Abstract
In recent years, natural polysaccharides have received growing attention and interest in view of their values in food, medical, cosmetics and other fields. Eleutherococcus senticosus (E. senticosus) is a medicine and food homologous plant that possess anti-tumor, anti-inflammatory, central nervous system and cardiovascular protection, anti-radiation, enhancement of human microcirculation, improvement of physical fatigue effects, mainly based on lignans, flavonoids and coumarin types. E. senticosus polysaccharides (ESPS), act as a kind of polysaccharide extracted and isolated from the root and rhizome of E. senticosus, have been found in many applications of medicine and food for their unique biological activity. Nevertheless, the existing studies are mostly concerned with small molecules of E. senticosus, less attention is paid to polysaccharides. Moreover, the types and structural characterization of ESPS reported in existing literature were also not summarized. In this paper, the research progress of ESPS is reviewed from the aspects of extraction, separation, structural characterization and biological activity, future perspectives from points of efficient extraction, resource utilization and quality control standards were also proposed, which provide reference for the further development and utilization of ESPS.
Keywords: extraction, purification, structure, bioactivities, Eleutherococcus senticosus polysaccharides (ESPS, )
Highlights
• The extraction and purification techniques of Eleutherococcus senticosus polysaccharides were summarized.
• Types and structural characterization of Eleutherococcus senticosus polysaccharides isolated and purified at present were preliminarily discussed.
• The modern pharmacological research and biological activity of Eleutherococcus senticosus polysaccharides were summarized, and its application prospect was proposed.
Introduction
Eleutherococcus senticosus, commonly known as “Ci-wu-jia” or “Acanthopanax senticosus”, is botanically from the dried root and rhizome or stem of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim [syn. Acanthopanax senticosus (Rupr. and Maxim.) Harms] belong to the Araliaceae. It is a medicine and food homologous plant that is widely distributed in northeast China, which contains multi-kinds of nutrients and components. Among variety of active ingredients, flavonoids (quercetin, hyperoside, quercetin, rutin, etc.), coumarins (isofraxidin, scopolamine, etc), lignans (ferulic acid glucoside, eleutheroside, carotin, syringin, etc.,) (Lee et al., 2012) played an important role in its activities. Moreover, some trace elements such as Ca, P, Mg, Fe, a variety of amino acids and polysaccharides also play a role in mediating the immune function of the body (Liu, 2010), antioxidant, anti-tumor, anti-inflammatory, central nervous system and cardiovascular protection, anti-radiation, enhancement of human microcirculation, improvement of physical fatigue, etc., (Luan, 2012; Pan, 2019a). As a medicinal and food homologous plant, various products such as oral liquid, tea, capsule preparation, wine of E. senticosus have been developed and commercialized, and favored by those who pay attention to health. The products of E. senticosus are listed in Figure 1.
FIGURE 1.
Eleutherococcus senticosus. Salad from leaves (A); tea (B); pulp (C); wine (D); tablets (E); cortex (F).
GRAPHICAL ABSTRACT.
In recent years, polysaccharides have demonstrated good therapeutic effects against tumors, hyperlipemia, diabetes and hypertensive diseases (Shen et al., 1991; Duan, 2015). The development and utilization of polysaccharides have also become a hot spot. E. senticosus polysaccharides (ESPS) are an important biologically active ingredient extracted from the roots and rhizomes of E. senticosus. The content of alkaline polysaccharides in E. senticosus is about 2–8%, and that of the water-soluble polysaccharides is 2.3–5.7% (Gao, 2019). Studies had shown that ESPS possessed immunoenhancement (Xu et al., 2005), anti-tumor (Hao and Nan, 2013), anti-virus (Zhou, 2018), anti-fatigue, anti-oxidation, anti-diabetes (Fu et al., 2012), hepatoprotective, and other biological activities (Chen and Huang, 2018; Ganesan and Xu, 2019; Zeng et al., 2019), which prompts the huge application and development potential of ESPS. In this paper, the extraction and purification process and structure characterization of ESPS were systematically introduced, and the studies on biological activities of ESPS were further reviewed in order to expand the application of ESPS.
Extraction and Purification
Extraction of Crude Polysaccharides
Hot Water Extraction
Polysaccharides are a category of substances with higher polarity, higher water soluble and insoluble in EtOH. Because of the nature of polysaccharides, hot water extraction in combination with EtOH-precipitated has been commonly used in laboratories and industrial production. EtOH-precipitated polysaccharides are suitable for almost all water-soluble polysaccharides. The principal principle is to separate the polysaccharides by reducing the dielectric constant of the aqueous solution to dehydrate the polysaccharides for precipitation. There are several ways to extract ESPS (Table 1). Feng et al. used a single factor test to optimize the extraction conditions of ESPS and obtained the optimal condition: pulverization degree above 80 mesh, solute extraction 50% EtOH, extraction pH = 6, soaking for 2 h, degreasing treatment with petroleum ether. Under these conditions, the extraction yield of crude ESPS could reach 3.27 ± 0.07% (Feng, 2016). An orthogonal experiment was developed from the experimental data in order to predict the ESPS yield by Cheng, the optimal condition was found to be: extraction temperature 80°C, extraction once, time of 150 min and solid-liquid ratio of 1:20 g/ml with a maximum pectin yield (23.3 mg g−1) (Chen, 2011a). Based on the single factor test, some researches adopted the Box-Behnken center combination design test and response surface method (RSM) to compare rhizomes, fruits, and leaves, and finally determined that the fruit was the best extraction part. This technique gives a maximal yield of 13.21% for material-liquid ratio of 31:1 in 116 min, extraction temperature 95°C (Shen, 2017). Some scholars also investigated the drying method and found that the freeze-drying method was better than the spray-drying method (Zhang and Sun, 2005).
TABLE 1.
Summary of the extraction process of ESPS.
| Method | Pros | Cons | Optimization Factors | Optimization Results | ESPS Content | References |
|---|---|---|---|---|---|---|
| Hot water extraction | The production cycle is short, the experiment cost is low, experiment operation is simple and the degree of damage to the structure of the extract is small. | Part of the protein will be extracted and protein must be removed; low selectivity, low extraction rate. | Material/liquid, extraction time, times, temperature, alcohol precipitation temperature, time, pH | 90°C, 150 min, material/liquid 1:18, extraction twice; alcohol precipitation condition -20°C, 36 h, ethanol extractant ratio 4:1, pH=7 | 263.3 mg/ml | Zhai (2007) |
| — | — | — | Extraction temperature, time, material/liquid l, times | 80°C, 150 min, material/liquid 1:20, extract once extraction time> extraction temperature> extraction times> material/liquid | 23.21 mg/g | Chen (2011a) |
| — | — | — | Material/liquid, extraction time, temperature, times | 90°C, 2.5 h, material/liquid 1:25 (g/mL), extract 3 times. | 23.95 mg/g | Chen (2015a) |
| — | — | — | E. senticosus pulverization degree, extraction solute, pH, soaking time degreasing solvent | degree of pulverization:>80 meshes, solute is extracted by 50% ethanol, pH = 6 (petroleum ether degreasing treatment soaking 2 h | 3.27±0.07% (n = 3) | Feng (2016) |
| — | — | — | Extraction parts (rhizome, leaves, fruits) extraction time, temperature, alcohol concentration, material/liquid | 95°C, 116 min, material/liquid 31:1 best extraction part fruit, alcohol concentration 85%, | 13.21% | Shen (2017) |
| — | — | — | Extraction temperature, time, material / liquid | 80°C, 1.5 h, material/liquid 1:21 | 10.14% | Yu (2019) |
| Ultrasonic extraction | More efficient, faster, high extraction yield, low energy consumption | Ultrasound will damage the structure of polysaccharides, resulting in difficulty in separation and difficult to apply to industrial production | Extraction temperature, material/liquid, time, ultrasonic power | 58°C, 73 min, material/liquid 1:25; ultrasonic power 85 W | 1.532±0.037% (n = 3) | Chen et al. (2018) |
| Ultrasonic power, material/liquid , extraction temperature, time | 57°C, 42 min, material/liquid 1:40 g/mL ultrasonic power 90 W | 3.86% | Chen (2015b) | |||
| Microwave extraction | Strong penetration, uniform and fast heating, high selectivity. | Polysaccharides often contain some pigments, so they need to be decolorized. | — | It was the first time to extract ESPS using microwave technology and determine its content. | 5.01% | Li and Liu (2003) |
| — | — | — | Extraction time, microwave power, material/liquid | 22.5 min, material/liquid 1:25 22.5 min, material/liquid 1:25 | 1.52% ± 0.09% (n = 3) | Zhang and Guo (2015) |
| Alkaline extraction | Higher total glucose content | The yield of polysaccharide was slightly lower, and it will damage the structure of acid polysaccharide and neutral polysaccharide | Alkali concentration, material/alkali ratio, alkali extraction time | Alkali extraction time>material-alkali ratio>alkali concentration 10 h, material/alkali 1:16. alkali concentration 4%, | 5.70% | Wang (2006) |
| — | — | — | Graded alcohol precipitation, step-by-step alcohol precipitation | Used step-by-step alcohol precipitation method, more and higher content of polysaccharides can be obtained | 6.01% | Li (2019) |
However, the hot water extraction method will introduce some impurities like protein, which needs to be removed. Sevag reagent, trifluoro trichloroethane, trichloroacetic acid, anion and cation exchange resin and the enzymatic hydrolysis are the commonly used mediums. In ESPS literature, protein is mostly removed by the Sevag method (Jiao, 2008; Li, 2019). Wang et al. compared the deproteinization effects of Sevag, trichloroacetic acid (TCA)-Sevag, chitosan coagulation and enzyme on ESPS. The results showed that the protein removal rate: enzyme 93.1% = chitosan coagulation 93.1% > TCA-Sevag 83% > Sevag 28%, polysaccharide loss rate: chitosan coagulation 47% > TCA-Sevag 43% > Sevag 18% > enzyme 7%. Results indicated that enzyme deproteinization has the best effect (Wang, 2009).
Ultrasound-Assisted Extraction (UAE)
The ultrasound-assisted extraction (UAE) method were employed for increasing the movement frequency and speed of macromolecules, strengthening the solvent penetration ability, and shortening the extraction period of polysaccharides by ultrasonic waves (Cui, 2012). In some experiments, the RSM was used to optimize the extraction of ESPS by ultrasound-assisted extraction (UAE), by which the optimum levels of the parameters were obtained as follows: extraction temperature 58°C, extraction time 73 min, liquid/solid ratio 25:1, ultrasonic power 85 W (Chen et al., 2018). However, the extraction time should not be too long because ultrasound has a mechanical shearing effect, which may damage the structure of ESPS. Moreover, the UAE method is difficult to be widely applied in the field of industrial production in view of the limitations of the instruments.
Microwave Extraction
Water molecules in the cell are rapidly heated and pressurized under microwave due to its strong penetrating power, which increased the extraction rate by breakage of cell wall and release of intracellular polysaccharides into the extract.
Li et al. removed fat-soluble impurities and interfering components gradually with petroleum ether, ether and EtOH and then ectracted E. senticosus crude polysaccharide by microwave technology (Li and Liu, 2003). Zhang et al. used the RSM based on Box-Behnken design to optimize the extraction of ESPS from three aspects of the microwave power, microwave processing time and material-to-liquid ratio parameters. The results showed that microwave extraction 22.5 min, microwave power 450 W, material-liquid ratio 1:25 (g·ml−1) achieved the best performance (Zhang and Guo, 2015). Furthermore, microwave extraction of ESPS often requires depigmentation. Ion exchange, adsorption, oxidation and metal complexation are common methods for subsequent depigmentation.
Alkali-Extraction
Polysaccharides could be divided into acidic, alkaline and neutral polysaccharides, which can be extracted by adjusting the acidity and alkalinity like some small molecule compounds. For example, Jiang et al. extracted lemon polysaccharides under alkaline conditions with an extraction rate of 8.81% (Jiang et al., 2017), Li et al. used alkaline leaching method to optimize the extraction of acidic Tuckahoe polysaccharides, the extraction rate was increased to 78.5% (li, 2018). Similarly, corn silk polysaccharide was comparatively extracted by acid extraction and water under the same experimental conditions. The extraction rate 33.36% obtained by acid was higher than the water extraction (7.44%) (Li and Zhou, 2020). Thus, polysaccharides could also be extracted by the acid and alkaline leaching method due to their hydrolysis. However, little related literature on extracting ESPS were obtained in view of the fact that the acid-base method may destroy the structure of the active polysaccharides.
