Abstract
Five new species (Gastrochilus yei, Gastrochilus minimus, Luisia simaoensis, Taeniophyllum xizangense, Tuberolabium subulatum) and two newly recorded species (Cleisostoma tricornutum, Luisia inconspicua) of Vandeae (Orchidaceae) from China are described and illustrated. Gastrochilus yei is similar to G. affinis and G. nepalensis, but differs from them by having an epichile not lobed, the apex of the hypochile not bilobed, and a tine on the apex of the leaf. Gastrochilus minimus is similar to G. acinacifolius, but can be distinguished from the latter by having a flabellate epichile that is densely hirsute on the adaxial surface and an inconspicuous central cushion; in addition, the hypochile of G. minimus has a keel that extends to the apex of the epichile. Taeniophyllum xizangense is similar to T. stella and T. radiatum, but it is distinguished from them by having much bigger flowers, inflorescences densely covered with short-bristly hairs, papillae on the external surface of sepals, and bigger triangular-ovate viscidium. Luisia simaoensis is similar to L. magniflora and L. ramosii, but can be easily distinguished from them by having lateral sepals longer than dorsal sepals and petals, lip with irregular and waved margins, and lip with bilobed apex. Luisia inconspicua is moved from Gastrochilus to Luisia based on phylogenetic analyses of plastid matK sequence data. Tuberolabium subulatum is similar to T. carnosum, but it can be easily distinguished from the latter by having an inflorescence much shorter than the leaves, yellow sepals and petals, and many small papillae outside the lip lobes.
Keywords: New species, New records, Orchidaceae, Vandeae, China
Highlights
-
•
Five new orchid species belonging to Vandeae were described based on morphological characters.
-
•
Two new recorded orchids of China were reported.
-
•
Phylogenetic position of Luisia inconspicua is confirmed based on molecular systematics.
1. Introduction
The orchid family is among the largest families of angiosperms, with approximately 200 genera and 1650 species in China (Jin et al., 2019). In 2019, The Orchid Survey of China was initiated by the Chinese National Forestry and Grassland Administration. This survey will cover all provinces, autonomous regions, and municipalities in China. During our botanical survey in south and southwest China between 2019 and 2020, many little known or unknown orchid species were discovered. The results of morphological comparison indicate that five species belonging to Vandeae are new to science and two are new records in China. Here we describe these new discoveries to the flora of China.
2. Material and methods
Specimens were collected and deposited at the Herbarium (HITBC) of Xishuangbanna Tropical Botanical Garden and in the Herbarium (KUN) of the Kunming Institute of Botany, Chinese Academy of Sciences. Flowers were also preserved in formalin-acetic acid-alcohol (FAA) and DNA samples were preserved in a freezer for further study. Specimens were studied in the laboratory under a stereomicroscope.
The phylogenetic position of one new record, Luisia inconspicua, is confusing due to its morphological characters, i.e., terete leaves, short inflorescence, raceme with several flowers and saccate hypochile. It has previously been placed in Cymbidium, Gastrochilus, Luisia, Luisiopsis, and Saccolabium. To determine its phylogenetic position, we analyzed two markers, plastid matK and nrITS. Thirty species of Gastrochilus, Holcoglossum and Luisia were included for phylogenetic analysis. Calanthe sieboldii was selected as outgroup. Total DNA was extracted from dry leaves using a TIANGEN DNA secure Plant Kit (TIANGEN BIOTECH (BEIJING) CO., LTD) following the manufacturer's instructions. Phylogenetic analyses were performed using IQ-TREE multicore v.1.6.10 for Windows 64-bit (Schrempf et al., 2019) with 1000 bootstrap replicates. Newly sequenced markers of L. inconspicua have been deposited in GenBank (Table 1).
Table 1.
Taxa and GenBank accession numbers of each species and outgroup in matK sequence analyses.
| Species | Accession no. |
|---|---|
| Gastrochilus acinacifolius | KJ733569.1 |
| G. calceolaris | MK357135.1 |
| G. formosanus | KJ733573.1 |
| G. guangtungensis | KJ733574.1 |
| G. intermedius | MK357151.1 |
| G. japonicus | KJ733575.1 |
| G. linii | MK357152.1 |
| G. minutiflorus | MK357153.1 |
| G. rantabunensis | MK357155.1 |
| G. raraensis | KJ733577.1 |
| G. tianbaoensis | MK357157.1 |
| G. yunnanensis | MK357158.1 |
| G. zhenyuanensis | MK357146.1 |
| Holcoglossum lingulatum | EU558949.1 |
| H. omeiense | HQ452917.1 |
| H. omeiense | JN106346.1 |
| H. quasipinifolium | HQ452924.1 |
| H. tsii | AB217732.1 |
| Luisia amesiana | KJ733580.1 |
| L. cordata | KJ733581.1 |
| L. filiformis | KF421852.1 |
| L. inconspicua | MW169039 |
| L. longispica | KJ733582.1 |
| L. magniflora | KJ733583.1 |
| L. trichorrhiza | EF655800.1 |
| L. zeylanica | JN004496.1 |
| Calanthe sieboldii | KF673815.1 |
3. Taxonomic treatment
3.1. Tuberolabium Yamam.
Tuberolabium is a small genus comprising about 11 species, mainly distributed in India, China, Thailand, Peninsular Malaysia, the Philippines, Indonesia, New Guinea, and Australia (Pridgeon et al., 2014). Tuberolabium is an epiphytic herb with few leaves, characterized by a pendulous inflorescence with many flowers and a spurred, 3-lobed lip (Chen and Wood, 2009; Kocyan and Schuiteman, 2013; Ormerod and Juswara, 2019).
Tuberolabium subulatum Jian W. Li & X. H. Jin, sp. nov.(勐腊管唇兰 Meng La Guan Chun Lan). Figs. 1 and 2: A—D.
Type: — CHINA. Mengla County, Yunnan Province. Epiphytic on tree in tropical seasonal rain forests in Yiwu State Nature Reserve, alt. 900 m, 4 May 2016, J.W. Li (holotype: HITBC!).
Diagnosis: Epiphytic herbs. Stems 3–5 cm long, base with several fleshy roots. Leaves 3–9, distichous, leaf blade oblong-lanceolate, 3.0–6.0 × 0.7–1.5 cm, mid-vein slightly concave adaxially, convex abaxially, apex unequally bilobed, lobes acute; base sheathing, jointed and twisted, almost lying in one plane. Inflorescence axillary, 1–5, descending, 1.0–2.5 cm long, ca. 1.5 mm in diam.; peduncle 0.5–1.0 cm long, with 2–4 sheaths, sheath triangular, ca. 1 × 3 mm; rachis 0.5–1.5 cm long, slightly flattened, with 2–7-flowered, 1–2 opening at same time. Sepals and petals orange-yellow, lip white with yellowish apex and several yellow spots at mouth of tube; pedicel and ovary 7–8 mm long, white. Dorsal sepal ovate-oblong, 7.0–7.2 × 4.0–4.2 mm, apex obtuse and mucronulate, 5-veined; lateral sepals oblique ovate, 7.0–7.2 × 4.8–5.0 mm, apex obtuse and mucronulate, 5-veined. Petals ovate-oblanceolate, 6.0–6.2 × 2.5–2.6 mm, apex obtuse, 3-veined. Lip immovably attached to end of column foot, spurred, 4.2–4.3 mm long, 3-lobed; outside of lobes with many small papillae; mid-lobe very small, triangular, ca. 1.0 × 1.4 mm, apex obtuse; lateral lobes triangular, ca. 2.5 × 1.4 mm, apex bilobed, upper lobelet oblong, ca. 1.5 × 0.7 mm, apex rounded, lower lobelet equilateral triangular, ca. 0.5 mm, apex obtuse; spur conical, 4.0–4.2 mm long, ca. 2 mm in diam., apex obtuse. Column stout, ca. 2 mm long, column wings enlarge, ca. 0.2 mm high; rostellum 2, linear, ca. 1 mm; column foot ca. 2.5 mm long, anther cap triangular, pollinia 2, viscidium equilateral triangular, ca. 0.5 mm long; stipe triangular, ca. 1.0 × 0.4 mm. Capsule cylinder, slender.
