Abstract
Background: Adalimumab, golimumab, infliximab, certolizumab, and etanercept are five anti-tumor necrosis factor (anti-TNF) medicines that have been approved for use in rheumatology. Apart from their well-established therapeutic usefulness, -it is unclear to what extent -they are linked to an increased risk of various side effects. The present meta-analysis was carried out to assess the risk of infection and other side effects after anti-TNF- α for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Methods: We searched PubMed, Cinahl (via Ebsco), Scopus, and Web of Sciences databases for trials comparing anti-TNF medications to placebo or no therapy in adult patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis from August 2006 to August 2020. A total of 23 articles were used for meta-analysis. The Cochrane Collaboration’s risk of bias tool was used to assess the methodological quality of the included studies. In addition, a random-effects model was used to calculate the pooled odds ratio, and Forest plots were constructed to determine the risk of infections and cancer following the use of anti-TNF treatment.
Results: Treatment with anti-TNFα agents resulted in an increase in the risk of serious infections (OR: 1.72, 95% CI: 1.56–1.90, p < 0.00001) and an increase in cancer risk (OR: 1.36, 95% CI: 1.20–1.53, p < 0.00001) whereas the risk of developing tuberculosis was not significantly different with anti-TNFα agents versus those without treatment with anti-TNFα agents (OR: 2.55, 95% CI: 0.40–16.23, p = 0.32) although the number of studies is limited to make a definitive conclusion. The risk of bias of the included studies was unclear to high across most domains, and there was evidence of publication bias for most outcomes.
Conclusion: The present meta-analysis suggests an increased risk of infectious adverse events, including overall adverse events and cancer following anti-TNFα treatment, whereas the risk of tuberculosis was not significantly different. Although anti-TNF agents have shown promise to treat inflammatory conditions, their use should be balanced by the risk-benefit ratio as suggested by the meta-analysis.
Keywords: anti TNF therapy, rheumatoid arthritis, psoriatic arthritis (artritis psoriatica), ankylosing spondylitis, risk of infections, malignancy
Highlights
The present evidence suggests an increased risk of infections and malignancy after anti-TNF treatment. However, the risk of tuberculosis after anti-TNFα alpha therapy was not significant in the present meta-analysis. Therefore, it is essential to balance the risk-benefit profile when treatment with anti-TNFα is initiated in patients with inflammatory conditions.
Data on the development of adverse events following anti-TNFα treatment from data registries and surveillance reports is necessary to understand the long-term implications of treatment with biologic agents that extend beyond the time frame of randomized controlled trials.
Introduction
Tumor necrosis factor (TNF) and interleukin-1 (IL-1) have been shown to play an essential role in the pathogenesis of inflammatory conditions such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) (Listing et al., 2005). Therefore, drugs targeting TNF and IL-1 have been developed to neutralize the effects of these pro-inflammatory cytokines. Five anti-TNF agents are currently available for clinical use in rheumatology: adalimumab, golimumab, infliximab, certolizumab, and etanercept. Adalimumab and golimumab are fully human monoclonal antibodies; infliximab is a chimeric monoclonal antibody with a murine variable region; certolizumab is a humanized Fab fragment conjugated with polyethylene glycol, while etanercept is a fusion protein of two TNF2 receptor extracellular domains and the Fc fragment of human immunoglobulin 1 (Curtis et al., 2007; Minozzi et al., 2016). Although these biologic agents differ in structure, they all act by neutralizing TNFα, which is implicated in early inflammatory events associated with several conditions. In addition, TNFα inhibitors have been used in rheumatological conditions that are unresponsive to treatment with disease-modifying anti-rheumatic drugs (DMARDs) such as sulfasalazine, chloroquine, hydroxychloroquine, D penicillamine, and azathioprine, among others. The Food and Drug Administration (FDA) has approved the use of biological TNFα blockers: Remicade® (infliximab), Enbrel® (etanercept), Humira® (adalimumab), Cimzia® (certolizumab pegol), and Simponi® (golimumab).
Though several randomized controlled trials have successfully demonstrated the efficacy of these TNFα inhibitors, there is still debate regarding potential untoward effects of these biologicals upon long-term use. However, long-term use of anti-TNFα agents has been associated with the risk of serious infections, malignancies, skin and soft tissue infections, and tuberculosis (Baghai et al., 2001; Kroesen et al., 2003; Colombel et al., 2004). A meta-analysis carried out in 2006 showed an increase in the risk of malignancies and serious infections in patients treated with infliximab and adalimumab, where a higher dose was associated with increased cancer risk (Galloway et al., 2011). Although there has been no consensus on the risk of infections associated with anti-TNFα treatments in published clinical trials, post-marketing surveillance has shown an increase in the risk of tuberculosis and other granulomatous infections (Keane et al., 2001; Wallis et al., 2004). A recent meta-analysis by Bonovas et al. examined the effect of TNF inhibitors on the occurrence of malignancies in adult patients with RA, PsA, or AS. This study showed that there was no effect of anti-TNF agents on cancer risk in patients with RA, PsA, or AS using either fixed-effects or random-effects models. Furthermore, subgroup analysis according to the type of anti-TNF agent, did not demonstrate any statistically significant association between adalimumab, golimumab, infliximab, certolizumab, or etanercept and cancer risk.
Many countries have also developed national database registries that compile treatment outcomes and complications related to the prescribed treatment. These databases help in analyzing the safety of complications with biological therapies. However, many studies, including RCTs, case reports, etc., are emerging, stressing the incidence of various complications after receiving biological therapy for these disorders (Singh et al., 2009; Higgins et al., 2011; Abbott Laboratories, 2012; Downey, 2016).
The primary aim of this meta-analysis was to determine the relationship between anti-TNF α treatment versus no anti-TNF α treatment (or treatment with DMARDs) and risk of development of adverse effects such as serious infections, skin, and soft tissue infections and malignancies using data from randomized controlled trials (RCTs) and database registries in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) normative recommendations in this study with the registration number NMU # RC/IRB/2020/3941.
Data Search
From August 2006 to August 2020, a systematic search of PubMed, Cinahl (via Ebsco), Scopus, and Web of Sciences bibliographic databases was undertaken. Anti-tumor necrosis factor, tumor necrosis factor(s), tumor necrosis factor-alpha antibody (ies), tumo(u)r necrosis factor antibody (ies), anti-TNF, TNF, biologic (al) agent(s), or biologic(s), combined with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. The search was restricted to observational studies, RCTs, and data from disease registries involving human participants. There were no limitations on grammar, date, or publishing status. We also looked through the Cochrane Library for any observational studies and RCTs that were part of the Cochrane Central Register of Controlled Trials, as well as any systematic reviews on the subject.
Two analysts (JL and ZZ) reviewed the search results separately and screened the titles and abstracts to exclude papers that were simply unrelated. The full text of the selected papers was scrutinized for eligibility. Their reference lists (and those of related reviews and meta-analyses) were manually checked for additional studies. Experts were surveyed for additional evidence, but no unpublished research or results were sought.
Data Extraction
Observational Studies and RCTs evaluating an anti-TNF agent (adalimumab, certolizumab, etanercept, golimumab, or infliximab) as induction or maintenance therapy for adults with RA, PsA, or AS and reporting the presence of infectious adverse events and any type of cancer were considered. Any bacteria, severe infections (infections requiring antimicrobial treatment and/or hospitalization), cancer, tuberculosis, or opportunistic infections were all eligible outcomes. In addition, we looked for trials that linked an anti-TNF therapy to placebo or no medication, as well as multi-interventional treatments where the anti-TNF treatment effect could be isolated (i.e., an add-on to conventional disease-modifying anti-rheumatic drugs).