Li et al. compared the extraction of ESPS with water extraction and alkali-extraction. The yield of crude polysaccharides from water extraction and EtOH precipitation was 20.8%, and total carbohydrate content in which was 5.19%. Alternatively, the value obtained from alkali-extraction and EtOH precipitation were 18.31 and 6.01%, respectively (Li, 2019).
Other Methods
Aside from the common technologies mentioned above, enzymatic hydrolysis, supercritical fluid extraction (SFE) (Wei, 2018), enzyme-microwave-ultrasonic-assisted extraction (Yin et al., 2018) were also employed in the extraction of plant polysaccharides. However, these methods have not been applied to the ESPS extraction at present, which needs further research. Moreover, the crude polysaccharides extracted by above methods need to remove proteins, fat-soluble ingredients, pigments, inorganic salts and other small molecule impurities before subsequent tests. Figure 2 summarized the diagram of extraction, purification and structural characterization of ESPS.
FIGURE 2.
Schematic diagram of extraction, purification and structural characterization of ESPS.
Purification of Eleutherococcus Senticosus Polysaccharides
Polysaccharides in medicinal materials often coexist with other active ingredients. The separation and purification of homogeneous polysaccharide from crude polysaccharides was usually achieved by EtOH precipitation according to the solubility differences in EtOH. Normally, EtOH precipitation could be divided into step-alcohol-sinking and graded-alcohol-sinking, respectively. Related experiments showed that the purification performance of ESPS by step-alcohol-sinking performed more perfect in view of the higher content (Li, 2019). Besides, the concentration of alcohol in the graded-alcohol-sinking method would also affect the biological activity of ESPS. Li et al. compared the immune enhancement effects of ESPS obtained from 20, 40, and 60% alcohol precipitations. After comparison, ESPS obtained from 40% EtOH-precipitated exhibited better resistance to the splenic lymphocyte proliferation induced by ConA and LPS in a dose-dependent manner (Li, 2020).
In recent years, new materials and technologies have been increasingly applied in the separation and purification of polysaccharides accompanied by the rapid development of the science and technology. Among which, ion exchange chromatography has been used for the preparation of various types of components of traditional Chinese medicine in view of their advantages of good separation effect, fast separation speed, wide application range, and simple operation. Usually, diethylaminoethyl (DEAE)-cellulose is first used as an exchange agent to purify the polysaccharide to be acidic or neutral. The separated one was further purified by Sephadex gel according to the difference in molecular size. Based on this principle, scholars have developed different classification and types of fillers to achieve the systematic separation of polysaccharides with different properties. For example, Wang et al. employed Q Sepharose Fast Flow ion-exchange chromatography column to purify three kinds of ESPS,NASC-1, ASH-1 and ASA-1 that extracted by cold alkali, hot alkali and acid solutions, respectively (Wang, 2006). Bai et al. classified the ESPS from the rhizomes of E. senticosus into neutral ASPN and acidic ASPA-1 and ASPA-2 by DEAE-cellulose column. Subsequently, Sepharose CL-6B was further used to separate ASPA-1 into two uniform grades, namely ASPA-1-A and ASPA-1-B (Bai, 2015). Similarly, Zhai et al. adopted D941 resin, DEAE-Sepharose Fast Flow to chromatograph the crude polysaccharide and then used Sephadex-G200 molecular sieve to obtain the purified neutral and acidic polysaccharide As-1 and As-2, respectively (Zhai, 2007).
Content Determination
Content determination is an important procedure to control and evaluate the quality of polysaccharide products. Phenol-sulfuric acid and anthrone-sulfuric acid methods were the most commonly used ones. The principle is that polysaccharides are hydrolyzed into monosaccharides and further dehydrated to furfural or carboxymethyl furfural due to the concentrated sulfuric acid. Subsequently, phenol or anthraquinone can undergo condensation reaction with furfural derivatives to produce orange-yellow and blue-green compounds. Within 100–200 mg, the color depth increases with the increase of polysaccharide content, which can be determined by colorimetry. The compound produced by the phenol-sulfuric acid method has a maximum absorption peak at a wavelength of 490 nm, so the absorbance of the sample is measured at λ = 490 nm (Bai, 2015).
A study compared the phenol-sulfuric acid and the anthrone-sulfuric acid methods for the determination of ESPS content and found that the results of the two methods were similar, but the phenol-sulfuric acid method was more convenient. Using phenol-sulfuric acid method, the content of ESPS after freeze-drying was 51.9–56.5%, while that obtained by spray-drying was 50.5–53.1% (Zhang and Sun, 2005).
Existing data from literatures reveal that the variation explained by the content of ESPS ranged from 1.52 to 13.21% due to the limitation of the extracted part and process of E. senticosus. How to further increase the content of ESPS is still the direction we need to work hard on.
Structural Characterization of Polysaccharides
The polysaccharide belongs to a kind of high molecular polymer with a relative molecular weight (Mw) ranging from tens of thousands to tens of millions connected by glycosidic bonds. The structure identification of polysaccharides could be accomplished by the determination of the purity, monosaccharide composition, Mw, position and sequence of carbohydrate connection, glycoside bond configuration and oxygen ring. Several technologies including acid hydrolysis, methylation, periodic acid oxidation, Smith degradation, alkaline degradation, enzymatic hydrolysis, biological analysis, physical analysis (including IR, MS, ESI-MS, MALDI-MS, NMR, CE, GC, etc.,) would be employed.
The early research on structure of ESPS was limited to incomplete and inaccurate characterization by equipment and technology. Mw and monosaccharide composition were the only detected items for ESPS. The Mw of polysaccharides was obtained by measuring the standard dextran with known Mw. Based on this approach, PES-A and PES-B with 7 and 76 kDa together with immunologically active polysaccharides As-II and As-II with 150 and 30 kDa were purified from the root of E. senticosus (Xu, 1983). In the 21st century, the structure research and chemical modification technology of ESPS become more mature, high performance gel permeation chromatography (HPGPC) analysis technology with more accurate and fast superiority is developed to determine the Mw distribution and uniformity of polysaccharides based on the molecular sieve principle. Accordingly, several ESPS with Mw ranging from 7 to 400 kDa were reported (Wang, 2006). Their structural information is provided in Table 2. It is worth noting that the separation range of the HPGPC column possess the significant influence on the determination results. Bai et al. compared the Mw of the same ESPS samples separated by two specifications of columns from TSK-gel company. Results showed that Mw of ESPS-1-A and ASPA-1-B were 48 and 11 kDa by G-3000PWXL, while the value were 37 and 10 kDa by G-4000PWXL, respectively (Bai, 2015). As ASPS-1-A possessed larger Mw, more significant influence on it was present. This reminds us that the choice of chromatographic column is very important.
TABLE 2.
Structural characterization of polysaccharides isolated from the different parts of Eleutherococcus senticosus.
| No | Compound Name | Medical Parts | Monosaccharide Composition a | Main chain composition/ structural domain | Branch composition | M.W. (KDa) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PES-A | Root | Glc: Gal: Ara =3.3:2:1 | — | — | 7 | Xu (1983) |
| — | PES-B | Root | — | — | — | 76 | Xu (1983) |
| — | PES-W | Root | — | — | — | — | Xu (1983) |
| 2 | As-II | Root | Glc | — | — | 150 | wang (1986) |
| — | As-III | Root | Ara: Xyl:4-O-methyl-D-GlcA =1:11:1 | — | — | 30 | wang (1986) |
| 3 | AS-2 | Root | Ara: Xyl: Rha: Gal: Glc =1.6:1.2:1.8:1.0:3.6 | β-(1→3)-Glc, β-(1→4)-Glc | (1→4)-Rha, (1→6)-Gal, (1→3)-Gal, (1→6)-Glc | 78 | Zhang et al. (1993) |
| 4 | ASP | Root | — | — | — | — | Han et al. (2003) |
| 5 | ASC-1 | Root | — | — | — | — | Wang et al. (2006) |
| — | ASC-2 | Root | Rha: Ara: Xyl: Glc: Gal | — | — | — | Wang et al. (2006) |
| — | ASC-2C | Root | — | — | — | 17 | Wang et al. (2006) |
| — | ASC-2S | Root | — | — | — | 11 | Wang et al. (2006) |
| 6 | ASC-1P2B | Root | Xyl | — | — | — | Wang et al. (2006) |
| 7 | ASH-1 | Root | — | — | — | — | Wang et al. (2006) |
| — | ASH-1P3 | Root | Rha: Ara: Xyl: Man: Glc: Gal | — | — | — | Wang et al. (2006) |
| 8 | ASA-1 | Root | — | — | — | 12 | Wang et al. (2006) |
| — | ASA-2 | Root | — | — | — | — | Wang et al. (2006) |
| 9 | ASA-1P3A | Root | Glc: Xyl: Gal= 22.4:5.2:1 | (1→6)-Glc | (1→4)-Glc, (1→3)/(1→4)-Xyl, | 38.3 | Wang et al. (2006) |
| — | ASA-1P3B | Root | Glc: Xyl: Gal =7.6:19.4:1 | (1→2)-Glc , (1→4)-Xyl | (1→3)-Xyl, (1→2), (1→4), (1→6)-Glc | 7.2 | Wang et al. (2006) |
| 10 | ASP-2-1 | Leaf | Rha: Xyl: Glc: Man: Ara: Gal: GlcA =7.45:18.63:25.15:0.93:8.35:2.79:5.69 | — | — | 14.6 | Chen (2011b) |
| 11 | ESPS | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara: Fuc=1.5:13.6:0.6:14.9:17.1:24.2:11.6:15.4:1.1 | — | — | 17 | Bai (2015) |
| — | — | — | GlcA: Rha: Xyl: Fru: Glc=1.10:6.38:2.30:2.83:12.34 | — | — | — | Zhang and Sun, (2015) |
| — | — | — | Ara: Glu: Gal: Xyl: GalA =51.4:24.5:10.2:5.7:4.9 | — | — | 74,38,45,23 | Wang et al. (2016) |
| — | — | — | Ara: Xyl: Gluc: Man=7.1:22.3:7.6:1.0 | — | — | — | Meng (2018) |
| 12 | ASPA | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara: Fuc= 0.3:13.5:1.9:27:3.6:22.3:15.1:15.7:0.6 | RG-1, AG-II, XGA | — | — | Bai (2015) |
| 13 | ASPN | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara: Fuc =4.3:4.5:1:1.7:25.7:32.9:7.1:22.6:0.2 | — | — | — | Bai (2015) |
| 14 | ASPA-1 | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara = 0.2:13:0.8:35.9:0.6:17:25.3:7.2 | RG-1, XGA | — | — | Bai (2015) |
| — | ASPA-2 | Root | Man: Rha: GlcA: GalA: Glc: Gal: Ara = 0.4:11.4:1.8:15.7:2.2:44.7:23.8 | RG-1 | — | 400 | Bai (2015) |
| 15 | ASPA-1-A | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara: Fuc=0.4:14.8:1.1:26.9:0.6:20.4:27.1:8.3:0.4 | RG-1, XGA | — | 48,40,37 | Bai (2015) |
| — | ASPA-1-B | Root | Man: Rha: GlcA: GalA: Glc: Gal: Xyl: Ara: Fuc=0.4:11.7:0.9:46.2:1:14.1:20.4:5.1:0.2 | RG-1, XGA | — | 11,10 | Bai (2015) |
| 16 | ABPS-21 | Stem | Gal: Glc: Rha: GalA =3.0:2.0:2.0:1.0 →4)-D-Galp-(1→4)-D-Glcp-(1→4)-D- | α-L-Rhap-(1→4)-β-D-Glcp-(1→, | 106 | — | Hu et al. (2015) |
| — | — | — | — | Galp-(1→4)-D-Galp-(1→2)-α-L-Rhap-(1→, | α-D-GalpA-(1→O-6 1,4,6-linked β-D-Glcp | — | — |
| — | — | — | — | — | α-L-Rhap-(1→4)-β-D-Glcp-(1→, | — | — |
| — | — | — | — | — | α-D-GalpA-(1→ O-6 1,4,6-linked β-D-Galp | — | — |
| — | ABPS-21P | Stem | Gal: Glc: Rha =3.0:1.0:1.0 | — | — | 8.25 | Hu et al. (2015) |
| 17 | ANP | Buds | Ara: Man: Glc: Gal=1.0:2.6:2.5:1.4 | — | — | 10.7 | Lee et al. (2015) |
| — | AAP | Buds | Ara: Gal: 4-O-methyl-D-GlcA = 5:10:1 | AG-II | — | 84 | Lee et al. (2015) |
| 18 | CASPs | Root | Ara: Man: Rha: Gal: Glc =1:1.1:3:4.7:5 | — | — | 70,38,120,19 | Xie et al. (2015) |
Ara, arabinose; Fuc, fucose; Fru, fructose; Gal, galactose; Glc, glucose; GalA, galacturonic acid; GlcA, glucuronic acid; Man, mannose; Rha, rhamnose; Rib, ribose; Xyl, xylose; AG, arabinogalactans; RG, rhamnogalacturonan; XGA, xylogalacturonan.