Phenology. Flowering from April to May.
Distribution and habitat.Tuberolabium subulatum was found in Mengla County, Yunnan Province, P. R. China, at the border between China and Laos. It is epiphytic on trees under tropical seasonal rain forests at elevations of 850−1000 m.
Etymology. The specific epithet ‘subulatum’ refers to the shape of spur.
Relationships. Morphologically, Tuberolabium subulatum is similar to T. carnosum Seidenf., but it can be easily distinguished from the latter by having an inflorescence much shorter than the leaves (vs. inflorescence as long as or longer than leaves), yellow sepals and petals (vs. sepals and petals whitish-green), many small papillae outside the lip lobes, very small, triangular mid-lobe, triangular lateral lobes, bilobed apex, oblong upper lobus, apex rounded apex, and equilateral triangular nether lobus (vs. lip with mid-lobe fleshy, with a very small triangular tip; lateral lobes fleshy, triangular, apex rounded, near front edge inside with a pair of squarish flanges meeting at base).
Conservation status. For the time being, Tuberolabium subulatum is only known from the type locality in a Nature Reserve. Accurate data on abundance and distribution is lacking; we here consider it as “Data Deficient (DD)” (IUCN 2012).
3.2. Gastrochilus D. Don
Gastrochilus comprises about 65 species mainly distributed in China, India and Sri Lanka through eastern Asia and southern Japan to southeast Asia (Liu et al., 2016; Wu et al., 2019). Gastrochilus is characterized by having the lip divided into a semi-globose to saccate hypochile and broadly triangular or flabellate, often hairy or papillose epichile (Tsi et al., 1996; Pridgeon et al., 2014; Liu and Gao, 2018).
3.2.1. Gastrochilus yei Jian W. Li & X.H. Jin, sp. nov. (叶氏盆距兰 Ye Shi Pen Ju Lan). Fig. 2: E–F; Fig. 3: A–E.
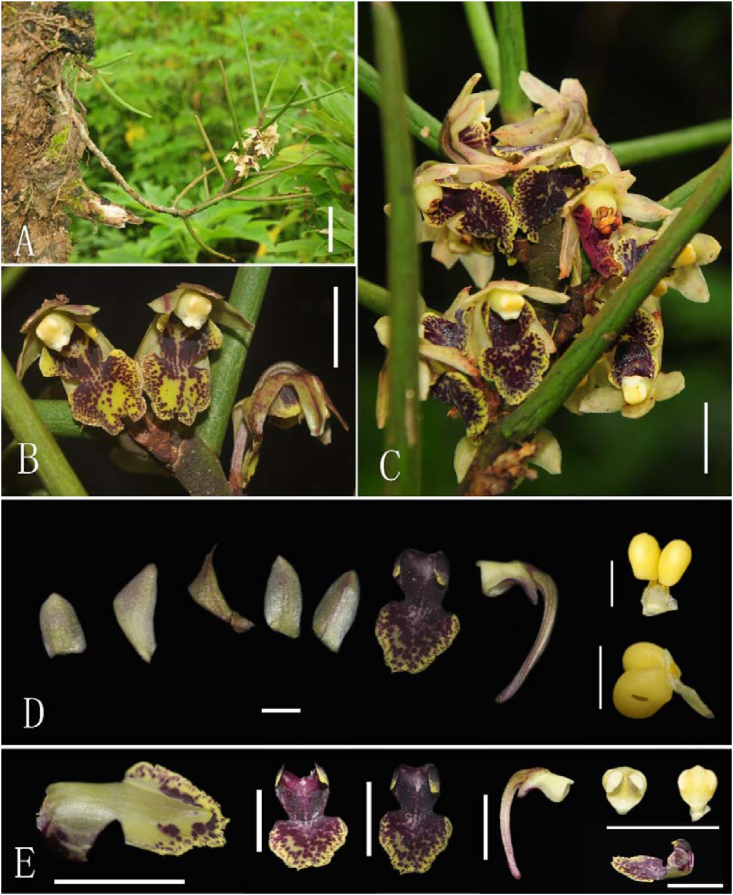
Fig. 2.
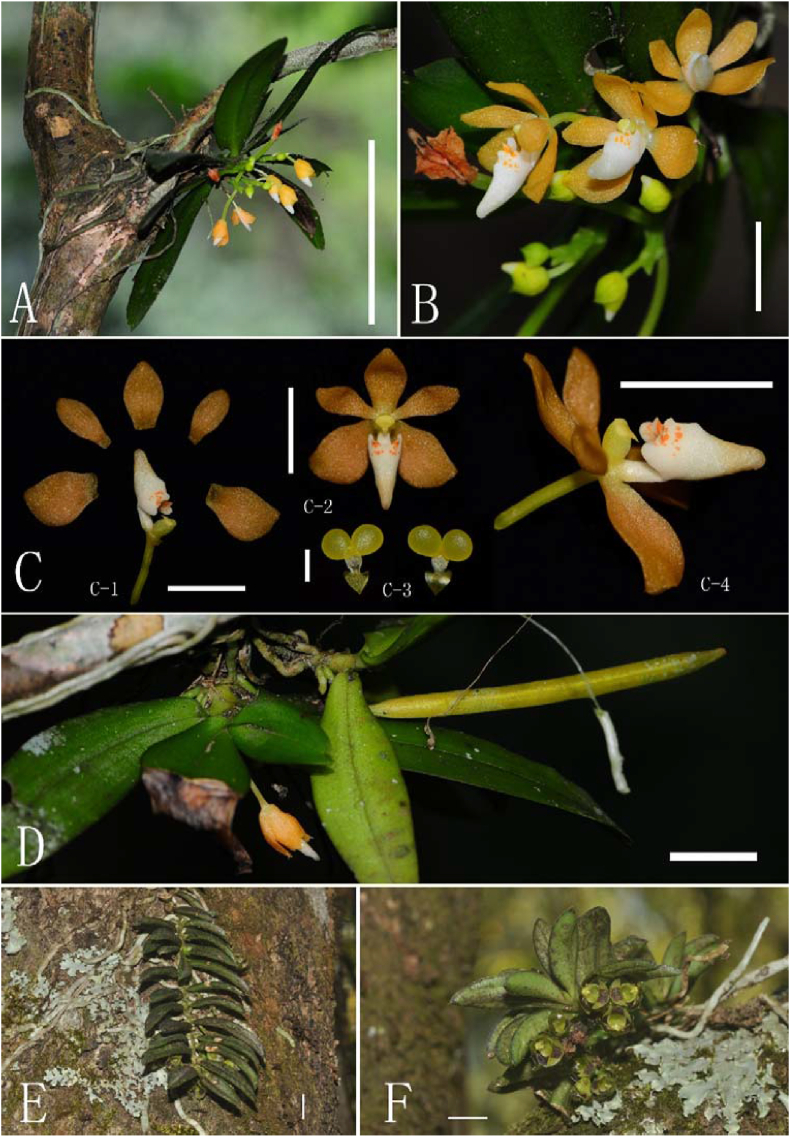
(A–D): Tuberolabium subulatum Jian W. Li & X.H. Jin, sp. nov. (A) Plant, bar = 5 cm. (B) Flowers, bar = 5 mm. (C) (C-1. Dissection of flower, bar = 5 mm; C-2. Face view of flower, bar = 5 mm; C-3. Pollinia, bar = 1 mm; C-4. Side view of flower, bar = 5 mm). (D) Plant with capsule, bar = 1 cm. (E–F): Plant of Gastrochilus yei Jian W. Li & X.H. Jin. (A–D photographed by Jian-Wu Li, E–F photographed by De-Ping Ye).
