Abstract
Background
A skilled health workforce is instrumental for the delivery of multidisciplinary cancer care and in turn a critical component of the health systems. There is, however, a paucity of data on the vast inequalities in cancer workforce distribution, globally. The aim of this study is to describe the global distribution and density of the health care workforce involved in multidisciplinary cancer management.
Methods
We carried out a systematic review of the literature to determine ratios of health workers in each occupation involved in cancer care per 100 000 population and per 100 cancer patients (PROSPERO: protocol CRD42018095414).
Results
We identified 33 eligible papers; a majority were cross-sectional surveys (n = 16). The analysis of the ratios of health providers per population and per patients revealed deep gaps across the income areas, with gradients of workforce density, highest in high-income countries versus low-income areas. Benchmark estimates of optimal workforce availability were provided in a secondary research analysis: mainly high-income countries reported workforce capacities closer to benchmark estimates. A paucity of literature was defined for critical health providers, including for pediatric oncology, surgical oncology, and cancer nurses.
Conclusion
The availability and distribution of the cancer workforce is heterogeneous, and wide gaps are described worldwide. This is the first systematic review on this topic. These results can inform policy formulation and modelling for capacity building and scaleup.
Key words: cancer workforce, global cancer policy, national cancer control planning, capacity-building, medical oncology workforce
Highlights
-
•
Workforce is an essential component of the health systems.
-
•
Stark inequalities are reported for the distribution of health workforce worldwide, but data are limited.
-
•
We aimed at portraying the first global figure of the comprehensive cancer workforce for cancer management.
-
•
Inequalities in density and distribution of the workforce regard all the key health personnel involved in cancer management.
-
•
These data will inform the development of evidence-informed policies for the workforce in low- and middle-income countries.
Introduction
Cancer is a leading cause of global morbidity, disability, and mortality.1 In 2020, 19.3 million people were diagnosed with cancer and 9.9 million cancer-related deaths occurred.1 Up to 60% of incident cases and 70% of cancer-related deaths were reported in low- and middle-income countries (LMs), where cancer prognosis is generally dismal.2,3 Disparities observed in global survival outcomes are multifactorial and generally relate to inefficiencies, weaknesses, and fragmentation of the cancer care continuum, including a paucity of health care workers.4,5 Investments in the workforce, however, have been chronically insufficient.
The health care workforce is an instrumental component of health systems and health systems can only function if there are quality trained health care providers avaliable.6 The health care workforce is subject to profound inequalities, with a projected shortfall of 18 million health workers, mostly in LM settings, by 2030.7 In order to tackle the disparities in cancer-related outcomes, a critical aspect is to strengthen the workforce for cancer care.
The United Nations Sustainable Development Goals (SDG) and the World Health Organization (WHO) Cancer Resolution emphasize the urgent need to develop and strengthen health system infrastructure, particularly related to human resources for health.3,4,6 Increasing health financing, recruitment, training, and retention of the workforce in LMs are key targets of the SDG agenda. The health workforce is a vital pillar of the health care system, and it is fundamental for delivering high-quality cancer care services. Currently, there is a paucity of data and tools that report or estimate the existing capacity and future needs of cancer care workers globally, and a comprehensive portrait of the cancer workforce is missing. This represents a relevant barrier in estimating workforce capacity for capacity building and scaling up.3 The aim of this study is to investigate the current global distribution and density of the cancer care workforce based on published estimates.
Methods
Review design
A systematic review was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in the PROSPERO database (protocol CRD42018095414).8, 9, 10
Data sources and search strategy
Three authors (MB, DT, AI) carried out a literature search in PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Database of Systematic Reviews, Ovid, PsycINFO, Political Science, Joanna Briggs Institute EBP Database, and SCOPUS, with no time and no language restrictions (i.e. from inception to date of search) on 1 April 2018. The research was updated in April 2021, with no new papers included. We searched for articles on occupations related to cancer management including medical and radiation oncologists, paediatrics, surgical oncologists, anatomic pathologists, radiotherapy technicians, medical physicists, dosimetrists, and cancer nurses. The mapping search and MeSH terms were combined with Boolean operators, and included ‘workforce’, ‘human resources,’ and ‘cancer’ arranged in research strings to accommodate different databases. The bibliographies of the relevant screened papers were also manually searched for snowballing.
