Abstract
Herbal remedies have been used in many cultures for decades to treat illnesses. These medicinal plants have been found to contain various phytochemical compounds that can help to cure mild to severe illnesses. The inadequacies of conventional medicines and their unusual side effects sparked a determined search for alternative natural therapeutic agents. Another reason for this hunt could be the availability and fewer side effects of natural products. T. arjuna is widely used in traditional medicine to alleviate various diseases like relieving pain, ameliorating diabetes, mitigating inflammation, and back-pedaling of depression. In this study, the ethanolic extract of T. arjuna possesses a promising effect on the animal model (p < 0.05/p < 0.01) as an antihyperglycemic, analgesic, anti-inflammatory, and antidepressant agent, but in a dose-dependent manner. The lower dose of T. arjuna was found to be capable of reversing the disturbed physiological state at a significant level (p < 0.05). However, a higher dose of T. arjuna exerts better therapeutic effects for those diseases. This animal study aims to evaluate the anti-diabetic, anti-depressant, and anti-inflammatory properties of T. arjuna compared to conventional marketed drugs. We will perform an in-silico study for active constituents of T. arjuna against their proposed targets and look for the molecular cascade on their claimed pharmacological properties. This study shows that different doses of T. arjuna bark extracts give similar therapeutic responses compared with established marketed drugs in managing hyperglycemia, stress-induced depression, and inflammation. Besides, our docking study reveals that flavonoids and triterpenoid active constituents of T. arjuna play an important role in its usefulness. This study, therefore, scientifically confirmed the traditional use of this medicinal plant in the management of several diseased conditions.
Keywords: Herbal remedy, Anti-depressant, Anti-diabetic, Analgesic, In-silico study, T.arjuna
Herbal remedy, Anti-depressant, Anti-diabetic, Analgesic, In-silico study, T.arjuna.
1. Introduction
Humans have a deep and fascinating history of diseases. Since the dawn of the history of mankind, lethal and life-threatening diseases have been known to occur.Peoples suffering from the severity of the diseases thrive them to look for the reason and remedies. Due to the changing environmental factors and food habits, nowadays, people are more susceptible to diseases like diabetes, depression, etc., and the rate is increasing day by day [1, 2]. Diabetes mellitus (DM) is a clinical and endocrinological disorder associated with prolonged hyperglycemia, raised glycated hemoglobin, and higher complexity on comorbid conditions [3, 4]. It was evaluated that 200 million individuals worldwide experienced DM in 2010, and it is estimated to arrive at 300 million by 2025 [5]. Depression is an extensive, multifactorial psychiatric ailment that intervenes with an individual's daily routine. World Health Organization alerts that depression could be the second-most reason for the global disability in 2020 and could impact the weight of diseases by 2030 [6].Acute to chronic pain is also a global concern as it increases patients' distress and long-term pain management is similarly expensive as cancer or heart diseases [7]. Apart from that, inflammation is closely linked with pain. Inflammation, the most common adaptive response of the body, involves eliminating harmful agents and removing damaged tissue parts to promote the healing process of the affected tissues, organs, or systems [8, 9]. These chronic diseases are alarming to the socio-economy of a nation and claim up to 75% of health care expenditure. By 2030, low and middle-income countries may spend 5% of GDP on chronic diseases, including diabetes and heart diseases [10].
Approximately 65–80% of the human population is largely dependent on consuming plant kingdom for their primary healthcare needs [11] owing to poverty, rising costs of conventional medicine [12], effectiveness, minimal side effects [13], and lack of available therapeutic alternatives [14]. In recent years, because of medicinal plants' great and unique biodiversity, their extracts in the invention of several natural medicines in treating different diseases have become increasingly popular. Medicinal plants contain a vast number of bioactive constituents responsible for giving diversified pharmacological effects. So, we may use a single plant to treat different types of diseases like depression and diabetes. Additionally, genetic modification can be implemented in plants to enhance the concentration of desired constituents [15].
Terminalia arjuna Roxb. (Combretaceae), one of the medicinally important large evergreen deciduous trees [16], is commonly found throughout the Indian subcontinent, Sri Lanka, Myanmar, and Mauritius forest [17, 18]. The different parts of the T. Arjuna such as stem bark, leaves, and fruit are widely used in traditional medicines to lower blood glucose [19], strengthen heart muscles, promote cardiac function, regulate blood pressure [20], cleanse sores, ulcers, or wounds as an astringent [14], treat rheumatoid arthritis or cancer [21]. The plant has been reported to possess antioxidant [22], antibacterial [23], antimicrobial [24], antifungal, anti-inflammatory [14], hepatoprotective [25], antitussive [26], antipyretic, anti dysentery [27] and abnormal platelet activity [28].Several studies showed that flavonoids in T. Arjuna gave satisfactory responses antihyperglycemic, analgesic, and antioxidant function while triterpenoids supply cardio-protective activity [29, 30, 31].In the present study, we aimed to exercise manifold test batteries to assess the therapeutic efficacy of T. Arjuna against several diseases. We also aimed to perform in-silico analysis to observe an interaction between natural ligands of T. Arjuna and few targets relevant to diabetes mellitus, cardiovascular, and inflammatory diseases. Satisfactory output regarding therapeutic activity may provide such influential justification so that the scientist may go for further study to isolate the therapeutic constituents and apply more precise and accurate components to mitigate the respective disease.
2. Method and materials
2.1. Plant collection and extract preparation
The Terminalia arjuna bark was collected from the garden of the Department of Pharmacy, University of Dhaka.The specimen was then certified by the Department of Pharmacy, University of Dhaka.
The wet T. Arjuna bark was air-dried and powdered coarsely. The powdered bark was then extracted with 50% ethanol for several days. The extract was filtered after every three days. The obtained extract was dried in a rotary evaporator at low temperature and pressure. Finally, the crude residue was used to conduct required pharmacological tests.
2.2. Botanical authentication
According to the rules of our National Herbarium, we deposited the sample of each part of our plant species, and the herbarium authority took the necessary steps. But due to the sudden devastating wave of the pandemic, the authority made the institute restrict the outsiders for a long time. For all these reasons, we couldn't receive the botanical authentication (accession number) yet.
2.3. Drugs and chemicals
Alloxan and Carrageenan were bought from the sigma company, USA. Standard anti-diabetic drug Metformin, anti-inflammatory drug Ibuprofen, analgesic drug Aspirin And antidepressant drug Citalopram were obtained from Incepta Pharmaceutical Ltd. as a gift sample. Acetic acid was procured from Chemical.co.uk.