Fig. 3.
(A–E) Gastrochilus yei Jian W. Li & X.H. Jin, sp. nov. (A) Plants, bar = 5 cm. (B) Leaves, bar = 1 cm. (C) Plant with flower, bar = 1 cm. (D–E) Dissection of flower, bar = 1 mm. (F–H) G. minimus Jian W. Li, D.P. Ye & X.H. Jin sp. nov. (F) Dissection of flower, bar = 1 mm. (G) Plant with flowers, bar = 1 cm. (H) flowers, bar = 1 cm. (A–E photographed by Jian-Wu Li, F–H photographed by De-Ping Ye).
Type: — CHINA. Jingdong County, Yunnan Province, epiphytic on trees in broad-leaved evergreen forests, E: 100.77°, N: 24.39°, alt. 1900 m, 24 Apr. 2020, J.W. Li 5464 (Holotype: HITBC!, Isotype: HITBC!)
Fig. 1.
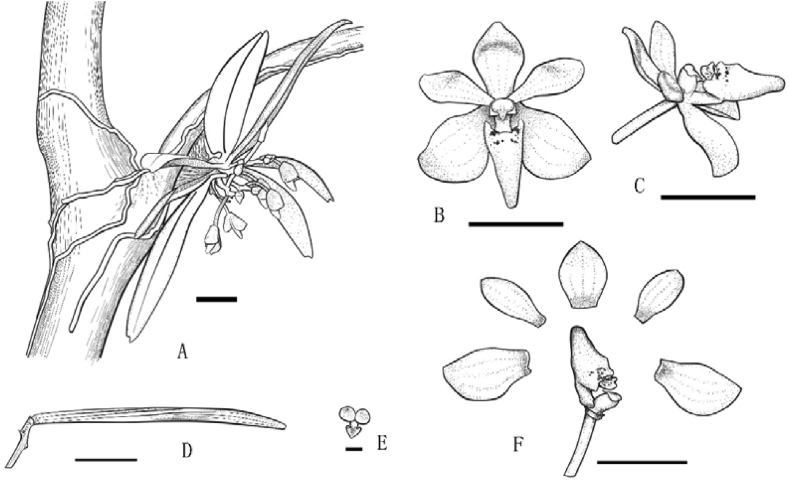
Line drawings of Tuberolabium subulatum Jian W. Li & X.H. Jin, sp. nov. (A) Plant, bar = 1 cm. (B) Flower (face view), bar = 5 mm. (C) Flower (side view), bar = 5 mm. (D) Capsule, bar = 1 cm. (E) Pollinia, bar = 1 mm. F. Dissection of flower, bar = 5 mm. (Draw from type specimen by Bo Pan).
Diagnosis: Mophologically, Gastrochilus yei Jian W. Li & X.H. Jin is similar to Gastrochilus affinis Schlechter and Gastrochilus nepalensis Raskoti, but differs from them by having epichile not lobed, apex of hypochile not bilobed, apex of leaf with a tine.
Epiphytic herb, creeping, 3–8 cm long. Roots several, come out from nodes of stem, 1.3 mm in diameter; stem 1.1 mm in diameter. Leaves distichous, leaf blade lanceolate, green with purple spots, 1.5–3.0 × 0.4–0.8 cm, apex acute, with a tine; base jointed amplexicaul-sheathing, twisted and almost lying in one plane; mid-vein slightly concave adaxially, slightly convex abaxially. Inflorescences 1–5 arising from nodes of stem, 1.0–1.5 cm long, rachis 0.3–0.7 mm long, raceme 2–6-flowered, flower yellowish-green, with purple spots, inside surface of petals and sepals purple with green margin. Floral bracts triangular, 0.9 × 1.0 mm, apex acute. Pedicel and ovary 0.7–1.0 cm long. Dorsal sepal oblong, 3.3 × 1.9–2.0 mm, apex rounded; lateral sepals oblong, 3.9–4.0 × 1.8–1.9 mm, apex obtuse. Petals oblong, 3.5 × 1.8 mm, apex rounded. Lip with an epichile and a saccate hypochile; epichile semi-rounded, 2.0–2.2 × 4.0–4.2 mm, glabrous, with a thicken central, rugose cushion, tint with purple, margin irregularly denticulate; hypochile subconical, 3 mm tall, 3 mm in diameter, apex rounded. Column stout, 1.5 mm long; rostellum bilobed; pollinia 2, viscidium peltate, stipe filiform. Capsule cylinder, 14 mm long, 4 mm in diameter, with 5 ridges.
Phenology. Flowering from April to May.
Distribution and habitat.Gastrochilus yei is only known from the type locality in Wuliangshan National Nature Reserve. G. yei is epiphytic on tree trunks in mossy broad-leaved evergreen forests at an elevation of 1850–2000 m.
Etymology. The specific epithet ‘yei’ is in honor of De-Ping Ye, who found this species.
Relationships. Morphologically, Gastrochilus yei is mostly similar to G. affinis Schltr. and G. nepalensis Raskoti, which share green leaves with purple spots, as well as the inside surface of sepals and petals purple with green margin. G. yei can be easily distinguished from G. affinis and G. nepalensis by having an acute leaf apex with a tine (vs. apex of leaf acute, with 2–3 denticulate in G. affinis; apex of leaf acute, without tine in G. nepalensis); rounded saccate apex (vs. apex of saccate bilobed both in G. affinis and G. nepalensis); semi-rounded epichile, with a thickened central, rugose cushion, and irregularly denticulate margin (vs. epichile triangular, central with 2 ridges ranging from base to apex, basal part of margin denticulate, apex slightly bilobed in G. affinis; epichile 3-lobed, lateral lobes subauriform, margin entire, midlobe suborbicular, base with rugose callus, margin entire in G. nepalensis).
Conservation status.Gastrochilus yei is only known from the type locality. In total, 150 individuals have been documented during our investigation. However, the habitat of this new species is widespread in the Jingdong Natural Reserve. We here tentatively consider it “Data Deficient (DD)” (IUCN 2012).
3.2.2. Gastrochilus minimus Jian W. Li, D.P. Ye & X.H. Jin, sp. nov. (小盆距兰 Xiao Pen Ju Lan). Fig. 3: F–H.
Type: — CHINA. Yixiang township, Simao District, Yunnan Province, epiphytic on trees near riverside in subtropical mixed coniferous broad-leaved forests, E: 100.97°, N: 22.79°, alt. 1287 m, 13 Sep. 2020, D.P. Ye (Holotype: HITBC!, Isotype: HITBC!)
Diagnosis: Epiphytic herb, small plant, 1.5–2.0 cm tall, stem ca. 1 mm in diameter. Roots several, ca. 1 mm in diameter. Leaves 3–6, ovate-lanceolate, 1.5–2.5 × 0.5–0.8 cm, apex acute, base jointed and amplexicaul-sheathing, twisted and almost lying in one plane, mid-vein concave adaxially, convex abaxially, green on both surfaces, sometimes tinted purple adaxially. Inflorescences come out from nodes from lower middle part, 0.5–0.9 cm long, ca. 1 mm in diameter, base with 1–2 sheaths, membranous, ca. 1 mm long, apex acute. Rachis 0.2–0.5 cm long, raceme 2–4-flowered, flowers yellowish-green, inside surface of sepals and petals with 2 longitudinal red-purple stripes, lip tinted red-purple spots. Floral bracts triangular, 0.9 × 0.9 mm, apex acute. Pedicel and ovary ca. 3 mm long. Dorsal sepal spatulate, concave, 3.2 × 1.7 mm, apex rounded; lateral sepals oblong-lanceolate, 3.5 × 1.2 mm, apex rounded, keel slightly convex abaxially. Petals oblanceolate, 2.8 × 1.4 mm, apex rounded, keel slightly convex abaxially. Lip with an epichile and a saccate hypochile; epichile flabellate, 2.0–2.2 × 4.0–4.2 mm, adaxially densely hirsute and inconspicuously with a central cushion, slightly reflexed; hypochile subconic, ca. 2 mm tall, ca. 2 mm in diameter, abaxially with a keel, extending to apex of epichile. Column stout, ca. 1.2 mm long; rostellum bilobed with acuminate tip; pollinia 2; viscidium oblong, ca. 0.3 mm long, stipe filiform, ca. 0.6 mm long.