Study selection
Papers were selected in two phases; in phase 1, papers were independently screened according to titles and abstracts by two authors (DT, AI). In phase 2, two authors (DT, AI) independently reviewed full-text articles for eligibility; discrepancies were discussed by a third reviewer (GC), who functioned as a tiebreaker. Peer-reviewed literature was primarily considered, including reviews and descriptive papers. We manually searched adjunctive materials from grey literature, using Google (e.g. government reports, health policy documents issued by ministries of health) and searched the repository of the National Cancer Control Plans provided by the International Cancer Control Partnership.11
Inclusion and exclusion criteria
Articles were considered eligible if they included descriptions of the distribution and density of the cancer workforce. We only included studies that reported ratios of health care providers per population (i.e. 100 000 population) and/or ratios per patients (i.e. 100 cancer patients). We did not estimate de novo ratios, therefore papers reporting absolute estimates of providers with no relative figures to the cancer burden or population size were excluded. The choice of a denominator for the ratio is a sensitive matter, and the risk of reporting subnational figures of the workforce was the primary caveat we addressed. We included any type of quantitative, qualitative, or mixed-method studies. We did not include pharmacy, palliative care, social, and psychology workforce; though highly relevant for the delivery of effective multidisciplinary cancer care, the complexity and broad scope of the social, psychological, and palliative care workforce has been comprehensively reviewed and published elsewhere and is outside the primary scope of this paper.12,13
Data extraction and data analysis
We extracted the ratios of providers per 100 000 population and/or ratios of providers per 100 cancer patients, for each country. The workforce ratios were analysed according to the country’s gross national income level [World Bank grouping: low- (LIC); lower-middle- (LMIC); upper-middle- (UMIC); high- (HIC) income country] and WHO Regions (AFRO, African Region; SEARO, South-East Asia Region; EURO, European Region; EMRO, Eastern Mediterranean Region; AMRO, Region of the Americas; WPRO, Western Pacific Region).14,15 Data were analyzed using descriptive statistics (Microsoft Excel 2019; Microsoft Corporation, Redmond, WA). For secondary analyses, we collected data on the workload estimates (e.g. time spent on clinical and/or research activities), and the minimal amount of training that is required to practice the profession (e.g. undergraduate or postdoctoral training).
Results
Overview
Our search identified 8735 articles and 33 papers were included, based on the eligibility criteria (Figure 1). The majority of the studies identified were cross-sectional surveys (n = 16) and health professional registry-based studies (n = 14 papers). For the surveys, the average response rate was 89%; three-quarters of the articles reported 80% response rates or higher. The median year of publication was 2013 (range 1996-2018), 12 were published before 2010 (36%), and all papers were in the English language. Most of the articles reported were single-country analysis (n = 27). The most common surveyed occupations were medical oncologist (n = 10 papers), radiation oncologist (n = 9 papers), and pathologist (n = 7 papers). Figures and references are listed in Supplementary Table S1 and Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100292, and the findings per WB income areas and WHO Regions summarized in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) systematic review flowchart.
Table 1.
Estimated median ratios of the cancer health providers per income areas
| APA | Clin Onc | Med Onc | Rad Onc | Med Phys | RTT | |
|---|---|---|---|---|---|---|
| Ratios per 100 000 population | ||||||
| HIC | 3.7 (1.5; 5.7) | 0.88a | 1.6 (0.9; 3) | 1.24 (0.28; 3) | 0.78 (0.13; 1.5) | 0.44 (0.18; 1.2) |
| UMIC | 0.35 (0.13; 1.2) | 0.2 (0.07; 0.9) | 0.41b | 0.4 (0.09; 0.96) | 0.3 (0.16; 0.6) | 0.28 (0.2; 1.6) |
| LMIC | 0.07 (0.03; 0.6) | 0.03 (0.01; 0.94) | 0.09d | 0.04 (0.01; 0.3) | 0.05 (0; 14) | 0.06 (0.015; 0.2) |
| LIC | 0.04 (0; 0.07) | 0.006 (0; 0.15) | — | — | 0.1 (0.04; 0.18) | — |
| Global | 0.06 (0; 5.7) | 0.028 (0; 0.94) | 1.25 (0.09; 3) | 0.28 (0.01; 3) | 0.23 (0; 1.5) | 0.19 (0.015; 1.6) |
| Ratios per 1000 cancer patients | ||||||
| HIC | 0.7 (Canada) | 0.86a-2.9c | 0.65 (0.04; 1.18) | 0.25 (0.07; 0.6) | 0.47e | 1.2a |
| UMIC | — | 0.37 (0.23; 0.85) | 0.45b | 0.19 (0.1; 0.43) | 0.28f | 0.7g |
| LMIC | — | 0.12 (2.9; 0.014) | — | 0.076 (0.01; 0.4) | — | — |
| LIC | — | 0.01 (0; 1.1) | — | — | — | — |
| Global | — | 0.27 (0; 2.9) | 0.48 (0.04; 1.37) | 0.15 (0.01; 0.6) | NA | NA |
All the ratios are reported as median value (interval: min; max).