The determination of monosaccharide composition of polysaccharides has been improved and perfected. In the initial stages, thin layer chromatography (TLC) method was used by comparing the specific Rf value with each monosaccharide standard. While TLC can only provide the type of monosaccharides like ASC-2 and ASH-1P3 (Wang, 2006), their proportion could not be given as TLC is an unquantifiable method. Instead, gas chromatography (GC) could realize the measurement of above two respects based on the derivatization method. After GC analysis, the proportion of monosaccharides of PES-A (Xu, 1983), ASA-1P3A and ASA-1P3B had been supplied, which also provides key information for the subsequent estimate of ESPS types. Besides, the results in Table 2 showed that GalA and Gal possessed relatively high proportion in ESPS.
In recent years, more in-depth research into chain sequencing and structural domain of carbohydrate is developed based on the requirement of pharmacology, toxicology and structural modification. According to the sequence, mode, and monosaccharide types of glycosylation in the main chain, the structure of ESPS could be divided into three types, namely RG-I, XGA and AG-II. 1) RG-I: Most of ESPS belong to the polysaccharides with RG-I structure due to high content of Rha and GalA connected through 1→4 linkage. Besides, the Rha/GalA ratios in different samples ranged from 0.25 to 0.73 indicated that they may be inducted as pectin. 2) XGA: XGA domain represent for some polysaccharides with higher Xyl content. Accordingly, the Xyl/GalA ratios 1.0 and 0.44 obtained from ASPA-1-A and ASPS-1-B suggest that XGA domain existed in both components. Moreover, several XGA domains also coexist with RG-I domain in other ESPS samples (Bai, 2015). 3) AG-II: Three kinds of ESPS named ASPA (Bai, 2015), ANP (Lee et al., 2015), and AAP (Lee et al., 2015)were found as AG-II pectin due to major connection of Ara and Gal through 1→3 linkage. However, the structure of ESPS was irregularly in branch composition.
Biological Activities
Anti-tumor Activity
Anti-lung Cancer Activity
Lung cancer remains the leading cause of cancer deaths all over the word. It is generally divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), respectively. However, SCLC was harder to cure due to its high invasiveness, low tumor differentiation, high deterioration as well as poor results of chemotherapy and surgery. Some studies had confirmed that ESPS could inhibit the proliferation of human SCLC cell line H446 using MTT, FCM and Hoechst33258 staining methods (Zhao, 2008a), and promote the apoptosis of H446 cells by up-regulating the expression of Bax and p53 genes and down-regulating the expression of Bcl-2 genes (Zhao, 2008b), and lead to G2/M phase arrest to interfere with H446 cell cycle progression by activating the ERK1/2 pathway (Zhao, 2010). Besides, the intervention effect of ESPS on the incidence of Lewis LC was also observed. In vivo, inhibition of tumor growth by ESPS was also manifested by decreasing the levels of PAI-1 and μPA in plasma, tumor and lung tissue. Moreover, combined administration of cyclophosphamide (CTX) and ESPS exhibited better effect than a single group of drugs (Zhang, 2001). Similarly, Sun et al. explored transwell and wound healing assay to evaluate the anti-tumor activity of ESPS on NCI-H520 cells after 24 and 48 h, decreased proliferation as well as repressed invasion and migration associated with Wnt/β-catenin pathway mediated-EMT provided new theoretical knowledge for NSCLC (Sun et al., 2019). In addition, Aidi injection is a proprietary Chinese medicine extracted from E. senticosus to treat stage IIIB/IV non-small cell lung cancer, and this therapeutic effect seems to be related to ESPS (Wang et al., 2018).
Anti-liver Cancer Activity
In recent years, some studies have found that ESPS also showed remarkable biological activity against liver cancer. In 1997, Zhang et al. found that ESPS had a significant inhibitory effect on hepatocellular carcinoma induced by chemical carcinogens (3'-me-DAB) (Zhang et al., 1997). In vitro experiments showed that ESPS could induce apoptosis and G0/G1 phase cell cycle growth arrest in HepG2 cells by targeting Wnt/β-catenin pathway (Wang et al., 2016). Anti-hepatic carcinoma assay experiment of ASC-1 and ESPS on mouse H22 transplanted tumors also showed a preventive effect. Compared with the white water group, the tumor suppression rate of ASC-1 reached 39.13% (Wang, 2006), and the tumor quality of the ESPS group was significantly lower than that of the control group (Meng, 2018). It can be inferred that ESPS indirectly suppressed hepatic carcinoma by enhancing the immunity of the body.
Other Anti-cancer Activity
Malignant tumors remain a serious disease that threatens human health. Currently, previously discovered ESPS have shown effects on cervical cancer, sarcoma, and laryngeal cancer of varying degrees along with the advantages of safety, efficacy and low toxicity. It can be used as antineoplastic and anti-tumor adjuvant drugs. As early as 1993, Cao et al. found that intragastric administration of ESPS at 10 mg/kg promoted the proliferation of mouse spleen cells and enhanced the activity of LAK cells, which provides an ideal biological response adjustment for LAK/IL-2 tumor cell therapy agents (Cao and Du, 1993). Meng et al. founded that different doses of ESPS (200, 100 and 50 mg/kg) could significantly inhibit the growth of sarcoma S180 and cervical cancer U14 on tumor-bearing mice and prolonged their survival time. Among which, 100 mg/ml dose possessed the best activity (Meng, 2018). Simultaneously, Tong et al. confirmed that ESPS has a direct anti-tumor effect on sarcoma S180 cells, whose inhibition rate exceeds 70% and half effective inhibitory concentration is 0.38 g/L (Tong, 1994). Besides, studies have also shown that ESPS can inhibit the proliferation of human cervical cancer HeLa cells via promoting the expression of Bax protein. At the same time, it may down-regulate the expression of survivin protein (Jin, 2014; Jin, 2016), thereby promoting the apoptosis of HeLa cells. ESPS inhibited the proliferation of CD133-positive stem cells in laryngeal cancer Hep-2, which may be related to the influence of ESPS on the expression levels of PD-L1 and Bcl-2 to promote stem cell apoptosis (Chen et al., 2019). This important discovery lays the foundation for the targeted therapy of laryngeal cancer stem cells.
Immunoregulatory Activity
Immunomodulation is considered to be one of the important physiological functions for the body to identify and remove antigenic foreign bodies as well as maintain physiological dynamic balance and relative stability. Abnormalities in the immune system could cause a variety of diseases such as infection, inflammation and cancer (Yu et al., 2018). In recent years, ESPS has been proved to have a certain regulatory effect on the immune system of mice. On the one hand, it can directly activate the immune function of T, B lymphocytes and macrophages by regulating immune organs; on the other hand, it can also play its biological activity by regulating the level of cytokines. Many studies have shown that intraperitoneal injection or intragastric administration of ESPS could increase the weight of thymus and spleen and improve immune function in mice (Chen, 1984; Xie, 1989a; Xu, 1990). Moreover, this regulatory effect may be positively correlated with the dose. Sun et al. found that the immune organ index, peripheral blood leukocyte count and macrophage phagocytosis of CTX-induced immunocompromised mice were increased by intragastric administration of different doses of ESPS aqueous solution (6 mg/10 g, 12 mg/10 g and 24 mg/10 g), and the efficacy of high dose group was significantly (Sun et al., 2018). Equally, Luo et al. also found that high-dose ESPS could significantly promote the proliferation of spleen lymphocytes and increase the ratio of CD3+CD4+/CD3+CD8+ (Luo, 2013a). In addition, ESPS can also improve the innate immune function of the body by reducing the levels of cytokines such as IFN-γ, IL-2, IL-6 and TNF-α (Diao, 2008a; Han et al., 2014; Zhang, 2015). In vitro, ESPS could significantly promote lymphocyte proliferation induced by concavalinA and LPS, and this effect is not related to endotoxin (Chen et al., 2011). These studies provide abundant evidence for ESPS as a natural immunostimulant agent. However, the present research on the immune activity of ESPS remains on the animal level, and whether it can be used in clinic needs more in-depth research.
Antioxidant Activity
As we all know, the production of free radicals has the beneficial effects of regulating cell growth and inhibiting viruses and bacteria (Diao, 2008c). Nevertheless, excessive free radicals will lead to excessive reactive oxygen species (ROS) formation and break the redox homeostasis. This is closely related to some human chronic diseases, such as cancer, arteriosclerosis and aging (Diao et al., 2010b). ESPS have been proved to have significant scavenging effects on a variety of free radicals, such as hydroxyl radicals, superoxide anions and hydrogen peroxide. In vitro, Chen and Zhao reported that ESPS had obvious scavenging activity evaluated by ferric reducing antioxidant power assay (FRAP), Fenton’s reaction, pyrogallol’s auto oxidation and DPPH radical scavenging experiments in a concentration-dependent manner (Jiao, 2008) (Meng et al., 2005; Diao, 2008d; Chen, 2011b). Xia et al. compared the antioxidant capacity of two water-soluble polysaccharides (ASP-B2 and ASP-B3) extracted from the leaves of E. senticosus. It is worth noting that the clearance rates of ASP-B2 and ASP-B3 for DPPH reached 91.75 ± 0.59% and 91.58 ± 1.58% at 2.0 mg/ml, respectively (Diao, 2008b). Likewise, ESPS extracted from fruits also showed antioxidant activity. Among the concentration range from 0.05 to 0.8 mg/ml, the scavenging rates of ESPS to DPPH and ABTS increased in a concentration-dependent manner, and the highest scavenging rates were 65.21 ± 1.85% and 58.02 ± 1.25%, respectively (Diao, 2009b).
Meantime, researchers also focused on the antioxidant activity of ESPS in vivo. It is reported that the activities of superoxide dismutase (SOD) and GSH-Px increased in a dose-dependent manner after 15 days of intragastric administration of ESPS (50, 100 and 200 mg/kg/d) in rats with cerebral ischemia-reperfusion. As SOD is the most important antioxidant enzyme for scavenging free radicals in vivo, the result suggests that ESPS may improve cerebral ischemia-reperfusion injury by improving the antioxidant capacity of brain tissue (Xie et al., 2015). Similarly, Long et al. found that ESPS could also improve the growth performance of chicks by increasing the activities of SOD and GSH-Px (Long et al., 2021). Besides that, some domestic scholars have also found that ESPS can significantly protect rat hippocampal neurons from oxidative stress damage induced by H2O2 (Diao et al., 2010b; Liu et al., 2013). Above studies indicated that ESPS seem to be used as a potential natural antioxidant. However, there is a big gap between the clinical application and fundamental research, which need further explore in the future.
Anti-inflammatory Activity
Inflammation is a response to pathogens and tissue damage. This process is mainly caused by a series of pathological reactions of the injured site by stimulating immune cells to release inflammatory mediators. However, long-term inflammation is the main cause of aging and serious diseases, such as inflammatory bowel disease, cardiovascular disease (CVD), hepatitis and cancer (Diao, 2009d). In the past decades, the natural polysaccharides extracted from E. senticosus showed significant anti-inflammatory activity. Many studies have shown that ESPS could regulate the level of inflammatory mediators in vivo and reduce the infiltration of inflammatory cells in multiple organs. It is reported that ESPS pretreatment reduced the secretion and expression of inflammatory cytokines such as IL-2, IL-4 and INF-γ, which has a certain protective effect on immune liver injury (Zhu et al., 1982; Xie, 1989c; Luo, 2013b; Han, 2013; Zhang, 2018; Zhai, 2020). Similarly, ESPS could also inhibit the activation of NF-κβ in mouse liver tissue and endotoxic shock induced by LPS/D-GalN (Diao, 2009c). Xie et al. also reported that intragastric administration of ESPS could reduce the contents of IL-1β and TNF-α in brain tissue and exhibit a certain protective effect on cerebral ischemia-reperfusion injury (Zhang et al., 2019). In addition, Han and Fan et al. have verified that ESPS could improve a variety of inflammatory bowel diseases induced by LPS, whose effect is mainly mediated by NF-κB/MLCK, HIF-1α/COX-2 and TLR4/NF-κB signal pathways (Yang et al., 1983; Yang et al., 1985; Poolsup et al., 2004; Luo, 2008; Mo, 2012a; Mo, 2012b; Han et al., 2016a; Han et al., 2016b; Lu, 2016; Yang, 2016; Han et al., 2017; Pan, 2019b; Zhang et al., 2020a). Clinically, Poolsup et al. conducted a statistical analysis of 433 patients with upper respiratory tract infection and found that E. senticosus was more effective than placebo. Whether this effect is related to ESPS needs to be further explored (Poolsup et al., 2004).
