Abstract
Objective
To assess the epidemiologic association between Kawasaki disease and common pediatric infectious diseases (PIDs) identified during the coronavirus disease 2019 (COVID-19) pandemic period to confirm whether the infection-triggered theory is a plausible hypothesis for the pathogenesis of Kawasaki disease.
Study design
A retrospective epidemiologic study was conducted using datasets obtained from Web-based surveillance of Kawasaki disease and PIDs in Japan. We compared weekly numbers of patients who developed Kawasaki disease and specific PIDs between 2020 and 2017-2019 and evaluated the association between the percent reduction in the number of patients with these diseases.
Results
A total of 868 patients developed Kawasaki disease in 2020. During the social distancing period in 2020, the number of patients with Kawasaki disease was approximately 35% lower than in 2017-2019. Time from the onset of Kawasaki disease until the first hospital visit did not differ significantly among the examined years. The proportion of older children with Kawasaki disease decreased more than that of infants with Kawasaki disease (age <1 year), resulting in a significant difference in the proportion of infant patients between 2020 and 2017-2019 (24% vs 19%; P < .01). The number of patients with incomplete Kawasaki disease was unchanged from that of previous years. The weekly percent reduction in patient numbers differed between Kawasaki disease and PIDs during 2020, with no strong correlation between the 2 diseases.
Conclusions
Our data indicate that parents of patients with Kawasaki disease did not avoid hospital visits during the COVID-19 pandemic period. The findings indicate the possibility that triggering Kawasaki disease might be associated with presently unidentified respiratory pathogen(s) that potentially might be acquired from both within and outside the household.
Keywords: Kawasaki disease, etiology, pediatric infectious disease, coronavirus disease-2019 pandemic
Abbreviations: COVID-19, Coronavirus disease 2019; GAS, Group A streptococcal; MIS-C, Multisystem inflammatory syndrome in children; PID, Pediatric infectious disease; RSV, Respiratory syncytial virus
See editorial, p 11
Kawasaki disease has been reported throughout the world and in children of all races and ethnicities.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 However, the etiology of Kawasaki disease has remained unknown since its initial description by Dr Tomisaku Kawasaki in 1967.11 Previous studies strongly support that infectious agents trigger an abnormal acute immune response of Kawasaki disease, according to epidemiologic characteristics such as the age distribution, with the highest susceptibility in children aged <2 years and the lowest susceptibility in those aged <6 months, compatible with infection by a ubiquitous agent resulting in increasing immunity with age and with transplacental immunity,1, 2, 3, 4 , 12, 13, 14 occurrence through potential sibling-to-sibling transmission inside households,13 , 15, 16, 17, 18 temporal- and municipal-level clustering,19, 20, 21 seasonal differences in occurrence,21, 22, 23, 24, 25, 26 and epidemics observed during the past 50 years in Japan (1979, 1982, and 1986).1, 2, 3, 4
Recent studies have reported that the incidence of Kawasaki disease decreased during the coronavirus disease 2019 (COVID-19) pandemic period compared with the corresponding period in previous years.27, 28, 29, 30 Several studies have found a dramatic reduction in the number of patients with Kawasaki disease, as well as a simultaneously large decrease in the number of those with common pediatric infectious diseases (PIDs) during the COVID-19 mitigation period, strongly supporting the hypothesis of an infection-triggered pathogenesis in Kawasaki disease.27 , 28 However, these studies included a small number of patients with Kawasaki disease that developed during the study period—66 patients in Japan27 and 13 in the US.28 Therefore, studies with larger numbers of patients are warranted to confirm these findings, as well as to evaluate the plausibility of the infection-triggered hypothesis in the pathogenesis of Kawasaki disease.
Web-based Kawasaki disease surveillance in Japan has been conducted since 2008 for timely reporting of the occurrence of Kawasaki disease in 227 hospitals nationwide.1, 2, 3, 4 , 31 Using data from the surveillance system, which includes a large number of patients who developed Kawasaki disease during the COVID-19 pandemic period, we aimed to assess the epidemiologic association between Kawasaki disease and common PIDs to confirm whether the infection-triggered theory is a plausible hypothesis for Kawasaki disease pathogenesis.
Methods
This retrospective epidemiologic study was conducted using 2 datasets obtained from the Japanese Web-based Kawasaki disease surveillance system and Japanese surveillance of infectious diseases. The Ethics Committee of Tokyo Women's Medical University approved the study and waived the requirement for informed consent from individual participants (approval 5774).
Web-Based Kawasaki Disease Surveillance System in Japan
Web-based Kawasaki disease surveillance has been conducted in Japan since 2008 to monitor patients who develop Kawasaki disease in registered hospitals nationwide. An overview of the surveillance system has been published previously.31 Currently, 227 hospitals are registered in the surveillance system, with timely reporting of information on patients with Kawasaki disease after diagnosis. The weekly number of patients with Kawasaki disease reported by the hospitals is updated on a Web site in a timely manner.32
The surveillance database contains information on patient characteristics including age, sex, date of Kawasaki disease onset, days of illness at the first hospital visit, and the number of principal signs of Kawasaki disease. The day of Kawasaki disease onset was defined as the first day that the patient presented with fever related to Kawasaki disease. All patients were diagnosed in accordance with the Japanese Kawasaki disease clinical guidelines.33 The 6 principal signs of Kawasaki disease are fever, bilateral conjunctival injection, oral mucosal changes, polymorphous skin rash, peripheral extremity changes, and nonsuppurative cervical lymphadenopathy. Patients with 5 or 6 principal signs were considered to have complete Kawasaki disease, and those with ≤4 principal signs were considered to have incomplete Kawasaki disease.