APA, anatomic pathologist; Clin Onc, clinical oncologist; HIC, high-income country; LIC, low-income country; LMIC, lower-middle-income country; Med Onc, medical oncologist; Med Phys, medical physicist; NA, cannot be estimated; Rad Onc, radiation oncologist; RTT, radiation therapy technician; UMIC, upper-middle-income country.
Only one estimate available, from United Arab Emirates.
Estimate from Iran.
Estimate from Israel.
Estimate from Morocco.
Estimate from Poland.
Estimate from Bulgaria.
Estimate from Thailand.
Anatomic pathologist
We estimated a median ratio of 0.06 (min 0; max 5.7) pathologists per 100 000 population across the studies retrieved in literature. In HICs, there is a median of 3.7 (1.5; 5.7) pathologists per 100 000 population, 0.35 (0.13; 1.2) in UMICs, 0.07 (0.03; 0.6) in LMICs, and 0.04 (0; 0.07) in LICs (Figure 2). The ratio of pathologists per 100 cancer patients is available only for Canada, and is equal to 0.7. Postgraduate training was required for anatomic pathologists in all the studies analysed.
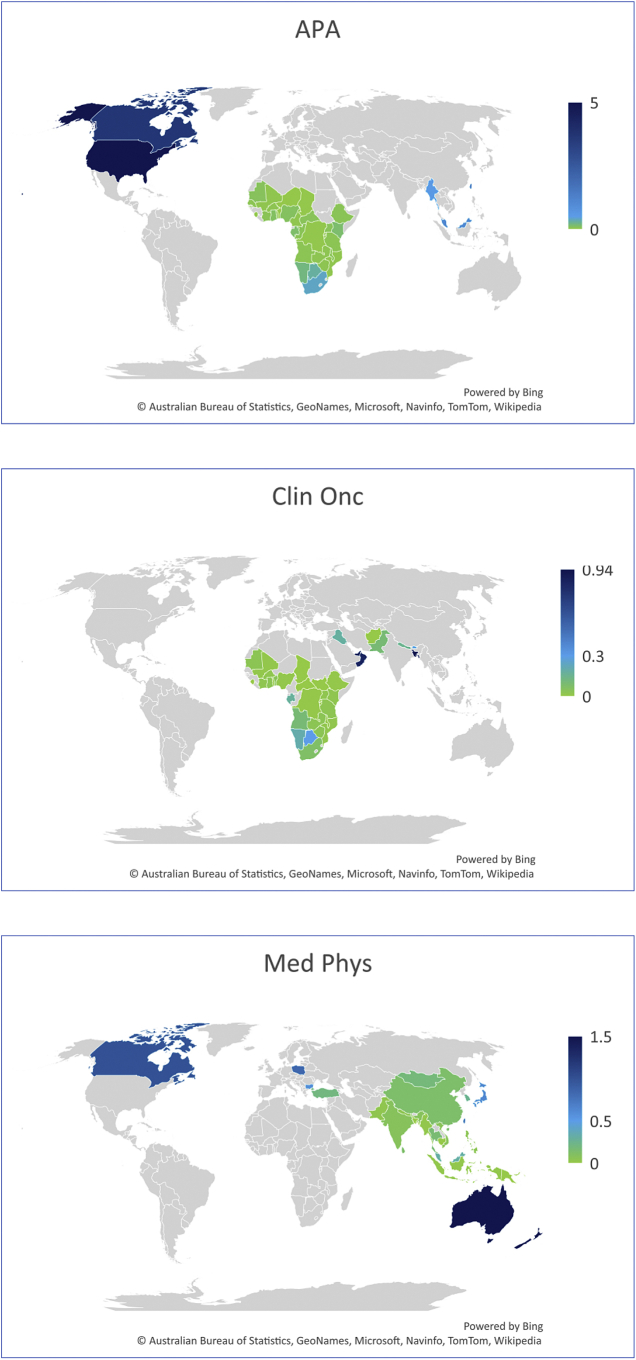
Figure 2.
Distribution of the estimated median ratios of health providers per population (A) and per cancer patients (B).
APA, anatomic pathologist; Clin Onc, clinical oncologist; HIC, high-income country; LIC, low-income country; LMIC, lower-middle-income country; Med Onc, medical oncologist; Med Phys, medical physicist; Rad Onc, radiation oncologist; RTT, radiation therapy technician; UMIC, upper-middle-income country.