Eligibility of Criteria
The articles were reviewed from the title or abstract. Case reports, adult studies, reviews, and editorial articles were excluded. However, articles concerning adults with at least one defined treatment group addressed various adverse effects written in English.
Information on the following aspects were included in the meta-analysis: risk of serious infections, risk of skin and soft tissue infections, tuberculosis, and cancer following treatment with anti-TNFα agents compared to non-biologics.
The primary search yielded 123 results. The articles were excluded based on the exclusion criteria. These included the 46 articles, out of which 23 articles were used for data analysis. The article search was limited to the English language, and no other limiting factor was used in finalizing the study. The details of the number of articles included are given in Figure 1.
FIGURE 1.
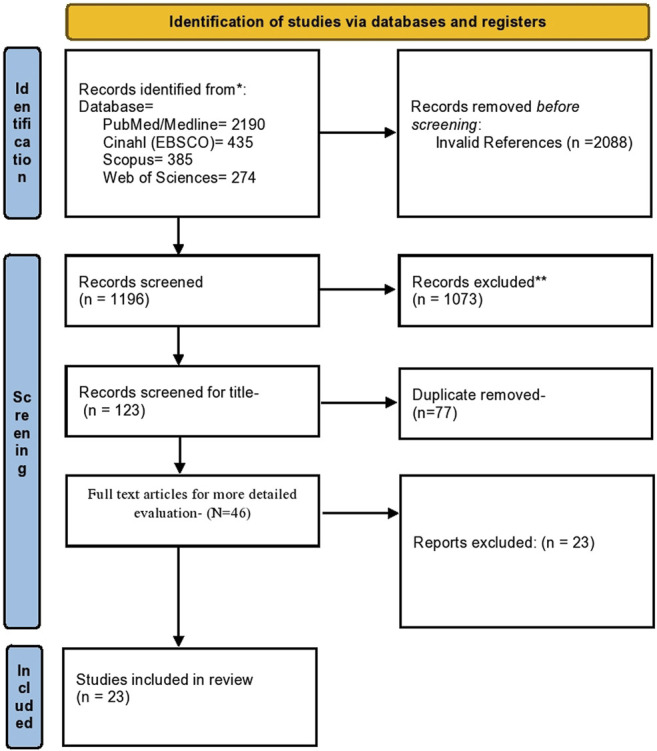
Flow chart for identification and inclusion of studies in the meta-analysis (PRISMA statement).
When there were several papers from the same sample, we chose the most recent one and extracted the results over the longest possible period. Independent researchers oversaw data extraction. Any inconsistencies were overcome by consensus regarding the original paper. In addition, each study’s first author’s last name, journal and year of publication, trial acronym, study design and duration, number of participants, disease studied (RA, PsA, or AS), patient characteristics (age, concurrent treatments, disease duration), intervention parameters (drug, dose, administration), and numbers of participants with events (serious infections) were extracted.
Quality Assessment
The Cochrane Collaboration’s risk of bias tool was used to assess the methodological quality of the included studies (Higgins et al., 2011). This tool includes the following criteria: randomization, allocation concealment, blinding, and completeness of follow-up. In addition, the risk of bias for each item was graded as high, low, or unclear risk.
Statistical Analysis
Meta-analysis was performed using Review Manager (RevMan, Version 5. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration. 2020). The total number of participants in each study developing an adverse event and the total number of participants in each group (intervention: anti-TNFα and control group: without anti-TNFα treatment) were used to calculate the odds ratio 95% confidence interval (CI). Meta-analyses were done using a random-effects model (Mantel-Haenszel method), and heterogeneity in the included studies was evaluated using the I 2 statistic, with small heterogeneity for I 2 values of 25%, moderate heterogeneity for I 2 values of 25–50%, and high heterogeneity for I 2 values >50% (Higgins et al., 2003). Forest plots were constructed, and p < 0.05 was statistically significant.
Evaluation of the Publication Bias
Begg’s Funnel plot was used to determine the publication bias of the included studies. The methodological validity of the included studies was evaluated by two reviewers (JL and ZZ) separately. XW and JZ were in charge of resolving any disagreements between authors.
Results
Literature Search Results
Through electronic scans, we found a total of 3284 studies. By reading titles and abstracts, we excluded 1,073 on reading titles and abstracts and 2088 invalid references. Out of 123 studies, around 77 studies were excluded based on duplicity. Full-text publications were required for final screening was 46, of which 23 were excluded based on the inclusion criteria. Thus, the study and meta-analysis contained 23 studies that met the inclusion criteria, i.e., based on the adverse events associated with anti-TNF treatment, as shown in Figure 1. Inappropriate comparison criteria and inadequate evidence to create 2 × 2 tables for review were the key reasons for the omission.
Table 1 shows the demographic details of the studies included in the present meta-analysis describing the study author, year of publishing, place where the study was conducted, total sample size with age, the individual sample size in each group using anti-TNF agents compared to non-biologic, type of adverse events reported in groups using anti-TNFα agents and controlled or placebo, and key findings of all the included study. All studies were released as full-text papers. Infliximab (n = 12), adalimumab (n = 18), golimumab (n = 10), certolizumab pegol (n = 8), or etanercept (n = 11) is tested as induction or maintenance therapies for adult patients with RA (n = 38), PsA (n = 6), or AS (n = 8) in these 23tudies. A total of 75,406 patients were included in the present meta-analysis.
TABLE 1.
Demographic Details of Included Studies.