Phenology. Flowering from July to September.
Distribution and habitat.Gastrochilus minimus was found in Simao District, Pu'er City, Yunnan Province, P.R. China. It is epiphytic in mixed coniferous broad-leaved evergreen forests at evations between 1200–1300 m.
Etymology. The specific epithet ‘minimus’ refers to smallest species in Gastrochilus s.l.
Relationships. Morphologically, Gastrochilus minimus is the smallest plant in Gastrochilus s.l. It is similar to G. acinacifolius Z.H. Tsi, but can be easily distinguished from the latter by being much smaller (1.5–2.0 cm tall in G. minumus. vs. 8–15 cm tall in G. acinacifolius); having yellowish-green flowers, with 2 longitudinal red-purple stripes on the inside surfaces of sepals and petals (vs. sepals and petals yellow with purplish red spots in G. acinacifolius); and an adaxially flabellate epichile that is densely hirsute and has an inconspicuous central cushion, which is slightly reflexed; a hypochile with a keel, on the abaxial surface that extends to apex of epichile (vs. epichile transversely oblong, adaxially sparsely papillate-hairy except on central cushion, margin irregularly denticulate, hypochile abaxially with 3 ridges.
Conservation status.Gastrochilus minimus was found only from the type locality. The habitat is in mixed coniferous broad-leaved evergreen forests along a river. Its habitat was greatly disturbed by plantations and deforestation. We estimate that the suitable habitat of G. minimus is less than 500 ha. In total, approximately 200 individuals were documented. Following IUCN (2012) guidelines we consider the conservation status of the new species Critically Endangered (CR: criteria B1ab(iii) + B2ab(iii)).
Notes. This species was included in the Wild Orchis in Yunnan (Xu et al., 2010) as Gastrochilus pumilus H. Jiang & D.P. Ye, sp. nov. ined. However, it was not validly published because the specific epithet ‘pumilus’ was already published as Gastrochilus pumilus Kuntze (1891), and also as Gastrochilus pumilus Hayata, which is now transferred to Holcoglossum pumilum (Hayata) X.H. Jin. According to the International Code of Nomenclature for algae, fungi and plants (Shenzhen Code) (Turland et al., 2018), it has no status in the Code. It may not be later homonym. Therefore, we proposed G. minimus Jian W. Li, D.P. Ye & X.H. Jin as a replaced name.
3.3. Taeniophyllum Blume
Taeniophyllum is a leafless and epiphytic genus with short stems and four divided pollinia (Carr, 1932). Taeniophyllum was divided into two subgenera based on having sepals and petals that are connate or free at the base; these subgenera were further subdivided into six sections (Schlechter, 1913a). Recent molecular studies indicated that leafy Microtatorchis is nested within Taeniophyllum and that Microtatorchis should be included in Taeniophyllum (Zou et al., 2015). Taeniophyllum s.l. comprises ca. 236 species widely distributed from tropical Africa (only one species) through tropical Asia to Australia and the Pacific islands (POWO, 2019); there are 4 species in China, with one Microtatorchis species (Chen and Wood, 2009).
3.3.1. Taeniophyllum xizangense J.D. Ya & C. Liu, sp. nov. (西藏带叶 兰Xi Zang Dai Ye Lan). Fig. 4.
Fig. 4.
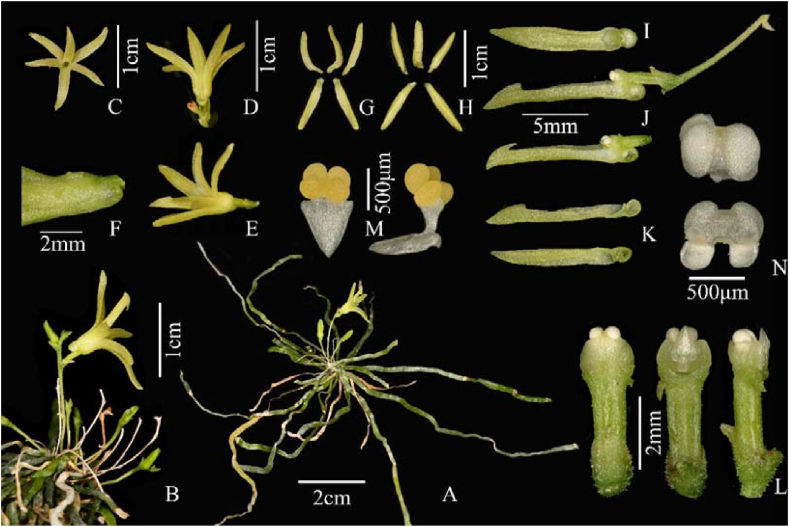
Taeniophyllum xizangense J.D. Ya & C. Liu, sp. nov. (A) Plant. (B) Inflorescence. (C) Front view of flower. (D) Dorsal view of flower. (E) Lateral view of flower. (F) Papillae on external surface of the sepals. (G) Adaxial sepals and petals. (H) Abaxial sepals and petals. (I) Front view of labellum. (J) Lateral view of column and labellum. (K) Lateral view of labellum (rip cutting). (L) Ovary and column. (M) Pollinarium. (N) Anther cap. (Photographed by Ji-Dong Ya).
Type: CHINA. Xizang Autonomous Region: Mêdog County, subtropical evergreen broad-leaved forest, 2000 m, 28 May 2019, J.D. Ya & C. Liu (Holotype: KUN!)
Diagnosis. Epiphytic herb. Roots up to 40, strongly flattened, 2–10 cm or longer, 1.5–2.0 mm in diameter. Inflorescences 3–7, erect, muricate, dense short-bristly hairs, 6–16 mm long, 1–3-flowered; peduncle filiform, 0.5–0.8 mm in diameter; bracts distichous, dense short-bristly hairs, broadly ovate-triangular, ca. 2.0 mm. Flowers open singly and widely, yellowish-green, sepals externally with papillae, peduncle and ovary ca. 4.0 mm long, sparse short-bristly hairs. Sepals and petals connate at the base into a tube ca. 2.0 mm long. Dorsal sepal narrowly lanceolate, obtuse, ca. 10.0 × 1.5 mm, incurved; lateral sepals narrowly lanceolate, obtuse, ca. 10.0 × 1.8 mm. Petals narrowly lanceolate, obtuse, ca. 8.0 × 1.8 mm, base clawed, apex acuminate. Labellum thick fleshy, narrowly lanceolate in outline, 10.0–11.0 mm × ca. 1.7 mm, with a transverse V-shaped lamella and shallowly saccate at the base of disk, with an inflexed spine ca. 0.25–0.35 mm long at the tip, base with retrorse square septum over spur entrance; spur subglobose, ca. 1.5 mm. Column short, stelidia rounded, ca. 1.4 × 1.4 mm. Anther cap white, ca. 0.75 × 0.65 mm, with 2 prominent humps. Pollinia 4, in two pairs, pale yellow, ovoid, ca. 0.4 × 0.3 mm; stipe white, ca. 0.5 mm long; viscidium large, white, triangular-ovate, ca. 0.9 × 0.6 mm.
Phenology. Flowering from May to June (based on cultivated material in the greenhouse).