∗ Estimate not available.
Clinical oncologist
Eighty percent of the ratios extracted about the clinical oncology workforce were derived from LMICs and LICs; three-quarters of the data were from the AFRO region. There is a median of 0.028 clinical oncologists per 100 000 population and 0.27 oncologists per 100 cancer patients, worldwide. Only two HICs reported data for clinical oncologists, United Arab Emirates (UAE) and Israel. UAE has a median of 0.88 providers per 100 000 population and 2.9 providers per 100 cancer patients. Israel has 0.86 providers per 100 cancer patients. In UMICs there are 0.2 (0.07; 0.9) clinical oncologists per 100 000 population and 0.37 (0.23; 0.85) per 100 cancer patients. In LICs, the median ratio was 0.006 clinical oncologists per 100 000 population and 0.01 per 100 cancer patients. In eight countries (Burundi, Central African Republic, Chad, Rwanda, Sierra Leone, Togo, South Sudan, and Afghanistan), 0 clinical oncologists per 100 000 population and/or 100 patients were reported. Postgraduate training in clinical oncology was required for all the countries surveyed.
Medical oncologist
Worldwide, a median of 1.25 (0.09; 3) medical oncologists per 100 000 population and 0.48 (0.04; 1.37) per 100 cancer patients exist. Almost 75% of the data were collected from HICs. In HICs, a median of 1.6 (0.9; 3) medical oncologists per 100 000 population and 0.65 (0.04; 1.18) per 100 cancer patients exist. Iran was the only UMIC with data available, reporting 0.41 providers per 100 000 population and 0.45 per 100 cancer patients. The only LMIC with data available was Morocco, which has 0.09 providers per 100 000 population. Medical oncologists were required to have postgraduate training in all the studies reporting this information.
Paediatric oncologist
In our analysis, we included any oncologist with postgraduate training in oncology, haematology, and paediatrics who delivers paediatric oncology care under the definition of paediatric oncologist. Published data reported experiences only from HICs. In the USA, a study from the year 2000 estimated 0.4 paediatric oncologists per 100 000 population;16 this figure was later updated in 2015 by the American Medical Association to a ratio of 2.6 paediatric oncology providers per 100 000 population.17 In Canada, 0.2 per 100 000 paediatric oncologists exist.18 A target ratio of 6.7 providers per 100 new cancer patients was projected as an optimal workforce ratio needed to care for paediatric cancer patients in North America.16, 17, 18 All paediatric oncology specialists were required to have completed postgraduate training.
Radiation oncologist
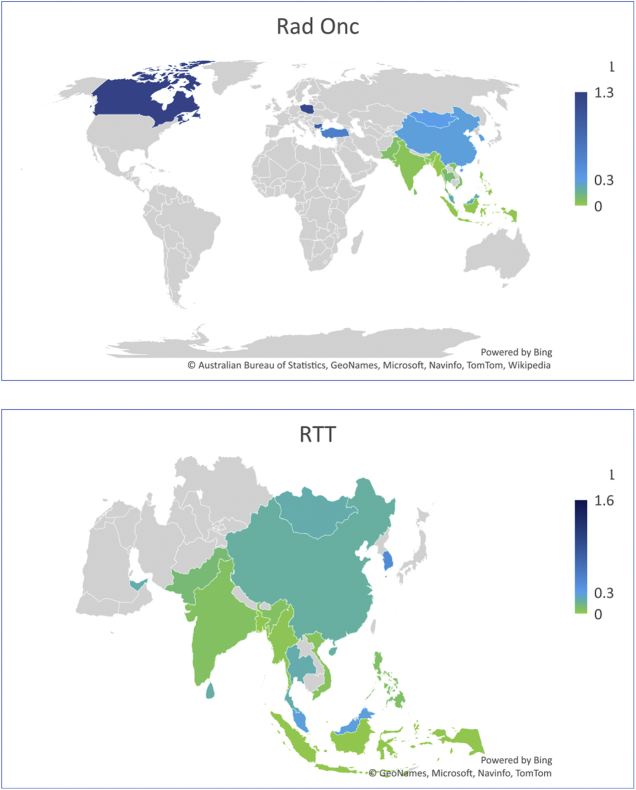
There are a median of 0.28 (0.01; 3) radiation oncologists per 100 000 population and 0.15 (0.01; 0.6) per 100 cancer patients, worldwide. In HICs, the median ratio of providers is 1.24 (0.28; 3) per 100 000 population, and 0.25 (0.07; 0.6) per 100 cancer patients. In UMICs, ratios are 0.4 (0.09; 0.96) per 100 000 and 0.19 (0.1; 0.43) per 100 cancer patients, respectively. In LMICs 0.04 (0.01; 0.3) providers per 100 000 and 0.076 (0.01; 0.4) per 100 cancer patients exist. No data are reported for density or distribution of the radiation oncology workforce in LICs in the extracted literature.19 Postgraduate training was required in all studies.