Other Biological Activities
Apart from to above biological activities, ESPS also possess the activities of analgesia, anti-radiation, anti-fatigue as well as compatibility with other drugs to treat diabetes and leukemia. As early as the end of the 20th century, ESPS was reported to increase the ability to produce IFNs in S801 and S7811 leukemia cell lines (Diao, 2008b; Diao, 2008c; Diao, 2008d; Diao, 2009a; Diao, 2009b). Similarly, the proliferation of leukemia cells K562 and Pmur388 original from human and mice were also inhibited by ESPS (Diao, 2009c; Jin, 2016). Moreover, Mo et al. reported that ESPS also had anti-radiation effect (Diao et al., 2010a; Diao et al., 2010b). ESPS has also shown a protective effect on the gastrointestinal tract, reproductive system, and peripheral blood cells of rats irradiated by 60Co gamma rays (Diao et al., 2010c). It’s worth noting that magical effect of ESPS as an adjuvant therapy for diabetes was discovered by the rapidly reduced blood glucose after combined use of ESPS with Metformin, whose efficacy on the level of blood lipids (TC and TG), thiobarbituric acid reactive substances (TBARS), AST, ALT, ALP, total bilirubin, creatinine and urea was better than that of Metformin (Fu et al., 2012).
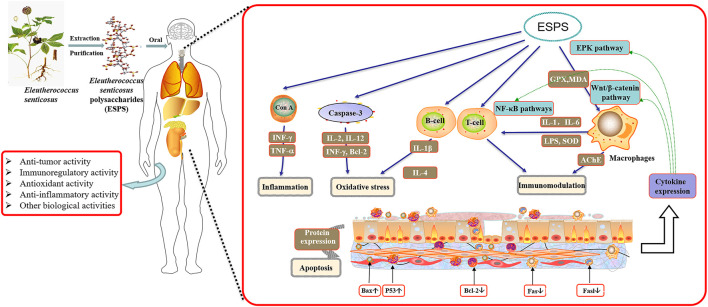
Besides, ESPS can also improve exercise-induced energy metabolism, increase serum creatinine level and reduce protein decomposition, which exhibits a significant anti-fatigue potential (Han, 2007; Bang et al., 2015). Others used metabonomics methods to investigate the mechanism of ESPS on Alzheimer’s disease and CVD rats by detecting the endogenous substances, 20 potential biomarkers such as 6-dimethylaminopurine and l-acetylcarnitine were screened out (Lu, 2015). Table 3 and Figure 3 summarize the biological activities and related mechanism of ESPS, respectively.
TABLE 3.
Pharmacological activities and involved mechanism of ESPS.
| Biological activity | Compound Name | Medical Parts | Model | Vivo/Vitro | Administration Route a | Dose | Duration | Cytokine/Enzyme /Protein | Results | Involved Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-lung cancer | ESPS | Root | Female C57/BLmice | In vivo | i.p. | 0.12 ml/piece | ─ | μPA ↓ | Intervene of lung cancer | PAI-1 ↓ | Zhang (2001) |
| ESPS | Root | H446 cells | In vitro | — | 240,480,960 μg/ml | 48 h | — | Cell proliferation ↓ | P53, Bax↑, Bcl-2 expression ↓ Apoptosis | Zhao (2018) | |
| ESPS | Root | H446 cells | In vitro | — | 240,480,960 μg/ml | 48 h | TNF-α, IL-1 | Cell proliferation ↓ | P53,Bax↑,Bcl-2↓p-38 expression EPK pathway, Apoptosis | Zhao (2018) | |
| ESPS | Root | H446 cells | In vitro | — | 240,480,960 mg/l | 48 h | — | G2/M arrest ↓ | EPK MAP kinase pathways | Zhao (2010) | |
| ESPS | Root | NSCLC NCI-H520 | In vitro | — | 10,20,40,80,160, 320 mg/ml | 12,24,48 h | MMP2, MMP9, FN1, wnt3a | Proliferation, metastasis ↓ | Wnt/β-catenin pathways | Sun et al. (2019) | |
| ASC-1 | Root | A-549 cells | In vitro | — | ASC-1 35.7 μM | 11 days | — | Cell viability ↓ | — | Wang, (2006) | |
| ASA-1 | Root | — | — | — | ASA-1 125 μM | — | — | — | — | — | |
| ASH-1 | Root | — | — | — | ASH-1 163.5 μM | — | — | — | — | — | |
| Anti-liver cancer | ESPS | Root | Mice, HEPA cells | In vivo | s.c. | 0.34 g/kg /d | 10 days | — | The life of mice ↑ | — | Chen (1984) |
| ASC-1 | Root | Male Kunming mice, | In vivo | i.g. | 2 mg/piece | 11 days | ASC-1 prevent seed tumors | — | — | Wang, (2006) | |
| ASA-1 | Root | — | H22 cells | i.p. | 0.04 mg/piece | — | — | ASA-1no anti-tumor effect | — | — | |
| ESPS | Root | Kunming mice, | In vivo | i.g. | 50,100,200 mg/kg/d | 10 days | TNF-α,TNF-γ, | Survival days ↑,regulate cytokines | — | Meng, (2018) | |
| — | — | H22cells | — | — | — | — | IL-2, IL-12 | — | — | — | |
| PAS | Root | C57BL/6 mice | In vivo | i.p. | 10 mg/kg /d | 14 days | IL-2R, LAK | LAK activity ↑, splenocyte ↑ | — | Cao and Du, (1993) | |
| ESPS | Root | Male Wister rat | In vivo | i.g. | 20% | 70 days | γ-GT ↑ | Inhibit tumor | — | Zhang et al. (1997) | |
| ESPS | Root | HepG2 cells | In vitro | — | 0,10,20,40,80 mg/L | 48 h | — | Apoptosis↑, G0/G1 phase arrest in | Wnt/β-catenin pathway | Wang et al. (2016) | |
| Anti-other tumors | ESPS | Root | Kunming mice, | In vivo | i.g. | 50,100,200 mg/kg/d | 10 days | IL-2,IL-12,INF-γ | Cell growth ↓ | Apoptosis | Meng et al. (2018) |
| ESPS | Root | Kunming mice, | In vivo | i.g. | 50,100,200 mg/kg/d | 10 days | TNF-α,TNF-γ, | Survival days ↑, regulate cytokines | Immunomodulation | Meng, (2018) | |
| — | — | U14 cells | — | — | — | — | IL-2, IL-12 | — | — | — | |
| ESPS | Root | Hela cells | In vitro | ─ | 1,2,4,8,16 mg/ml | 24,48,72 h | ─ | Cell proliferation ↓ | Bax expression ↑ , Apoptosis | Jin (2014) | |
| ESPS | Root | Hela cells | In vitro | ─ | 1,2,4,8,16 mg/ml | 24,48,72 h | ─ | Cell proliferation ↓ | Survivin expression ↓, Apoptosis | Jin (2016) | |
| ESPS | Root | Kunming mice, | In vivo | i.g. | 50,100,200 mg/kg /d | 10 days | TNF-α,TNF-γ, | Survival days ↑, regulate cytokines | Immunomodulation | Meng, (2018) | |
| — | — | S180cells | — | — | — | — | IL-2, IL-12 | — | — | — | |
| ESPS | Root | Mice S37,S180 cells | In vivo | s.c | 0.34 g/kg/d | 10 days | — | Excited reticuloendothelial system | Immunomodulation | Chen (1984) | |
| ESPS | Root | S180cells | In vitro | — | 1,10,100,500, | 24 h | — | Cell proliferation ↓ | Apoptosis | Tong (1994) | |
| — | — | — | — | — | 1000 mg/l | — | — | — | — | — | |
| ESPS | Root | Hep 2 CD133 | In vitro | — | 100 mg/l | 24,48,72 h | Bcl-2,PD-L1 | CD133 stem cells ↓ | Apoptosis | Chen et al. (2019) | |
| Immuno-modulatory | PES-W | Root | Female C57BL mice | In vivo | i.p. | 100 mg/kg /d | 5 days | — | Immunity↑, hyperplasia of | Immunomodulation | Xu (1983) |
| PES-A | — | — | — | — | — | — | spleen and thymus ↑ | — | — | — | |
| PES-B | — | — | — | — | — | — | — | — | — | — | |
| PES-W | Root | Female C58BL mice | In vivo | i.p. | 100 mg/kg/d | 4 days | ─ | The phagocytic function of mice | Immunomodulation | Xu (1983) | |
| PES-A | — | — | — | — | — | — | — | Peritoneal macrophages ↑ | — | — | |
| PES-B | — | — | — | — | — | — | — | — | — | — | |
| ESPS | Root | Mice | In vivo | i.p. | 30 mg/kg/d | 3 days | — | Sleep time↑, number of thymus ↓ | Immunomodulation | Chen (1984) | |
| ESPS | Root | Mice | In vivo | i.p. | 30 mg/kg /d | 3 days | — | Sleep time↑, white blood cells↑ | Immunomodulation | Chen (1984) | |
| ESPS | Root | Mice | In vivo | s.c. | 0.067 g/kg/d | 3 days | — | Formation of antibodies in the spleen ↑ | Immunomodulation | Chen (1984) | |
| ASII | Root | Mice | In vivo | — | — | — | — | Strong phagocytosis ↑ | Immunoregulation | Wang, (1986) | |
| ASIII | — | — | — | — | — | — | — | — | — | — | |
| ESPS | Root | Male BALB/c, C57BL/6 mice | In vivo | i.p. | 100 mg/kg/d | 10 days | ConA, LPS | Stimulate T-cell proliferation | — | Xie et al. (1989a) | |
| Immuno-Modulatory | ESPS | Root | Male BALB/c, C57BL/7 mice | In vivo | i.p. | 100 mg/kg/d | 15 days | ConA, LPS | Number of antibody secreting cells↑ C57BL/7 mice delayed-type hypersensitivity↑ | Immunomodulation | Xie (1989b) |
| ESPS | Root | LACA mice | In vivo | i.p. | 12.5,25,50,100 mg/kg | 4/9 days | — | Humoral immune response↑ | — | Xu, (1990) | |
| PES | Root | Female C57BL/JCR | In vitro | s.c. | 100 mg/kg | 4 days | LPS | PES possesses mitogenic activities | Immunomodulation | Shen et al. (1991) | |
| — | — | mice | — | — | — | — | — | Enhanced LPS, lymphocyte | — | — | |
| — | — | — | — | — | — | — | — | Transformations ↓, stimulates B-cells | — | — | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 25,50,100 mg/kg | 7 days | CD3+,CD4+, | The proliferation of splenic | Immunomodulation | Luo (2013a) | |
| — | — | — | — | — | — | — | CD8+ | lymphocytes ↑, | — | — | |
| — | — | — | — | — | — | — | — | CD3+CD4+/CD3+CD8+↑ | Immunomodulation | — | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 25,50,100 mg/kg | 7 days | CD4+/CD8+, | The body's cellular immune | Immunomodulation | Luo (2013b) | |
| — | — | — | — | — | — | — | IL-2 | function ↑ | — | ||
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 14 days | ─ | The phagocytic function of | Immunomodulation | Zhai (2020) | |
| — | — | — | — | — | — | — | — | immunosuppressed mice ↑ | — | — | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 7 days | — | The production of hemolysin HC50↑ | Immunomodulation | Zhai (2020) | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 7 days | — | The weight of the thymus ↓ | Immunomodulation | Zhang, (2015) | |
| ESPS | Root | BALB/c, DTH mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 7 days | CD4+/CD8+ | Immunosuppressive effect | — | Zhang, (2015) | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 7 days | IL-2,IL-4,IFN-γ | Cytokine content ↓ | Cytokine expression | Zhang, (2015) | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36,25,72.5,145 mg/kg | 14 days | HC50 | Sheep red blood cell production | Immune stress | Zhang, (2015) | |
| — | — | — | — | — | — | — | — | Hemolysin ↑ | — | — | |
| ESPS | Root | Weaned piglets | In vitro | ─ | 0,40,80,160, 320 μg/ml | 21 days | NO, iNOS, NF-κB, | Cooperate with ConA to promote | Apoptosis | Han, (2013) | |
| — | — | — | — | — | — | — | Th1 | T- cell proliferation | — | — | |
| ESPS | Root | Weaned piglets | In vitro | ─ | 800 mg/kg | 14d | IL-2,IL-6, TNF-α, | Modulate the release of | Cytokine expression | Han et al. (2014) | |
| — | — | — | — | — | — | — | α-AGP | Pro-inflammatory cytokines | — | — | |
| ESPS | Root | ICR mice | In vivo | i.g. | 6,12,24 mg/10g/d | 21 days | TNF-α, INF-γ | Regulate the serum hemolysin level, | Apoptosis | Sun et al. (2018) | |
