. Trials 17 (2), 72–77. 10.1080/15284336.2016.1141468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoswa W. N. (2021). The Role of HIV Infection in the Pathophysiology of Gestational Diabetes Mellitus and Hypertensive Disorders of Pregnancy. Front. Cardiovasc. Med. 8, 613930. 10.3389/fcvm.2021.613930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell P. A., Jackson A. G., Boffito M., Back D. J., Tjia J. F., Taylor J., et al. (2010). Effect of Boosted Fosamprenavir or Lopinavir-Based Combinations on Whole-Body Insulin Sensitivity and Lipids in Treatment-Naive HIV-Type-1-Positive Men. Antivir. Ther. 15 (8), 1125–1132. 10.3851/IMP1675 [DOI] [PubMed] [Google Scholar]
- Rosso R., Parodi A., d'Annunzio G., Ginocchio F., Nicolini L., Torrisi C., et al. (2007). Evaluation of Insulin Resistance in a Cohort of HIV-Infected Youth. Eur. J. Endocrinol. 157 (5), 655–659. 10.1530/EJE-07-0414 [DOI] [PubMed] [Google Scholar]
- Saag M. S., Gandhi R. T., Hoy J. F., Landovitz R. J., Thompson M. A., Sax P. E., et al. (2020). Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA 324, 1651–1669. 10.1001/jama.2020.17025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfo F. S., Norman B., Nichols M., Appiah L., Osei Assibey S., Tagge R., et al. (2021). Prevalence and Incidence of Pre-diabetes and Diabetes Mellitus Among People Living with HIV in Ghana: Evidence from the EVERLAST Study. HIV Med. 22 (4), 231–243. 10.1111/hiv.13007 [DOI] [PubMed] [Google Scholar]
- Sedgwick P. (2015). Intention to Treat Analysis versus Per Protocol Analysis of Trial Data. BMJ 350, h681. 10.1136/bmj.h681 [DOI] [PubMed] [Google Scholar]
- Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 350, g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- Siwek J., Gourlay M. L., Slawson D. C., Shaughnessy A. F. (2002). How to Write an Evidence-Based Clinical Review Article. Am. Fam. Physician 65 (2), 251–258. [PubMed] [Google Scholar]
- Stanley T. L., Joy T., Hadigan C. M., Liebau J. G., Makimura H., Chen C. Y., et al. (2009). Effects of Switching from Lopinavir/Ritonavir to Atazanavir/Ritonavir on Muscle Glucose Uptake and Visceral Fat in HIV-Infected Patients. AIDS 23 (11), 1349–1357. 10.1097/QAD.0b013e32832ba904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Tiozzo E., Rodriguez A., Konefal J., Farkas G. J., Maher J. L., Lewis J. E. (2021). The Relationship Between HIV Duration, Insulin Resistance and Diabetes Risk. Int. J. Environ. Res. Public Health 18 (8), 3926. 10.3390/ijerph18083926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A. K., Koster J. C., Tzekov A., Hruz P. W. (2010). Effects of the HIV Protease Inhibitor Ritonavir on GLUT4 Knock-Out Mice. J. Biol. Chem. 285 (47), 36395–36400. 10.1074/jbc.M110.176321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2021). Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Geneva, Switzerland. WHO. Available at: https://www.who.int/publications/i/item/9789240027077 [Google Scholar]
- World Health Organization (WHO) (2016). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd Edn. Geneva, Switzerland: WHO. Available at: https://apps.who.int/iris/handle/10665/208825. [PubMed] [Google Scholar]
- Ye Y., Shrestha S., Burkholder G., Bansal A., Erdmann N., Wiener H., et al. (2020). Rates and Correlates of Incident Type 2 Diabetes Mellitus Among Persons Living with HIV-1 Infection. Front. Endocrinol. 11, 555401. 10.3389/fendo.2020.555401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha B. S., Wan X., Zhang X., Zha W., Zhou J., Wabitsch M., et al. (2013). HIV Protease Inhibitors Disrupt Lipid Metabolism by Activating Endoplasmic Reticulum Stress and Inhibiting Autophagy Activity in Adipocytes. PLoS One 8 (3), e59514. 10.1371/journal.pone.0059514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.