2.4. Experimental animal procurement, nursing, and grouping
Total 630 male rats weighing between (120–150) gm were purchased from Jahangirnagar University, Savar, Dhaka, Bangladesh. Each of them was kept in the Institute of Nutrition & Food Science in a well-controlled environment (temperature 25 ± 3 °C, relative humidity 55 ± 5%, and 12 h light/dark cycle) at the University of Dhaka. They were treated with a standard eating regimen and permitted admittance to cleaned water. All of the animals were kept in this environment for at least one week before the experiment. All of the experimental methods were performed according to the Institutional Animals Ethics Committee (IEAC).
Six hundred thirty rats were constantly dispensed to 63 groups of ten rats in each. In all experiments, rats were randomly picked for each group. The sample size of each group was calculated by following Eq. (1).
Initially, we took 490 rats that were constantly dispensed to 49 groups. After Completing the assessment of anti-diabetic, anti-depressant, anti-inflammatory, and analgesic activity, then we took 140 rats again, and intending to predict the mechanism of action of anti-diabetic properties of plant extract; we assessed the insulin level and Liver glycogen content of those rats after treating them with the respective specimen.
2.5. Animal model sample size detection
We applied the Power "Analysis Method" for the calculation of sample size (shown in Eq. (1)) in every single animal model study. There is a formula to carry out the calculation manually. This formula is given below [32].
| Sample size = 2 SD2 (Zα/2 + Zβ)2/d2 | (1) |
where Standard deviation = from previous studies or pilot study.
Zα/2 = Z 0.05/2 = Z 0.025 = 1.96 (From Z table) at type 1 error of 5%
Zβ = Z 0.20 = 0.842 (From Z table) at 80% power
d = effect size = difference between mean values.
Expected attrition/death of animals: We adjusted the final sample size for expected attrition. According to our previous experience, we observed that 10% of rats might die after treating them with alloxan. So we calculated the sample size by dividing the whole by 0.9 to get the actual sample size.
For the pilot study, we took five rats and used them as Diabetic Control (alloxan-induced group). According to the calculation mentioned above, we took five rats in every single pre-clinical study.
After injecting alloxan at a dose of 150 mg/kg body weight through the intraperitoneal route; we keep the rats untreated for four weeks to increase blood glucose levels significantly.
After four weeks, the mean value of blood glucose was found 24.74 mmol/L and a Standard deviation: 5.17.
According to our previous study, it can be assumed that; if the mean blood glucose level will be found 18.05 mmol/L after treatment with the extract, then it can be estimated that the plant extract can significantly reduce the elevated blood sugar level (p < 0.05).
The standard deviation of the Pilot study: 5.17.
Zα/2 = 1.96, Zβ = Z 0.20 = 0.842, d = 24.74-18.05 = 6.85.
So, Sample size = 2 SD2 (Zα/2 + Zβ)2/d2 = 2× (5.17)2× (1.96 + 0.842)2/(24.74-17.906)2 = 8.98.
Now we have to adjust the final sample size for expected attrition.
So our sample Size will be = 8.98/0.9 = 9.98. For this reason, we took ten rats [32].
Our rats were bred in the animal house during the pandemic lockdown, and the lab curator was the only one who was taking care of the animal, and we, the researcher, visited the lab twice a week. Apart from the pandemic time, we used to monitor the rat every day throughout breeding time. Most probably, some factors affecting issues regarding the pandemic situation can influence the elevation of sample size. According to our calculation, to bring about more validity in our experiment, we need to take 10 rats per group.
2.6. Dose selection for respective study
A pilot study was conducted before initiating the study. It was observed that the test extract started showing pharmacological activity at dose 500 mg/kg, indicating the minimum effective concentration (MEC) value at above 500 mg/kg. In addition, the activity was continuous with the increase of dose. Finally, when the dose was increased from 1000 mg/kg to 1200 mg/kg, the effect didn't increase much. It indicates the receptors associated with concern pharmacological activity started getting saturated at a dose of 1000 mg/kg. Also, the doses of standard drugs were selected in the same manner.
2.7. Evaluation of anti-diabetic activity
For this experiment, 140 rats were randomly picked and equally divided into fourteen groups.
| Group Number | Group Specification | Treatment species | Dose Treatment species (mg/kg) | Abbreviation of Groups |
|---|---|---|---|---|
| 1 | Negative Control | Physiological Saline | 10 ml/kg | N |
| 2 | Alloxan Control | N/A | N/A | A |
| 3 | Alloxan + Metformin | Metformin | 100 | A + M100 |
| 4 | Alloxan + Metformin | Metformin | 250 | A + M250 |
| 5 | Alloxan + Metformin | Metformin | 500 | A + M500 |
| 6 | Alloxan + Terminalia arjuna | Terminalia arjuna | 500 | A + TA500 |
| 7 | Alloxan + Terminalia arjuna | Terminalia arjuna | 750 | A + TA750 |
| 8 | Alloxan + Terminalia arjuna | Terminalia arjuna | 1000 | A + TA1000 |
| 9 | Metformin | Metformin | 100 | M100 |
| 10 | Metformin | Metformin | 250 | M250 |
| 11 | Metformin | Metformin | 500 | M500 |
| 12 | Terminalia arjuna | Terminalia arjuna | 500 | TA500 |
| 13 | Terminalia arjuna | Terminalia arjuna | 750 | TA750 |
| 14 | Terminalia arjuna | Terminalia arjuna | 1000 | TA1000 |
Alloxan was administered in the rats of the group (2–8) at a dose of 150 mg/kg through an intraperitoneal route to induce diabetes [33]. On the other hand, alloxan was not injected in the rats of groups 1 and 9–14. Later, blood glucose level was measured in all groups to check whether diabetes was induced or not. The duration of treatment was six weeks, and blood glucose level was measured once a week in the fasting condition. Both the drugs and the extracts were given orally.
