Abstract
Aim
To evaluate the cost-effectiveness of [177Lu]Lu-DOTA-TATE versus relevant comparators for the treatment of neuroendocrine tumours located in the gastrointestinal tract (GI-NETs) and the pancreas (P-NETs).
Materials and methods
A three-state partitioned survival model was developed to perform a cost-utility analysis of [177Lu]Lu-DOTA-TATE versus standard of care (high dose Octreotide LAR), everolimus and sunitinib. Effectiveness data for SoC, everolimus and sunitinib were obtained from published Kaplan–Meier survival curves. Given a lack of head-to-head effectiveness data, matching adjusted indirect comparisons (MAICs) were performed to population-adjust [177Lu]Lu-DOTA-TATE survival data based on prognostic factors and derive estimates of relative effectiveness. Health state utilities were estimated from real-world evidence. Drug acquisition costs were taken from nationally published sources (BNF, NICE), and administration costs were based on treatment protocols in [177Lu]Lu-DOTA-TATE studies, combined with nationally published unit costs (PSSRU, DoH reference costs). Incidence of adverse events were estimated using published sources. A discount rate of 3.5% was applied to both utilities and costs, and deterministic and probabilistic sensitivity analyses were performed. Costs were included from an NHS perspective and presented in 2017/18 GBP (and PPP Euros for base case).
Results
In GI-NETs, the incremental cost-effectiveness ratio (ICER) of [177Lu]Lu-DOTA-TATE compared to SoC and everolimus was £26,528 (€27,672) and £24,145 (€25,186) per QALY, respectively. In P-NETs, the ICER of [177Lu]Lu-DOTA-TATE compared to SoC was £22,146 (€23,101) or £28,038 (€29,251) dependent on matched population, and £21,827 (€22,766) and £15,768 (€16,445) compared to everolimus and sunitinib, respectively.
Conclusions
At a willingness to pay threshold of £30,000, [177Lu]Lu-DOTA-TATE is likely to be a cost-effective treatment option for GI-NET and P-NET patients versus relevant treatment comparators (NHS perspective).
Keywords: Gastro-enteropancreatic neuroendocrine tumours (GEP-NETs), 177Lu-DOTA-octreotate, [177Lu]Lu-DOTA-TATE, Everolimus, Sunitinib, Quality-Adjusted Life Years (QALYs)
Highlights
-
•
A three-state partitioned survival model of GEP-NET patients was developed.
-
•
Base case ICERs for [177Lu]Lu-DOTA-TATE were under £30,000 per QALY for all comparators.
-
•
[177Lu]Lu-DOTA-TATE is cost-effective for the treatment of GEP-NETs from a NICE perspective.
1. Introduction
Gastroenteropancreatic neuroendocrine tumours (GEP-NETs) are a heterogeneous group of tumours originating in neuroendocrine cells of the gastrointestinal tract (GI-NETs) or pancreas (P-NETs) [1]. To date, the only curative treatment for GEP-NETs is surgery of resectable and localised tumours. In patients who are ineligible for surgery, targeted ablation therapies and transarterial embolisation can achieve regional control of liver metastases [2]. In patients with advanced, unresectable GEP-NETs, therapeutic options are limited, with somatostatin analogues (e.g. Octreotide LAR (Sandostatin®; Novartis International AG), Somatuline Autogel often used as best supportive care to limit symptoms and, in recent times, they have been shown to have some anti-tumour benefit [3,4]. Two biologically targeted therapies, everolimus (Afinitor®; Novartis International AG) and sunitinib (Sutent®; Pfizer Inc.) were approved in 2011 for the treatment of patients with advanced, progressive, well-differentiated P-NETs changing the treatment paradigm. Chemotherapy is usually reserved for high-grade progressive NETs but historically, streptozocin regimens have been utilised for advanced well-differentiated P-NETs and more recently temozolomide regimens for P-NETs. There is very little role for chemotherapy in well-differentiated GI-NETs. [177Lu]Lu-DOTA-TATE (Lutathera®; Advanced Accelerator Applications), a peptide receptor radionuclide therapy (PRRT) that selectively targets somatostatin receptors, received marketing authorisation for the treatment of GEP-NETs in 2017, with orphan designation from the European Medicines Agency (EMA) [5].