Distribution and habitat.Taeniophyllum xizangense is currently known only from the type locality Mêdog County, Xizang, China. It is epiphytic on trunks under evergreen broadleaf forest at an elevation of 1350 m.
Etymology. The specific epithet ‘xizangense’ refers to the type locality where the new species occurs, Xizang, China.
Relationships.Taeniophyllum xizangense is similar to T. stella Carr and T. radiatum J.J. Sm. in morphological structure and shape of the flowers (Smith, 1918; Carr, 1932), but can be easy distinguished from them by having much bigger flowers, longer inflorescences densely covered with short-bristly hairs, sepals with papillaeon on the external surface, and bigger triangular-ovate viscidium (see Table 2).
Table 2.
Differences between Tainiophyllum xizangense, T. stella and T. radiatum.
| Characters | Taeniophyllum xizangense | T. stella | T. radiatum |
|---|---|---|---|
| Roots in diam. | 1.5–2.0 mm | 0.5 mm | 2.5–3.5 mm |
| Inflorescences | 6.0–16.0 mm long, 1–3-flowered, densely short-bristly hairs | 4.0–8.0 mm long, many flowered, muriculate | 5.0–6.0 mm long, many flowered, papillosa |
| Bracts | ca. 2.0 mm, densely short-bristly hairs | ca. 0.7 mm, minutely muriculate | ca. 0.7 mm, minutely muriculate |
| Flowers | yellowish green | pale yellowish white to bright yellow or salmon pink | pale orange or pale salmon pink |
| Sepals | 10.0 × 1.5 mm, externally with papillae | 5.5 × 1.0 mm, externally without papillae | 5.8–7.6 × 1.0–1.3 mm, externally without papillae |
| Petals | ca. 8.0 × 1.8 mm | ca. 5.5–7.5 × 1.0–0.75 mm | ca. 5.0 × 0.9 mm |
| Labellum | 10.0–11.0 × ca. 1.7 mm, with an inflexed spine at the tip, disk without keel | ca. 7.0 × 1.8 mm, without spine at tip, keeled in the disk | ca. 5.5–8.0 × 1.3 mm, with an inflexed spine at the tip, disk without keel |
| Pollinia | ovoid | pyriform | oblique obovoid |
| Viscidium | triangular-ovate, 0.9 mm | oval | lanceolate, 0.7 mm |
Conservation status. During our 2-week field survey, only one population was found; thus, we regard its status as “Data Deficient (DD)” (IUCN, 2012).
3.4. Luisia Gaudich.
Luisia comprises about 40 species distributed through Sri Lanka, India, Bhutan, China, Southeast Asia, to Japan, New Guinea, the Pacific Islands and Australia (Khuraijam and Roy, 2015; Karuppusamy and Ravichanderan, 2019; Mishra et al., 2020a, b). There are eleven species in China, five of which are endemic.
3.4.1. Luisia simaoensis D.P. Ye & H. Jiang, sp. nov. (裂唇钗子股 Lie Chun Chai Zi Gu). Fig. 5.
Fig. 5.
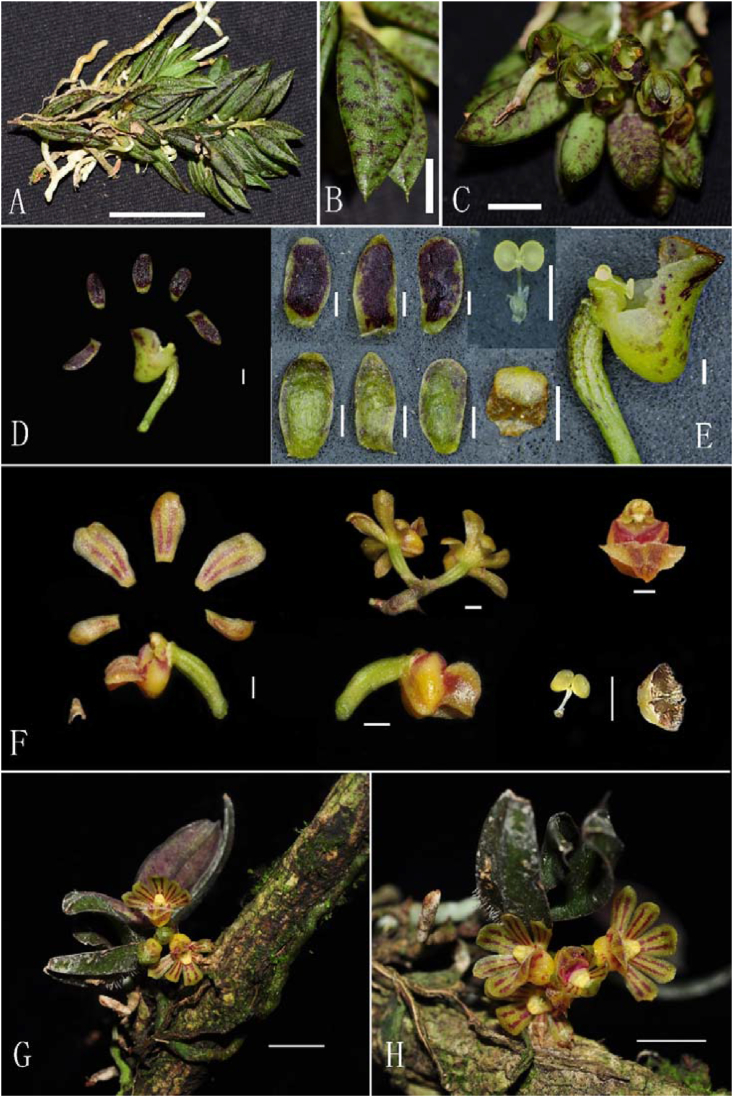
Luisia simaoensis D.P. Ye & H. Jiang ex Jian W. Li & D.P. Ye, sp. nov. (A) Plant, bar = 5 cm. (B–C) Flowers, bar = 1 cm. (D–E) Dissection of flower (D. bar = 5 mm; E. bar = 1 cm). (A–C photographed by Jian-Wu Li, D–E photographed by De-Ping Ye).
Type: CHINA. Sigangli village, Ximeng County, Pu'er City, Yunnan Province, epiphytic on trees in Karst region, alt. 1700 m, 30 Aug. 2011, Jianwu LI 897 (Holotype: HITBC!, Isotype: HITBC!)
Diagnosis. Epiphytic herb, stem suberect, or pendulous and ascending, rigid, simple or sometimes basally branched, 10–50 cm tall, 0.6–0.7 cm in diameter, internodes 1.5–2.0 cm long, perennial covered with persistent leaf sheath, brownish. Roots several, basally, slightly depressed, ca. 5 mm in diameter. Leaves several on upper part of stem, nearly distichous, obliquely upward, 1.5–2.0 cm apart from each other, terete, 10–14 × ca. 0.4 cm, apex acute, base enlarged into amplexicaul-sheathing, sheath 1.5–2.0 cm long. Inflorescence 1–5 came out from nodes of stem, penetrating leaf sheath, 0.7–1.0 cm long, base with 2–3 cannular sheaths, sheath 2–3 mm long, apex acute; rachis 3–5 mm long, raceme 2–5-flowered, sepals and petals whitish, outside tinged with purplish-red, lip yellowish-green, dense dark purple spots. Flora bracts triangular, 5.0–5.5 × 6.5–7 mm, apex sharp contractive and acuminate. Pedicel and ovary 1.7 cm long. Dorsal sepal ovate-elliptic, 8.5–9.0 × 5.0–5.2 mm, 7-veined, apex rounded; lateral sepals oblong, slightly oblique, 12 × 5 mm, 5-veined, abaxially with a keel, heightening gradually towards tip, forming an awn, 1.1 mm tall at tip, apex acute; petals oblong, 10 × 5 mm, 5-veined, apex obtuse. Lip broadly oblong, 1.5 cm long, middle strongly constricted and forming hypochile and epichile, with a distinct boundary between hypochile and epichile; epichile subcordate, 9 × 11 mm, margin irregular waved, apex bilobed, with a sinus 2 mm deep, apex of lobus rounded and irregular waved; hypochile transverse ovate, 6 × 14 mm, with lateral lobes ascending and embracing column, apex rounded. Column 7 mm long, anther cap galeiform, pollinia 2, viscidium rounded, ca. 2 mm in diameter, stipe lorate, 2 × 1.2 mm, rostellum lorate, ca. 1 mm long.