2.8. Evaluation of anti-depressant activity
Overnight starved rats were chosen arbitrarily for the experiment for the administration of vehicle, standard medication, and test extract. Depression was induced in rats by infusing reserpine for 14 consecutive days at a dose of 0.2 mg/kg.
| Group Number | Group Specification | Treatment species | Dose Treatment species (mg/kg) | Abbreviation of Groups |
|---|---|---|---|---|
| 1 | Negative Control | Physiological Saline | 10 ml/kg | N |
| 2 | Reserpine | N/A | N/A | R |
| 3 | Reserpine + Citalopram | Citalopram | 5 | A + C5 |
| 4 | Reserpine + Citalopram | Citalopram | 10 | A + C10 |
| 5 | Reserpine + Citalopram | Citalopram | 15 | A + C15 |
| 6 | Reserpine + Terminalia arjuna | Terminalia arjuna | 500 | A + TA500 |
| 7 | Reserpine + Terminalia arjuna | Terminalia arjuna | 750 | A + TA750 |
| 8 | Reserpine + Terminalia arjuna | Terminalia arjuna | 1000 | A + TA1000 |
| 9 | Citalopram | Citalopram | 5 | C5 |
| 10 | Citalopram | Citalopram | 10 | C10 |
| 11 | Citalopram | Citalopram | 15 | C15 |
| 12 | Terminalia arjuna | Terminalia arjuna | 250 | TA500 |
| 13 | Terminalia arjuna | Terminalia arjuna | 500 | TA750 |
| 14 | Terminalia arjuna | Terminalia arjuna | 750 | TA1000 |
2.9. Tail suspension test
A discernible behavioral model declared as the tail suspension test is generally operated to appraise anti-depressant corollary in rats [34]. Each rat's immobilizing condition was independently perceived by suspending the body base up position noticeable in the air where tail on a wire adjusted utilizing tacky tape. After early fierce, combative movement to get away from the unpleasant position, the rodent hangs latently and out peaceful by abating to twist and curl. A thorough manual process of around 6 min is used to track the whole episode of immobility. The reduced unmoving period was checked after 21 days of anti-depressant implementation.
2.10. Sucrose preference test
The depression states in rats can be assessed by a basic sucrose preference test (SPT) in which rats have access to water and the solution sweetened with sucrose, and the preference rates were then analyzed. Notwithstanding setting up and completing SPT, a reasonable trigger to actuate depression is required. After seven days of reserpine-induced depression, all rats were given 1 h of admittance to one container of 1% (w/v) sucrose solution and one container of standard water. Each tube is weighed before and after the test. This test quantifies the amount of sweet-tasting sucrose that the rats ingest across a fixed period. In the SPT, disorders of reward behavior are characterized as a decrease in sucrose preference ratio obtained during the preference test, compared with a control group. The reclamation of sucrose preference was uncovered after 21 days of anti-depressant usage.
2.11. Evaluation of anti-inflammatory activity
Carrageenan was used to induce inflammation in the rodents to examine the anti-inflammatory activity of the reference drug and the extract of T. Arjuna. The percentage of inhibition of inflammatory mediation was measured by collecting rat's paw before and after injection shown in Eq. (2).
| Group Number | Group Specification | Treatment species | Dose Treatment species (mg/kg) | Abbreviation of Groups |
|---|---|---|---|---|
| 1 | Negative Control | Physiological Saline | 10 ml/kg | N |
| 2 | Carrageenan Control | N/A | N/A | Car |
| 3 | Carrageenan + Ibuprofen | Ibuprofen | 10 | Car + Ib10 |
| 4 | Carrageenan + Ibuprofen | Ibuprofen | 20 | Car + Ib20 |
| 5 | Carrageenan + Ibuprofen | Ibuprofen | 25 | Car + Ib25 |
| 6 | Carrageenan + Terminalia arjuna | Terminalia arjuna | 500 | Car + TA500 |
| 7 | Carrageenan + Terminalia arjuna | Terminalia arjuna | 750 | Car + TA750 |
| 8 | Carrageenan + Terminalia arjuna | Terminalia arjuna | 1000 | Car + TA1000 |
| 9 | Ibuprofen | Ibuprofen | 10 | Ib10 |
| 10 | Ibuprofen | Ibuprofen | 20 | Ib20 |
| 11 | Ibuprofen | Ibuprofen | 25 | Ib25 |
| 12 | Terminalia arjuna | Terminalia arjuna | 500 | TA500 |
| 13 | Terminalia arjuna | Terminalia arjuna | 750 | TA750 |
| 14 | Terminalia arjuna | Terminalia arjuna | 1000 | TA1000 |
2.12. Carrageenan-induced acute inflammatory model
The habitually utilized technique for deciding the effectiveness of anti-inflammatory agent is Carrageenan prompted rodent paw edema to examine [35, 36]. A special kind of instrument called a plethysmometer was utilized to perform the anti-inflammatory test. Initially, the Paw volume of each rodent was taken. After 30 min of the administration of various doses of the test drug and extracts, 1% of the freshly formulated carrageenan solution was infused into the sub plantar tissue of the left rear paw rat at a dose of 0.1 mL per 100 g body weight to incited edema. Estimating the paw volume was carried out at 0, 20, 40, 60, 80, 100, 120, 140, 160, 180 min after infusion of Carrageenan utilizing a plethysmometer. The rate of the hindrance of edema was then determined using the accompanying formula [35, 36].
| (2) |
Here.
Vt = volume of animals' paw after injection.
V0 = volume of animals' paw before injection.
2.13. Evaluation of analgesics activity
The rodent is stimulated with pain through the acetic acid-induced writhing test and tail-flick method. Percent of writhes, one of the parameters to demonstrate analgesic activity was calculated by following Eq. (3).
| Group Number | Group Specification | Treatment species | Dose Treatment species (mg/kg) | Abbreviation of Groups |
|---|---|---|---|---|
| 1 | Acetic Acid Control | Physiological Saline | 10 ml/kg | Ace |
| 2 | Acetic Acid + Aspirin | Aspirin | 100 | As100 + Acetic Acid |
| 3 | Acetic Acid + Aspirin | Aspirin | 150 | As150 + Acetic Acid |
| 4 | Acetic Acid + Aspirin | Aspirin | 200 | As200 + Acetic Acid |
| 5 | Acetic Acid + Terminalia arjuna | Terminalia arjuna | 500 | TA500 + Acetic Acid |
| 6 | Acetic Acid + Terminalia arjuna | Terminalia arjuna | 750 | TA750 + Acetic Acid |
| 7 | Acetic Acid + Terminalia arjuna | Terminalia arjuna | 1000 | TA1000 + Acetic Acid |
2.13.1. Acetic acid-induced writhing test
Writhing test intervened by acidic acid was utilized to identify peripheral analgesic activity [37, 38]. Plant extract and test drug (Aspirin) was administered 30 min before the intraperitoneal conveyance of acidic acid. The intraperitoneal injection of 1% acidic acid (10 ml/kg) was offered to ascend to the writhes in rats. The number of writhes (muscular contraction ions) was accounted for across a time of 20 min, beginning at 5 min after infusion of acidic acid. Comparing the number of writhes of each group with the control group, the percent decrease of writhes counts was resolved demonstrated as follows [38]:
| (3) |
where = the mean number of the writhing of each test group.