Separate from Web-based Kawasaki disease surveillance, the Japanese nationwide Kawasaki disease survey has been conducted independently biennially since 1970 to collect information on patients who developed Kawasaki disease during the preceding 2 years.1, 2, 3, 4 The latest survey was completed in 2019, obtaining information on patients who developed Kawasaki disease between January 1, 2017, and December 31, 2018.1 The survey covers nearly all medical facilities across the country in which patients with Kawasaki disease are eventually hospitalized. Therefore, the survey can be considered a census survey of patients with Kawasaki disease among the Japanese general population.1 Of all hospitals contributing to the nationwide Kawasaki disease survey, hospitals that reported large numbers of patients were typically selected as eligible hospitals for the Web-based Kawasaki disease surveillance system, resulting in 227 hospitals registered in the current surveillance.31 , 32 Web-based Kawasaki disease surveillance has been demonstrated to have high validity compared with data from the nationwide Kawasaki disease survey, thus reflecting the nationwide occurrence of Kawasaki disease in Japan.31
Japanese Surveillance of Infectious Diseases
Since 1999, the Japanese government has conducted the National Epidemiologic Surveillance of Infectious Diseases program and has obtained information on diseases specified in the Infectious Diseases Control Law. The numbers of patients with specific infectious diseases are updated weekly on a Web site presented by the National Institute of Infectious Diseases, in which the relevant datasets are publicly available.34 Among all infectious diseases specified in the surveillance, common PIDs are reported by approximately 3000 sentinel medical facilities across Japan (sentinel surveillance).35 Common PIDs comprise respiratory syncytial virus (RSV) infection; pharyngoconjunctival fever; group A streptococcal (GAS) pharyngitis; infectious gastroenteritis; varicella; hand, foot, and mouth disease; erythema infectiosum; exanthema subitum; herpangina; and mumps.35 Among these 10 common PIDs, the present study focused on RSV infection and exanthema subitum because the susceptible age for these 2 infectious diseases is similar to that of Kawasaki disease. In addition, RSV infection and exanthema subitum have a viral etiology and are most often introduced into the household by parents or siblings, potentially leading to infection of infants.36, 37, 38, 39, 40, 41 GAS pharyngitis was also included in the analysis because of its bacterial nature and because patients with GAS pharyngitis can manifest similar signs and symptoms to those of Kawasaki disease.5
Statistical Analyses
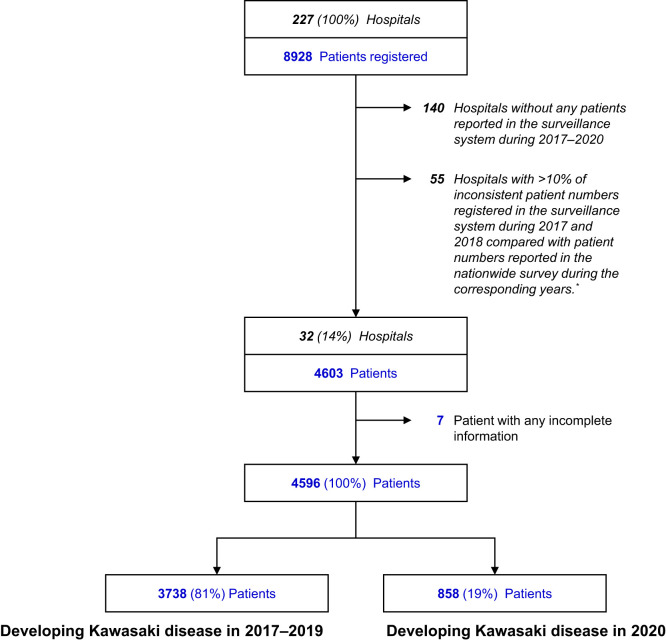
Patient registration in the Web-based Kawasaki disease surveillance system completely depends on voluntary reporting from each registered hospital, which may result in an inconsistent number of reported patients compared with the actual number of patients with Kawasaki disease. To obtain a reliable dataset from the surveillance system, we compared the number of patients registered in the surveillance system during 2017-2018 with the number of patients reported in the latest nationwide Kawasaki disease survey that identified patients during the corresponding period for all 227 hospitals registered in the surveillance system. If the patient numbers registered by a hospital in the surveillance system during 2017-2018 had >10% inconsistency with patient numbers reported in the nationwide survey during the corresponding years, the hospital was excluded from the analysis because of potentially incomplete information on patients registered during 2020 (Figure 1; available at www.jpeds.com). We also excluded hospitals without any patients registered in the surveillance system during 2017-2020.
Figure 1.
Selection of hospitals and patients with Kawasaki disease. ∗Potentially incomplete reporting information during 2020.
First, we assessed the basic characteristics of patients who developed Kawasaki disease during 2020 and compared these with patients who developed Kawasaki disease during 2017-2019, using the χ2 test for week number (weeks 1-10, 11-20, 21-30, 31-40, and 41-52), sex, age group (<1, 1-5, 6-10, and ≥11 years), days of illness at the first hospital visit (1-4, 5-7, 8-10, and ≥11 days), and number of principal signs of Kawasaki disease (5 or 6, and ≤4). Median years of age and days of illness at the first hospital visit were also compared using the Mann-Whitney U test.