| — | — | — | — | — | — | — | — | Cell apoptosis ↓ to enhance the | — | — | |
| — | — | — | — | — | — | — | — | Body's humoral immune function. | — | — | |
| PES | Root | Mice | In vivo | i.p. | 125 mg/kg/d | 5 days | BSAIgG↑ | Enhance the defense. | Antioxidant index | Zhu et al. (1982) | |
| Anti-inflammatory | ESPS | Root | Kunming mice | In vivo | i.g. | 20 mg/kg/d | 10 days | IL-1, IL-2, | Improve immune cytokines | Cytokine expression | Meng, (2018) |
| — | — | — | — | — | — | — | IL-10, TNF-α | — | — | — | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36.25,72.5,145 mg/kg | 7 days | IL-1, IL-1β, IL-2, IL-4 | Number of enzymes ↓,protect liver | Antioxidant index | Zhang (2018) | |
| CASPs | Root | Wistar male rats | In vivo | i.g. | 50,100,200 mg/kg | 15d | IL-10↑, IL-1β, TNF-α↓ | Inflammatory cytokines↓ | — | Xie et al. (2015) | |
| ESPS | Root | Male BALB/c mice | In vivo | i.g. | 14.5 mg/ml/d | 7 days | IL-4, Cyclic GMP | Improve the body’s immunity, | Glutathione metabolism, purine | Yang, (2016) | |
| — | — | — | — | — | — | — | — | Anti-inflammatory, Antioxidant, | Generation metabolism, cysteine , | — | |
| — | — | — | — | — | — | — | — | and supply the body's energy | methionine metabolism pathways | — | |
| ASPS-1-1 | Root | Male BALB/c mice | In vivo | i.g. | 6.9 mg/ml | 7 days | IL-1β, TNF-α, IL-2, | Liver cell damage ↓ | — | Yang, (2016) | |
| ASPS-2-1 | — | — | — | — | 4.6 mg/ml | — | IL-4, IL-6 | — | — | — | |
| ASPS-3-1 | — | — | — | — | 2.0 mg/ml | — | — | — | — | — | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36.25,72.5,145 mg/kg | 7 days | IL-2, IL-4, | Secretion and expression of | Cytokine expression | Zhang (2018) | |
| — | — | — | — | — | — | — | — | inflammatory cytokines ↓ | — | — | |
| ESPS | Root | BALB/c mice | In vivo | i.g. | 36.25,72.5,145 mg/kg | 7 days | MDA, IL-1β, TNF-α, | The activity of inflammatory | Cytokine expression | Zhang et al. (2019) | |
| — | — | — | — | — | — | — | NO, GSH-Px, SOD, | Cytokines, adhesion factors↓, | — | — | |
| — | — | — | — | — | — | — | ICAM-1, iNOS, | The secretion and expression of | — | — | |
| — | — | — | — | — | — | — | NF-κB | Inflammatory cytokines↓ | — | — | |
| ESPS | Root | KM mice | In vivo | i.g. | 14.5 mg/ml/d | 7 days | IL-2, IL-6 | By enhancing the body's immune function, scavenging free radicals, liver cell apoptosis↓, and other mechanisms | Bile secretion, Cysteine metabolism, Ubiquinone and other terpenoid quinone biosynthesis, Citrate cycle regulating the body's cytokines Purine metabolism, Riboflavin metabolism, Primary bile acid biosynthesis, Biosynthesis of unsaturated fatty acids, Cysteine and methionine metabolism | Lu (2016) | |
| Anti-leukemia | ESPS | Root | Mice, L615 cells | In vivo | i.p. | 0.34 g/kg/d | 5 d | — | No significant effect on survival time | Oxidative stress | Chen (1984) |
| ESPS | Root | S801, S7811 cells | In vitro | — | 10 μg/ml | single | — | Improve the ability of cells to produce interferon | Oxidative stress | Yang et al. (1983) | |
| ESPS | Root | S801,S7811 cells | In vitro | — | 10 μg/ml | single | — | The life span of the transcribed dry money mRNA↑ or mRNA inactivation rate↓ | Apoptosis | Yang et al. (1985) | |
| ESPS | Root | K562 cells | In vitro | — | 1,10,100,500, 1000 mg/l | 24 h | — | Cell proliferation↓ | Apoptosis | Tong (1994) | |
| ESPS | Root | K562 cells | In vitro | — | 0.405, 0.810, 1.620, 2.430, 3.240 mg/mL | 24 h | — | K562 cell apoptosis ↑ | Apoptosis | Luo (2008) | |
| ASC-1 | Root | P-388 cells | In vitro | ─ | 35.69 μM | single | — | Mouse white blood cell activity↓ | — | Wang, (2006) | |
| ASH-1 | — | — | — | — | 164.23 μM | single | — | — | — | — | |
| Anti-oxidation | ESPS | Root | ·OH, O2- | In vitro | ─ | 3 mg/l | single | — | Elimate ·OH and O2- | Oxidative stress | Meng et al. (2005) |
| ESPS | Root | ·OH, O2- | In vitro | ─ | 3 mg/l | single | — | Elimate ·OH and O2- | Oxidative stress | Jiao, (2008) | |
| — | — | — | — | — | — | — | — | Inhibit lipid peroxidation of red blood | — | — | |
| — | — | — | — | — | — | — | — | cell membrane↓ | — | — | |
| ASP-2-1 | Leaf | Mice spleen cells | In vitro | — | 0.2 mg/ml | single | — | Elimate ·OH, O2-, DPPH· | Oxidative stress | Chen (2011a) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 g/ml | 7 days | SOD,NOS,MDA | Improve cell resistance to oxidative stress damage | Bax expression ↓,Bcl-2 expression ↑ | Diao (2008a) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | — | Improve the anti-apoptotic ability | Bax expression ↓,Bcl-2 expression ↑ | Diao (2008b) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | LDH,SOD,MDA | Improve the anti-apoptotic ability | P53 expression | Diao (2009a) | |
| ESPS | Root | SD rats hippocampal | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | NO, iNOS mRNA↓ | The expression of iNOSmRNA↓ | iNOSmRNA expression | Diao (2008c) | |
| ESPS | Root | SD rats hippocampal | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | — | Anti-apoptotic ability ↑ | P53 expression ↓ | Diao (2009b) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | SOD, CAT, GSH-Px, MDA | Activity of Antioxidant ↑ | Antioxidant index | Diao (2008d) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | MDA, ROS, H2O2 | The content of oxygen free radicals | Oxidative stress ↓ | Diao (2009c) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 1.25, 2.5, 5, 10 μg/ml | 7 days | SOD, GSH-Px, OGG1mRNA | OGG1mRNA expression ↑ | OGG1mRNA expression | Diao (2009d) | |
| CASPs | Root | Wistar male rats | In vitro | i.g. | 50, 100, 200 mg/kg | 15 days | SOD, GSH-Px↑, MDA↓ | Oxidative damage ↓ | Oxidative stress | Xie et al. (2015) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 2.5,5,10 μg/ml | 7 days | Fas, Fasl↓ | Cell apoptosis↓ | Fas,Fasl↓, Apoptosis | Diao et al. (2010a) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 2.5,5,10 μg/ml | 7 days | — | Gene expression↓, the activity↑ | C-fos,p53 expression | Diao et al. (2010b) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 2.5,5,10 μg/ml | 7 days | NF-κB↓ | Gene expression↓ | NF-κB pathways | Diao et al. (2010c) | |
| ESPS | Root | SD rats hippocampal neurons | In vitro | — | 2.5,5,10 μg/ml Caspase-3 mRNA | 24 h | Caspase-3 | Resist oxidative stress damage | Oxidative stress | Liu et al. (2013) | |
| Other biological activities | ESPS | Root | Kunming mice | In vivo | — | 300 mg/kg | 14 days | DAO, occludin-1, zonula occludens-1 | Improves intestinal integrity | TLR4/NF-κB signaling pathway | Han et al. (2016a) |
| ESPS | Root | Kunming mice | In vivo | i.g. | 300 mg/kg | 14 days | Occludin-1, HSP70 | Gene mRNA expression of epidermal growth factor and its receptor ↑ | mRNA expression | Han et al. (2016b) | |
| ESPS | Root | Male Wistar rat | In vivo | i.g. | 50,100,200 mg/kg | 10 days | — | Protect the body weight of irradiated rats | — | Mo (2012a) | |
| ESPS | Root | Male Wistar rat | In vivo | i.g. | 50,100,200 mg/kg | 10 days | WBC, RBC, PLT | Protect peripheral blood cells from radiation exposure | — | Mo (2012b) | |
| ESPS | Root | Adult flies | In vivo | i.g. | 15,30 mg/ml | 5 days | — | Protects the intestinal tract from DSS | EGFR, JNK, Notch pathways | Zhang et al. (2020a) | |
| ESPS | Root | BALB/C mice | In vivo | i.g. | 300 mg/kg | 7 days | MLCK, TJ, NF-κB, LPS | Associated with inhibition of the NF-κB/MLCK pathway | NF-κB/MLCK pathway | Han et al. (2017) | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 50,100,200 mg/kg | 7 days | IL-6, TNF-α, IL-1β | Improve immune organ index and regulate cytokine levels | — | Pan (2019b) | |
| ESPS | Root | Male SD rat | In vivo | i.g. | 100 mg/kg | 20 days | — | Neuroprotection, anti-apoptosis | Anti-apoptosis | Lu (2015) | |
| ESPS | Root | Male kunming mice | In vivo | i.g. | 450,150 mg/kg/d | 14 days | — | Ameliorate energy metabolism, creatinine, improve the level of serum the breakdown of protein↓ free radical content of the body | Energy metabolism | Han, (2007) | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 50,100,200 mg/kg/d | 5 days | IL-6, TNF-α | Inhibit swelling, improve immune organs, immune cytokines | Immunomodulation | Meng, (2018) | |
| ESPS | Root | Female SD rat | In vivo | s.c. | 100 mg/kg/d | 2/5 days | sICAM-1, TGF-β1, LYM, RT, GLU, LDH | Improve pleural effusion | Immunomodulation | Meng, (2018) | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 50,100,200 mg/kg/d | 7 days | IL-1β, TNF-α | Improve immune organs, immune cytokines | Immunomodulation | Meng, (2018) | |
| ASP | Root | Male Wistar rats | In vivo | i.g. | 200 mg/kg | 28 days | SOD, GPX, TC, TG, TBARS | Adjust the pathophysiological | Antioxidant index parameters of diabetic rats | Fu et al. (2012) | |
| ESPS | Root | Kunming mice | In vivo | i.g. | 50,100,200 mg/kg | 5 days | SOD, GSH-PX, | Improve the Antioxidant capacity CAT, MDA | Antioxidant index caused by D-Gal | Meng, (2018) | |
| ESPS | Root | Female SD rat | In vivo | i.p. | 50,100,200 mg/kg/d | 5 days | SOD, MDA, AChE, ChAT | Regulate enzyme activity in brain tissue and serum | Immunomodulation | Meng, (2018) | |
| ANP | Buds | RAW-Blue TM cells | In vitro | — | — | — | — | Promoted by the interaction through | — | Lee et al. (2015) | |
| AAP | — | (RAW 264.7) | — | — | — | — | — | the membrane receptors | — | — | |
| PEA | Root | — | In vitro | i.g. | 1.75 g/kg | 24 h | CRP | Suppress hypoglycemia and | Inflammation inflammation to relieve alcohol | Bang et al. (2015) |
i. g. intragastric administration; i. v. intravenous injection; i. p. intraperitoneal injection; s. c. subcutaneous injection.