= The mean number of the writhing of acetic acid control groups.
The analgesic activity of the extract is then also assessed via the "Tail Flick Method" on the same experiment rat model after giving a break for seven days. The effect of injected acetic was terminated by this time.
| Group Number | Group Specification | Treatment species | Dose Treatment species (mg/kg) | Abbreviation of Groups |
|---|---|---|---|---|
| 1 | Tail Flick Stress (control) | Physiological Saline | 10 ml/kg | TFS |
| 2 | Aspirin + Tail Flick Stress | Aspirin | 100 | As100 + TFS |
| 3 | Aspirin + Tail Flick Stress | Aspirin | 150 | As150 + TFS |
| 4 | Aspirin + Tail Flick Stress | Aspirin | 200 | As200 + TFS |
| 5 | Terminalia arjuna + Tail Flick Stress | Terminalia arjuna | 500 | TA500 + TFS |
| 6 | Terminalia arjuna + Tail Flick Stress | Terminalia arjuna | 750 | TA750 + TFS |
| 7 | Terminalia arjuna + Tail Flick Stress | Terminalia arjuna | 1000 | TA1000 + TFS |
2.13.2. Tail flick method
An accessible method familiar as the tail-flick assay portrayed by Love and Smith, 1941 [39] is utilized to appraising assessing pain-relieving personal behavior patterns, making slight variety simultaneously. Operating a radiant heat programmed tail-flick analgesia meter (UGO BASILE®, Germany), response latencies of the rats are estimated. The nichrome wire of the device was warmed to a suitable temperature and kept up with the assistance of heat controllers, where a consistent current of 4 Amps is coursed through the exposed nichrome. Here, radiant heat is given to the tail of the mice 5 cm away from the tip of the tail to provoke discomfort. Response time has been documented for control rats or animals treating with a test medication and plant extract. The test was dispatched at 0, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h after the use of the test substance. Once more, the rats were noticed for contemplating remaining activity following 24 h.
2.14. Statistical analysis
All our findings (raw data) belong to several groups regarding numerous research parameters recorded and analyzed on a broadsheet using MS excel program. Data were subjected to descriptive statistics, and results were represented as mean ± SD. We employed the "One Way Anova Test" of SPSS 16″ software for interpreting the inter-group heterogeneity in terms of diverse biological parameters to determine the statistical significance. We consider the events as statistically significant while the 'p' value was detected as less than 0.05 (p < 0.5).
2.15. Experimental guideline
All experiments were performed according to the ethical standards laid down in the Declaration of Helsinki 2013. Animals were handled and treated according to the principles of the Swiss Academy of Medical Sciences and Swiss Academy of Sciences. Animals were euthanized according to the Guidelines for the Euthanasia of Animals: 2013 edition.
2.16. Ethical approval for this study
We obtained ethical approval from the Research Ethics Committee of the Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Bangladesh. All of the experiments of this study were done under their supervision.
3. Results
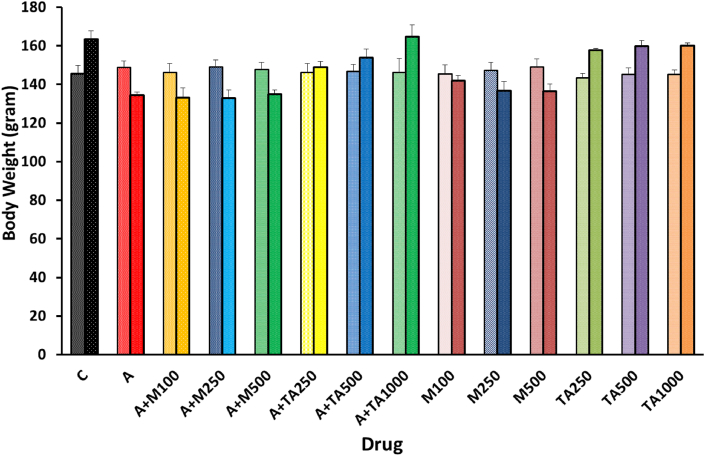
A significant elevation of body weight was observed after administering T. arjuna extract in alloxan-induced diabetic rats [Figure 1]. In the negative control group, an escalation in body weight was obtained at the final stage. However, alloxan treatment prompted a decrease in terminal body weight in the positive control group. Though groups receiving metformin in low dose, medium dose, and high dose following alloxan induction imitated the weight-reducing pattern as displayed by the positive control group, a reversal of Alloxan mediated bodyweight decline was reported upon treatment with different doses of T. arjuna extract. Groups fed with only plant extract followed a similar trend as the negative control group, whereas a contrasting scenario was observed in groups given metformin only.
Figure 1.
Body weight of rats of 14 groups before and after completing the experiment in diabetic rats.
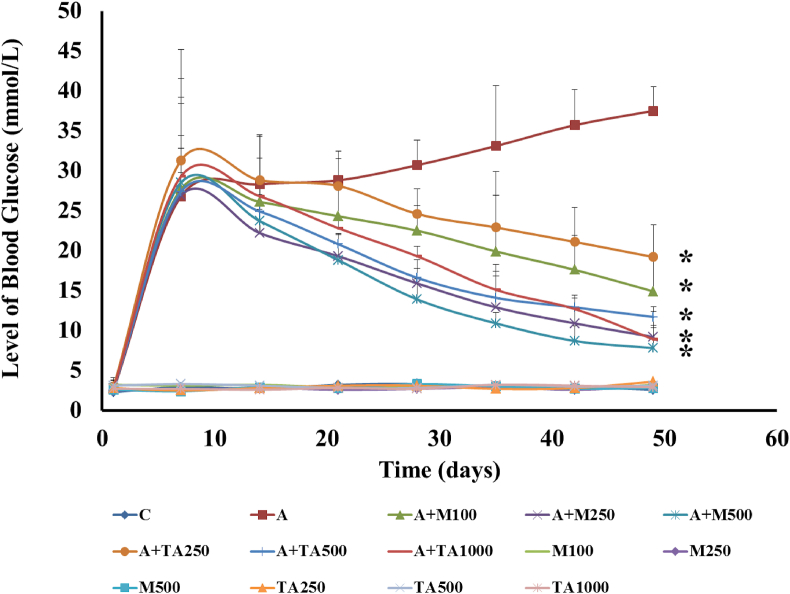
The difference between the positive control and negative control curves demonstrated statistically significant data (p ≤ 0.05) [Figure 2]. In the former case, the curve was a straight line, while the curve for the Alloxan-induced group was found to be higher than the normal level representing a hyperglycemic condition.