In addition to demonstrating clinical effectiveness, cost-effectiveness of new treatments plays a pivotal role in the decision-making process overseen by the National Institute of Health and Care Excellence (NICE) in England. A de novo partitioned survival model was constructed to evaluate the cost-effectiveness of [177Lu]Lu-DOTA-TATE for the NHS in England for the treatment of GEP-NETs. This paper details the structure, input parameters, and results of the model, including consideration of uncertainty in modelled outputs.
2. Methods
The model reflects a population of adult patients with inoperable, progressive GEP-NETs. In this analysis, the GEP-NET patient population was broken down into GI-NETs and P-NETs to reflect different effectiveness data and comparators. Most patients would have initially been treated first-line with somatostatin analogues and, thus, [177Lu]Lu-DOTA-TATE is usually used as a second- or third-line therapy. In the GI-NET patient subpopulation, [177Lu]Lu-DOTA-TATE was compared to standard of care (SoC), consisting of increased dose octreotide, and everolimus; and in the P-NET patient subpopulation, [177Lu]Lu-DOTA-TATE was compared to SoC, everolimus and sunitinib. It should be noted that everolimus is only indicated in non-functional GI-NETs and P-NETs and sunitinib solely for P-NETs [6,7].
Evidence for the efficacy of [177Lu]Lu-DOTA-TATE was derived from the ERASMUS single arm study for both GI-NET and P-NET patients and data in GI-NETs for everolimus and SoC were derived from the RADIANT-4 study [8,9]. For everolimus and SoC, data in P-NETs were derived from the RADIANT-3 study [10,11] and for sunitinib, data from NCT00428597 trial [12,13] were used.
The model comprises effectiveness, health-related quality of life (HRQoL) and cost data to perform a cost-utility analysis. Outcomes are presented as the cost per quality-adjusted life-years (QALYs).
2.1. Intervention and comparators
A summary of intervention and comparator regimen included in the analysis is presented in Table 1.
Table 1.
Intervention and comparators.
| Treatment | Indication | Regimen | Price |
|---|---|---|---|
| [177Lu]Lu-DOTA-TATE (Lutathera®) | GI-NETs and P-NETs | 4 administrations of 7.4 GBq (200 mCi), once every 8 weeks [28] | 29.6 GBq (800 mCi) = £71,500 |
| Standard of care (SoC) | GI-NETs and P-NETs | 60 mg Octreotide LAR (Sandostatin®), once every 28-day cycle | 30 mg vial = £998.41 |
| Everolimus (Afinitor®) | GI-NETs and P-NETs | 10 mg administered once daily, until progression [6] | 30-tab, 10 mg packs = £2673 |
| Sunitinib (Sutent®) | GI-NETs and P-NETs | 37.5 mg administered once daily, until progression [7] | 30-tab, 12.5 mg packs = £784.70 |
GI-NETs, neuroendocrine tumours located in the gastrointestinal tract; P-NETs, neuroendocrine tumours located in the pancreas.
2.2. Model structure
The model structure is illustrated in Fig. 1. In a partitioned survival model, survival is separated into distinct health states, which are associated with state specific estimates of HRQoL and/or costs. In oncology trials, progression-free survival (PFS) and overall survival (OS) constitute common study endpoints that are routinely collected, which can be used to construct partitioned survival models [14]. A partitioned approach was favoured over a state transition approach primarily because it enables the use of comparator trials where only aggregate level survival data are available from published Kaplan–Meier curves [15].
Fig. 1.
Partitioned survival model structure (stylised).
The model health states were defined as
-
1.
Progression-free survival: PFS(t) = P(PFS ≥ t)
-
2.
Post-progression survival: PPS(t) = P(OS ≥ t) − P(PFS ≥ t)
-
3.
Death: D(t) = 1 − P(OS ≥ t)
The modelled cohort starts in the progression-free state at year time zero. State membership is determined by PFS and OS curves, estimated using parametric models fitted to patient-level data (PLD) or Kaplan–Meier data. The proportion of the cohort in the progression-free state is determined by the PFS curve. The proportion of the cohort in the death state equals 1, minus the survival probability associated with the OS curve at any given time interval. The difference between the PFS curve and OS curves yields the proportion of modelled cohort in the post-progression state in any given time interval. Therefore, PFS and OS are implicitly modelled independently. Explored parametric fits included Weibull, Gompertz, lognormal and exponential. Clinical plausibility, visual inspection and AIC/BIC were used to determine the most appropriate parametric curves.