FIGURE 3.
Schematic diagram of biological activity mechanism of ESPS.
Conclusions and Future Perspectives
Consult the related literature and data about E. senticosus, we can find that the related studies mainly focused on small molecule components, which ignored macromolecular substances such as protein, polysaccharides, pectin, and cellulose. As the effective ingredient of E. senticosus, polysaccharide possess a wide range of pharmacological effects with the advantages of non-toxic, two-way immune regulation, and has great potential for clinical application. However, research on polysaccharides has increased resistance due to its high molecular weight, complex structure, low content, difficulty of separation and low purity. The research work of its future perspectives is mainly reflected in the following aspects.
1) From aspects of efficient extraction, the existing methods expose the disadvantages of low yield and long extraction time, and therefore cannot be industrialized in mass production. Instead, a wide range of green environmental technologies such assisted extraction with enzymes and chelating agents have not been studied and applied in the extraction of ESPS.
2) As for the resource utilization, the residues of herbs and fruits after extraction of small molecules are rich in a large number of macromolecular substances, such as pectin, polysaccharides and cellulose. Thus, recirculated extraction is conducive to the sustainable development and maximum utilization of traditional Chinese medicine. It has been reported that relative high yield of sugars and pectin was separately extracted from residues of Ginseng (Zhang et al., 2020b) and Sweet Potato Peels (Hamidon et al., 2020). Meanwhile, Chen et al. found that a large amount of ESPS could be extracted from petioles, leaves and fruits (Chen, 2009). However, no literature on the reuse and expansion of E. senticosus resource were found. Collectively, extraction of ESPS from the residue and other medical parts will provide us future research direction.
3) Recently, the rapid development and application of biological macromolecules in new drug delivery system and edible film provide people new directions and technologies for pharmaceutical and food preservation. If ESPS could pack or interact with other ingredients (flavonoids, saponins, protein) and then act as drug delivery system is relatively empty areas.
4) At present, several products derived from E. senticosus such as capsules, wine and tea have long been developed and marketed in view of their excellent health-care function and clinical therapeutic effect. However, ESPS has not been put into clinical use in view of the absence of quality control standards and reference substance. How to establish a comprehensive quality management system is a problem worth thinking about.
In conclusion, health has received more and more attention with the development of science and the improvement of living standard, and the exploitation of functional food has become a research hotspot and development trend. E. senticosus, a homology of medicine and food as the main raw materials, has displayed the most economic and developing value. Meanwhile, the emergence of ESPS can better inherit, innovate and develop traditional Chinese medicine culture and products. It is believed that ESPS will be widely applied soon in the fields of clinical and functional food with the deepening of research and the maturity of technology.
Author Contributions
XL: Investigation, Resources, Writing-original draft. CC: Writing-original draft. JQ: Conceptualization, Writing-review and editing, Funding acquisition. AL: Writing-review and editing, Funding acquisition.
Funding
This work was financially supported by grants from National Natural Science Foundation of China (No. 81703675), China Postdoctoral Science Foundation (2021M692398) and Natural Science Foundation of Liaoning Province (No. 2019-ZD-0631 and 2019-ZD-0914).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AchE
acetylcholinesterase
- AG
arabinogalactans
- ALP
Alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- Ara
arabinose
- ChAT
choline acetyl-transferase
- ConA
Concanavalin A
- CVD
cardiovascular disease
- CTX
cyclophosphamide
- EGFR
epidermal growth factor receptor
- ERK
regulated protein kinase
- ESPS
Eleutherococcus senticosus polysaccharides
- Fuc
fucose
- Fru
fructose
- FT-IR
Fourier transform infrared spectroscopy
- Gal
galactose
- GC
gas chromatography
- Glc
glucose
- GalA
galacturonic acid
- GlcA
glucuronic acid
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- HPLC
high-performance liquid chromatography
- i.g.
intragastric administration
- i.v.
intravenous injection
- i.p.
intraperitoneal injection
- IFN
interferon
- ICAM
intercellular adhesion molecule
- iNOS
inducible nitric oxide synthase
- IL
interleukin
- JNK
c-jun N-terminal kinase
- LAK
lymphokine-activated killer cell
- LC
liquid chromatography
- LC
Lung cancer
- LPS
lipopolysaccharide
- Man
mannose
- MAPK
mitogen-activated protein kinase
- MDA
malonaldehyde
- MMP
mitochondrial membrane potential
- MS
mass spectrometry
- NF-κB
nuclear factor-kappa B
- NSCLC
non-small cell lung cancer
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- OGG1
8-hydroxyguanine DNA glycosidase
- PLT
platelets
- Rha
rhamnose
- RBC
red blood cells
- RG
rhamnogalacturonan
- Rib
ribose
- SCLC
small cell lung cancer
- s.c.
subcutaneous injection
- SFE
supercritical fluid extraction
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- TCA
trichloroacetic acid
- TLR
toll-like receptor
- TC
serum total cholesterol
- TG
triglyceride
- TNF
tumor necrosis factor
- UAE
ultrasound-assisted extraction
- WBS
white blood cells
- XGA
xylogalacturonan
- Xyl
xylose.
References
- Bai Y. M. (2015). Study on Separation, Purification and Structure of Acanthopanax Senticosus Polysaccharide. Northeast Normal University. [Google Scholar]
- Bang J. S., Chung Y. H., Chung S. J., Lee H. S., Song E. H., Shin Y. K., et al. (2015). Clinical Effect of a Polysaccharide-Rich Extract of Acanthopanax Senticosus on Alcohol Hangover. Pharmazie 70 (4), 269–273. 10.1691/ph.2015.4786 [DOI] [PubMed] [Google Scholar]
- Cao G. W., Du P. (1993).Astragalus Polysaccharides, Acanthopanax Senticosus Polysaccharides and Lycium Barbarum Polysaccharides Regulate the Anti-tumor Activity of LAK Cells In Vivo . J. Second Mil. Med. Univ. 14 (1), 10–13. [Google Scholar]
- Chen F. F. (2015). Study on the Extraction Process of Acanthopanax Senticosus Polysaccharide. Beijing, China: China Feed; 2015 (1), 22–24. 10.15906/j.cnki.cn11-2975/s.20150106 [DOI] [Google Scholar]
- Chen H. T. (2011). Studies on the Extraction and Purification Techniques of Polysaccharides from Acathopanax Senticosus. JOURNAL JIAMUSI UNIVERSITY(NATURAL SCIENCE EDITION) 29 (02), 311–313. 10.3969/j.issn.1008-1402.2011.02.04610.7202/1012856ar [DOI] [Google Scholar]
- Chen J. E. (2015). Optimization of Ultrasound-Assisted Extraction of Manyprickle Acathopanax Root Polysaccharides Using Response Surface Methodology. North. Hortic. (07), 123–127. 10.11937/bfyy.201507036 [DOI] [Google Scholar]
- Chen J. Y., Yu S. J., Xiao D. (2019). The Effection of Acanthopanax Senticosus Polysaccharide on Cancer Stem Cells. Chin. J. Lab. Diagn. 23 (07), 1233–1238. 10.3969/j.issn.1007-4287.2019.07.04410.1177/1071100719879438 [DOI] [Google Scholar]
- Chen K., M. (2009). Delirium und Wahn. Psychiat Prax, 36 (02), 98–99. Ankang, China: J. Ankang Univ. 10.3969/j.issn.1674-0092.2009.02.03110.1055/s-0029-1220834 [DOI] [Google Scholar]
- Chen L., Huang G. (2018). Antitumor Activity of Polysaccharides: An Overview. Curr. Drug Targets 19 (1), 89–96. 10.2174/1389450118666170704143018 [DOI] [PubMed] [Google Scholar]
- Chen R., Liu Z., Zhao J., Chen R., Meng F., Zhang M., et al. (2011). Antioxidant and Immunobiological Activity of Water-Soluble Polysaccharide Fractions Purified from Acanthopanax Senticosu. Food Chem. 127 (2), 434–440. 10.1016/j.foodchem.2010.12.143 [DOI] [PubMed] [Google Scholar]
- Chen R. Z. (2011). Study on Separation, Purification and Antioxidant and Immune Activity of Acanthopanax Senticosus Polysaccharide. Indonesia: Bali Island. [Google Scholar]
- Chen X. J. (1984). The Anti-tumor and Immune Effects of Acanthopanax Senticosus Polysaccharide. Cancer 3 (3), 191–193. [Google Scholar]
- Chen Y., Zhang J., Li Q., Wu J., Sun F., Liu Z., et al. (2018). Response Surface Methodology for Optimizing the Ultrasound-Assisted Extraction of Polysaccharides from Acanthopanax Giraldii. Chem. Pharm. Bull. (Tokyo) 66 (8), 785–793. 10.1248/cpb.c18-00030 [DOI] [PubMed] [Google Scholar]
- Cui N. (2012). Research Review on Polysaccharides in Traditional Chinese Drug. QILU PHARMACEUTICAL AFFAIRS 31 (11), 659–662. [Google Scholar]
- Diao B. (2008). A Study on the Antioxidative Effect of Acanthopanacis Senticosi Polysaccharides. THE JOURNAL PRACTICAL MEDICINE 22 (01), 1102–1104. 10.3969/j.issn.1006-5725.2008.07.008 [DOI] [Google Scholar]
- Diao B. (2009). Effect of Acanthopanacis Senticosi Polysaccharides on Oxygen Free Radical in Hippocampal Neures Impaired by H2O2. CHINESE JOURNAL HOSPITAL PHARMACY 29 (01), 7–11. 10.3321/j.issn:1001-5213.2009.01.003 [DOI] [Google Scholar]
- Diao B. (2009). Effect of Acanthopanax Senticosus Polysaccharide on H_2O_2 Induced Apoptosis of Hippocampal Neurons and Apoptosis Genes. Guangdong Med. 30 (01), 35–36. 10.3969/j.issn.1001-9448.2009.01.015 [DOI] [Google Scholar]
- Diao B. (2009). Effects of Acanthopanacis Senticosi Polysaccharides on Antioxidant Enzyme System in Hippocampal Neurons. HERALD MEDICINE 28 (01), 21–24. 10.3870/yydb.2009.01.006 [DOI] [Google Scholar]
- Diao B. (2008). Effects of Acanthopanacis Senticosi Polysaccharides on Expression of iNOS mRNA in Hippocampal Neurons Damaged by Oxidative Stress. MILITARY MEDICAL JOURNAL SOUTH CHINA 19 (11), 15–17. [Google Scholar]
- Diao B. (2009). Effects of Acanthopanacis Senticosi Polysaccharides on Expression of OGG1 mRNA in Hippocampal Neurons Damaged by Oxidative Stress. CHINESE JOURNAL GERONTOLOGY 29 (04), 396–399. 10.3969/j.issn.1005-9202.2009.04.005 [DOI] [Google Scholar]
- Diao B. (2008). Effects of Acanthopanacis Senticosi Polysaccharides on Oxidative Stress Damage and Bcl-2 Family in Hippocampal Neurons Impaired by H2O2. LISHIZHEN MEDICINE MATERIA MEDICA RESEARCH 24 (7), 2705–2707. 10.3969/j.issn.1008-0805.2008.11.061 [DOI] [Google Scholar]
- Diao B. (2008). Protective Effect of Acanthopanax Senticosus Polysaccharide on H_2O_2 Induced Apoptosis of Hippocampal Cells. South China Natl. Defense Med. J. 22 (4), 4–6+17. [Google Scholar]