Figure 2.
The blood glucose level (mmol/dl) of rats of 14 groups after receiving 42 days of respective treatments. The data were expressed as mean ± standard deviation. (∗indicates statistically significant change).
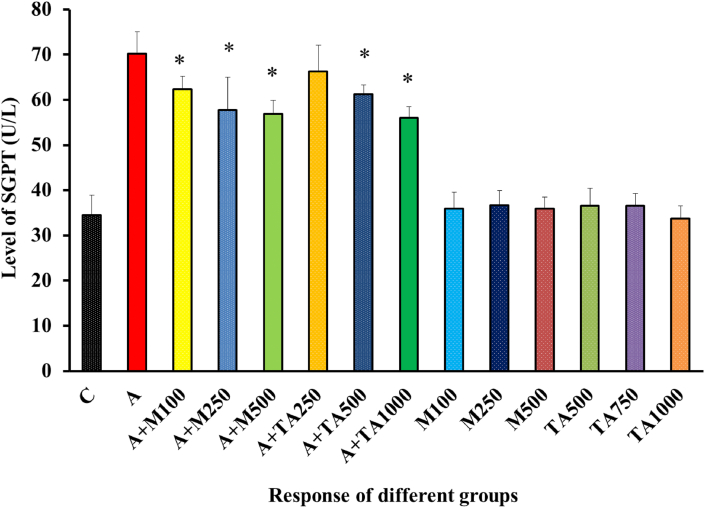
The SGPT level of the alloxan-treated group was increased to a higher level than that of the negative control [Figure 3].
Figure 3.
SGPT (U/L) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
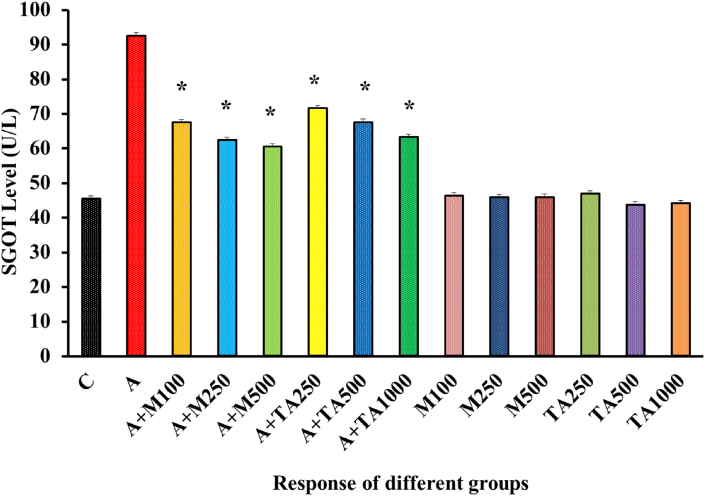
A striking contrast was seen in the SGOT level of positive control and negative control group [Figure 4]. SGOT levels were negotiably higher for T. Arjuna treated diabetic rats in comparison to metformin-treated diabetic rats.
Figure 4.
SGOT (U/L) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
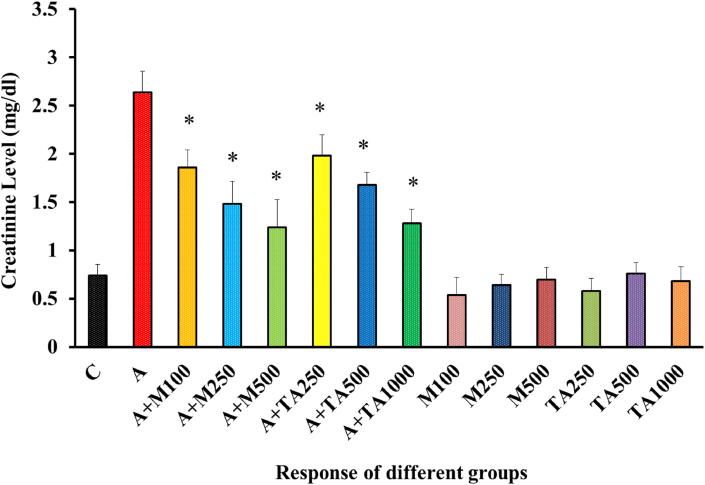
Both drug and extracts showed statistical significance and ensured the effectiveness of T. arjuna extract [Figure 5]. No significant deviation could be spotted from the negative control group in the creatinine level yielded by the remaining six non-alloxan induced groups.
Figure 5.
Creatinine (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
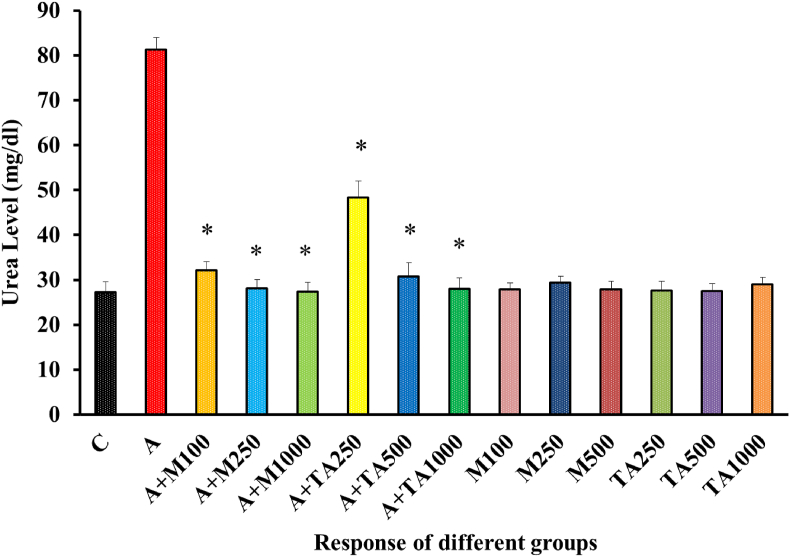
A large increase in the urea level was displayed by the positive control group, which was in stark contrast with the negative control group [Figure 6].
Figure 6.