| Studies with year | Place | Study design | Sample size | Anti-TNF agent | Inflammatory condition | Follow-up | Mean age (yrs) | Sample size in anti TNF-α v/s controlled or placebo | Adverse events reported | Sample size with adverse events | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti TNF-α | Without anti TNF-α | Anti TNF-α | Without anti TNF-α | ||||||||||
| Galloway et al. (2011) | United Kingdom | Prospective Observational Study | 15,396 | Adalimumab, etanercept, infliximab | RA | 6 months | 58 years | 11,798 | 3598 | Serious Infection | 1,512 | 296 | 1.64 (1.44–1.87) |
| Curtis et al. (2007) | United States | Retrospective Cohort Study | 5,326 | Adalimumab, etanercept, infliximab | RA | 17 months | 52.5 ± 12.5 | 2,393 | 2,933 | Serious bacterial Infection | 65 | 58 | 1.38 (0.97–1.98) |
| Dixon et al. (2006) | United Kingdom | Prospective Observational Study | 9,018 | Adalimumab, etanercept, infliximab | RA | 6 months | 58 years | 7,664 | 1,354 | Serious Infection | 525 | 56 | 1.70 (1.29–2.26) |
| Carmona et al. (2007) | Spain | Registry For Active Long-Term Follow-Up | 1,578 | Infliximab, etanercept | RA | 2 years | 60 | 789 | 789 | Serious Infection | 114 | 63 | 1.54 (1.15–2.07) |
| Malignancies | 11 | 23 | 0.47 (0.23–0.97) | ||||||||||
| Bejarano et al. (2008) | United Kingdom | Multicenter, Randomized, Controlled Trial | 148 | Adalimumab | RA | 1 year | 47 ± 9 | 75 | 73 | Serious Infection | 13 | 11 | 1.18 (0.49–2.84) |
| Breedveld et al. (2006) | United States | Multicenter, Randomized, Double-Blind Clinical Trial | 799 | Adalimumab | RA | 2 years | 52 ± 14 | 274 | 257 | Serious Infection | 3 | 7 | 0.40 (0.10–1.55) |
| Malignancies | 2 | 4 | 0.47 (0.08–2.56) | ||||||||||
| TB | 1 | 0 | 2.82 (0.11–69.65) | ||||||||||
| Chen et al. (2009) | Taiwan | Randomized Double-Blind, Placebo-Controlled, Comparative Study | 47 | Adalimumab | RA | 12 weeks | 53 | 35 | 12 | Adverse events | 28 | 11 | 0.36 (0.04–3.31) |
| Detert et al. (2013) | Germany | Comparative Study | 172 | Adalimumab | RA | 48 weeks | 59.5 | 87 | 85 | Serious Infection | 3 | 4 | 0.72 (0.16–3.33) |
| Malignancies | 0 | 3 | 0.13 (0.01–2.65) | ||||||||||
| Kavanaugh et al. (2013) | United States | Randomised, Controlled Study | 1,032 | Adalimumab | RA | 20 months | 50.5 | 515 | 517 | Serious Infection | 13 | 6 | 2.21 (0.83–5.85) |
| Malignancies | 1 | 0 | 3.02 (0.12–74.24) | ||||||||||
| TB | 1 | 0 | 3.02 (0.12–74.24) | ||||||||||
| Huang et al. (2014) | China | Randomised, Controlled Trial | 344 | Adalimumab | AS | 12 weeks | 29.8 | 229 | 115 | Serious Infection | 1 | 0 | 1.52 (0.06–37.52) |
| Miyasaka (2008) | Japan | MulticenterDouble-Blind Study | 352 | Adalimumab | RA | 24 weeks | 54.4 | 265 | 87 | Serious Infection | 13 | 4 | 1.07 (0.34–3.37) |
| Malignancies | 0 | 2 | 0.06 (0.00–1.35) | ||||||||||
| Takeuchi et al. (2014) | Japan | Randomised, Double-Blind, Placebo-Controlled, Multicentre Study | 334 | Adalimumab | RA | 70 days | 54.0 ± 13.1 | 171 | 163 | Serious Infection | 2 | 1 | 1.92 (0.17–21.35) |
| Choy et al. (2012) | United Kingdom | Randomized, Double-Blind Study | 247 | Certolizumab pegol | RA | 24 weeks | 54.4 | 126 | 121 | Serious Infection | 3 | 2 | -1.45 (0.24–8.84) |
| Fleischmann et al. (2009) | 2009 | Randomised Double-Blind Study | 220 | Certolizumab pegol | RA | 24 weeks | 53.8 | 111 | 109 | Serious Infection | 4 | 0 | -9.17 (0.49–172.34) |
| Landewé et al. (2014) | Netherlands | A Double-Blind Randomised Placebo-Controlled phase 3 Study | 325 | Certolizumab pegol | AS | 24 weeks | 39.4 | 218 | 107 | Serious Infection | 2 | 0 | -2.48 (0.12–52.17) |
| Mease et al. (2014) | United States | Double-Blind Randomised Placebo-Controlled Study | 409 | Certolizumab pegol | PsA | 24 weeks | 47.5 | 273 | 136 | Serious Infection | 4 | 1 | 2.01 (0.22–18.14) |
| Smolen et al. (2009) | United States | Multicentre, Randomised, Double-Blind, Placebo-Controlled, phase III Trial | 461 | Golimumab | RA | 24 weeks | 54.5 | 306 | 155 | Serious Infection | 4 | 3 | 0.67 (0.15–3.04) |
| Malignancies | 1 | 0 | 1.53 (0.06–37.70) | ||||||||||
| Weinblatt et al. (2003) | United States | Randomised Double-Blind Study | 271 | Adalimumab | RA | 24 weeks | 55.5 | 209 | 62 | Serious Infection | 2 | 0 | 1.51 (0.07–31.78) |
| Malignancy | 1 | 0 | 0.90 (0.04–22.35) | ||||||||||
| Galloway et al. (2013) | United Kingdom | Cohort Study | 15,554 | Etanercept, adalimumab, infliximab | RA | - | 58 | 11,881 | 3673 | Skin And Soft Tissue Infection | 275 | 45 | 2.1428 |
| Listing et al. (2005) | Germany | Case Control Study | 1,529 | Etanercept and infliximab | RA | 12 months | 55 | 928 | 601 | Serious infections | 200 | 39 | 3.96 (2.76–5.68) |
| TB | 1 | 0 | 1.95 (0.08–47.84) | ||||||||||
| Mercer et al. (2015) | United Kingdom | Prospective Cohort Study | 15,016 | Adalimumab, etanercept, infliximab | RA | 3 years | 58 | 11,767 | 3249 | Cancer | 239 | 93 | -0.70 (0.55–0.90) |
| Axelrad et al. (2016) | United States | Retrospective Cohort Study | 255 | - | - | - | 33 | 106 | 149 | Cancer | 22 | 46 | -0.59 (0.33–1.05) |
| Scott et al. (2014) | United States | Cohort Study | 6,841 | Adalimumab, etanercept, infliximab, golimumab, certolizumab pegol | RA | 12 months | - | 932 | 5,909 | Cancer | 367 | 1,465 | -1.97 (1.71–2.28) |
Meta-Analysis Results
Overall Adverse Effects
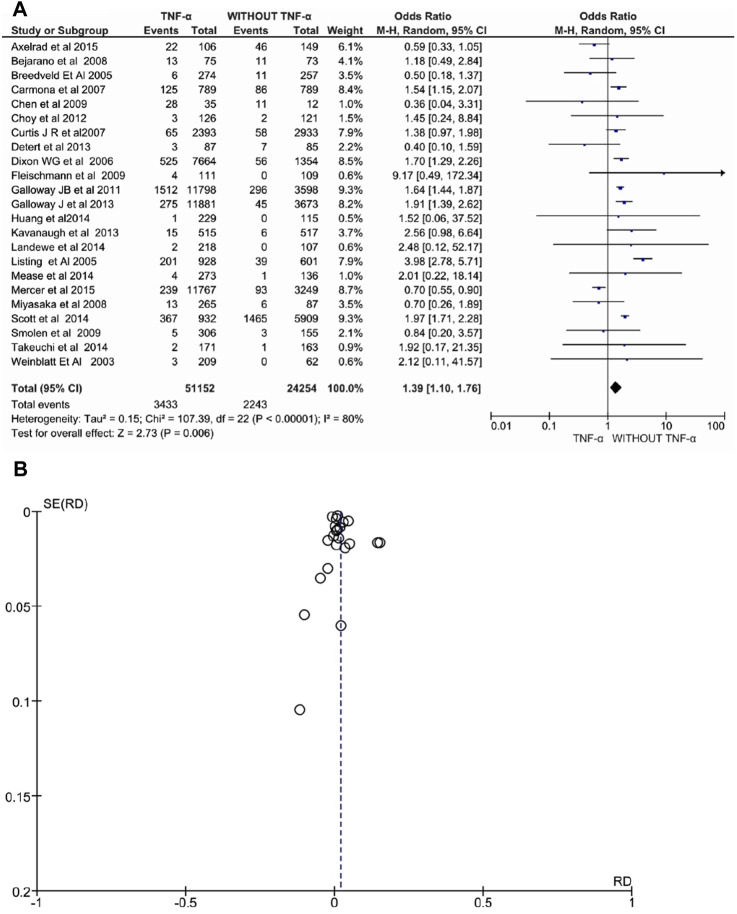
Twenty-three studies involving 75.406 patients with RA, PsA, or AS evaluated anti-TNFα drugs and reported a significant increase in overall adverse events. Exposure to anti-TNFα agents was associated with an increased risk of overall adverse events under the random-effects model (OR: 1.39, 95% CI: 1.10–1.76, p = 0.006, Figure 2). Additionally, the heterogeneity was high, I 2 = 80% (p < 0.00001). Finally, the funnel plot showed asymmetry, indicating a possible risk of publication bias.