Second, to evaluate exposure to social distancing measures in relation to Kawasaki disease occurrence, we compared the number of patients who developed Kawasaki disease after week 11 in 2020 with the mean number of patients who developed Kawasaki disease in the corresponding period during 2017-2019. The Japanese government formally began to close nationwide schools, including daycare centers for younger children from week 10 in 2020, and subsequently announced a state of emergency from week 15. School closure continued until the state of emergency was officially lifted in week 19. Therefore, the period comprising weeks 10-19 was defined as the “school closure” period. This period was highlighted in the present study because movement among children as well as adults was largely restricted during this period. Social distancing measures were implemented continuously throughout 2020, even after the school closure period. Therefore, we determined that patients who developed Kawasaki disease from week 11 to week 52 in 2020 were exposed to social distancing measures. Mask wearing was typically required for all people when going outside and in public places. In contrast, putting masks on children aged <2 years was avoided because of the potential risk of suffocation.
Third, we evaluated differences in weekly trends in the number of patients with Kawasaki disease as well as PIDs between the year 2020 and previous years 2017-2019. In this analysis, the number of patients who developed Kawasaki disease and PIDs during 2017-2019 are presented as mean, with range, for each week using error bars in charts. The school closure period is highlighted in the charts.
Fourth, we evaluated any reduction in the number of patients with Kawasaki disease and PIDs during 2020 in comparison with these patient numbers during 2017-2019. The weekly percent reduction in the number of patients with Kawasaki disease and PIDs was estimated for each week. In this analysis, percent reductions in the number of patients who developed Kawasaki disease and PIDs in 2020 were compared with the mean (with range) for the corresponding weeks during 2017-2019, described using error bars in charts.
Finally, we assessed the associations of weekly percent reductions in patient numbers between Kawasaki disease and PIDs using Pearson correlation analysis. Scatterplots were used to determine distributions of the associations between percent reductions in the number of patients with Kawasaki disease and those with PIDs. Correlation coefficients were deemed significant when the absolute value of the lowest limit of the 95% CI was >0. The 2.5th and 97.5th percentiles were used to express the 95% CIs. All analyses were performed using SPSS version 25 (IBM) and Python version 3.7.4 (Python Software Foundation). Categorical variables are presented as percentage, and median and IQR are used for numeric variables.
Results
Of a total 227 hospitals registered in the surveillance system, 32 hospitals (14%) met the inclusion criteria (Figure 1). Of a total 4603 patients with Kawasaki disease identified in these hospitals, 4596 were finally included in the analysis, including 868 (19%) patients who developed Kawasaki disease in 2020 and 3738 (81%) who did so in 2017-2019.
Comparisons of the basic characteristics of patients who developed Kawasaki disease in 2020 and 2017-2019 are shown in the Table . The proportion of patients aged <1 year was significantly larger in 2020 compared with that in 2017-2019 (24% vs 19%; P < .01). In the additional analysis, the number of patients aged <1 year who developed Kawasaki disease was 12% lower in 2020 (n = 209) compared with 2017-2019, and the number of patients aged ≥1 year who developed Kawasaki disease was 36% lower in 2020 (n = 649) compared with 2017-2019. For sex and number of principal Kawasaki disease signs, no differences were found in the proportion of patients who developed Kawasaki disease in 2020 and 2017-2019. The median days of illness when patients with Kawasaki disease first visited a hospital also did not significantly differ between these time periods, with most patients first visiting a hospital at 1-4 days from Kawasaki disease onset (67% in 2020 vs 66% in 2017-2019; P = .64). During weeks 11-52 in 2020, 657 patients (77%) who were exposed to social distancing measures developed Kawasaki disease (Table). This number was 34% lower than the mean number of patients who developed Kawasaki disease during weeks 11-52 in 2017-2019.
Table.
Comparison of basic characteristics among patients who developed Kawasaki disease during 2017-2019 and in 2020 (N = 4596)
| Characteristics | Survey period |
P value† | |
|---|---|---|---|
| 2017-2019 (N = 3738) | 2020 (N = 858) | ||
| Weeks, n (%)∗ | |||
| 1-10 | 753 (20) | 201 (24) | .03 |
| 11-20 | 763 (21) | 186 (22) | |
| 21-30 | 700 (19) | 126 (15) | |
| 31-40 | 669 (18) | 144 (17) | |
| 41-52 | 819 (22) | 189 (22) | |
| Sex, n (%) | |||
| Male | 2115 (57) | 472 (55) | .40 |
| Female | 1623 (43) | 386 (45) | |
| Age, y, median (IQR) | 2 (1-3) | 2 (1-3) | <.01‡ |
| Age, y, n (%) | |||
| <1 | 711 (19) | 209 (24) | <.01 |
| 1–5 | 2747 (73) | 578 (67) | |
| 6–10 | 261 (7) | 65 (8) | |
| ≥11 | 19 (1) | 6 (1) | |
| Day of illness at first hospital visit | |||
| Median (IQR) | 4 (3-5) | 4 (3-5) | .09‡ |
| 1-4, n (%) | 2481 (66) | 573 (67) | .64 |
| 5-7, n (%) | 1147 (31) | 266 (31) | |
| 8-10, n (%) | 93 (2) | 15 (2) | |
| ≥11, n (%) | 17 (0) | 4 (0) | |
| Number of principal signs of Kawasaki disease | |||
| 5 or 6 (complete Kawasaki disease) | 3077 (82) | 692 (81) | .25 |
| ≤4 (incomplete Kawasaki disease) | 661 (18) | 166 (19) | |
Excluding 46 patients who developed Kawasaki disease in week 53.
χ2 test.
Mann–Whitney U test.