Phenology. Flowering from August to September.
Distribution and habitat.Luisia simaoensis was found in Pu'er City, Yunnan Province, P.R. China. It is an epiphytic herb, epiphytic on trees in karst regions at elevations between 1100–1800 m.
Etymology. The specific epithet ‘simaoensis’ refers to the type locality of this species, Liushun Township, Simao District, Pu'er City, Yunnan Province, P.R. China.
Relationships. Morphologically, Luisia simaoensis is similar to L. magniflora Z.H. Tsi & S.C. Chen and L. ramosii Ames. All three share lateral sepals with a keel on the abaxial surface, a lip with a distinct boundary between hypochile and epichile, a hypochile in which the lateral lobes embrace the column. L. simaoensis can be easily distinguished from these species by having a lateral sepals longer than the dorsal sepals and petals (Dorsal sepal ovate-elliptic, 8.5–9.0 mm long; lateral sepals oblong, slightly oblique, 12 mm long; petals oblong, 10 mm long. vs. dorsal sepal ovate-oblong, 9–12 mm long, lateral sepals suboblong, 10–12 mm long, margin incurved and embracing epichile, petals subelliptic, 10–11 mm long in L. magniflora; Dorsal sepal elliptic, 5 mm long, lateral sepals oblong, slightly oblique, 6 mm long, petals ovate, 6.2 mm long in L. ramosii); margins of lip irregular waved (vs. margins entire, recurved in L. magniflora; margins entire in L. ramosii), apex of lip bilobed (vs. apex of lip emarginate in L. magniflora; apex of lip obtuse in L. ramosii).
Luisia simaoensis is also similar to L. teres (Thunb.) Blume, but differs from the latter by having a lip with a distinct boundary between hypochile and epichile, a subcordate epichile, 9 × 11 mm, and an irregular waved margin.
Conservation status.Luisia simaoensis was found in Simao district, Lancang County, Ximeng County, Yunnan Province. Although it is distributed in several counties, the number of populations and range of distribution are still not clear. We here consider L. simaoensis as “Data Deficient (DD)” (IUCN, 2012).
Additional specimens examined (Paratypes). CHINA. Laba Township, Lancang County, Pu'er City, Yunnan Province, epiphytic on trees near riversides in karst regions, alt. 1230, 13 Sep. 2020, D.P. Ye 1923 (HITBC!, PE!)
Notes.Xu et al. (2010) published an inedita name as Luisia simaoensis D.P. Ye & H. Jiang, sp. nov. ined. in the Wild Orchids in Yunnan; however, only a diagnosis was provided, no type specimens designated. According to the International Code of N. for algae, fungi and plants (Shenzhen Code) (Turland et al., 2018), it is an invalid name (Art. 40.1, Turland et al., 2018). Here, the name of L. simaoensis is validly published. In addition, an illustration with color photos, distribution, IUCN conservation status and description are provided.
3.4.2. Luisia inconspicua (Hook.f.) King & Pantl., in Ann. Roy. Bot. Gard. (Calcutta) 8: 203. 1898. (兜唇钗子股 Dou Chun Chai Zi Gu). Fig. 6: A–C.
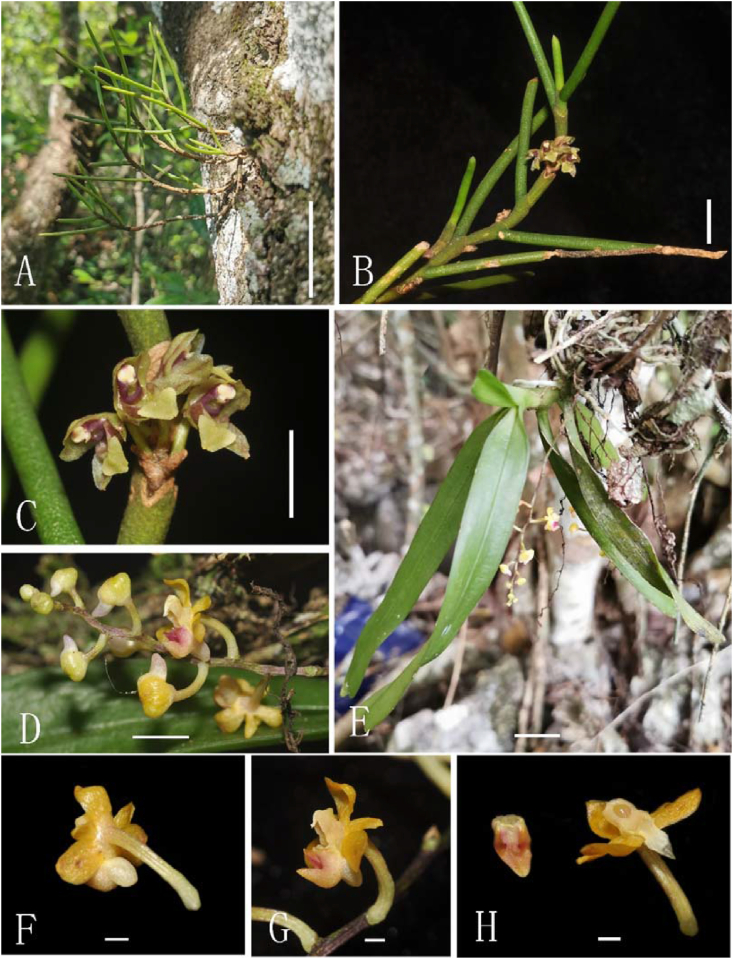
Fig. 6.
(A–C): Luisia inconspicua (Hook.f.) King & Pantl. (A) Plant, bar = 5 cm. (B) Plant with flowers, bar = 1 cm. (C) Flowers, bar = 1 cm. (D–H) Cleisostoma tricornutum Aver. (D) Inflorescence, bar = 1 cm. (E) Plant, bar = 1 cm. (F) Back view of flower, bar = 1 mm. (G) Side view of flower, bar = 1 mm. (H) Dissection of flower, bar = 1 mm (A photographed by Jian-Wu Li, B–H photographed by De-Ping Ye).
––Cymbidium inconspicuum Wall. ex Hook.f., Ann. Roy. Bot. Gard. (Calcutta) 5(1): 46. 1895; Gastrochilus inconspicuus (Hook.f.) Kuntze. Revis. Gen. Pl. 2: 661. 1891; Luisiopsis inconspicua (Hook.f.) Sath. Kumar & P.C.S. Kumar, Rheedea 15(1): 48. 2005; Saccolabium inconspicuum Hook.f., Fl. Brit. India. 6(17): 56. 1890.