FIGURE 2.
(A) Forest plot for overall adverse events after treatment with anti-TNFα agents versus control group (without anti-TNFα agents) using a random-effects model. Odds ratios and 95% confidence intervals are shown (B) Funnel plot for assessment of publication bias.
Serious Infections
Eighteen studies involving 37,693 patients with RA, PsA, or AS evaluated anti-TNFα drugs and reported a significant increase in serious infections. Exposure to anti-TNFα agents was associated with an increased risk of serious infections under the random-effects model (OR: 1.72, 95% CI: 1.56–1.90, p < 0.00001, Figure 3). Additionally, the heterogeneity was moderate, I 2 = 49% (p = 0.01). The funnel plot showed asymmetry with the left corner of the pyramidal part of the funnel missing indicating a possible risk of publication bias.
FIGURE 3.
(A) Forest plot for serious infection after treatment with anti-TNFα agents versus control group (without anti-TNFα agents) using a random-effects model. Odds ratios and 95% confidence intervals are shown (B) Funnel plot for assessment of publication bias.
Tuberculosis
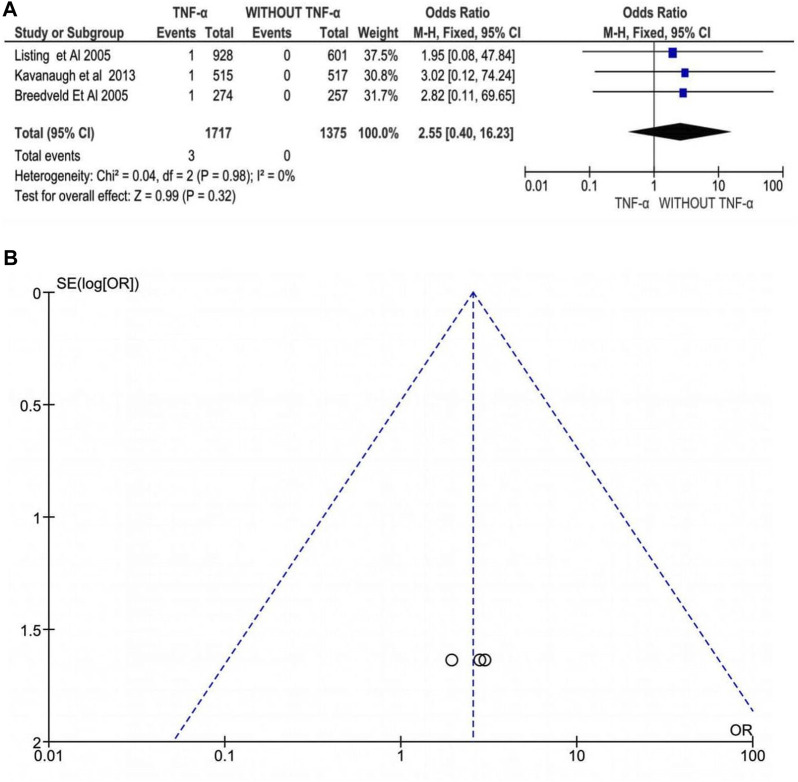
Three studies involving 3,092 patients with RA, PsA, or AS evaluated anti-TNFα drugs and reported an insignificant difference in the risk of tuberculosis following treatment with anti-TNFα agents. Exposure to anti-TNFα agents was not associated with an increased risk of tuberculosis under the random-effects model (OR: 2.55, 95% CI: 0.40–16.23, p = 0.32, Figure 4). Additionally, there was no heterogeneity between the studies, I 2 = 0% (p = 0.98). Thus, the funnel plot does not show significant publication bias, although the number of studies is too low to make a definitive conclusion.
FIGURE 4.
(A) Forest plot for tuberculosis infection after treatment with anti-TNFα agents versus control group (without anti-TNFα agents) using a random-effects model. Odds ratios and 95% confidence intervals are shown (B) Funnel plot for assessment of publication bias.
Cancer
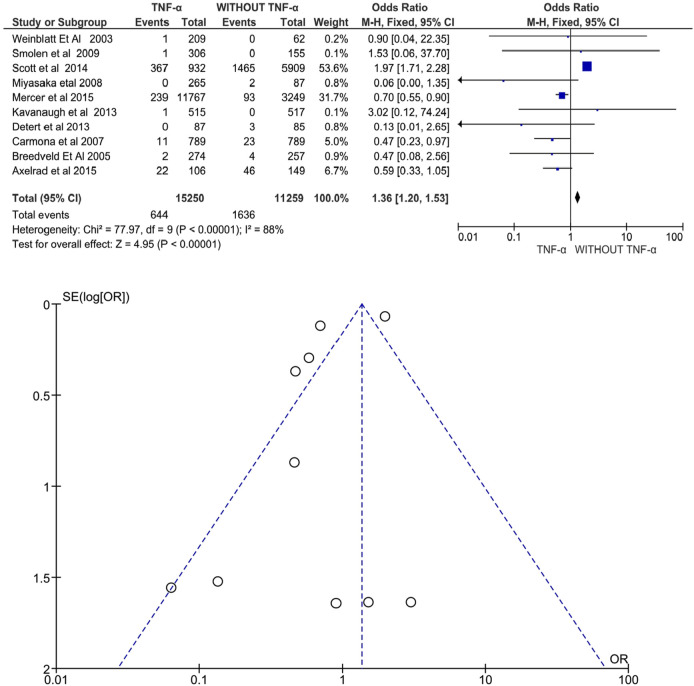
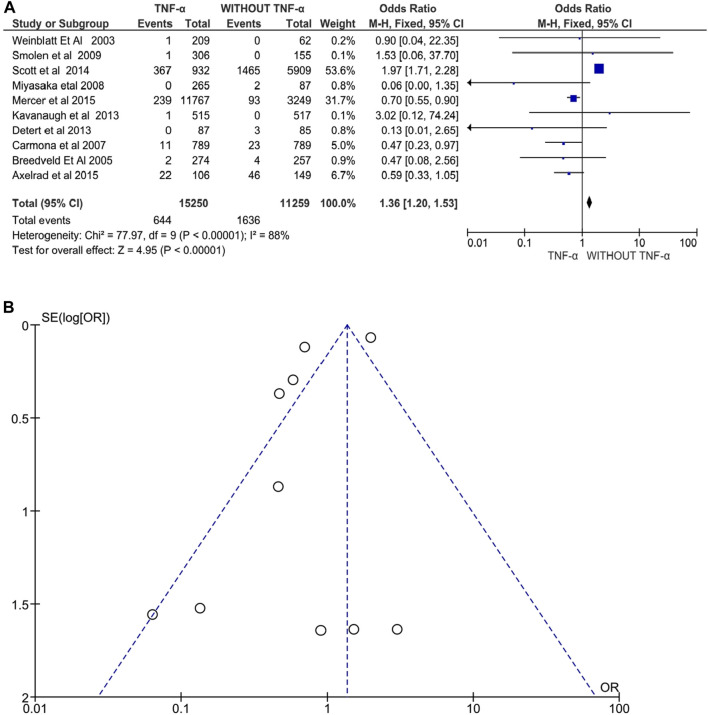
Ten studies involving 26,509 patients with RA, PsA, or AS evaluated anti-TNFα drugs and reported a significant increase in cancer risk following treatment with anti-TNFα agents. Exposure to anti-TNFα agents was associated with an increased risk of cancer under the random-effects model (OR: 1.36, 95% CI: 1.20–1.53, p < 0.00001, Figure 5). Additionally, there was high heterogeneity between the studies, I 2 = 88% (p < 0.00001). The funnel plot does not show significant publication bias. Only three studies showed an increased risk of cancer development and were all conducted in patients with RA (Smolen et al., 2009; Scott et al., 2014 and Kavanaugh et al., 2013).