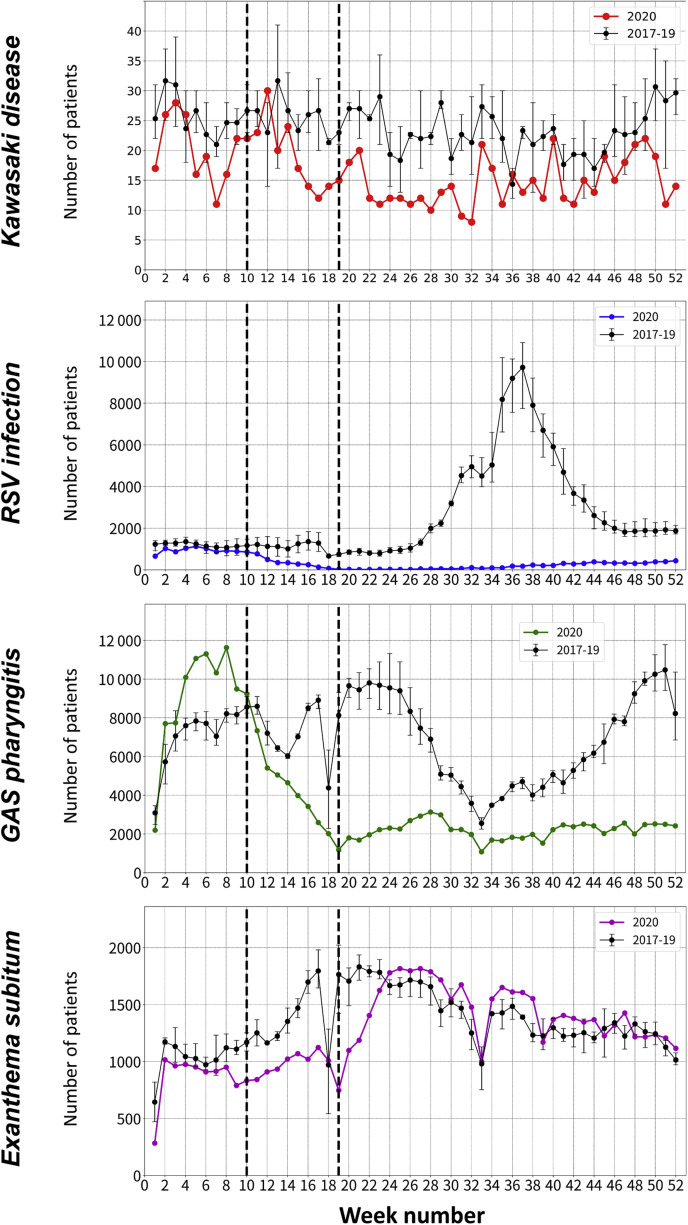
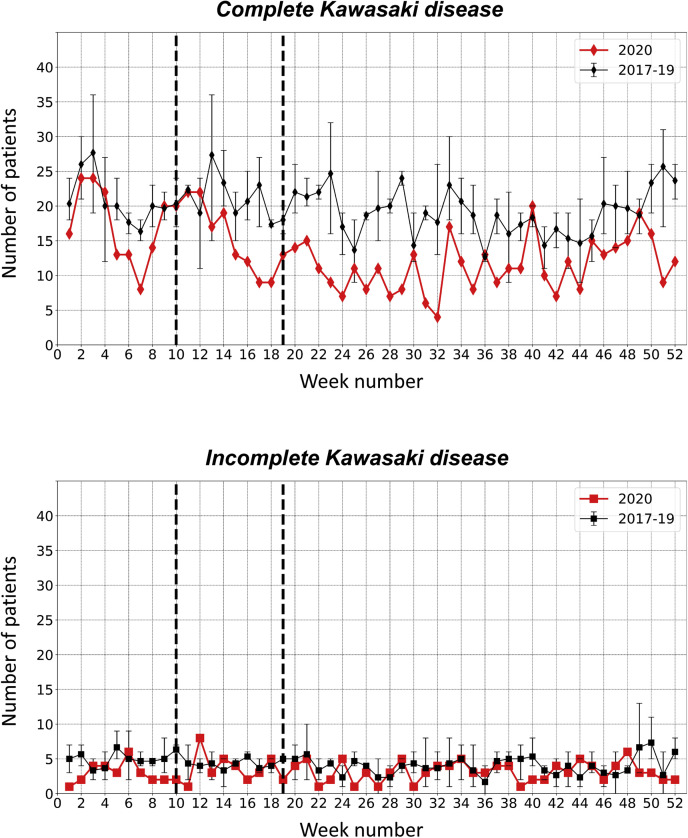
Figure 2 shows the differences in weekly trends in the number of patients with Kawasaki disease and PIDs between 2020 and 2017-2019. During weeks 15-35, the weekly number of patients who developed Kawasaki disease in 2020 showed a decreasing trend and was lower than the minimum weekly patient numbers in previous years. After week 36, there was a moderate increasing trend, although the weekly number of patients with Kawasaki disease was almost lower than the weekly numbers in previous years. When assessing complete and incomplete Kawasaki disease types (Figure 3 ), trends in the weekly number of patients with incomplete Kawasaki disease was unchanged in 2020 compared with 2017-2019, with a consistently low number (range, 1-8) throughout the 52 weeks in each of these years.
Figure 2.
Differences in weekly trends in the numbers of patients with Kawasaki disease and PIDs between 2020 and 2017-2019. Numbers of patients who developed Kawasaki disease and PIDs in 2017–2019 are presented as mean and range for each week using error bars. The school closure period (weeks 10-19) is highlighted using dashed lines in all panels.
Figure 3.