Urea (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
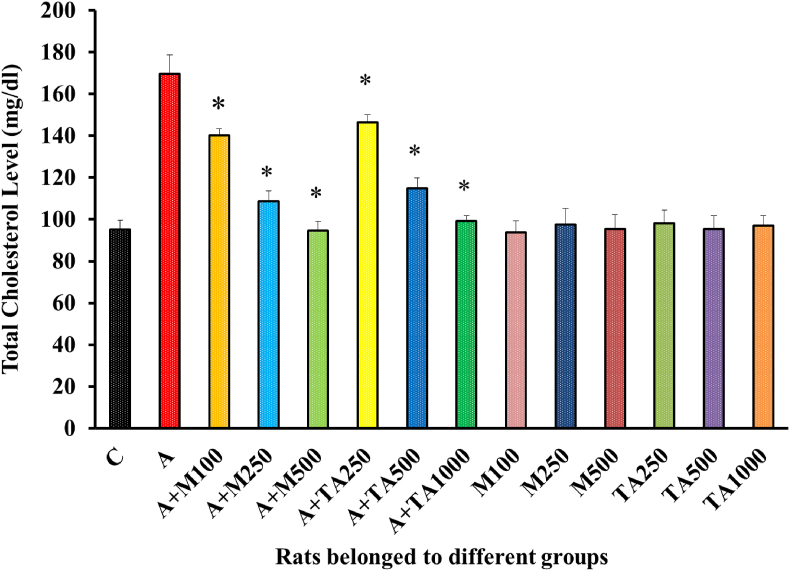
Supplementation of Alloxan successfully elevated cholesterol levels in all groups except the first one (negative control group) [Figure 7]. A dose-dependent fall in total cholesterol level was noted following the treatment of diabetic rats with both metformin and plant extract.
Figure 7.
Total Cholesterol (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
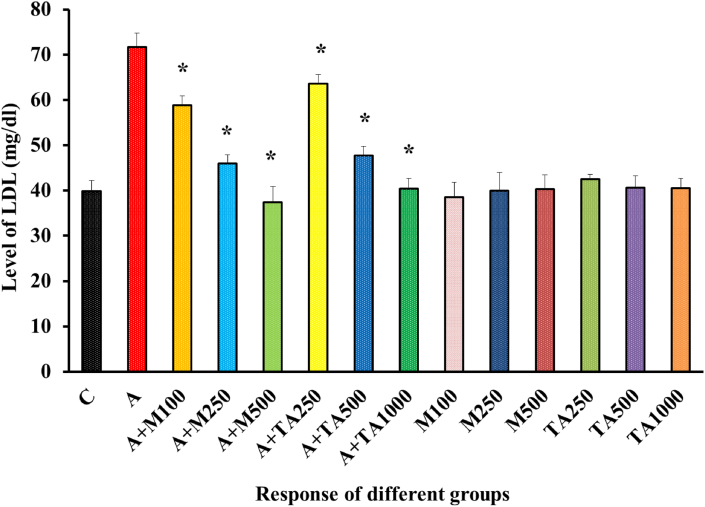
In stark contrast with that of the negative control group, the positive control group was yielding a higher level of LDL following alloxan administration [Figure 8].Treatment of diabetic rats with either metformin or T. arjuna extract in differing doses effectively reversed alloxan mediated rise in LDL level to normal.
Figure 8.
LDL (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
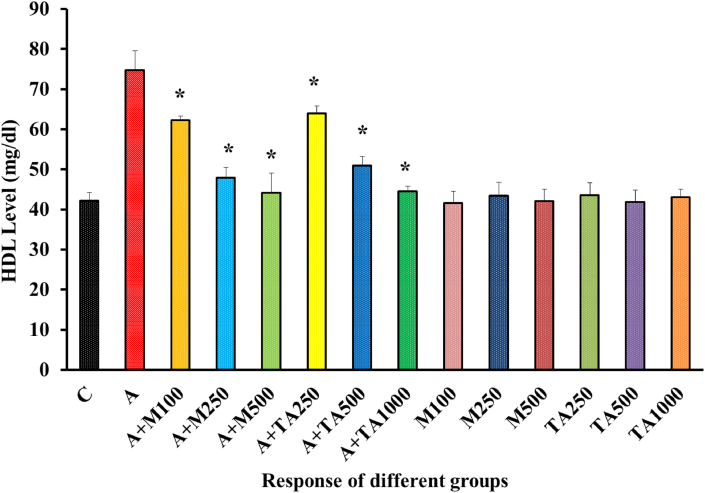
Induction of diabetes lowered the HDL level like plasma periostin [40] in the diabetic specimen. T. arjuna extract successfully increased the HDL level in a dose-dependent manner [Figure 9].
Figure 9.
HDL (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
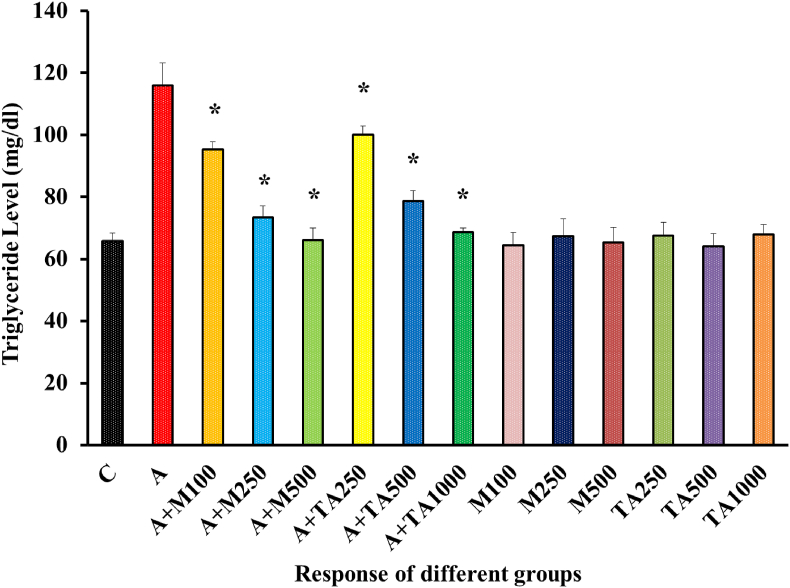
A noticeable difference has been observed in the triglyceride level of the positive and the negative control groups, as evident from the elevated triglyceride level after rats were subjected to alloxan induction [Figure 10].
Figure 10.
Triglyceride (mg/dl) Level of rats from 14 groups. The data were expressed as mean ± standard deviation. (∗ indicates statistically significant change).
It has been observed that level of insulin was increased significantly (p < 0.05) in diabetic rats after a six weeks treatment with extract in 3 different doses. In contrast, no significant change was found in the insulin level of rats that belonged to other groups. Also, the liver glycogen content was increased significantly in the same groups and non-significant change.