Medical physicist
Our data-based estimate of the median number of medical physicists per 100 000 population is 0.23 (0; 1.5). Reports of providers per 100 cancer patients were available in only two countries, Poland and Bulgaria, and were 0.47 and 0.28, respectively. Graduate education was required in half of the countries for which this information was available (n = 28 in total), while the remaining half (n = 14 countries) required postgraduation education.
Radiation therapy technician
A median ratio of 0.19 (0.015; 1.6) per 100 000 population radiation therapy technician providers is estimated across the extracted papers. In HICs, UMICs, and LMICs there is a median of 0.44 (0.18; 1.2), 0.28 (0.2; 1.6), and 0.06 (0.015; 0.2) per 100 000 population of radiation therapy technicians, respectively. No data were reported from LICs. Only two countries have data for providers per 100 cancer patients, UAE and Thailand, with reported ratios of 1.2 and 0.7, respectively. The educational requirements for radiation therapy technicians are variable across countries; generally graduate or postgraduate training is required.
Other occupations
Our systematic research returned a paucity of papers for ‘surgical oncology’ (n = 2 papers), ‘dosimetrist’ (n = 1 paper), and ‘oncology nurse’ (n = 1 paper). For the surgical oncology workforce, an analysis from Iran reported a ratio of 0.06 cancer surgeons per 100 000 population and 0.06 per 100 cancer patients (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100292). Postgraduation training was required. A survey carried out in the USA in 2003 reported 0.35 dosimetrists per 100 000 population (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100292). University education was required. A health professional registry-based study carried out in 1996 in the UK reported a ratio of 2.4 cancer nurses per 100 000 population and 1 cancer nurse per 100 cancer patients.
Discussion
To our knowledge, this is the first study to assess the distribution and density of the multidisciplinary cancer care workforce. Previous reports have primarily focused on physician providers.20 Our systematic research suggests wide disparities and gaps in the availability and accessibility of the cancer care workforce worldwide, especially in LMs (Figures 3 and 4). This is in stark contrast with the cancer burden sharing, which is relatively higher in LMs, and where the highest cancer mortality is described. Severe shortages are described in LMICs and LICs, for all health care professionals. HICs report ratios that fall within the ranges of an optimal workforce capacity to meet the needs of a health system, based on various exercises and staffing estimates.16, 17, 18, 19, 20 For example, a modelling exercise from Spain provided a benchmark estimation of 2.79 full-time equivalent (FTE) medical oncologists per 100 000 inhabitants and 0.63 FTEs per 100 patients, respectively.20 These benchmark ratios are similar to the ones reported by HICs in our analysis, suggesting that HICs are more likely to meet population health needs for cancer care and have nominally an adequate provision of human resources.16, 17, 18, 19, 20
Figure 3.
Graphical mapping of the ratios of health providers per 100 000 population.
Occupations for which at least estimates from 10 countries were available were plotted.
APA, anatomic pathologist; Clin Onc, clinical oncologist; Med Phys, medical physicist; Rad Onc, radiation oncologist; RTT, radiation therapy technician.
Figure 4.
Graphical mapping of the ratios of health providers per 100 patients.
Occupations for which at least estimates from 10 countries were available were plotted.
Clin Onc, clinical oncologist; Med Onc, medical oncologist; Rad Onc, radiation oncologist.