Comparison of weekly trends in the number of patients with complete and incomplete Kawasaki disease between 2020 and 2017-2019. The numbers of patients who developed complete and incomplete Kawasaki disease in 2017-2019 are presented as mean and range for each week using error bars. The school closure period (weeks 10-19) is highlighted using dashed lines in all panels.
Trends in the weekly number of patients in 2020 differed among the 3 PIDs examined in the study (Figure 2). Dramatic reductions were observed in the number of patients with RSV infection and GAS pharyngitis after week 10 in 2020, owing to prompt response to the national school closure. Although RSV infection had a single seasonal peak during weeks 30-40 in previous years, the weekly number of patients who developed RSV infection after week 11 in 2020 were consistently minimized, with no surge trends during the peak seasons. For GAS pharyngitis, the weekly number of patients was almost greater in 2020 than in previous years, until week 10. However, dramatic reductions in the weekly number of patients were observed after week 11, which had the smallest weekly number of patients since 2017 among the examined years. Furthermore, no subsequent seasonal peaks were found throughout 2020. As opposed to RSV infection and GAS pharyngitis, the weekly number of patients who developed exanthema subitum was nearly unchanged between 2017-2019 and 2020, although the weekly number of patients decreased during periods encompassing the national school closure period (weeks 8-23).
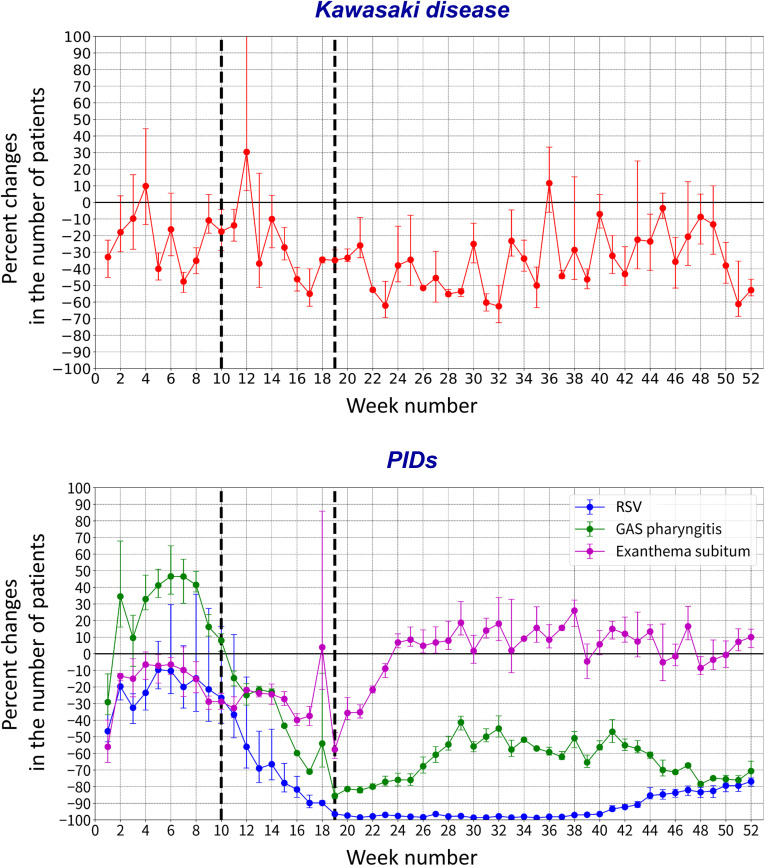
Figure 4 (available at www.jpeds.com) shows the percent reductions in the weekly number of patients who developed Kawasaki disease and PIDs during 2020 compared with 2017-2019. The weekly percent reduction in patients with Kawasaki disease decreased moderately from week 12, reached a >60% reduction at week 32, and then increased moderately after week 33. In contrast, the percent reductions in the weekly number of patients with RSV infection and GAS pharyngitis clearly decreased from week 10 with the rapid response to national school closure. The largest reduction in the weekly number of patients with RSV infection occurred from weeks 19 to 43, with a >90% reduction. After week 15, the weekly number of patients with GAS pharyngitis consistently decreased by >40% each week.
Figure 4.
Percent reduction in the weekly numbers of patients with Kawasaki disease and PIDs in 2020 compared with 2017-2019. The mean and range of weekly percent reductions in the numbers of patients who developed Kawasaki disease and PIDs are compared between 2020 and 2017-2019, shown using error bars in the charts. The school closure period (weeks 10-19) is highlighted using dashed lines in all panels.
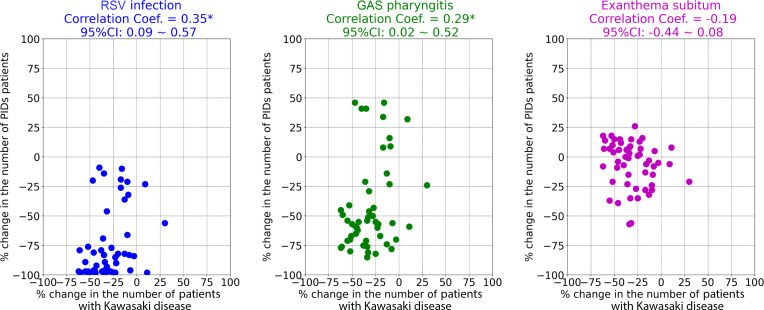
In Figure 5 , scatterplots show the associations between percent reduction in the weekly number of patients with Kawasaki disease and PIDs in 2020. Slight correlations were found for the percent reduction in weekly patient numbers between Kawasaki disease and RSV infection (Pearson correlation coefficient, 0.35; 95% CI, 0.09-0.57) and GAS pharyngitis (0.29; 0.02-0.52). No significant correlation was found in the percent reductions between Kawasaki disease and exanthema subitum.