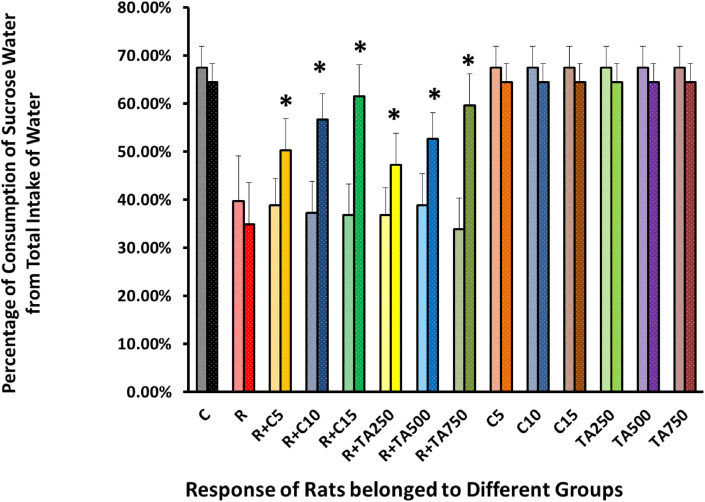
In the sucrose preference test, rats of the positive control group have demonstrated a substantial decline in the desire for sucrose, which indicates the depressive state of rodents [Figure 11].
Figure 11.
Comparison of Sucrose water intake before the treatment and after administering the drug/extract in rats belonged to 14 groups.
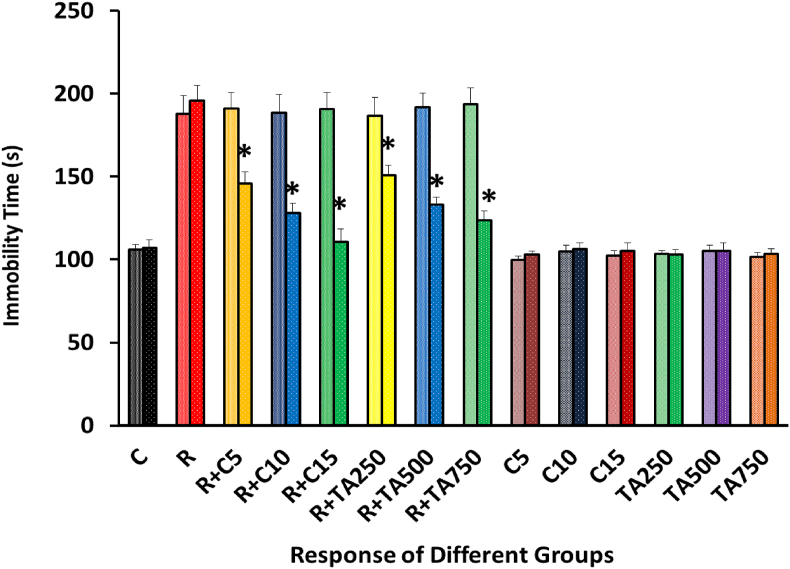
In the tail suspension test, a significant statistical difference was detected in the treatment groups after administering test extract and the standard drug, as the rate of immobility was found to be decreased [Figure 12].
Figure 12.
Comparison of Immobility time in seconds belonged to 14 groups before the treatment and after administering the drug or extract in rats.
3.1. Anti-inflammatory response
A statistically significant difference was observed in edema conditions between the positive control group and treatment groups. Like the standard drug, T. arjuna extract significantly reduces edema formation in low, medium, and high doses [Table 1].
Table 1.
Assessment of anti-inflammatory activity of Terminalia arjuna extract and Ibuprofen through paw edema test in a rat model (∗ presents the level of significance of result). Here, the percentage of inhibition of inflammatory mediation was measured by collecting rat's paw before and after injection shown in Eq. (2).
| Group | Time |
||||
|---|---|---|---|---|---|
| 0 Minute (Just before carrageenan injection) | 1 hour (just before treatment) | 2 Hours | 3 hours | 4 hours | |
| N.C | 116.28 ± 2.68 | 116.24 ± 2.49 | 116.28 ± 2.68 | 116.28 ± 2.68 | 116.28 ± 2.68 |
| Car | 117.28 ± 5.05 | 145.06 ± 3.67 | 159.82 ± 4.98 | 171.38 ± 2.21 | 184.54 ± 4.83 |
| Car + Ib10 | 114.9 ± 1.86 | 143.5 ± 4.14 | 132.44 ± 13.3∗∗ (17.1%) | 122.08 ± 2.21∗∗ (28.77%) | 118.32 ± 6.10∗∗ (35.88%) |
| Car + Ib20 | 119.54 ± 3.51 | 151.62 ± 4.77 | 127.48 ± 4.58∗∗ (20.23%) | 120.12 ± 5.82∗∗ (29.9%) | 117.02 ± 5.96∗∗ (36.59%) |
| Car + Ib25 | 114.2 ± 4.17 | 150.76 ± 3.55 | 122.16 ± 7.14∗∗ (23.56%) | 117.02 ± 4.73∗∗ (31.72%) | 114.42 ± 4.17∗∗ 37.99% |
| Car + TA500 | 117.86 ± 5.15 | 142.8 ± 4.92 | 136.5 ± 2.56∗∗ (14.59%) | 126.34 ± 3.58∗∗ (26.28%) | 122.98 ± 3.62∗∗ (33.36%) |
| Car + TA750 | 109.98 ± 3.42 | 148 ± 2.98 | 131.26 ± 2.19∗∗ (17.87%) | 124.36 ± 2.19∗∗ 27.44% |
116.16 ± 3.85∗∗ 37.05% |
| Car + TA1000 | 111 ± 2.09 | 146.92 ± 4.16 | 122.96 ± 2.89∗∗ (23.06%) | 117.56 ± 2.25∗∗ (31.40%) | 112.68 ± 2.97∗∗ (39.26%) |
| Ib10 | 114.9.±1.26 | 116.2 ± 2.33 | 114.55 ± 1.45 | 114.7.28 ± 2.05 | 114.77 ± 1.58 |
| Ib20 | 118.6.±3.1 | 117.8 ± 2.8 | 117.8. ±2.62 | 117.8 ± 2.64 | 116.9 ± 2.03 |
| Ib25 | 115.53 ± 2.3 | 114.9 ± 2.5 | 115.7 ± 2.35 | 115.5 ± 2.29 | 115.49 ± 2.52 |
| TA500 | 118.7 ± 2.66 | 117.9 ± 2.0 | 118.4 ± 3.2 | 118.55 ± 2.25 | 118.6 ± 2.61 |
| TA750 | 113.59 ± 2.4 | 113.59 ± 2.6 | 113.48 ± 1.94 | 114.33 ± 2.24 | 114.24 ± 2.23 |
| TA1000 | 113.34 ± 2.2 | 113,29 ± 1.35 | 113.44 ± 2.1 | 112.99 ± 1.79 | 113.54 ± 2.10 |
3.2. Analgesic activity of Terminalia arjuna
T arjuna extract was observed to reduce the abdominal contraction induced by acetic acid but in a dose-dependent manner [Table 2]. Low dose of extract reduced the pain, but medium and high doses cause a significant reduction in pain, whereas Aspirin reduced the pain significantly in low, medium, and high doses.