Epiphytic, stems erect, rare ramose, branched at base and tufted, 5–15 cm tall, 0.3 cm in diameter, with internodes 0.5–0.7 cm long, perennial stems covered by persistent leaf sheaths, brownish, base with several roots, root depressed, 1.5–2.0 mm in diameter. Leaves several on upper part of stem, obliquely upward, irregular distichous, 6–9 mm apart from each other. Leaf blade terete, 2–5 cm long, ca. 2 mm in diam., base jointed and sheathing, 6–9 mm long, apex acute. Inflorescences come out from node of stem, penetrating leaf sheaths, green to dark purple, 1–3 every stem, 4–6 mm long, with 2–3 sheaths, sheath triangular, 1.3 × 3.0 mm, apex obtuse. Rachis 2–3 mm long, 4–5-flowered, flowers pale green. Flora bracts triangular, 2 × 2 mm, apex acute. Pedicel and ovary 5–6 mm long. Dorsal sepal oblong, 2.5 × 1.8 mm, apex obtuse to rounded; lateral sepals ovate-oblong, 3.2–3.4 × 1.5–1.6 mm, mid-vein slightly raised abaxially, apex acute. Petals oblong, 2.8–3.0 × 1.3–1.5 mm, apex obtuse to rounded. Lip ovate-oblong, slightly fleshy, with an epichile and a saccate hypochile, dense with purple spots abaxially; epichile subtriangular, recurved, 1.8 × 2.5 mm, apex obtuse and inconspicuous emarginate; hypochile nearly subglobose-cucullate, 2 mm tall, 1.5–2.0 mm in diam., lateral edges with an semicircular lobe above the oral area, ca. 0.9 mm tall, fleshy at bottom of saccate. Column stout, ca. 2.2 mm long, purple; rostellum bifid; anther cap cucullate; pollinia 2, ovate-orbicular; viscidium oblong, 0.5 × 0.1 mm; stipe linear, ca. 1 mm long.
Distribution. China (Yunnan), India, Nepal and Bhutan.
Phenology. Flowering from July to September.
Habitat. Epiphytic on trees in karst regions.
Specimens Examined. CHINA. Yunnan, Jinping County, Jinshuihe Town, alt. 400 m, 11 Aug. 2020, epiphytic on trees in karst regions. J.W. Li 6085 (HITBC!).
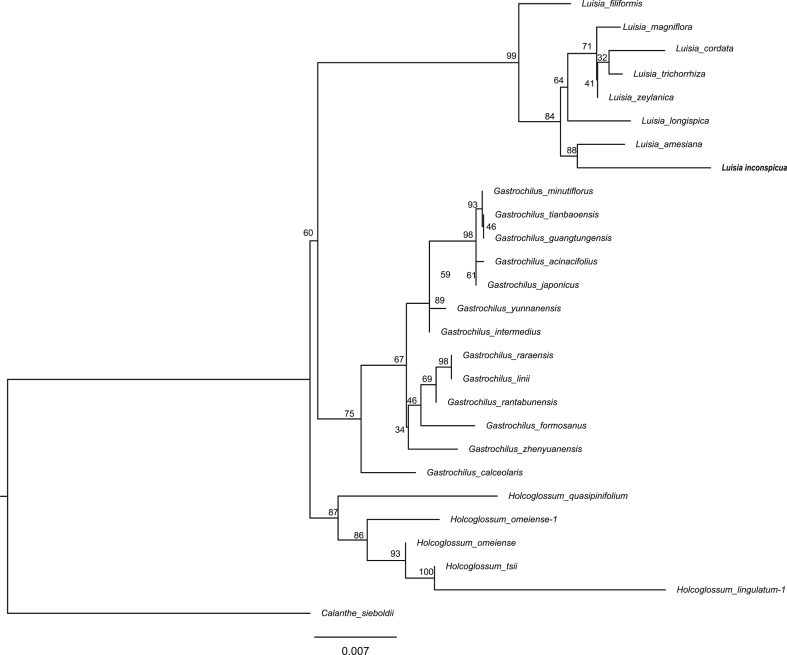
Note. Our phylogenetic analyses indicate that Luisia inconspicua belongs to Luisia with high support (Fig. 7). L. inconspicua is sister to Luisia amesiana.
Fig. 7.
Bayesian tree obtained from analysis of the combined data set showing the detailed relationships of Luisia inconspicua.
3.5. Cleisostoma Blume
Cleisostoma comprises approximately 110 species widely distributed from mainland tropical and subtropical regions of Asia, Malaysia, Indonesia, and the Philippines to Japan, Western Pacific islands and Australia (Ponert et al., 2016). There are 18 species in China four of which are endemic (Huang et al., 2020).
3.5.1. Cleisostoma tricornutum Aver. in Taiwania 60 (3): 108. 205. (角状隔距兰Jiao Zhuang Ge Ju Lan). Fig. 6: D–H.
––Type: Vietnam: Thanh Hoa Province, Thuong Xuan district, Van Xuan municipality, Hang Cao village, Xuan Lien Natural Reserve. 8 Nov. 2013, remnants of primary and secondary broad-leaved evergreen forest on highly eroded rocky limestone hills, Averyanov et al. CPC 6894 (Holotype: LE!)
Epiphytic herb. Roots several, basal, terete, ca. 1 mm in diameter. Stem pendulous and ascending, 1–6 cm tall, ca. 3 mm in diameter. Leaves several, distichous, flat, slightly V-shape, 9–18 × 1.1–2.0 cm, leathery, apex unequally bilobed, lobes acute to rounded, base jointed and amplexicaul-sheathing. Inflorescence 1–5, come out from basal axillary, unbranched, slender, 5–30 cm long, ca. 0.9 mm in diameter, with 1–4 cannular sheaths, sheath membranous, 2–3 × 1–2 mm, apex acute; rachis 4–18 cm long, slightly zig-zag curved, sparsely with many flowers, flower yellowish-white, lip with purple lateral lobes. Flora bracts triangular, 0.5–0.7 × 0.7–1.0 mm, apex acute. Pedicel and ovary 5–6 mm long. Dorsal sepal oblong, 4.0–4.5 × 1.3–1.5 mm, apex obtuse, slightly cymbiform, 5-veined; lateral sepals broadly ovate, 3.5–3.7 × 2.5–2.6 mm, apex obtuse, 5-veined; petals slightly oblique spatula, 2.6–2.8 × 1.1–1.2 mm, apex obtuse, 1-veined. Lip spurred, 3-lobed, lateral lobes erect, narrowly conic, parallel and forward protruding, ca. 1.0 × 1.5 mm, apex acute; mid-lobe sagittate-triangular, 2.5–2.6 × 1.9–2 mm, apex obtuse, central with a longitudinal groove, disc with 3 fleshy low indistinct keels, median slightly longer; spur conical, 1.6–1.7 mm long, apex rounded, inside with longitudinally septate. Back-wall callus globrous, simple. Column stout, 2.2–2.3 mm long, base slightly papillate, wings inconspicuous; anther cap beaked, pollinia 4, in 2 pair; rostellum filiform, ca. 1 mm long; viscidium oblong, ca. 0.3 mm long, stipe filiform, 1.6 mm long.
Distribution. China (Yunnan), Vietnam.
Phenology. Flowering from August to October.
Habitat. Epiphytic on trees or lithophytic on rocks in karst regions in tropical seasonal rain forests, at elevations between 100–1600 m.
Specimens Examined. CHINA. Tukahe village, Qushui township, Jiangcheng County, Yunnan Province, epiphytic on tree trunks in karst regions in tropical seasonal forest. E:102.28, N: 22.59, alt. 370 m, 8 Oct. 2019, Jianwu LI 5121 (HITBC!).
Notes.Averyanov et al. (2015) described flowers “not widely opening’. We observe that this species has flowers slightly spreading. It seems that plants and flowers are larger.
Declaration of competing interest
None declared.