FIGURE 5.
(A) Forest plot for cancer incidence after treatment with anti-TNFα agents versus control group (without anti-TNFα agents) using a random-effects model. Odds ratios and 95% confidence intervals are shown. (B) Funnel plot for assessment of publication bias.
Risk of Bias
The results of the risk of bias evaluation of the RCTs or comparative studies included in the meta-analysis are shown in Figure 6 (n = 14). Overall, there was a high risk of bias due to unclear or high risk due to randomization, selection, performance, and selection bias.
FIGURE 6.
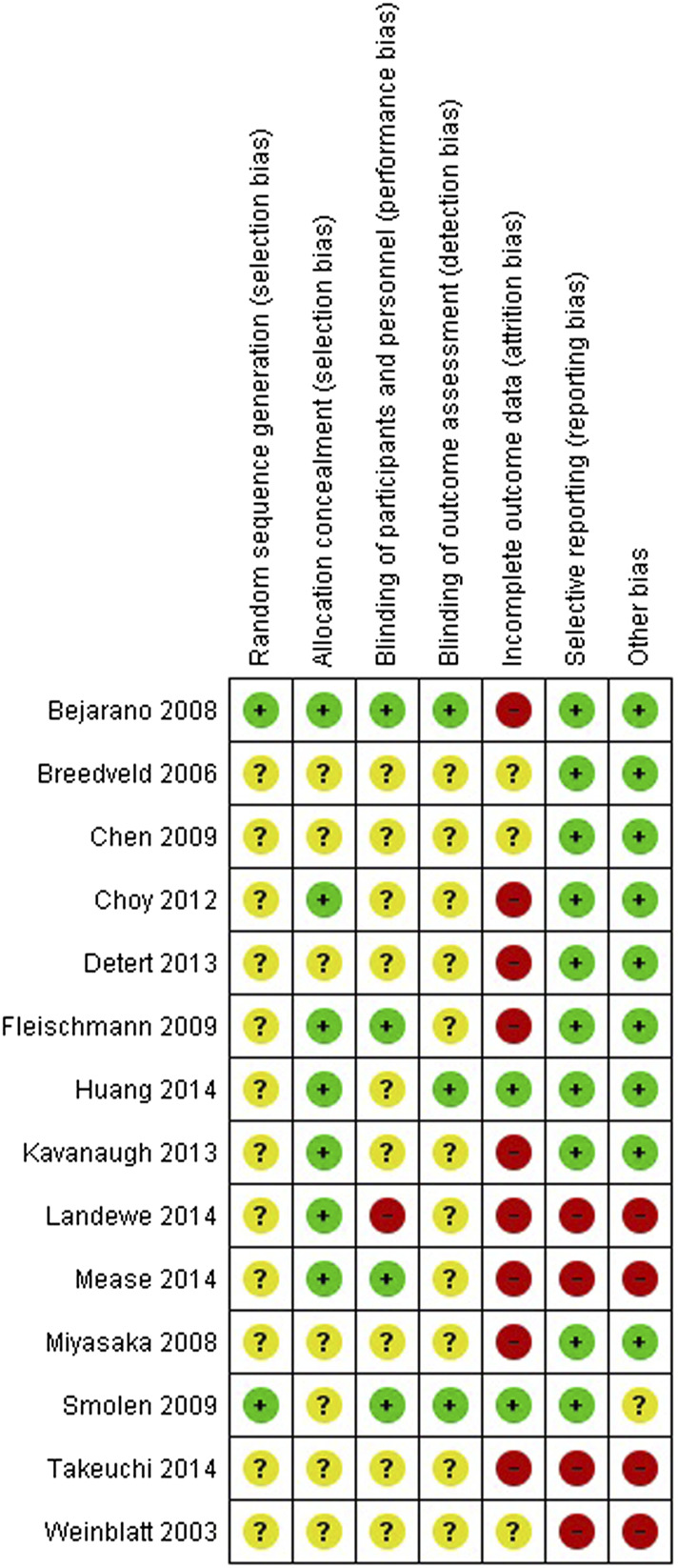
Risk of bias summary for randomized controlled trials and prospective studies included in the meta-analysis (+): low risk of bias (+): high risk of bias (?): unclear risk of bias.
Discussion
Tumor necrosis factor (TNF) is a pro-inflammatory agent formed in the macrophages, T cells, and synovial fibroblasts and is responsible for joint destruction and synovitis (Seymour et al., 2000). Elevation of TNF-α levels has been observed in synovial fluid and the synovium of patients with RA (Fütterer et al., 1998; Edwards et al., 2007). The development in biotechnology contributes to the development of enhanced biological agents, like anti-TNFα monoclonal antibodies, a potent treatment drug for chronic inflammatory diseases. However, the advancement of such drugs brings serious side effects along with its treatment potentials. The present meta-analysis was an effort to assess the various adverse effects after anti-TNF α therapy to treat rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. The findings of our meta-analyses pose questions about the application of anti-TNFα use, especially in infectious disease patients. The present meta-analysis showed a statistically significant increase in overall adverse events, serious infections, and malignancies associated with the use of anti-TNFα agents.
Serious Infections
The present meta-analysis shows that there is an increased risk of various infections after treatment with anti-TNF agents. A case-control study carried out by Doran et al. (Doran et al., 2002) showed an increase in the risk of developing infections in patients with rheumatoid arthritis compared to non-RA subjects, particularly infections of the bone, joints, skin, soft tissues, and respiratory tract. The high frequency of infections in the RA group was attributed to the immunomodulatory effects of RA, or immunosuppressive agents used for RA treatment. Studies have also reported an increased risk of infections in older people, leukopenia, people on steroids, and smokers (Maini et al., 2004; Wolfe et al., 2006; Bernatsky et al., 2007; Edwards et al., 2007; Schneeweiss et al., 2007).
The Anti-TNF Trial in Rheumatoid Arthritis with Combination Therapy (ATTRACT) trial concluded that the frequency of serious infections was comparable between those that received MTX/infliximab and those treated with MTX38. A similar trial by Goekoop-Ruiterman YP et al. found an increase in the risk of serious infections following treatment with anti TNF agents (Goekoop-Ruiterman et al., 2005)Schneeweiss et al. performed a prospective cohort study and noted that the risk of serious bacterial infections in those treated with anti-TNF agents was high compared to users of DMARDs and MTX (Schneeweiss et al., 2007).