The availability and accessibility of a quality skilled health care workforce is critical to deliver multidisciplinary cancer care.21,22 There is global acknowledgement that the paucity of the oncology workforce has a detrimental impact on patient outcomes and population health.21,22 This has been recognized by multiple stakeholders, including the ‘Lancet Commission on the Access to Radiotherapy’, which estimated that only 40%-60% of patients with cancer have access to radiotherapy services, in the world.22 The high cost and time commitment of training, assumed by countries, was a key barrier identified in the ‘Commission’. Additionally, Wilson et al.23 modelled the chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040. The authors estimated that 65 000 cancer physicians were required worldwide to deliver optimal chemotherapy in 2018, anticipating this to rise to 100 000 by 2040, especially marked in LMs. Our study illustrates this shortage currently exists for radiotherapy providers and demonstrates the severe shortage of radiotherapy health care workforce, with only 0.06 (0.015; 0.2) per 100 000 population of radiotherapy technicians in LMICs and 0.04 (0.01; 0.3) radiation oncologists per 100 000 and 0.076 (0.01; 0.4) per 100 cancer patients, with a benchmark estimation of an optimal ratio of 0.38 radiation oncologists per 100 cancer patients, in one exercise from Japan.19 The deep shortages of health providers result in various consequences that affect the workforce itself, as well.24, 25, 26 For example, a rise in cancer cases with a paucity of providers can produce an increased workload for the single providers and lower job satisfaction.27,28, 29, 30, 31, 32, 33, 34, 35 One study has shown that the annual case volume in LMs is 2.4 times higher than in HICs, where 40% and 7% of medical oncologists see >500 annual consults, respectively.28 This is one determinant of the high attrition rate of health providers in LMs.28
Whereas large differences across World Bank income areas might be expected when the health care workforce is computed per 100 000 population rates, we believe that disparities in the ratios per 100 cancer patients could be more accurate in informing policymakers on actual health system capacity for cancer management. Cancer services are framed within all levels of a health system; therefore, cancer care must be envisioned across the continuum of care, with some services intended to reach the population through a primary health care service and linked to referral pathways to secondary and tertiary level facilities.3,12 Accordingly, the ratio of providers per 100 cancer patients may need to be more precise to understand the capacity and needs of the health system to deliver specialized cancer care. Conversely, a ratio per 100 000 population may be more important for cross-cutting clinical services, like prevention services for non-communicable diseases or palliative care, especially when delivered through the primary health care platform. Based on this review, we propose that measurements per 100 cancer patients may be the best indicator for cancer-specific providers. As a metric, the variability of the ratio per 100 cancer patients is less than the ratio per 100 000 population, in regions with consistent health system capacities and/or income and cancer epidemiology. The ratio per cancer patients does not carry the problem of being affected by the population age (i.e. a major factor to adjust for when comparing countries), and is sensitive of the cancer burden. However, the ratio per 100 000 population is easier to implement, giving equal access to populations, and does not depend on the existence of good cancer registry—that is a common issue in LMs, therefore retaining utility in some cancer services and settings. The use of both metrics, per patients and per population, can be influenced by the dynamic of cancer epidemics. Therefore, the use for comparison purposes is perhaps more explicit, but the implementation in cancer planning should be more careful, and not the only metric to account for. We propose that countries should consider utilizing these indicators to accurately measure the cancer burden and workforce capacity needed.4,6 The establishment and alignment of resource allocation and disease burden is key for efficient cancer planning, financing, and well adapted implementation of cancer programs.3,4,6
Another point that emerged in our review is in the definition and scope of the occupations, and the minimum training required. Variability in the training requirements (e.g. educational level) was reported for non-physician providers, and mid-level professionals. For physician providers, medical, radiation, and clinical oncologists can overlap partially in core competencies. In general, clinical oncologists are more commonly trained to provide medical and radiation oncology interventions, for several tumor types. Whereas the scope of medical and radiation oncology practice is well established,25, 26, 27,36 our research has demonstrated that in many LMs, oncology practice is a relatively young field, with low to modest population coverage in the public sector.29 Accordingly, clinical oncologists were more prevalent in LMs, perhaps, as a result of less consolidated or not fully implemented curricula in oncology, for a workforce undergoing transitions toward more specifalized health professionals, like medical and radiation oncologists. Of interest, in some countries, the coexistence of both the medical and clinical oncology workforce was identified (i.e. Bangladesh, Canada, China, Indonesia, Pakistan, Sri Lanka), possibly mirroring a transition in the education of cancer health providers to a more specialized workforce. Of note, one case study has been reported for Morocco, where medical oncology is an emerging field, officially recognized in 2008: pre-, and post-2008 trained cancer providers are registered as clinical and medical oncologists, respectively, and they presently co-exist in overlapping professional scopes.30
The scope of this paper is limited for the surgical oncology workforce, related to variable training programs and core curricula: in fact, multiple surgeons not formally accounted as surgical oncologists, including general surgeons, may perform most of the cancer operations globally.31 The lack of uniform definitions and training programs, and professional registries of surgical oncologists represents a barrier, resulting in challenges in national health planning for cancer control. This includes key barriers for the workforce retention, including the brain drain, low empowerment, professional dissatisfaction, and poor incentives for career building. We could not identify a benchmark estimate ratio for surgical oncologists and portray a global picture. We suggest that the need to standardize international definitions and core curricula of the oncology workforce is instrumental to monitor global trends, which can in turn enhance the accountability of the workforce and inform national health care planning and policymaking.3,6,20,32,33
A limitation of this research is that we had few data on the competencies required of health providers at the country level and metrics of quality training, as the scopes of occupations were not generally described. This can be especially relevant for clinical oncologists, as discussed above. Albeit we intended to portray a global picture of the workforce distribution, the data points extracted more often are relevant to country-level or regional capacities. Therefore, this cannot be considered a definitive global picture on the workforce, but only the best data currently available, based on our research aims. Also, given the limited literature on this topic, a third of the papers were published before 2010, that may report a figure that is not updated, for some countries. While the aim of the paper was to investigate the global distribution of the workforce, major shortcomings are derived from the lack of data in some of the countries, based on the paucity of the workforce research, overall. The narrow scope for the surgical oncology workforce and the few data retrieved on selected mid-level workers, especially cancer nurses, demonstrates the need for research as a priority in these areas. The registries of surgical oncologists sometimes include all the providers with some cancer-related practice, resulting in inflated ratios. This is similar for pediatric oncologists. Also, the ratios per population and per patients were not analyzed at subnational levels, to address internal heterogeneities in some settings and the entity of attrition and migration from rural areas. Eventually, workforce capacity building and scaleup is a dynamic process and the current country reality may differ from our collected data.