- Diao B., Tang Y., Zhu Y. L. (2010). Effect of Acanthopanax Senticosus Polysaccharide on the Expression of Fas and Fasl in Hippocampal Neurons Injured by H_2O_2. Chin. J. Clin. Neurosurg. 15 (07), 416–418. 10.3969/j.issn.1009-153X.2010.07.010 [DOI] [Google Scholar]
- Diao B., Tang Y., Zhu Y. L. (2010). The Effect of Acanthopanax Senticosus Polysaccharide on the Expression of C-Fos and P53 Genes in Hippocampal Neurons Injured by H_2O_2. Chin. J. Clin. Neurosurg. 15 (06), 347–349. 10.3969/j.issn.1009-153X.2010.06.010 [DOI] [Google Scholar]
- Diao B., Tang Y., Zhu Y. L. (2010). The Effect of Acanthopanax Senticosus Polysaccharide on the Expression of NF-Κb in Hippocampal Neurons Injured by H_2O_2. Chin. J. Clin. Neurosurg. 15 (06), 350–352. 10.3969/j.issn.1009-153X.2010.06.011 [DOI] [Google Scholar]
- Duan X. L. (2015). Research Progress of Acanthopanax Senticosus' Immunomodulatory Function. Chin. J. Traditional Vet. Sci. 2015 (6), 72–75. 10.3969/j.issn.1003-8655.2015.06.043 [DOI] [Google Scholar]
- Feng Y. (2016). Study on Optimizing Extraction Conditions of Acanthopanax Senticosus Polysaccharide by Single Factor Test. Shandong J. Anim. Sci. Vet. Med. 37 (08), 8–9. 10.3969/j.issn.1007-1733.2016.08.004 [DOI] [Google Scholar]
- Fu J., Fu J., Yuan J., Zhang N., Gao B., Fu G., et al. (2012). Anti-diabetic Activities of Acanthopanax Senticosus Polysaccharide (ASP) in Combination with Metformin. Int. J. Biol. Macromol 50 (3), 619–623. 10.1016/j.ijbiomac.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Ganesan K., Xu B. (2019). Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 24 (14). 10.3390/molecules24142556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. Y. (2019). Research Progress on Chemical Constituents and Pharmacological Effects of Acanthopanax Senticosus. Inf. Traditional Chin. Med. 36 (02), 113–116. 10.19656/j.cnki.1002-2406.190060 [DOI] [Google Scholar]
- Hamidon N. H., Abang Zaidel D. N., Mohd Jusoh Y. M. (2020). Optimization of Pectin Extraction from Sweet Potato Peels Using Citric Acid and its Emulsifying Properties. Recent Pat Food Nutr. Agric. 11 (3), 202–210. 10.2174/2212798411666200207102051 [DOI] [PubMed] [Google Scholar]
- Han J., Bian L., Liu X., Zhang F., Zhang Y., Yu N. (2014). Effects of Acanthopanax Senticosus Polysaccharide Supplementation on Growth Performance, Immunity, Blood Parameters and Expression of Pro-inflammatory Cytokines Genes in Challenged Weaned Piglets. Asian-australas J. Anim. Sci. 27 (7), 1035–1043. 10.5713/ajas.2013.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Li J. H., Bai G., Shen G. S., Chen J., Liu J. N., et al. (2017). Acanthopanax Senticosus Polysaccharides-Induced Intestinal Tight junction Injury Alleviation via Inhibition of NF-Κb/MLCK Pathway in a Mouse Endotoxemia Model. World J. Gastroenterol. 23 (12), 2175–2184. 10.3748/wjg.v23.i12.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Liu L., Yu N., Chen J., Liu B., Yang D., et al. (2016). Polysaccharides from Acanthopanax Senticosus Enhances Intestinal Integrity through Inhibiting TLR4/NF-Κb Signaling Pathways in Lipopolysaccharide-Challenged Mice. Anim. Sci. J. 87 (8), 1011–1018. 10.1111/asj.12528 [DOI] [PubMed] [Google Scholar]
- Han J., Xu Y., Yang D., Yu N., Bai Z., Bian L. (2016). Effect of Polysaccharides from Acanthopanax Senticosus on Intestinal Mucosal Barrier of Escherichia coli Lipopolysaccharide Challenged Mice. Asian-australas J. Anim. Sci. 29 (1), 134–141. 10.5713/ajas.15.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. (2013). Study on the Regulation and Mechanism of Acanthopanax Senticosus Polysaccharide on Immune Function of Weaned Piglets. Shenyang, China: Shenyang Agricultural University. [Google Scholar]
- Han S. B., Yoon Y. D., Ahn H. J., Lee H. S., Lee C. W., Yoon W. K., et al. (2003). Toll-like Receptor-Mediated Activation of B Cells and Macrophages by Polysaccharide Isolated from Cell Culture of Acanthopanax Senticosus. Int. Immunopharmacol 3 (9), 1301–1312. 10.1016/s1567-5769(03)00118-8 [DOI] [PubMed] [Google Scholar]
- Han X. G. (2007). Changes in Blood Biochemical Indicators of Loaded-Swimming Mice after the Intervention of Acanthopanax Root Polysaccharides. JOURNAL CLINICAL REHABILITATIVE TISSUE ENGINEERING RESEARCH 11 (51), 10300–10303. 10.3321/j.issn:1673-8225.2007.51.034 [DOI] [Google Scholar]
- Hao C. Y., Nan J. X. (2013). Research Progress of Acanthopanax Senticosus against Liver Injury. J. Jilin Med. Coll. 34 (02), 112–115. 10.3969/j.issn.1673-2995.2013.02.015 [DOI] [Google Scholar]
- Hu H., Liang H., Wu Y. (2015). Isolation, Purification and Structural Characterization of Polysaccharide from Acanthopanax Brachypus. Carbohydr. Polym. 127, 94–100. 10.1016/j.carbpol.2015.03.066 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Jiang T., T., Yue N. (2017). Extraction of Polysaccharide from Lemon by Alkaline Solution. Chem. World 58 (05), 257–261. 10.11882/j.issn.0254-5071.2018.10.031 [DOI] [Google Scholar]
- Jiao Y. (2008). Study on Extraction of Acanthopanax Senticosus Polysaccharide and its Antioxidant Activity. Changchun, China: Jilin University. [Google Scholar]
- Jin W. (2016). Acanthopanax Senticosus Polysaccharide Down-Regulates Survivin Protein Expression and Induces HeLa Cell Apoptosis. Chin. Traditional Patent Med. 38 (04), 902–904. 10.3969/j.issn.1001-1528.2016.04.040 [DOI] [Google Scholar]
- Jin W. (2014). Experimental Study of Acanthopanax Senticosus Polysaccharide on the Proliferation and Apoptosis of Human Cervical Cancer HeLa Cells In Vitro . Chin. Traditional Patent Med. 36 (01), 162–164. [Google Scholar]
- Lee D., Park J., Yoon J., Kim M. Y., Choi H. Y., Kim H. (2012). Neuroprotective Effects of Eleutherococcus Senticosus Bark on Transient Global Cerebral Ischemia in Rats. J. Ethnopharmacol 139 (1), 6–11. 10.1016/j.jep.2011.05.024 [DOI] [PubMed] [Google Scholar]
- Lee J. B., Tanikawa T., Hayashi K., Asagi M., Kasahara Y., Hayashi T. (2015). Characterization and Biological Effects of Two Polysaccharides Isolated from Acanthopanax Sciadophylloides. Carbohydr. Polym. 116, 159–166. 10.1016/j.carbpol.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Li F. R., Liu B. (2003). Microwave Extraction and Content Determination of Acanthopanax Senticosus Polysaccharide. XINJIANG JOURNAL TRADITIONAL CHINESE MEDICINE 21 (1), 11–12. 10.3969/j.issn.1009-3931.2003.01.009 [DOI] [Google Scholar]
- Li L. H. (2019). Study on the Extraction Technology for Acanthopanax Senticosus Polysaccharide. Feed Res. 42 (06), 93–96. 10.13557/j.cnki.issn1002-2813.2019.06.026 [DOI] [Google Scholar]
- Li X., J. (2018). Extraction Process Optimization of Acidic tuckahoe Polysaccharides. China Brewing 37 (10), 158–161. 10.11882/j.issn.0254-5071.2018.10.031 [DOI] [Google Scholar]
- Li X. H. (2020). The Effect of Graded Alcohol Precipitation of Acanthopanax Senticosus Polysaccharide at Different Concentrations on Mouse Spleen Cell Proliferation and IL-2 Expression. Heilongjiang Anim. Sci. Vet. Med. 2020 (5), 127–130+133. 10.13881/j.cnki.hljxmsy.2019.06.0286 [DOI] [Google Scholar]
- Li Y. P., Zhou H. L. (2020). Acidic Extraction and Antioxidant Activity of Polysaccharides from Corn Silk. Cereals & Oils 33 (08), 86–90. 10.3969/j.issn.1008-9578.2020.08.024 [DOI] [Google Scholar]
- Liu Y. (2010). Extraction, Separation and Micronization of the Main Active Components of Acanthopanax Senticosus. Harbin, China: Northeast Forestry University. [Google Scholar]
- Liu Y., Huang Q. C., Cao J. H. (2013). The Protective Effect of Acanthopanax Senticosus Polysaccharide on H_2O_2 Induced Apoptosis of Rat Hippocampal Neurons. Chin. J. Clin. Neurosurg. 18 (11), 681–683. [Google Scholar]
- Long L. N., Zhang H. H., Wang F., Yin Y. X., Yang L. Y., Chen J. S. (2021). Research Note: Effects of Polysaccharide-Enriched Acanthopanax Senticosus Extract on Growth Performance, Immune Function, Antioxidation, and Ileal Microbial Populations in Broiler Chickens. Poult. Sci. 100 (4), 101028. 10.1016/j.psj.2021.101028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F. (2015). Application of Metabonomics Method to Identify Possible Effect of Acanthopanax Senticosus Polysaccharide on AD. Chin. J. Exp. Traditional Med. Formulae 21 (22), 150–153. 10.13422/j.cnki.syfjx.2015220150 [DOI] [Google Scholar]
- Lu F. (2016). Investigation of Protective Effect of Acanthopanax Senticosus Polysaccharides on Immunological Liver Injury in Mice by Metabolomics Methods. New Chin. Med. Clin. Pharmacol. 27 (06), 823–829. 10.19378/j.issn.1003-9783.2016.06.016 [DOI] [Google Scholar]
- Luan J. G. (2012). A Precious Medicinal and Edible Plant-Acanthopanax Senticosus. New Agric. 2012 (15), 53–54. 10.3969/j.issn.1002-4298.2012.15.074 [DOI] [Google Scholar]
- Luo Q. (2013). Effects of Acanthopanax Senticosus Polysaccharide on Immune Function of normal Mice. Heilongjiang Anim. Sci. Vet. Med. (13), 120–122. 10.13881/j.cnki.hljxmsy.2013.13.052 [DOI] [Google Scholar]
- Luo Q. (2013). Effects of Acanthopanax Senticosus Polysaccharide on Lymphocyte Transformation Rate and Peripheral Lymphocyte Subsets in Normal Mice. J. Henan Agric. Sci. 42 (08), 137–140. 10.3969/j.issn.1004-3268.2013.08.034 [DOI] [Google Scholar]
- Luo Q. (2008).Study on Cell K562 Apoptosis Induced by Acanthopanax Senticosus In Vitro . JOURNAL HEBEI NORTH UNIVERSITY(MEDICAL EDITION) 05, 17–19. [Google Scholar]
- Meng Q., Pan J., Liu Y., Chen L., Ren Y. (2018). Anti-tumour Effects of Polysaccharide Extracted from Acanthopanax Senticosus and Cell-Mediated Immunity. Exp. Ther. Med. 15 (2), 1694–1701. 10.3892/etm.2017.5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q. F., Yu X. K., Xu M. Y., Li M. L., Gao Z. H., Fan H., et al. (2005). Extraction of Acanthopanacis Senticosi Polysaccharides and Their Antioxidative Effect. J. Jilin Univ. Inf. Sci. Ed. 43 (5), 683–686. 10.3321/j.issn:1671-5489.2005.05.031 [DOI] [Google Scholar]
- Meng Q. L. (2018). Basic Pharmacological Research on the Development of Changbai Mountain Acanthopanax Senticosus Polysaccharide. Changchun, China: Jilin Agricultural University. [Google Scholar]
- Mo L. F. (2012). Protective Effect of Acanthopanax Senticosus Polysaccharide on the Gastrointestinal Tract and Reproductive System of Rats Irradiated by ∼(60)Coγ Rays. China Radiat. Health 21 (2), 153–155. 10.3760/cma.j.issn.1009-6906.2012.01.004 [DOI] [Google Scholar]
- Mo L. F. (2012). Protective Effects of Acanthepanax Senticosus Polysaccharide on Gastrointestinal and Reproductive System of 60Coγ-ray Irradiated Rats. Chin. J. Nautical Med. Hyperb. Med. (01), 17–20. [Google Scholar]