Figure 5.
Correlation between percent reductions in the weekly numbers of patients with Kawasaki disease and those with PIDs in 2020. The percent reduction in the number of patients for each disease in 2020 were evaluated to compare mean patient numbers for these diseases in 2017-2019. ∗ Significant correlation.
Discussion
Two previous studies have reported that the numbers of patients with Kawasaki disease and common PIDs have decreased during the COVID-19 pandemic period in 2020 compared with previous years, supporting an infectious etiology of Kawasaki disease.27 , 28 Using data for a larger number of patients with Kawasaki disease than in the aforementioned studies, we found results consistent with their findings. However, the present study yielded additional findings. First, the distribution of days from onset of fever until the first hospital visit did not differ among the years examined, indicating that the parents of patients with Kawasaki disease did not avoid visiting a hospital during the pandemic period. Second, the number of patients who developed incomplete Kawasaki disease in 2020 was unchanged from that of previous years. Third, the weekly percent reduction in patient numbers differed between Kawasaki disease and specific PIDs during 2020, with no strong correlation in the association between the 2 diseases for a reduction in patient numbers. Finally, there was a greater decrease in the number of older children with Kawasaki disease compared with the number of infants with Kawasaki disease (aged <1 year), resulting in a significant difference in the proportion of infant patients between 2020 and previous years.
Shulman et al suggested that parental behaviors during the COVID-19 pandemic, such as refraining from visiting a hospital, were unlikely to contribute significantly to the striking reduction in cases of Kawasaki disease, primarily because of the severity of clinical presentation in Kawasaki disease, characterized by prolonged fever, rash, and other features, including marked irritability.28 Our study findings are in line with that reasoning. Our results indicate that compared with previous years, no significant differences in the distribution of days from Kawasaki disease onset until first hospital presentation. These results support the hypothesis that the parents of patients with Kawasaki disease did not hesitate to visit a hospital because of the Kawasaki disease clinical presentations, even during the COVID-19 mitigation period in Japan. Changes in healthcare-seeking behavior, such as avoiding hospitals, would not introduce bias in terms of the reduction in patients with Kawasaki disease during 2020, indicating that our results could reflect a true reduction in the number of patients who developed Kawasaki disease during the pandemic period.
Since the start of the COVID-19 pandemic, multisystem inflammatory syndrome in children (MIS-C) has been increasingly recognized as a serious health condition associated with COVID-19.42, 43, 44, 45 Although clinical manifestations vary widely, some patients with MIS-C manifest principal signs of Kawasaki disease, which may result in misdiagnosis in some patients.44 , 46 To date, very few patients with MIS-C have been observed in Japan during the COVID-19 pandemic.47 In our study, if the number of patients with MIS-C had increased and these patients had been misdiagnosed with Kawasaki disease during 2020, then the number of patients diagnosed with incomplete Kawasaki disease would be larger than those in previous years. However, our results did not indicate an increased number of patients with incomplete Kawasaki disease during 2020, which suggests that the incidence of MIS-C in Japan may be extremely low compared with the incidence in Western countries.
Rowley et al12 , 46 proposed that presently unidentified infectious agents may cause Kawasaki disease, according to immunopathologic evidence.48, 49, 50 In support of their reasonable hypothesis, we further hypothesized that such infectious agents could be transmitted from human to human. Because children aged 9-11 months are most susceptible to Kawasaki disease,1, 2, 3, 4 these infectious agents may be transmitted inside households from parents (or siblings) to children, who may subsequently develop Kawasaki disease. This infectious pathway is applicable to RSV infection.36, 37, 38, 39 In our study, we found that the number of patients with RSV infection decreased dramatically during the COVID-19 pandemic period, suggesting that social distancing contributed to reducing human-to-human RSV transmission among adults. In contrast, the reduction in the number of patients with Kawasaki disease was much smaller than that in patients with RSV during 2020. One possible reason for this is that Kawasaki disease might be triggered by infectious agents latent in parents (or siblings) over time, with subsequent transmission to infants inside the household rather than by infectious agents whose transmission among adults would be minimized by social distancing measures.
During COVID-19 mitigation period, there was a greater decrease in cases of Kawasaki disease in children compared with infants (36% vs 12%), resulting in a significant difference in the proportion of patients with Kawasaki disease aged <1 year between 2020 and previous years. Children aged <2 years basically should not wear a mask because of suffocation risk, which might contribute to these results. A large decrease in the number of older children with Kawasaki disease during the COVID-19 mitigation period might be associated with mask-wearing, potentially resulting in reduced inhalation of a respiratory pathogen(s) triggering Kawasaki disease that has eluded identification to date, as suggested by Rowley et al.12 , 51
The primary limitation of the present study is an as-yet insufficient number of Kawasaki disease cases that developed during 2020. The upcoming nationwide Japanese Kawasaki disease survey can confirm the results of this study using a much larger dataset of patients who developed Kawasaki disease in 2020.1, 2, 3, 4 Second, children with PIDs who lacked severe signs and symptoms likely would not have presented to a hospital during the COVID-19 pandemic period, possibly contributing to the decreased numbers of patients with PIDs identified in 2020. Third, correlation analyses were performed using only 52 weeks of data, which might have introduced statistical inaccuracy in the correlation coefficients between the percent reductions in the number of patients with Kawasaki diseases and those with PIDs. Finally, the study did not include information on infectious and illness status reported from family members of patients with Kawasaki disease.