Conclusion
Our research portrayed estimates of the current landscape of the cancer health care workforce in different regions, based on a systematic research of the literature. With the rising global cancer burden, we reported that there is a significant shortage of health care workforce caring for cancer patients, especially in LMs. In the era of Universal Health Coverage and with the rising global cancer burden, it is critical for health systems to develop capacity for competent health care professionals trained in cancer care, to tackle to the global cancer burden and address disparities in health care.3,4,6
Acknowledgements
We acknowledge Dr Catherine Lam and Daniel Moreira from St Jude Children’s Hospital for the help provided and inputs in developing the research strategy for the childhood cancer workforce. We also acknowledge the contribution of Dr Elena Fidarova from WHO, for the important inputs on the radiation therapy workforce. We thank the European Society for Medical Oncology, for supporting this work, and the ESMO leadership for providing valuable comments and edits on the final proof, including an articulated revision from Prof G. Pentheroudakis, under the coordination or Mrs Vyas and Mrs Bricalli.
Funding
None declared.
Disclosure
GC reports consultancy from Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche, Seagen, and Daiichi Sankyo; employment at the University of Milano, Istituto Europeo di Oncologia, IRCCS, Milano, Italy; and speaker's bureau participation for Lilly, Pfizer, Roche, Seagen, and Daiichi Sankyo. AI and MB are employees of WHO. The other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37513025patients diagnosed with one of 18 cancers from 322 population -basedregistries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization WHO report on cancer: setting priorities, investing wisely and providing care for all. 2020. https://apps.who.int/iris/handle/10665/330745 Available at.
- 4.World Health Organization Seventieth World Health Assembly. Cancer prevention and control in the context of an integrated approach. 2017. https://apps.who.int/iris/handle/10665/275676 Available at.
- 5.Prades J., Remue E., van Hoof E., Borras J.M. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy (New York) 2015;119(4):464–474. doi: 10.1016/j.healthpol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Seventieth World Health Assembly. Human resources for health and implementation of the outcomes of the United Nations’ High-Level Commission on Health Employment and Economic Growth. 2017. https://www.who.int/health-topics/health-workforce#tab=tab_1 Available at.
- 7.Limb M. World will lack 18 million health workers by 2030 without adequate investment, warns UN. Br Med J. 2016;354:i5169. doi: 10.1136/bmj.i5169. [DOI] [PubMed] [Google Scholar]
- 8.Haby M.M., Chapman E., Clark R., Barreto J., Reveiz L., Lavis J.N. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016;14(1):83. doi: 10.1186/s12961-016-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo J.H. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 11.Given L.S., Hohman K., Kostelecky B., Vinson C. Cancer control planning: self-assessment for pre-planning, development, implementation and evaluation of national cancer control plans. Cancer Causes Control. 2018;29(12):1297–1303. doi: 10.1007/s10552-018-1123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasson F., Nicholson E., Muldrew D., Bamidele O., Payne S., McIlfatrick S. International palliative care research priorities: a systematic review. BMC Palliat Care. 2020;19(1):16. doi: 10.1186/s12904-020-0520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates I., John C., Seegobin P., et al. An analysis of the global pharmacy workforce capacity trends from 2006 to 2012. Hum Resour Health. 2018;16:3. doi: 10.1186/s12960-018-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Definition of regional groupings. https://www.who.int/healthinfo/global_burden_disease/definition_regions/en/#:∼:text=WHO%20regions%3A%20WHO%20Member%20States,Region%2C%20and%20Western%20Pacific%20Region Available at.