Specific estimates of HRQoL and background medical costs were attributed progression-free and post-progression health states reflective of severity of symptoms experienced by patients and utilisation of different healthcare resources as disease progresses.
2.3. Model inputs
2.3.1. Effectiveness
The pivotal Phase III (NETTER-1) clinical trial showed increased progression-free and overall survival in the [177Lu]Lu-DOTA-TATE arm compared to Octreotide LAR (60 mg) [16]. However, this trial only involved GI-NET patients with midgut NETs and does not allow for direct comparisons with other potentially relevant treatments, which have emerged in recent years.
The investigator-sponsored single-arm study ERASMUS provides an additional source for effectiveness data. This study was conducted at the Erasmus Medical Centre, Rotterdam, Netherlands, evaluating the efficacy of [177Lu]Lu-DOTA-TATE administered intravenously to patients with somatostatin receptor–positive tumours [9]. The study constitutes the longest available follow-up data of patients undergoing treatment with [177Lu]Lu-DOTA-TATE and included patients with GI-NET and P-NETs, with a high proportion of patients with observed PFS and OS events. However, this study does not contain a control arm; it was, therefore, necessary to perform indirect comparisons.
Two matching adjusted indirect comparisons (MAICs) were performed for GI-NET and P-NET patient populations using patient-level data (PLD) from the ERASMUS study for [177Lu]Lu-DOTA-TATE, published data from the RADIANT-4 [8] (GI-NETs) and RADIANT-3 [11] (P-NETs) trials for everolimus, and published data from the NCT00428597 trial for sunitinib [12]. Full details of these methods have been published previously (refer to MAIC publication). In brief, patient-level data from ERASMUS were reweighted based on prognostic factors and effect modifiers of survival, identified through engagement with clinicians, published literature and empirical investigation of the relationships in the ERASMUS PLD (age, gender, ECOG, previous radiotherapy and previous chemotherapy). This reweighting procedure produced survival data based on a population matched to the patient population in the comparator trials, for which only aggregate data were available. For OS comparisons in GI-NET, comparator data were only available in a GI-NET and lung NET population. To avoid potential bias from matching to this population, post-progression survival was assumed to be the same in SoC and everolimus arms as in [177Lu]Lu-DOTA-TATE, in line with the approach suggested by Hoyle and colleagues [17].
After reweighting survival outcomes, parametric time-to-event curves were fitted, mainly to extrapolate beyond the duration of the comparator randomised trials until the time horizon of the cost-effectiveness analysis. Parametric curves were preferred over reweighting, as this can produce long tails in Kaplan–Meier survival curves. Of these, the Weibull curve generally provided best fit to the observed data visually and based on AIC and BIC, except in the comparison versus sunitinib (see supplementary data Table A1.7). The Weibull curve was operationalised with functional form S (t) = exp {−(λt) β} where S = survival; t = time (interval); λ = location (scale); β = shape. The exponential curve was implemented as S (t) = exp {−(αt)}, where: S = survival; t = time (interval); α = rate parameter. Full parameterisation of the survival curves is given in supplementary data Table A1.1 to A1.6.
2.3.2. Adverse events
All active comparators are associated with an incidence of adverse events. These events may have both HRQoL and cost implications. Data were taken from clinical trials according to individual trial reporting criteria, accounting for more severe, at least grade III adverse events likely to have a HRQoL impact and/or cost consequence. Full details of adverse events and cost and utilities applied in the cost-effectiveness analysis are shown in supplementary data Table A2.1 to A2.2.
Given incidences are expressed as a proportion of patients experiencing adverse events during a specified treatment period, and assuming a patient is likely to only experience one of each of these events, costs and disutilities were accounted for in the first modelled interval.
2.3.3. Resource use and unit costs
Costs accounted for in the model were drug acquisition costs, drug administration costs, monitoring costs, costs of managing adverse events, and costs of palliative care (included in a scenario analysis). All costs are presented in 2017/18 prices. Where necessary, unit costs were inflated using PSSRU indices [18].