Acknowledgments
This work was supported by Grant from National Forestry and Grassland Administration (No. 2019073018, 2019073019, 2019073002, 2019073003), Science and Technology Basic Resources Investigation Program of China “Survey and Germplasm Conservation of Plant Species with Extremely Small Populations in South-west China” (Grant No. 2017FY100100), National Natural Science Foundation of China (No. 31870195), Strategic Biological Resources Capacity Building Project from the Chinese Academy of Sciences “Seed Plants Research in Xishuangbanna Karst Region” (Grant No. KFJ-BRP-017-36), the Large-scale Scientific Facilities of the Chinese Academy of Sciences (Grant No. 2017-LSFGBOWS-02) and the National Wild Plant Germplasm Resource Center, National Science & Technology Infrastructure. We are grateful to Guoping YANG (Xishuangbanna tropical Botanical Garden) for his help in the field work, to Congrui AI for her to showing us the literature, and to Lian-Yi Li for his help with image processing.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Contributor Information
Jian-Wu Li, Email: ljw@xtbg.org.cn.
Xiao-Hua Jin, Email: xiaohuajin@ibcas.ac.cn.
References
- Averyanov L.V., Tich N.T., Canh N.V. New species of the genus Cleisostoma in the flora of Vietnam. Taiwania. 2015;60:107–116. doi: 10.6165/tai.2015.60.107. [DOI] [Google Scholar]
- Carr C.E. The genus Taeniophyllum in the Malay peninsula. The gardens’ bulletin Singapore. 1932;7:61–86. [Google Scholar]
- Chen S.C., Wood J.J. In: Wu Z.Y., et al., editors. vol. 25. Science Press; Beijing: 2009. Tuberolabium; pp. 504–505. (Flora of China). [Google Scholar]
- Hayata B. vol. 6. Bureau of Productive Industries, Government of Formosa; Taihoku: 1917. Gastrochilus pumilus; p. 78. (Supplement to Icones Plantarum Formosanarum). [Google Scholar]
- Huang M.Z., Liu D.K., Yin J.M., et al. Cleisostoma hainanense, a new species (Orchidaceae: Epidendroideae) from Hainan, China: evidence from morphological and DNA analyses. Phytotaxa. 2020;428:263–270. doi: 10.11646/phytotaxa.428.3.7. [DOI] [Google Scholar]
- IUCN . second ed. IUCN; Gland and Cambridge: 2012. IUCN Red List Categories and Criteria, Version 3.1; p. 32. [Google Scholar]
- Jin X.H., Li J.W., Ye D.P. Henan Science and Tecnology Press; 2019. Atlas of Native Orchids in China. [Google Scholar]
- Karuppusamy S., Ravichanderan V. A new species of Luisia (Orchidaceae: Epidendroideae: Vandeae) from the western Ghats of India. Phytotaxa. 2019;387(4):295–299. doi: 10.11646/phytotaxa.387.4.3. [DOI] [Google Scholar]
- Khuraijam J.S., Roy R.K. A new species of Luisia Gaud. (Orchidaceae) from northwestern Bihar, India. Biodivers. J. 2015;6:699–702. [Google Scholar]
- King G., Pantling R. VIII. Annals of the Royal Botanic Garden; Calcutta: 1898. p. 203. [Google Scholar]
- Kocyan A., Schuiteman A. New combinations in Aeridinae (Orchidaceae) Phytotaxa. 2013;161:61–85. doi: 10.11646/phytotaxa.161.1.3. [DOI] [Google Scholar]
- Kumar C.S., Kumar P.C.S. An orchid digest of Manipur, Northeastern India. Rheedea. 2005;15:1–70. [Google Scholar]
- Kuntze O. 1891. Revisio Generum Plantarum: Vascularium Omnium Atque Cellularium Multarum Secundum Leges Nomeclaturae Internationals Cum Enumeratione Plantarum Exoticarum in Itinere Mundi Collectarum. Pars II; p. 661. [Google Scholar]
- Liu Q., Gao J.Y. Gastrochilus dulongjiangensis (Aeridinae, Vandeae, epidendroideae, Orchidaceae), a new species from Yunnan province, China. Phytotaxa. 2018;340:293–296. doi: 10.11646/phytotaxa.340.3.11. [DOI] [Google Scholar]
- Liu Q., Tan Y.H., Gao J.Y. A new species of Gastrochilus (Aeridinae, Vandeae, Orchidaceae) and a new record species from Yunnan, China. Phytotaxa. 2016;282:66–170. doi: 10.11646/phytotaxa.282.1.8. [DOI] [Google Scholar]
- Mishra S., Jalal J.S., Vivek C.P., et al. A note on the occurrence of Luisia unguiculata (Orchidaceae) in Andaman and Nicobar islands, India. Nelumbo. 2020;62:50–53. doi: 10.20324/nelumbo/v62/2020/152396. [DOI] [Google Scholar]
- Mishra S., Jalal J.S., Paulose V.C., et al. Two new species of Luisia (Vandeae, Orchidaceae) from the Andaman and Nicobar islands, India. Phytotaxa. 2020;453:255–264. doi: 10.11646/phytotaxa.453.3.7. [DOI] [Google Scholar]
- Ormerod P., Juswara L. New names in Indonesian orchids. Harvard Paters in Botany. 2019;24:27–30. doi: 10.3100/hpib.v24iss1.2019.n5. [DOI] [Google Scholar]
- Ponert J., Trávníček P., Vuong T.B., et al. A new species of Cleisostoma (Orchidaceae) from the Hon Ba nature Reserve in Vietnam: a multidisciplinary Assessment. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWO . 2019. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew.http://www.plantsoftheworldonline.org/ Published on the Internet. [Google Scholar]
- Pridgeon A.M., Cribb P.J., Chase M.W., Rasmussen F.N. vol. 6. Oxford University Press; 2014. (Genera Orchidacearum). [Google Scholar]
- Raskoti B.B. A new species of Gastrochilus and new records for the orchids of Nepal. Phytotaxa. 2015;233:179–184. doi: 10.11646/phytotaxa.233.2.5. [DOI] [Google Scholar]
- Schlechter R. Die Gattungen Gastrochilus D. Don and Gastrochilus Wall. Repertorium Specierum Novarum Regnivegetabilis. 1913;12:313–317. doi: 10.1002/fedr.19130121713. [DOI] [Google Scholar]
- Schlechter R. Die Orchidaceen von Deutsch-Neu-Guinea: sarcanthinae. Repertorium Specierum Novarum Regni Vegetabilis Fedde, Beihefte. 1913;1:953–1039. [Google Scholar]
- Schrempf D., Minh B.Q., Haeseler A.V., et al. Polymorphism-aware species with advanced mutation models, bootstrap, and rate Heterogeneity. Mol. Biol. Evol. 2019;36:1294–1301. doi: 10.1093/molbev/msz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J. Die Orchidaceen von Java. Bull. Jard. Bot. Buitenzorg, sér. 1918;2:127–128. [Google Scholar]
- Tsi Z.H. A preliminary revision of Gastrochilus (Orchidaceae) Guihaia. 1996;16:123–154. [Google Scholar]
- Turland N.J., Wiersema J.H., Barrie F.R., et al., editors. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten. Koeltz Botanical Books; 2018. [DOI] [Google Scholar]
- Wu X.F., Ye D.P., Pan B., et al. Validation of Gastrochilus prionophyllus (Vandeae, Orchidaceae), a new species from Yunnan province, China. PhytoKeys. 2019;130:161–169. doi: 10.3897/phytokeys.130.34555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X.G., Li D.Z., Jin X.H., et al. Monophyly or paraphyly– The taxonomy of Holcoglossum (Aeridinae: Orchidaceae) PLoS One. 2012;7:e52050. doi: 10.1371/journal.pone.0052050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.H., Jiang H., Ye D.P., Liu E.D. Yunnan Science & Technology Press; 2010. The Wild Orchids in Yunnan. [Google Scholar]
- Zou L.H., Huang J.X., Zhang G.Q., et al. A molecular phylogeny of Aeridinae (Orchidaceae: epidendroideae) inferred from multiple nuclear and chloroplast regions. Mol. Phylogenet. Evol. 2015;85:247–254. doi: 10.1016/j.ympev.2015.02.014. [DOI] [PubMed] [Google Scholar]