In conclusion, parents of children with Kawasaki disease did not avoid hospital visits, resulting in a true reduction in the number of patients who developed Kawasaki disease in 2020. During the social distancing period in 2020, there was an approximate 35% reduction in the number of patients with Kawasaki disease compared with the corresponding period in 2017-2019. Weekly trends in the percent reduction in patient numbers differed between Kawasaki disease and PIDs during 2020, with no strong correlation in the association of the 2 diseases for reducing patient numbers, indicating that the specific PIDs are unlikely to be associated with the trigger of Kawasaki disease. Mask-wearing in older children might contribute to reducing Kawasaki disease, indicating that the triggering of Kawasaki disease might be associated with presently unidentified respiratory pathogen(s) that potentially might be acquired from both within and outside the household.
Acknowledgments
We thank all the pediatricians who contributed to the Web-based Kawasaki disease surveillance system in Japan. We thank Analisa Avila, MPH, ELS, of Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Supported by the Japan Kawasaki Disease Research Center, a nonprofit organization. The authors declare no conflicts of interest.
Appendix
References
- 1.Ae R., Makino N., Kosami K., Kuwabara M., Matsubara Y., Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the Nationwide Survey in Japan, 2017-2018. J Pediatr. 2020;225:23–29.e2. doi: 10.1016/j.jpeds.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Makino N., Nakamura Y., Yashiro M., Kosami K., Matsubara Y., Ae R., et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015-2016. Pediatr Int. 2019;61:397–403. doi: 10.1111/ped.13809. [DOI] [PubMed] [Google Scholar]
- 3.Makino N., Nakamura Y., Yashiro M., Sano T., Ae R., Kosami K., et al. Epidemiological observations of Kawasaki disease in Japan, 2013-2014. Pediatr Int. 2018;60:581–587. doi: 10.1111/ped.13544. [DOI] [PubMed] [Google Scholar]
- 4.Makino N., Nakamura Y., Yashiro M., Ae R., Tsuboi S., Aoyama Y., et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25:239–245. doi: 10.2188/jea.JE20140089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 6.Lin M.T., Wu M.H. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. 2017;2017:e201720. doi: 10.21542/gcsp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uehara R., Belay E.D. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holman R.C., Belay E.D., Christensen K.Y., Folkema A.M., Steiner C.A., Schonberger L.B. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29:483–488. doi: 10.1097/INF.0b013e3181cf8705. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y., Yanagawa H. The worldwide epidemiology of Kawasaki disease. Prog Pediatr Cardiol. 2004;19:99–108. [Google Scholar]
- 10.Singh S., Vignesh P., Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100:1084–1088. doi: 10.1136/archdischild-2014-307536. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. (in Japanese) [PubMed] [Google Scholar]
- 12.Rowley A.H., Shulman S.T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowley A.H. Is Kawasaki disease an infectious disorder? Int J Rheum Dis. 2018;21:20–25. doi: 10.1111/1756-185X.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley A.H., Shulman S.T. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8:197–203. doi: 10.1586/eri.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banday A.Z., Bhattacharya D., Pandiarajan V., Singh S. Kawasaki disease in siblings in close temporal proximity to each other—what are the implications? Clin Rheumatol. 2021;40:849–855. doi: 10.1007/s10067-020-05328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita Y., Nakamura Y., Sakata K., Hara N., Kobayashi M., Nagai M., et al. Kawasaki disease in families. Pediatrics. 1989;84:666–669. [PubMed] [Google Scholar]
- 17.Nagao Y. Decreasing fertility rate correlates with the chronological increase and geographical variation in incidence of Kawasaki disease in Japan. PLoS One. 2013;8:e67934. doi: 10.1371/journal.pone.0067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagao Y., Urabe C., Nakamura H., Hatano N. Predicting the characteristics of the aetiological agent for Kawasaki disease from other paediatric infectious diseases in Japan. Epidemiol Infect. 2016;144:478–492. doi: 10.1017/S0950268815001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano T., Makino N., Aoyama Y., Ae R., Kojo T., Kotani K., et al. Temporal and geographical clustering of Kawasaki disease in Japan: 2007-2012. Pediatr Int. 2016;58:1140–1145. doi: 10.1111/ped.12970. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi S., Yanagawa H., Kawasaki T., Yanase Y. An outbreak of Kawasaki disease in Miyako Island in Okinawa prefecture. Pediatr Int. 1983;25:436–437. [Google Scholar]
- 21.Burns J.C., Cayan D.R., Tong G., Bainto E.V., Turner C.L., Shike H., et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Z.D., Zhao D., Du J., Zhang Y.L., Lin Y., Liu C., et al. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. 2007;26:449–451. doi: 10.1097/01.inf.0000261196.79223.18. [DOI] [PubMed] [Google Scholar]
- 23.Kido S., Ae R., Kosami K., Matsubara Y., Makino N., Sasahara T., et al. Seasonality of i.v. immunoglobulin responsiveness in Kawasaki disease. Pediatr Int. 2019;61:539–543. doi: 10.1111/ped.13863. [DOI] [PubMed] [Google Scholar]
- 24.Kim G.B., Park S., Eun L.Y., Han J.W., Lee S.Y., Yoon K.L., et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012-2014. Pediatr Infect Dis J. 2017;36:482–485. doi: 10.1097/INF.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 25.Ozeki Y., Yamada F., Kishimoto T., Yashiro M., Nakamura Y. Epidemiologic features of Kawasaki disease: winter versus summer. Pediatr Int. 2017;59:821–825. doi: 10.1111/ped.13293. [DOI] [PubMed] [Google Scholar]