Listing et al. conducted a cohort study and reported a two times higher risk of serious upper and lower respiratory tract infection in patients treated with etanercept and infliximab (Listing et al., 2005). Similarly, the present meta-analysis observed that the odds of serious infection were 1.72 times higher in the anti-TNFα agent treatment group (95% CI: 1.56–1.90, p < 0.00001). A moderate heterogeneity of I 2 = 49% can be attributed to multiple inflammatory conditions and different anti-TNFα agents and comparators that were all pooled together in the same analysis.
Contrastingly, a meta-analysis of randomized controlled trials of the safety of TNF blockers in over 8,800 RA patients did not identify an increased risk of serious bacterial infection in the standard recommended dose. However, a dose-response increase in sepsis was observed with high dose biological therapy− (Leombruno et al., 2009). Another meta-analysis by Alonso-Ruiz et al. (2008) showed an increased risk of serious infections upon treatment with adalimumab and etanercept versus controls.
Tuberculosis
It is critical to develop an effective latent tuberculosis infection screening technique until starting anti-TNF therapy in patients with immune-mediated inflammatory diseases. Therefore, implementing guidelines for latent tuberculosis infection screening and (prophylactic) treatment before starting anti-TNF therapy could lower infection rates. The tuberculosis prevalence rate was consistently higher for all anti-TNF drugs than in the general population, with infliximab and adalimumab showing higher rates compared to etanercept. Monoclonal anti-TNF α such as infliximab or adalimumab antibodies pose a higher risk of TB incidence than soluble TNF-α receptor, etanercept due to differential abilities to bind to the TNF receptor. Studies have found that apoptosis occurs upon binding of infliximab to membrane TNF on T cells and monocytes which may lead to reduction in number of antimycobacterial effector cells and/or dissolution of granulomas. In contrast, etanercept does not cause apopotosis of cells that express membrane TNF (Minozzi et al., 2016). Our results showed a non-significant difference in the incidence of tuberculosis following treatment with anti-TNFα agents (OR: 2.55, 95% CI: 0.40–16.23, p = 0.32). However, the number of studies included was low (n = 3 studies), making it necessary to interpret the results cautiously as they may not be reliable.
Various biologic registries have shown an increased risk of TB infection in patients treated with monoclonal antibodies than TNF blockers (Leslie et al., 2013). The Brazilian Society of Rheumatology’s guidelines states that all patients should have baseline chest X-ray and tuberculin skin test (PPD) before treatment with biologic DMARDs (Da Mota et al., 2012). They have also prescribed a 6-month course of isoniazid a month before anti-TNFα therapy in patients with a PPD of ≥5 mm with the previous TB on chest X-ray or who have had contact with tuberculosis patients. Strict adherence to guidelines for prescribing TNF-α blockers led to a decrease in tuberculosis’s incidence rate ratios to that of the normal-population (Bongartz et al., 2006).
Cancer Risk
According to Bongartz et al., the risk of malignancy increases three times when treated with infliximab and adalimumab (Bongartz et al., 2006). Similarly, the present meta-analysis observed that the odds of cancer were 1.36 times higher in the anti-TNFα agent treatment group (95% CI: 1.20–1.53, p < 0.00001). High heterogeneity of I 2 = 88% can be attributed to multiple inflammatory conditions and different anti-TNFα agents and comparators that were all pooled together in the same analysis. The cancer risk is higher in three of 10 studies, namely Smolen et al., 2009, Scott et al., 2014, and Kavanaugh et al., 2013 which were all conducted in patients with RA. In contrast, Bonovas et al. have reported no significant effect of anti-TNF agents (adalimumab, golimumab, infliximab, certolizumab, or etanercept) on cancer risk in adult patients with rheumatologic disease. However, this meta-analysis indicates the risk of publication bias via funnel plot asymmetry suggesting an overestimation of the pooled risk for cancer.
Limitations
Although the present meta-analyses raise concerns about the use of anti-TNFα agents to treat inflammatory conditions based on higher incidences of serious infections and cancer, it is important to interpret these results with caution. The number of studies is limited, and the event rate is low, particularly regarding the development of tuberculosis, raising concern about the reliability of the pooled estimate. The risk of bias was unclear to high across most domains in the included RCTs, limiting the results obtained. Further, the development of specific adverse events such as cancer may not have been appropriately captured in the follow-up period of most studies leading to a possible underestimation of the true odds ratio. Subgroup analysis by anti-TNFα agent type, comparator, or inflammatory condition would also address high to moderate heterogeneity observed for some of the meta-analysis results.
Conclusion
Synthesis of current evidence from RCTs, data registries, and prospective studies involving the use of anti-TNFα agents for the treatment of inflammatory conditions such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis suggests an increased risk of serious infections and malignancies. However, long-term surveillance and monitoring of patients on anti-TNFα treatment via data registries and long-term epidemiological studies are necessary to capture any long-term complications, particularly the development of cancers that can occur long after the follow-up time of RCTs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee of the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University and Huaian Hospital of Huaian City. And comply with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Furthermore, the written informed consent was taken from all the patients.
Author Contributions
JL has designed the concept; ZZ, Data acquisition; XW drafted the manuscript; JZ and DM literature search; PZ Final proof reading and editing. All Authors read and approved the manuscript before submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TNF, Tumor Necrosis Factor; RA, Rheumatoid Arthritis.
References
- Abbott Laboratories (2012) Humira (Adalimumab) [highlights of Prescribing Information]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/ (accessed December 15, 2020).