- 15.World Bank Country classifications by income. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Available at.
- 16.DeAngelis C., Feigin R., DeWitt T., et al. Final report of the FOPE II Pediatric Workforce Workgroup. Pediatrics. 2000;106(5):1245–1255. [PubMed] [Google Scholar]
- 17.Hord J., Shah M., Badawy S.M., et al. The American Society of Pediatric Hematology/Oncology workforce assessment: Part 1 – Current state of the workforce. Pediatr Blood Cancer. 2018;65(2):e26780. doi: 10.1002/pbc.26780. [DOI] [PubMed] [Google Scholar]
- 18.Gruskin A., Williams R.G., McCabe E.R., et al. Final report of the FOPE II Pediatric Subspecialists of the Future Workgroup. Pediatrics. 2000;106(5):1224–1244. [PubMed] [Google Scholar]
- 19.Teshima T., Numasaki H., Shibuya H., et al. Japanese structure survey of radiation oncology in 2007 based on institutional stratification of patterns of care study. Int J Radiat Oncol Biol Phys. 2010;78(5):1483–1493. doi: 10.1016/j.ijrobp.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Rivera F., Andres R., Felip E., et al. Medical oncology future plan of the Spanish Society of Medical Oncology: challenges and future needs of the Spanish oncologists. Clin Trans Oncol. 2017;19(4):508–518. doi: 10.1007/s12094-016-1595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed A.J., Ghebreyesus T.A. Healthy living, well-being and the sustainable development goals. Bull World Health Organ. 2018;96(9):590. doi: 10.2471/BLT.18.222042. 590A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atun R., Jaffray D.A., Barton M.B., et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- 23.Wilson B.E., Jacob S., Yap M.L., Ferlay J., Bray F., Barton M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol. 2019;20(6):769–780. doi: 10.1016/S1470-2045(19)30163-9. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization State of the world’s nursing. 2020. https://www.who.int/publications/i/item/9789240003279 Available at.
- 25.Popescu R.A., Schäfer R., Califano R., et al. The current and future role of the medical oncologist in the professional care for cancer patients: a position paper by the European Society for Medical Oncology (ESMO) Ann Oncol. 2014;25(1):9–15. doi: 10.1093/annonc/mdt522. [DOI] [PubMed] [Google Scholar]
- 26.Leon-Ferre R.A., Stover D.G. Supporting the future of the oncology workforce: ASCO medical student and trainee initiatives. J Oncol Pract. 2018;14(5):277–280. doi: 10.1200/JOP.17.00088. [DOI] [PubMed] [Google Scholar]
- 27.Vanderpuye V., Hammad N., Martei Y., et al. Cancer care workforce in Africa: perspectives from a global survey. Infect Agent Cancer. 2019;14:11. doi: 10.1186/s13027-019-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fundytus A., Sullivan R., Vanderpuye V., et al. Delivery of Global Cancer Care: An International Study of Medical Oncology Workload. J Glob Oncol. 2018;4(4):1–11. doi: 10.1200/JGO.17.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization WHO Global Health Observatory. https://www.who.int/ncds/surveillance/ncd-capacity/en/ Available at.
- 30.Boutayeb S., Taleb A., Belbaraka R., et al. The Practice of Medical Oncology in Morocco: The National Study of the Moroccan Group of Trialist in Medical Oncology (EVA-Onco) Int Sch Res Notices. 2013;2013(4) doi: 10.1155/2013/341565. 341565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelassi F. 2010 SSO presidential address: subspecialty certificate in advanced surgical oncology. Ann Surg Oncol. 2010;17(12):3094–3103. doi: 10.1245/s10434-010-1286-7. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization Global strategy on human resources for health: Workforce 2030. https://www.who.int/hrh/resources/globstrathrh-2030/en/ Available at.
- 33.Mathew A. Global survey of clinical oncology workforce. J Glob Oncol. 2018;4:1–12. doi: 10.1200/JGO.17.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seruga B., Sullivan R., Fundytus A., et al. Medical oncology workload in Europe: one continent, several worlds. Clin Oncol (R Coll Radiol) 2020;32(1):e19–e26. doi: 10.1016/j.clon.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Raphael M.J., Fundytus A., Hopman W.M., et al. Medical oncology job satisfaction: results of a global survey. Semin Oncol. 2019;46(1):73–82. doi: 10.1053/j.seminoncol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 36.International Cancer Control Partnership Human Resources for Health. https://www.iccp-portal.org/human-resources-health Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.