NETTER-1 and ERASMUS studies informed resources used to administer octreotide LAR and [177Lu]Lu-DOTA-TATE. Cost of the multidisciplinary approach, including physicist, radiographer, consultant time and an overnight stay (c. 90% are overnight, even though it is possible to perform as a day case) were estimated for Lutathera administration. Drug acquisition costs were taken from the British National Formulary (BNF) or manufacturers list price indicated in NICE guidance (see Table 1 and supplementary data Table A2.3). Only Octreotide LAR in-hospital along side [177Lu]Lu-DOTA-TATE and [177Lu]Lu-DOTA-TATE were assumed to have administration costs, the other treatments being oral medication with assumed use in an out-patient setting.
Monitoring resource use was based on NETTER-1 protocol, and unit costs were taken from Department of Health (DoH) NHS Reference Costs (2017/18) [19], see Table 3. Adverse event unit costs were also sourced from NHS Reference Costs, with associated resource use inferred from Common Terminology Criteria for Adverse Events (CTCAE) grading guidance notes [20]. It was assumed that grade III–V events would require a short stay hospital admission, except in the case of blood cell count events (neutropenia, lymphocyte count disease, lymphopenia) and asthenia/fatigue which were assumed to result in an outpatient attendance (see supplementary data Table A2.2).
Table 3.
Monitoring costs.
| Resource use item | Frequency | Unit cost (SE) | Source |
|---|---|---|---|
| CT/MRI | Every 12 weeks | £122.91 | NHS reference costs 2017/18 [19] |
| ECG | Every 8 weeks | £107.84 | NHS reference costs 2017/18 [19] |
| CBC with differential | Every 4 weeks | £2.51 | NHS reference costs 2017/18 [19] |
| Blood chemistry screen | Every 4 weeks | £2.51 | NHS reference costs 2017/18 [19] |
| Urinalysis | Every 4 weeks | £1.11 | NHS reference costs 2017/18 [19] |
To provide a benchmark and aid cross-country comparisons, costs were converted into Euros using purchasing power parity rates for the UK and Euro Area [21]. Base case results were presented in Euros (€) alongside cost-effectiveness expressed in GBP (£) (see Table 5, Table 6).
Table 2.
Drug administration costs.
| Resource use item | Frequency | Unit Costs (SE) | Source | Notes |
|---|---|---|---|---|
| Lutathera® | ||||
| Physicist | 30 min used in preparation of Lutathera® | £53 (53) | PSSRU | Band 7 hospital based professional per hour |
| Consultant | 15 min, administering Lutathera® and concomitant treatment | £108 (108) | PSSRU | General medicine consultant per hour |
| Radiographer | 1.5 h for preparation and administration of Lutathera® | £37 (37) | PSSRU | Band 5 radiographer per hour |
| Hospitaladmission (hotel costs) | One overnight stay | £431 (431) | NHS reference costs 2017/18 | Elective inpatient excess bed day |
| Octreotide LAR | ||||
| Day ward nurse | 30 min, administering Octreotide LAR | £37 (37) | NHS reference costs 2017/18 | Band 5 Nurse per hour |
| Outpatient day attendance | 0 h for administering Octreotide LAR | £698 (698) | NHS reference costs 2017/18 | Day case- assumed 12 h admission |
Table 5.
GI-NET results: [177Lu]Lu-DOTA-TATE versus SoC, [177Lu]Lu-DOTA-TATE versus everolimus.
| Treatment modalities | Costs | QALYs | Incremental costs | Incremental QALYs | ICER (cost/QALY) | P (cost-effective) at WTP £30,000 |
|---|---|---|---|---|---|---|
| SoC | £67,454 | 2.94 | ||||
| [177Lu]Lu-DOTA-TATE | £100,073 | 4.17 | (€34,040) | 1.23 | £26,528 (€27,672) | 77% |
| Everolimus | £74,687 | 3.1 | ||||
| [177Lu]Lu-DOTA-TATE | £100,584 | 4.17 | (€27,015) | 1.07 | £24,145 (€25,186) | 88% |
GI-NETs, neuroendocrine tumours located in the gastrointestinal tract; P-NETs, neuroendocrine tumours located in the pancreas; QALYs, quality-adjusted life-years.
P stands for probability, SoC for standard of care and WTP for Willingness to Pay. In brackets the ICER values are provided for the costs in euros based on Purchasing Power Parity rates.
Table 6.