- 26.Ozeki Y., Yamada F., Saito A., Kishimoto T., Yashiro M., Makino N., et al. Epidemiologic features of Kawasaki disease distinguished by seasonal variation: an age-specific analysis. Ann Epidemiol. 2018;28:796–800. doi: 10.1016/j.annepidem.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Iio K., Matsubara K., Miyakoshi C., Ota K., Yamaoka R., Eguchi J., et al. Incidence of Kawasaki disease before and during the COVID-19 pandemic: a retrospective cohort study in Japan. BMJ Paediatr Open. 2021;5:e001034. doi: 10.1136/bmjpo-2021-001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shulman S., Geevarghese B., Kim K.Y., Rowley A. The impact of social distancing for COVID-19 upon diagnosis of Kawasaki disease. J Pediatr Infect Dis Soc. 2021 doi: 10.1093/jpids/piab013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J.M., Kim Y.E., Huh K., Hong J., Kim D.W., Kim M.Y., et al. Reduction in Kawasaki disease after nonpharmaceutical interventions in the COVID-19 era: a nationwide observational study in Korea. Circulation. 2021;143:2508–2510. doi: 10.1161/CIRCULATIONAHA.121.054785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey L.C., Razzaghi H., Burrows E.K., Bunnell H.T., Camacho P.E.F., Christakis D.A., et al. Assessment of 135 794 pediatric patients tested for Severe Acute Respiratory Syndrome Coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura Y., Yashiro M., Ae R., Chihara I., Sadakane A., Aoyama Y., et al. Characteristics and validity of a web-based Kawasaki disease surveillance system in Japan. J Epidemiol. 2010;20:429–432. doi: 10.2188/jea.JE20100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Web-based Kawasaki disease surveillance system in Japan. http://www.kawasaki-disease.net/kawasakidata/ (in Japanese) [DOI] [PMC free article] [PubMed]
- 33.Kobayashi T., Ayusawa M., Suzuki H., Abe J., Ito S., Kato T., et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition) Pediatr Int. 2020;62:1135–1138. doi: 10.1111/ped.14326. [DOI] [PubMed] [Google Scholar]
- 34.National Institute of Infectious Diseases Japan [Web site of IDWR Surveillance Data] https://www.niid.go.jp/niid/en/data.html
- 35.Ministry of Health, Labour and Welfare, Japan Implementation manual for the National Epidemiological Surveillance Infectious Diseases program. https://www.mhlw.go.jp/english/policy/health-medical/health/dl/implementation_manual.pdf
- 36.Crowcroft N.S., Zambon M., Harrison T.G., Mok Q., Heath P., Miller E. Respiratory syncytial virus infection in infants admitted to paediatric intensive care units in London, and in their families. Eur J Pediatr. 2008;167:395–399. doi: 10.1007/s00431-007-0509-9. [DOI] [PubMed] [Google Scholar]
- 37.Hall C.B., Geiman J.M., Biggar R., Kotok D.I., Hogan P.M., Douglas G.R., Jr. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 38.Heikkinen T., Valkonen H., Waris M., Ruuskanen O. Transmission of respiratory syncytial virus infection within families. Open Forum Infect Dis. 2015;2:ofu118. doi: 10.1093/ofid/ofu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munywoki P.K., Koech D.C., Agoti C.N., Lewa C., Cane P.A., Medley G.F., et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209:1685–1692. doi: 10.1093/infdis/jit828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerr D.M., Meier A.S., Selke S.S., Frenkel L.M., Huang M.L., Wald A., et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 41.Asano Y., Yoshikawa T., Suga S., Kobayashi I., Nakashima T., Yazaki T., et al. Clinical features of infants with primary human herpesvirus 6 infection (exanthem subitum, roseola infantum) Pediatrics. 1994;93:104–108. [PubMed] [Google Scholar]
- 42.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrams J.Y., Godfred-Cato S.E., Oster M.E., Chow E.J., Koumans E.H., Bryant B., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54.e1. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrams J.Y., Oster M.E., Godfred-Cato S.E., Bryant B., Datta S.D., Campbell A.P., et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belay E.D., Abrams J., Oster M.E., Giovanni J., Pierce T., Meng L., et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.0630. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowley A.H. Multisystem inflammatory syndrome in children and Kawasaki disease: two different illnesses with overlapping clinical features. J Pediatr. 2020;224:129–132. doi: 10.1016/j.jpeds.2020.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda S., Kaneta M., Miyake M., Ohya T., Miyakawa K., Iwamoto M., et al. A case of multisystem inflammatory syndrome in children in a Japanese boy: with discussion of cytokine profile. Mod Rheumatol Case Rep. 2021;5:442–447. doi: 10.1080/24725625.2021.1920140. [DOI] [PubMed] [Google Scholar]
- 48.Rowley A.H., Baker S.C., Shulman S.T., Fox L.M., Takahashi K., Garcia F.L., et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192:1757–1766. doi: 10.1086/497171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowley A.H., Baker S.C., Shulman S.T., Garcia F.L., Guzman-Cottrill J.A., Chou P., et al. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004;190:856–865. doi: 10.1086/422648. [DOI] [PubMed] [Google Scholar]
- 50.Rowley A.H., Baker S.C., Shulman S.T., Rand K.H., Tretiakova M.S., Perlman E.J., et al. Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a "new" virus associated with Kawasaki disease. J Infect Dis. 2011;203:1021–1030. doi: 10.1093/infdis/jiq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowley A.H., Baker S.C., Arrollo D., Gruen L.J., Bodnar T., Innocentini N., et al. A protein epitope targeted by the antibody response to Kawasaki disease. J Infect Dis. 2020;222:158–168. doi: 10.1093/infdis/jiaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]