- Alonso-Ruiz A., Pijoan J. I., Ansuategui E., Urkaregi A., Calabozo M., Quintana A. (2008). Tumor Necrosis Factor Alpha Drugs in Rheumatoid Arthritis: Systematic Review and Metaanalysis of Efficacy and Safety. BMC Musculoskelet. Disord. 9, 52. 10.1186/1471-2474-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad J., Bernheim O., Colombel J. F., Malerba S., Ananthakrishnan A., Yajnik V., et al. (2016). Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-tumor Necrosis Factor Agents. Clin. Gastroenterol. Hepatol. 14 (1), 58–64. 10.1016/j.cgh.2015.07.037 [DOI] [PubMed] [Google Scholar]
- Baghai M., Osmon D. R., Wolk D. M., Wold L. E., Haidukewych G. J., Matteson E. L. (2001). Fatal Sepsis in a Patient with Rheumatoid Arthritis Treated with Etanercept. Mayo Clin. Proc. 76, 653–656. 10.4065/76.6.653 [DOI] [PubMed] [Google Scholar]
- Bejarano V., Quinn M., Conaghan P. G., Reece R., Keenan A. M., Walker D., et al. (2008). Effect of the Early Use of the Anti-tumor Necrosis Factor Adalimumab on the Prevention of Job Loss in Patients with Early Rheumatoid Arthritis. Arthritis Rheum. 59, 1467–1474. 10.1002/art.24106 [DOI] [PubMed] [Google Scholar]
- Bernatsky S., Hudson M., Suissa S. (2007). Anti-rheumatic Drug Use and Risk of Serious Infections in Rheumatoid Arthritis. Rheumatology (Oxford) 46, 1157–1160. 10.1093/rheumatology/kem076 [DOI] [PubMed] [Google Scholar]
- Bongartz T., Sutton A. J., Sweeting M. J., Buchan I., Matteson E. L., Montori V. (2006). Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-Analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA 295 (19), 2275–2285. 10.1001/jama.295.19.2275 [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Weisman M. H., Kavanaugh A. F., Cohen S. B., Pavelka K., van Vollenhoven R., et al. (2006). The PREMIER Study: A Multicenter, Randomized, Double-Blind Clinical Trial of Combination Therapy with Adalimumab Plus Methotrexate versus Methotrexate Alone or Adalimumab Alone in Patients with Early, Aggressive Rheumatoid Arthritis Who Had Not Had Previous Methotrexate Treatment. Arthritis Rheum. 54, 26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- Carmona L., Descalzo M. A., Perez-Pampin E., Ruiz-Montesinos D., Erra A., Cobo T., et al. (2007). All-cause and Cause-specific Mortality in Rheumatoid Arthritis Are Not Greater Than Expected when Treated with Tumor Necrosis Factor Antagonists. Ann. Rheum. Dis. 66, 880–885. 10.1136/ard.2006.067660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Y., Chou S. J., Hsieh T. Y., Chen Y. H., Chen H. H., Hsieh C. W., et al. (2009). Randomized, Double-Blind, Placebo-Controlled, Comparative Study of Human Anti-TNF Antibody Adalimumab in Combination with Methotrexate and Methotrexate Alone in Taiwanese Patients with Active Rheumatoid Arthritis. J. Formos. Med. Assoc. 108, 310–319. 10.1016/S0929-6646(09)60071-1 [DOI] [PubMed] [Google Scholar]
- Choy E., McKenna F., Vencovsky J., Valente R., Goel N., Vanlunen B., et al. (2012). Certolizumab Pegol Plus MTX Administered Every 4 Weeks Is Effective in Patients with RA Who Are Partial Responders to MTX. Rheumatology (Oxford) 51, 1226–1234. 10.1093/rheumatology/ker519 [DOI] [PubMed] [Google Scholar]
- Colombel J. F., Loftus E. V., Jr, Tremaine W. J., Egan L. J., Harmsen W. S., Schleck C. D., et al. (2004). The Safety Profile of Infliximab in Patients with Crohn's Disease: the Mayo Clinic Experience in 500 Patients. Gastroenterology 126, 19–31. 10.1053/j.gastro.2003.10.047 [DOI] [PubMed] [Google Scholar]
- Curtis J. R., Patkar N., Xie A., Martin C., Allison J. J., Saag M., et al. (2007). Risk of Serious Bacterial Infections Among Rheumatoid Arthritis Patients Exposed to Tumor Necrosis Factor Alpha Antagonists. Arthritis Rheum. 56 (4), 1125–1133. 10.1002/art.22504 [DOI] [PubMed] [Google Scholar]
- Da Mota L. M., Cruz B. A., Brenol C. V., Pereira I. A., Rezende-Fronza L. S., Bertolo M. B., et al. (2012). 2012 Brazilian Society of Rheumatology Consensus for the Treatment of Rheumatoid Arthritis. Rev. Bras Reumatol 52, 152–174. 10.1590/s0482-50042012000200002 [DOI] [PubMed] [Google Scholar]
- Detert J., Bastian H., Listing J., Weiß A., Wassenberg S., Liebhaber A., et al. (2013). Induction Therapy with Adalimumab Plus Methotrexate for 24 Weeks Followed by Methotrexate Monotherapy up to Week 48 versus Methotrexate Therapy Alone for DMARD-Naive Patients with Early Rheumatoid Arthritis: HIT HARD, an Investigator-Initiated Study. Ann. Rheum. Dis. 72, 844–850. 10.1136/annrheumdis-2012-201612 [DOI] [PubMed] [Google Scholar]
- Dixon W. G., Watson K., Lunt M., Hyrich K. L., Silman A. J., Symmons D. P. (2006). Rates of Serious Infection, Including Site-specific and Bacterial Intracellular Infection, in Rheumatoid Arthritis Patients Receiving Anti-tumor Necrosis Factor Therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 54 (8), 2368–2376. 10.1002/art.21978 [DOI] [PubMed] [Google Scholar]
- Doran M. F., Crowson C. S., Pond G. R., O'Fallon W. M., Gabriel S. E. (2002). Frequency of Infection in Patients with Rheumatoid Arthritis Compared with Controls: a Population-Based Study. Arthritis Rheum. 46 (9), 2287–2293. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- Downey C. (2016). Serious Infection during Etanercept, Infliximab and Adalimumab Therapy for Rheumatoid Arthritis: A Literature Review. Int. J. Rheum. Dis. 19, 536–550. 10.1111/1756-185X.12659 [DOI] [PubMed] [Google Scholar]
- Edwards C. J., Cooper C., Fisher D., Field M., van Staa T. P., Arden N. K. (2007). The Importance of the Disease Process and Disease-Modifying Antirheumatic Drug Treatment in the Development of Septic Arthritis in Patients with Rheumatoid Arthritis. Arthritis Rheum. 57, 1151–1157. 10.1002/art.23003 [DOI] [PubMed] [Google Scholar]
- Fleischmann R., Vencovsky J., van Vollenhoven R. F., Borenstein D., Box J., Coteur G., et al. (2009). Efficacy and Safety of Certolizumab Pegol Monotherapy Every 4 Weeks in Patients with Rheumatoid Arthritis Failing Previous Disease-Modifying Antirheumatic Therapy: the FAST4WARD Study. Ann. Rheum. Dis. 68, 805–811. 10.1136/ard.2008.099291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer A., Mink K., Luz A., Kosco-Vilbois M. H., Pfeffer K. (1998). The Lymphotoxin Beta Receptor Controls Organogenesis and Affinity Maturation in Peripheral Lymphoid Tissues. Immunity 9, 59–70. 10.1016/s1074-7613(00)80588-9 [DOI] [PubMed] [Google Scholar]
- Galloway J. B., Hyrich K. L., Mercer L. K., Dixon W. G., Fu B., Ustianowski A. P., et al. (2011). Anti-TNF Therapy Is Associated with an Increased Risk of Serious Infections in Patients with Rheumatoid Arthritis Especially in the First 6 Months of Treatment: Updated Results from the British Society for Rheumatology Biologics Register with Special Emphasis on Risks in the Elderly. Rheumatology (Oxford) 50 (1), 124–131. 10.1093/rheumatology/keq242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J. B., Mercer L. K., Moseley A., Dixon W. G., Ustianowski A. P., Helbert M., et al. (2013). Risk of Skin and Soft Tissue Infections (Including Shingles) in Patients Exposed to Anti-tumour Necrosis Factor Therapy: Results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 72 (2), 229–234. 10.1136/annrheumdis-2011-201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop-Ruiterman Y. P., de Vries-Bouwstra J. K., Allaart C. F., van Zeben D., Kerstens P. J., Hazes J. M., et al. (2005). Clinical and Radiographic Outcomes of Four Different Treatment Strategies in Patients with Early Rheumatoid Arthritis (The BeSt Study): a Randomized, Controlled Trial. Arthritis Rheum. 52, 3381–3390. 10.1002/art.21405 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Gu J., Zhu P., Bao C., Xu J., Xu H., et al. (2014). Efficacy and Safety of Adalimumab in Chinese Adults with Active Ankylosing Spondylitis: Results of a Randomised, Controlled Trial. Ann. Rheum. Dis. 73, 587–594. 10.1136/annrheumdis-2012-202533 [DOI] [PubMed] [Google Scholar]