P-NET results: [177Lu]Lu-DOTA-TATE versus SoC, [177Lu]Lu-DOTA-TATE versus everolimus, [177Lu]Lu-DOTA-TATE versus sunitinib.
| Treatment modalities | Costs | QALYs | Incremental costs | Incremental QALYs | ICER (cost/QALY) | P (cost-effective) at WTP £30,000 |
|---|---|---|---|---|---|---|
| SoC (RADIANT-3) | £60,326 | 3.12 | ||||
| [177Lu]Lu-DOTA-TATE | £111,289 | 4.94 | £50,963 (€53,169) | 1.82 | £28,038 (€29,251) | 65% |
| SoC (NCT00428597) | £53,033 | 2.96 | ||||
| [177Lu]Lu-DOTA-TATE | £118,525 | 5.64 | £65,491 (€68,315) | 2.96 | £22,146 (€23,101) | 99% |
| Everolimus | £72,497 | 3.25 | ||||
| [177Lu]Lu-DOTA-TATE | £113,103 | 5.11 | £40,606 (€42,352) | 1.86 | £21,827 (€22,766) | 96% |
| Sunitinib | £81,350 | 3.55 | ||||
| [177Lu]Lu-DOTA-TATE | £117,915 | 5.87 | £36,617 (€38,135) | 2.32 | £15,768 (€16,445) | 100% |
GI-NETs, neuroendocrine tumours located in the gastrointestinal tract; P-NETs, neuroendocrine tumours located in the pancreas; QALYs, quality-adjusted life-years.
P stands for probability, SoC for standard of care and WTP for Willingness to Pay.
2.3.4. Utilities
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) instrument [22] was used to collect QoL data directly from patients in the NETTER-1 and ERASMUS studies. A further source of UK-specific real-world evidence (RWE) was identified from EORTC data collected at St Guys and Thomas's Hospital (London, UK), a large centre treating UK GEP-NET cases [23]. However, the EORTC QLQ-C30 does not explicitly provide utility values required to compute QALYs. Data collected in these three studies were mapped to the EQ-5D-3L for use in the cost-effectiveness model using the algorithm published by Longworth et al. [24].
Data from RWE was used to inform progression-free HRQoL in the GI-NET population. Given the data collection points and time until progression in these studies, post-progression utility data were only available for a small number of patients (<5) in the NETTER-1 study or RWE. HRQoL data for GI-NET patients in the ERASMUS study were therefore used for the post-progression health state in the model. For P-NETs, the Erasmus dataset constituted the largest source of P-NET-specific HRQoL data [25]. Health state utility values used in the model are shown in Table 4.
Table 4.
Partitioned health state utility values.
| Health state | GI-NET utility value (95%CI) | Source | P-NET utility value (95%CI) | Source |
|---|---|---|---|---|
| Progression-free | 0.793 (0.771–0.815) | RWE [23] | 0.805 (0.793–0.816) | ERASMUS [25] |
| Post-progression | 0.740 (0.721–0.759) | ERASMUS [25] | 0.790 (0.758–0.823) | ERASMUS [25] |
| Death | 0 | Assumption | 0 | Assumption |
GI-NETs, neuroendocrine tumours located in the gastrointestinal tract; P-NETs, neuroendocrine tumours located in the pancreas.
A disutility associated with each of the adverse events was applied in the relevant model arm. Disutilities were sourced through a pragmatic literature review, or obtained from previous NICE technology appraisals, as shown in supplementary data Table A2.2.
2.4. Cost-effectiveness analysis
The cost-utility analysis was operationalised in Microsoft Excel©, with intervals of a month for the partitioned survival model. A discount rate of 3.5% was applied to both utilities and costs in line with UK recommendations [26], with half-cycle correction using a life table approach [27]. The model was run for a time horizon of 20 years approximating a lifetime analysis, given the average cohort age of 63.7 years at the start of the model.
Deterministic sensitivity analysis was performed, with lower and upper bounds informed by 95% confidence intervals, or a wide interval used (±10% or 25%). Five thousand iterations were run to conduct probabilistic sensitivity analysis (PSA), with Gamma distributions used for costs and Beta distribution for utilities and relative dose intensity. Where an appropriate measure of dispersion was not available, standard error was assumed equal to the mean value. Survival probabilities at any given time interval were drawn from appropriate variance covariance matrices, calculated using the Cholesky decomposition. Results are presented as an incremental cost-effectiveness ratio (ICER), with associated probability of [177Lu]Lu-DOTA-TATE being cost-effective at a willingness to pay threshold of £30,000 per QALY.