- Pan J. Z. (2019). Research Progress on Chemical Constituents and Pharmacological Activities of Acanthopanax Senticosus. Sci. Tech. Food Industry 40 (23), 353–360. 10.13386/j.issn1002-0306.2019.23.058 [DOI] [Google Scholar]
- Pan J. Z. (2019). Study on analgesic and anti-inflammatory effect and immunoregulatory mechanism of polysaccharides in Radix et Caulis Acanthopanacis Senticosi. Shanghai J. Traditional Chin. Med. 53 (10), 74–82. 10.16305/j.1007-1334.2019.10.019 [DOI] [Google Scholar]
- Poolsup N., Suthisisang C., Prathanturarug S., Asawamekin A., Chanchareon U. (2004). Andrographis Paniculata in the Symptomatic Treatment of Uncomplicated Upper Respiratory Tract Infection: Systematic Review of Randomized Controlled Trials. J. Clin. Pharm. Ther. 29 (1), 37–45. 10.1046/j.1365-2710.2003.00534.x [DOI] [PubMed] [Google Scholar]
- Shen M. L., Zhai S. K., Chen H. L., Luo Y. D., Tu G. R., Ou D. W. (1991). Immunomopharmacological Effects of Polysaccharides from Acanthopanax Senticosus on Experimental Animals. Int. J. Immunopharmacol 13 (5), 549–554. 10.1016/0192-0561(91)90075-i [DOI] [PubMed] [Google Scholar]
- Shen Y. (2017). Response Surface Methodology to Optimize the Extraction Process of Acanthopanax Senticosus Polysaccharide. Heilongjiang Med. Pharm. 40 (03), 75–77. [Google Scholar]
- Sun D., Chen J., Hu H., Lin S., Jin L., Luo L., et al. (2019). Acanthopanax Senticosus Polysaccharide Suppressing Proliferation and Metastasis of the Human Non-small Cell Lung Cancer NCI-H520 Cells Is Associated with Wnt/β-Catenin Signaling. Neoplasma 66 (4), 555–563. 10.4149/neo_2018_180913N689 [DOI] [PubMed] [Google Scholar]
- Sun S. K., Song T., Sun Y. (2018). Immunomodulatory Effects of Acanthopanax Senticosus Acidic Polysaccharides in Cyclophosphamide-Induced Immunocompromised Mice. Immunological J. 34 (10), 863–868. 10.13431/j.cnki.immunol.j.20180133 [DOI] [Google Scholar]
- Tong L. (1994). Experimental Study on the Anti-tumor Effect and Mechanism of Acanthopanax Senticosus Polysaccharide. Chin. Pharmacol. Bull. 10 (2), 105–109. [Google Scholar]
- Wang F. F. (2006). Study on the Structure and Chemical Modification of Acanthopanax Senticosus Polysaccharide. Qingdao, China: Ocean University of China. [Google Scholar]
- Wang H., Sun B., Zhang Z., Chen J., Hao Q., Sun Y., et al. (2016). Effects of Acanthopanax Senticosus Polysaccharide on the Proliferation, Apoptosis and Cell Cycle in Human HepG2 Cells. Pharmazie 71 (4), 201–204. 10.1691/ph.2016.5807 [DOI] [PubMed] [Google Scholar]
- Wang J., Li G., Yu L., Mo T., Wu Q., Zhou Z. (2018). Aidi Injection Plus Platinum-Based Chemotherapy for Stage IIIB/IV Non-small Cell Lung Cancer: A Meta-Analysis of 42 RCTs Following the PRISMA Guidelines. J. Ethnopharmacol 221, 137–150. 10.1016/j.jep.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Wang J. P. (2009). Comparison of Different Methods for Removal of Protein from Extracted Polysaccharide of Acanthopanax Senticosus. NATURAL PRODUCT. RESEARCH DEVELOPMENT 21 (01), 155–158+162. 10.3969/j.issn.1001-6880.2009.01.036 [DOI] [Google Scholar]
- Wang Z. Z. (1986a).Immune Active Polysaccharides of Acanthopanax Senticosus. Shanghai, China: Foreign Medicine. Pharmacy Section, 309. [Google Scholar]
- Wang Z. Z. (1986b). Immunologically Active Polysaccharide from Acanthopanax Senticosus. Foreign Med. Sci. Sec Pharm. 24 (11), 309. [Google Scholar]
- Wei X. J. (2018). Optimization of Supercritical CO_2 Fluid Extraction of Kudingcha Polysaccharides. Guangxi Plant 38 (05), 590–595. 10.11931/guihaia.gxzw201706021 [DOI] [Google Scholar]
- Xie S. S. (1989). Immunoregulatory Effect of Polysaccharide of Acanthopanax Senticosus (PAS). I. Immunological Mechanism of PAS against Cancer. Zhonghua Zhong Liu Za Zhi 11 (5), 338–340. [PubMed] [Google Scholar]
- Xie S. S. (1989). Effect of Acanthopanax Senticosus Polysaccharide on Immune Function Reconstruction in Mice with Allogeneic Bone Marrow Transplantation. J. Beijing Med. Univ. (04), 289–291. [Google Scholar]
- Xie S. S. (1989). Experimental Study on the Immunomodulatory Effects of Acanthopanax Senticosus Polysaccharides-Ⅰ. A Preliminary Study on the Anti-tumor Mechanism of Acanthopanax Senticosus Polysaccharides. Chin. J. Oncol. (05), 338–340. [Google Scholar]
- Xie Y., Zhang B., Zhang Y. (2015). Protective Effects of Acanthopanax Polysaccharides on Cerebral Ischemia-Reperfusion Injury and its Mechanisms. Int. J. Biol. Macromol 72, 946–950. 10.1016/j.ijbiomac.2014.09.055 [DOI] [PubMed] [Google Scholar]
- Xu F., Zhao J. Y., Liu T. S. (2005). Study on Anti-fatigue Function of Acanthopanax Senticosus Extract. FOOD SCIENCE 26 (9), 453–456. 10.3321/j.issn:1002-6630.2005.09.120 [DOI] [Google Scholar]
- Xu R. S. (1983). Study on the Components of Acanthopanax Senticosus Polysaccharide. Chin. Sci. Bull. (03), 185–187. [Google Scholar]
- Xu S. K. (1990). Effect of Acanthopanax Senticosus Polysaccharide (ASPS) on Immune Function of Mice. Chin. patent Med. (03), 25–26. [Google Scholar]
- Yang J. C., Liu J. S., Xu H. Z. (1983). The Effect of Acanthopanax Senticosus Polysaccharide and Sodium Carboxymethyl Starch on the Induction of Interferon in S_(801) and S_(7811) Cell Lines. J. Suzhou Med. Coll. (03), 8–10. [Google Scholar]
- Yang J. C., Liu J. S., Xu H. Z. (1985). The Effect of Acanthopanax Senticosus Polysaccharide on the Induction of Interferon in S_(801) and S_(7811) Leukemia Cell Lines. Traditional Chin. Med. Inf. (02), 36. [Google Scholar]
- Yang X. D. (2016). Protective Effects of Acanthopanax Senticosus Polysaccharide on Immune Liver Injury in Mice. Harbin, China: Heilongjiang University of Traditional Chinese Medicine. [Google Scholar]
- Yin C., Fan X., Fan Z., Shi D., Gao H. (2018). Optimization of Enzymes-Microwave-Ultrasound Assisted Extraction of Lentinus Edodes Polysaccharides and Determination of its Antioxidant Activity. Int. J. Biol. Macromol 111, 446–454. 10.1016/j.ijbiomac.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Yu M. (2019). Reaserch on Extraction Technology of Polysaccharides from Acanthopanax Senticosus Waste Residue by Response Surface Methodology. Chin. Traditional Herbal Drugs 50 (07), 1610–1613. 10.7501/j.issn.0253-2670.2019.07.017 [DOI] [Google Scholar]
- Yu Y., Shen M., Song Q., Xie J. (2018). Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 183, 91–101. 10.1016/j.carbpol.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Zeng P., Li J., Chen Y., Zhang L. (2019). The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl Sci. 163, 423–444. 10.1016/bs.pmbts.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S. P. (2007). Optimization and Establishment of the Extraction Process of Acanthopanax Senticosus and Pleurotus Ostreatus Polysaccharides. Shijiazhuang, China: Hebei Normal University. [Google Scholar]
- Zhai X. N. (2020). Effect of Acanthopanax Senticosus Polysaccharide on Immune Function of Mice. Traditional Chin. Med. Inf. 37 (06), 42–45. 10.19656/j.cnki.1002-2406.200158 [DOI] [Google Scholar]
- Zhang H., Wang S., Jin L. H. (2020). Acanthopanax Senticosus Polysaccharide Regulates the Intestinal Homeostasis Disruption Induced by Toxic Chemicals in Drosophila. Phytother Res. 34 (1), 193–200. 10.1002/ptr.6522 [DOI] [PubMed] [Google Scholar]
- Zhang J. Y. (2001). Experimental Intervention Effect of Acanthopanax Senticosus Polysaccharide on Lewis Lung Cancer in Mice. CARCINOGENESIS, TERATOGENESIS AND MUTAGENESIS (04), 270. 10.3969/j.issn.1004-616X.2001.04.109 [DOI] [Google Scholar]
- Zhang L. P., Zhang L. Y., Zhang Y. K. (1993). Study on the Water-Soluble Polysaccharide of Acanthopanax Senticosus ——The Separation, Purification and Structure of AS-2. J. Biol. Chem. (01), 1–5. 8416916 [Google Scholar]
- Zhang N., Zhao L. Y., Mao D., Du Z. Y., Zhang X. J., Zhai X. N., et al. (2019). Modulation Effect of Acanthopanax Senticosus Polysaccharides through Inflammatory Cytokines in Protecting Immunological Liver-Injured Mice. Zhongguo Zhong Yao Za Zhi 44 (14), 2947–2952. 10.19540/j.cnki.cjcmm.20190129.001 [DOI] [PubMed] [Google Scholar]
- Zhang N. (2018). Modulation of Acanthopanax Senticosus Polysaccharides on Expression of IL-2,IL-4, INF-γ Cytokines, and mRNA in BALB/c Immunological Liver-Injured Mice. Drug Eval. Res. 41 (04), 557–561. 10.7501/j.issn.1674-6376.2018.04.010 [DOI] [Google Scholar]
- Zhang N. (2015). Study on the Immune Regulation of Acanthopanax Senticosus Polysaccharide and its Protective Effect on Immune Liver Injury. Harbin, China: Heilongjiang University of Traditional Chinese Medicine. [Google Scholar]
- Zhang N. Z., Zhang W. Z., Chen X. J. (1997). Pathological Study of the Influence of Acanthopanax Senticosus Polysaccharide and GST on Experimental Liver Cancer. Cancer Res. (02), 78–80. [Google Scholar]
- Zhang R., Lv C., Lu J. (2020). Studies on Laccase Mediated Conversion of Lignin from Ginseng Residues for the Production of Sugars. Bioresour. Technol. 317, 123945. 10.1016/j.biortech.2020.123945 [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Guo Q. T. (2015). Optimization of Microwave Extraction Technology for Acanthopanax Senticosus Polysaccharide by Box-Behnken Response Surface Methodology. China Pharmacist 18 (09), 1502–1505. 10.3969/j.issn.1008-049X.2015.09.022 [DOI] [Google Scholar]
- Zhang Y. M., Sun T. (2005). Comparative Study on Content Determination of Acanthopanax Senticosus Polysaccharide by Different Dry Methods. HEILONGJIANG MEDICINE JOURNAL (03), 160–162. 10.3969/j.issn.1006-2882.2005.03.003 [DOI] [Google Scholar]
- Zhao J. X. (2008). Acanthopanax Senticosus Polysaccharide's Inhibitory and Immunomodulatory Effects on Human Small Cell Lung Cancer H446 Cells. Shijiazhuang, China: Hebei Medical University. [Google Scholar]
- Zhao J. X. (2010). ASPS Induces G_2/M Arrest of H446 Cells by Activation of ERK Signal Pathway. BASIC CLINICAL MEDICINE 30 (01), 59–62. [Google Scholar]
- Zhao J. X. (2008). Induces Apoptosis of Acanthopanax Senticosus Polysaccharides on H446 Cells. CHINESE JOURNAL CELL BIOLOGY (02), 239–242. 10.3969/j.issn.1674-7666.2008.02.021 [DOI] [Google Scholar]
- Zhou L. (2018). Active Ingredients, Pharmacological Actions, and Clinical Applications of Acanthopanax Senticosus in the Central Nervous System. J. Traditional Chin. Med. Univ. Hunan 38 (8), 961–964. 10.3969/j.issn.1674-070X.2018.08.028 [DOI] [Google Scholar]
- Zhu Q., Tu G. R., Shen M. L. (1982). Effects of Acanthopanax Senticosus Polysaccharides on Specific Antibodies in Mouse Serum. Chin. Pharm. J. (03), 50. [Google Scholar]