- Kavanaugh A., Fleischmann R. M., Emery P., Kupper H., Redden L., Guerette B., et al. (2013). Clinical, Functional and Radiographic Consequences of Achieving Stable Low Disease Activity and Remission with Adalimumab Plus Methotrexate or Methotrexate Alone in Early Rheumatoid Arthritis: 26-week Results from the Randomised, Controlled OPTIMA Study. Ann. Rheum. Dis. 72, 64–71. 10.1136/annrheumdis-2011-201247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., et al. (2001). Tuberculosis Associated with Infliximab, a Tumor Necrosis Factor Alpha-Neutralizing Agent. N. Engl. J. Med. 345, 1098–1104. 10.1056/NEJMoa011110 [DOI] [PubMed] [Google Scholar]
- Kroesen S., Widmer A. F., Tyndall A., Hasler P. (2003). Serious Bacterial Infections in Patients with Rheumatoid Arthritis under Anti-TNF-alpha Therapy. Rheumatology (Oxford) 42, 617–621. 10.1093/rheumatology/keg263 [DOI] [PubMed] [Google Scholar]
- Landewé R., Braun J., Deodhar A., Dougados M., Maksymowych W. P., Mease P. J., et al. (2014). Efficacy of Certolizumab Pegol on Signs and Symptoms of Axial Spondyloarthritis Including Ankylosing Spondylitis: 24-week Results of a Double-Blind Randomised Placebo-Controlled Phase 3 Study. Ann. Rheum. Dis. 73, 39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leombruno J. P., Einarson T. R., Keystone E. C. (2009). The Safety of Anti-tumour Necrosis Factor Treatments in Rheumatoid Arthritis: Meta and Exposure-Adjusted Pooled Analyses of Serious Adverse Events. Ann. Rheum. Dis. 68, 1136–1145. 10.1136/ard.2008.091025 [DOI] [PubMed] [Google Scholar]
- Leslie G., Jewell T., Laversuch C., Samantha A. (2013). A Systematic Review of the Influence of Anti-TNF on Infection Rates in Patients with Rheumatoid Arthritis. Rev. Bras Rheumatol. 53 (6), 501–515. 10.1016/j.rbr.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Listing J., Strangfeld A., Kary S., Rau R., von Hinueber U., Stoyanova-Scholz M., et al. (2005). Infections in Patients with Rheumatoid Arthritis Treated with Biologic Agents. Arthritis Rheum. 52 (11), 3403–3412. 10.1002/art.21386 [DOI] [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Furst D., Weisman M. H., et al. (2004). Sustained Improvement over Two Years in Physical Function, Structural Damage, and Signs and Symptoms Among Patients with Rheumatoid Arthritis Treated with Infliximab and Methotrexate. Arthritis Rheum. 50, 1051–1065. 10.1002/art.20159 [DOI] [PubMed] [Google Scholar]
- Mease P. J., Fleischmann R., Deodhar A. A., Wollenhaupt J., Khraishi M., Kielar D., et al. (2014). Effect of Certolizumab Pegol on Signs and Symptoms in Patients with Psoriatic Arthritis: 24-week Results of a Phase 3 Double-Blind Randomised Placebo-Controlled Study (RAPID-PSA). Ann. Rheum. Dis. 73, 48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer L. K., Lunt M., Low A. L., Dixon W. G., Watson K. D., Symmons D. P., et al. (2015). Risk of Solid Cancer in Patients Exposed to Anti-tumour Necrosis Factor Therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 74, 1087–1093. 10.1136/annrheumdis-2013-204851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S., Bonovas S., Lytras T., Pecoraro V., González-Lorenzo M., Bastiampillai A. J., et al. (2016). Risk of Infections Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: a Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 15 (Suppl. 1), 11–34. 10.1080/14740338.2016.1240783 [DOI] [PubMed] [Google Scholar]
- Miyasaka N. (2008). Clinical Investigation in Highly Disease-Affected Rheumatoid Arthritis Patients in Japan with Adalimumab Applying Standard and General Evaluation: the CHANGE Study. Mod. Rheumatol. 18, 252–262. 10.1007/s10165-008-0045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweiss S., Setoguchi S., Weinblatt M. E., Katz J. N., Avorn J., Sax P. E., et al. (2007). Anti-tumor Necrosis Factor Alpha Therapy and the Risk of Serious Bacterial Infections in Elderly Patients with Rheumatoid Arthritis. Arthritis Rheum. 56 (6), 1754–1764. 10.1002/art.22600 [DOI] [PubMed] [Google Scholar]
- Scott F. I., Mamtani R., Brensinger C., Haynes K., Chiesa-Fuxench Z. C., Zhang J., et al. (2014). Risk of Recurrent Non-melanoma Skin Cancer with Methotrexate and Anti-TNF Use in Rheumatoid Arthritis. Arthritis Rheumatol. 66, S808.10.1001/jamadermatol.2015.3029. [Google Scholar]
- Seymour H. E., Worsley A., Smith J. M., Thomas S. H. (2000). Anti-TNF Agents for Rheumatoid Arthritis. Br. J. Clin. Pharmacol. 51, 201–208. 10.1046/j.1365-2125.2001.00321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. A., Christensen R., Wells G. A., Suarez-Almazor M. E., Buchbinder R., Lopez-Olivo M. A., et al. (2009). Biologics for Rheumatoid Arthritis: an Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 7 (4), CD007848. 10.1002/14651858.CD007848.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S., Kay J., Doyle M. K., Landewé R., Matteson E. L., Wollenhaupt J., et al. (2009). Golimumab in Patients with Active Rheumatoid Arthritis after Treatment with Tumor Necrosis Factor Alpha Inhibitors (GO-AFTER Study): a Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase III Trial. Lancet 374, 210–221. 10.1016/S0140-6736(09)60506-7 [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Yamanaka H., Ishiguro N., Miyasaka N., Mukai M., Matsubara T., et al. (2014). Adalimumab, a Human Anti-TNF Monoclonal Antibody, Outcome Study for the Prevention of Joint Damage in Japanese Patients with Early Rheumatoid Arthritis: the HOPEFUL 1 Study. Ann. Rheum. Dis. 73, 536–543. 10.1136/annrheumdis-2012-202433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Broder M. S., Wong J. Y., Hanson M. E., Beenhouwer D. O. (2004). Granulomatous Infectious Diseases Associated with Tumor Necrosis Factor Antagonists. Clin. Infect. Dis. 38, 1261–1265. 10.1086/383317 [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Keystone E. C., Furst D. E., Moreland L. W., Weisman M. H., Birbara C. A., et al. (2003). Adalimumab, a Fully Human Anti-tumor Necrosis Factor Alpha Monoclonal Antibody, for the Treatment of Rheumatoid Arthritis in Patients Taking Concomitant Methotrexate: the ARMADA Trial. Arthritis Rheum. 48, 35–45. 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- Wolfe F., Caplan L., Michaud K. (2006). Treatment for Rheumatoid Arthritis and the Risk of Hospitalization for Pneumonia: Associations with Prednisone, Disease-Modifying Antirheumatic Drugs, and Anti-tumor Necrosis Factor Therapy. Arthritis Rheum. 54, 628–634. 10.1002/art.21568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.