3. Results
The cost-effectiveness results for GI-NETs and P-NETs are shown in Table 5, Table 6, respectively. For all comparisons, [177Lu]Lu-DOTA-TATE produced additional QALYs at an additional cost. The health benefit ranged from 1.07 to 2.96 QALYs, with incremental costs ranging from £25,896 (€27,015) to £65,491 (€68,315) translating into ICERs between £15,768 (€16,445) and £28,038 (€29,251) per QALY gained (Table 5, Table 6).
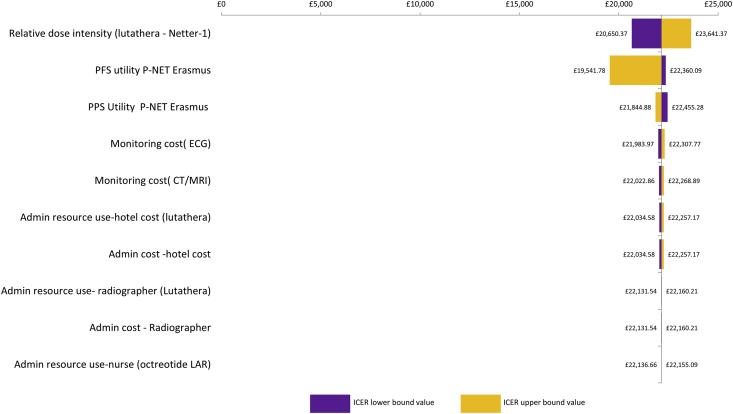
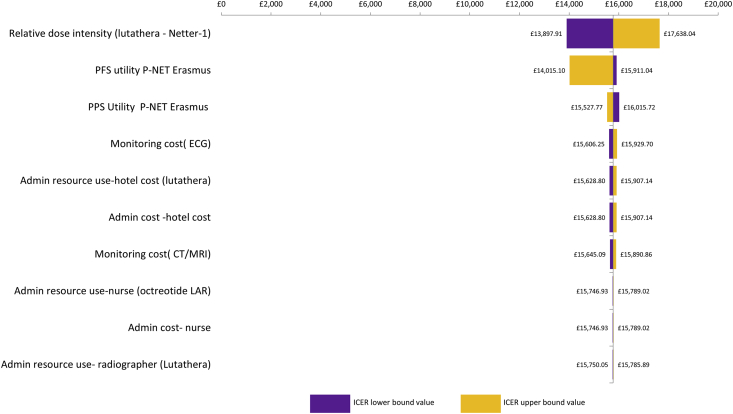
Results of one-way sensitivity analysis for P-NETs are shown in Tornado diagrams (Fig. 2, Fig. 3, Fig. 4, Fig. 5) and demonstrate that the ICER is most sensitive to the relative dose intensity of [177Lu]Lu-DOTA-TATE and to a lesser extent to health state utility values. In GI-NET and P-NET analyses, results were robust to changes in input parameters, with most ICERs remaining below £30,000 and the highest ICER being £30,469.
Fig. 2.
Tornado diagram: P-NET [177Lu]Lu-DOTA-TATE versus SoC - RADIANT-3 MAIC. P-NETs, neuroendocrine tumours located in the pancreas.
Fig. 3.
Tornado diagram: P-NET [177Lu]Lu-DOTA-TATE versus everolimus. P-NETs, neuroendocrine tumours located in the pancreas.
Fig. 4.
Tornado diagram: P-NET [177Lu]Lu-DOTA-TATE versus SoC–NCT00428597 MAIC. P-NETs, neuroendocrine tumours located in the pancreas.
Fig. 5.
Tornado diagram –P-NET [177Lu]Lu-DOTA-TATE versus sunitinib. P-NETs, neuroendocrine tumours located in the pancreas.
When all input parameter uncertainty is jointly considered, probabilistic sensitivity analyses suggest a high likelihood that [177Lu]Lu-DOTA-TATE is cost-effective at a willingness to pay threshold of £30,000 per QALY and probability of [177Lu]Lu-DOTA-TATE being cost-effective in P-NETs ranged from 65% compared with SoC to 100% compared to sunitinib. The respective probability of [177Lu]Lu-DOTA-TATE being cost-effective in GI-NETs was 77% compared with SoC and 88% compared to everolimus (CEP versus SoC and CEAC versus everolimus) (see Figs. 6 and 7).
Fig. 6.
Cost-effectiveness plane (CEP)–GI-NET [177Lu]Lu-DOTA-TATE versus SoC. GI-NETs, neuroendocrine tumours located in the gastrointestinal tract.
Fig. 7.
Cost-effectiveness Acceptability Curve (CEAC) – GI-NET [177Lu]Lu-DOTA-TATE versus everolimus. GI-NETs, neuroendocrine tumours located in the gastrointestinal tract,
4. Discussion
Demonstrating cost-effectiveness constitutes an important aspect of reimbursement for multiple decision-making bodies worldwide, including NICE. Cost-effectiveness analyses for [177Lu]Lu-DOTA-TATE were performed using a partitioned survival model, reflecting PFS and OS [15]. Analyses demonstrate cost-effectiveness of [177Lu]Lu-DOTA-TATE at a willingness to pay threshold of £30,000 per QALY for all GI-NET and P-NET model comparators and at a threshold of £20,000 per QALY in P-NET versus sunitinib, with all results robust to uncertainty and changes in model inputs. This is the first study to present data on the cost-effectiveness of [177Lu]Lu-DOTA-TATE relating to the UK.
It should, however, be noted that heterogeneity of trials included in the conducted MAIC partly contributed to the uncertainty of derived relative efficacies. Furthermore, no overall survival data were available for GI-NET in RADIANT-4 (it included lung NET patients in addition to GI-NET). In line with Hoyle et al. [17], post-progression was assumed equal to that of [177Lu]Lu-DOTA-TATE for SoC and everolimus. MAICs, especially those based on single-arm unanchored comparisons require multiple assumptions, whereby all variation in survival outcomes is assumed to be accounted for by included covariates, which is rarely likely to be the case. Matching of study populations in terms of prognostic factors may also lead to a substantial decrease in effective sample size.
The modelling approach detailed in this paper formed the basis of the manufacturer submission for the NICE Technology Appraisal TA539 which resulted in a positive recommendation, subject to a non-disclosed commercial arrangement (simple discount). Some aspects of the modelling have been updated since TA539, including unit costs, to reflect 2017/2018 prices and present more robust unit cost data; MAIC analyses, to include more covariates following engagement with clinicians; and adverse events, to account for occurrence of one event based on reported rates, rather than assuming occurrence of multiple events during treatments, as per NICE Economic Review Group analyses in TA539.
Base case results were presented in Euros alongside GBP, based on adjustment using purchasing power parity rates to aid cross country comparisons. However, these should be interpreted with extreme caution given country-specific health systems, payment mechanisms, drug reimbursement procedures and assessment bodies and specific treatment costs across jurisdictions, including the impact of negotiations and discounts. Evaluations based on local costs should be conducted to determine cost-effectiveness.
5. Conclusion
Based on these analyses it can be concluded that [177Lu]Lu-DOTA-TATE is likely to be a cost-effective treatment option compared to alternatives used in GEP-NET patients from an England NHS perspective. Performed sensitivity analyses demonstrate the robustness of cost-effectiveness estimates to input parameter uncertainty.
Role of the funding sources
PHMR received financial support from AAA for the work, including for the pragmatic review, developing the model and preparation of the manuscript.
Disclosure
This paper is part of a supplement supported by Advanced Accelerator Applications (AAA), a Novartis company.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Matthew Glover and Louise Longworth reports being employees at PHMR. PHMR reports financial support from Advanced Accelerator Applications for the work, including the development of the models and preparation of the manuscript.
Martyn Caplin reports research, speaker and advisory board honoraria from, Advanced Accelerator Applications, Novartis, Ipsen, Lexicon and Pfizer.
Oscar R Leeuwenkamp Novartis and having Novartis shares reports being employed by Advanced Accelerator Applications/Novartis and shares in Novartis.
Acknowledgments
The authors would like to acknowledge Dr Persefoni Ioannou for providing editorial support from the outset of this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejcsup.2021.06.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Oberg K., Knigge U., Kwekkeboom D., Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2012 Oct;23(Suppl 7):vii124–v130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 2.Basu B., Sirohi B., Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer. 2010 Mar;17(1):R75–R90. doi: 10.1677/ERC-09-0108. [DOI] [PubMed] [Google Scholar]
- 3.Caplin M.E., Pavel M., Ćwikła J.B., Phan A.T., Raderer M., Sedláčkova E., et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016 Mar;23(3):191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinke A., Wittenberg M., Schade-Brittinger C., Aminossadati B., Ronicke E., Gress T.M., et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 2017;104(1):26–32. doi: 10.1159/000443612. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency . 2017. Recommendation for maintenance of orphan designation at the time of marketing authorisation. EMA/552373/2017. [Google Scholar]
- 6.European Medicines Agency. Afinitor - summary of product characteristics.
- 7.European Medicines Agency. Sutent - summary of product characteristics.
- 8.Singh S., Carnaghi C., Buzzoni R., Pommier R.F., Raderer M., Tomasek J., et al. Efficacy and safety of everolimus in advanced, progressive, nonfunctional neuroendocrine tumors (NET) of the gastrointestinal (GI) tract and unknown primary: a subgroup analysis of the phase III RADIANT-4 trial. J Clin Oncol. 2016 Feb;34(4_suppl):315. [Google Scholar]
- 9.Brabander T., van der Zwan W.A., Teunissen J.J.M., Kam B.L.R., Feelders R.A., de Herder W.W., et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017 Aug;23(16):4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 10.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E., et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao J.C., Pavel M., Lombard-Bohas C., Van Cutsem E., Voi M., Brandt U., et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol. 2016 Nov;34(32):3906–3913. doi: 10.1200/JCO.2016.68.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faivre S., Niccoli P., Castellano D., Valle J.W., Hammel P., Raoul J.-L., et al. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol Off J Eur Soc Med Oncol. 2017 Feb;28(2):339–343. doi: 10.1093/annonc/mdw561. [DOI] [PubMed] [Google Scholar]
- 13.Raymond E., Dahan L., Raoul J.-L., Bang Y.-J., Borbath I., Lombard-Bohas C., et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 14.Llovet J.M., Di Bisceglie A.M., Bruix J., Kramer B.S., Lencioni R., Zhu A.X., et al. Design and endpoints of clinical trials in hepatocellular carcinoma. JNCI J Natl Cancer Inst. 2008 May;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 15.Woods B., Sideris E., Palmer S., Latimer N., Soares M. 2017. Nice Dsu technical support Document 19: partitioned survival analysis for decision modelling in health care: a critical review report by the decision support unit. [Google Scholar]
- 16.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017 Jan;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyle M., Hamilton W., Rudin C. When it may not Be necessary to model overall survival for economic evaluations of anti-cancer drugs. Value Heal J Int Soc Pharmacoeconomics Outcomes Res. 2014 Nov;17(7):A584. doi: 10.1016/j.jval.2014.08.1983. [DOI] [PubMed] [Google Scholar]
- 18.Curtis L., Burns A. Unit costs of health and social care 2018. Pers Soc Serv Res Unit. 2018 [Google Scholar]
- 19.NHS Improvement . 2018. NHS reference costs 2017/18. National schedule of reference costs. [Google Scholar]
- 20.U.S Department of Health and Human Services . 2017. Common Terminology criteria for adverse events (CTCAE) version 5.0. [Google Scholar]
- 21.Conversion rates - Purchasing power parities (PPP) - OECD Data [Internet]. [cited 2021 Mar 28]. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.
- 22.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 23.AAA Ltd. Guy's and St Thomas's study EORTC QLQC30 data (data on file).
- 24.Longworth L., Yang Y., Young T., Mulhern B., Hernandez Alava M., Mukuria C., et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014 Feb;18(9):1–224. doi: 10.3310/hta18090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AAA Ltd. ERASMUS study QLQC30 data (data on file).
- 26.NICE . 2013. Guide to the methods of technology appraisal. [PubMed] [Google Scholar]
- 27.Barendregt J.J. The half-cycle correction: banish rather than explain it. Med Decis Making. 2009;29(4):500–502. doi: 10.1177/0272989X09340585. [DOI] [PubMed] [Google Scholar]
- 28.EMA. Lutathera | European Medicines Agency.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.