Table 2.
An evaluation of the Analgesic effect of different doses of Terminalia arjuna and Aspirin by acetic acid writhing test (∗presents the level of significance of result). Here, the percent of writhes, one of the parameter to demonstrate analgesic activity was calculated by following Eq. (3).
| Group specification | Dose | Number of writhing | % Inhibition |
|---|---|---|---|
| Ace | 94.62 | ||
| As100 + Acetic Acid | Low | 69.84∗∗ | 26.19% |
| As150 + Acetic Acid | Medium | 50.4∗∗ | 46.73% |
| As200 + Acetic Acid | High | 33.94∗∗ | 64.13% |
| TA500 + Acetic Acid | Low | 82.08∗ | 13.25% |
| TA750 + Acetic Acid | Medium | 57.88∗∗ | 38.82% |
| TA1000 + Acetic Acid | High | 37.14∗∗ | 60.75% |
A high dose of T. arjuna produced statistically significant data (P < 0.01) after 45 min than the low and medium dose of the extract (P < 0.05). On the contrary, the standard drug produced high significant data in both medium and high doses [Table 3].
Table 3.
An evaluation of the analgesic activity of Terminalia arjuna and Aspirin by the tail-flick test method.
| Group No | Group Specification | Basal Reaction | Reaction time in second |
|||
|---|---|---|---|---|---|---|
| After 15 minutes | After 30 minutes | After 45 minutes | After 60 Minutes | |||
| 1 | C | 2.96 ± 0.76 | 3.2 ± 0.88 | 3.46 ± 1.28 | 4.26 ± 0.60 | 5.12 ± 0.72 |
| 2 | As100 + TFS | 3.4 ± 1.05 | 3.76 ± 1.02 | 4.88 ± 0.72 | 5.48 ± 0.53∗ | 6.56 ± 0.97∗ |
| 3 | As150 + TFS | 3.78 ± 0.65 | 4.36 ± 1.04 | 5.46 ± 0.54∗∗ | 7.02 ± 1.15∗∗ | 7.98 ± 1.05∗∗ |
| 4 | As200 + TFS | 4.1 ± 0.53 | 4.5 ± 0.97 | 6.28 ± 0.66∗∗ | 8.04 ± 0.78∗∗ | 10.32 ± 0.51∗∗ |
| 5 | TA500 + TFS | 3.18 ± 1.20 | 3.44 ± 1.00 | 3.84 ± 0.79 | 5.4 ± 1.32 | 6.72 ± 1.73 |
| 6 | TA750 + TFS | 3.52 ± -.78 | 3.96 ± 0.80 | 5.22 ± 0.92∗ | 5.98 ± 1.30∗ | 7.02 ± 1.28∗ |
| 7 | TA1000 + TFS | 3.64 ± 0.55 | 4.084 ± 0.74 | 5.76 ± 1.04∗ | 7.7 ± 0.51∗∗ | 9.3 ± 1.11∗∗ |
4. Discussion
4.1. Anti-diabetic activity of Terminalia arjuna
Diabetes is one of the major health problems that affect the major population worldwide. A wide range of tests is available to assess diabetes in the rat model. Due to the relative affordability and availability, alloxan is being used widely for inducing DM in an animal model [41, 42, 43]. The anti-diabetic, anti-inflammatory, analgesic, and anti-depressant activity of T. arjuna were evaluated in this study.
In Figure 1, a significant elevation of body weight was observed after administering T. arjuna extract in alloxan-induced diabetic rats. A similar result was observed after administering Aloe megalacantha Baker extract, Sigesbeckiaorientalis, Panicum maximum, Anacardium occidentale L, Ricinuscommunis, Chloroxylonswietenia, Perseaamericana, and Tithoniadiversifolia [44, 45, 46, 47, 48, 49] but, T. arjuna extract was observed to show a better result than Calpurnia aurea leaves [50].
In Figure 2 Blood glucose levels dropped significantly following the treatment of the alloxan-induced rats with T. Arjuna extract in low, medium, and high doses. Groups treated with metformin following induction of diabetes exerted a similar behavioral pattern like the (alloxan + T. Arjuna extract) treatment group revealing a gradual decline in blood glucose level. Metformin was shown to exert better results than the test extract at the same dose, as test extract is assumed to contain less amount of active compound responsible for reducing blood glucose level. The reducing pattern is also different in different doses of test extract. As we can see in the graph, a high dose (1000 mg) results in a drastic fall in blood glucose level, where a low dose (250 mg) causes a gradual decrease. Administration of non-alloxan induced groups with only plant extract or only drug did not result in marked fluctuation compared to the negative control group as all the curves were found to be overlapped with the negative control curve, declaring the fact that neither the drug nor the plant is associated with any hazardous effects. Similar effects were observed in the case of Calpurnia aurea leaves, Zizyphus mauritiana, Aloe megalacantha bark extract Sigesbeckia orientalis. Panicum maximum, Anacardium Occidentale L, Ricinus communis, Chloroxylon swietenia, persea Americana, Catharentus roseus, Eucalyptus globulus, Sargassum longiotom, Streblus asper and Sedum adenotrichum [3, 39, 41, 42, 43, 45, 47, 48, 49, 50, 51, 52].
In Figure 3, a dose-related lowering of SGPT level was demonstrated upon treatment of the alloxan-induced rats with metformin or plant extract though the lowering effect was slightly greater in the case of metformin as compared to the plant. The SGPT levels of the other six non-alloxan treated groups didn't deviate much when we made a comparison with the negative control group nullifying any possibility of severe effects to occur upon ingestion of metformin or the plant. Similar results were obtained in the case of Anacardium occidentale L, Sargassum longiotom, Tithonia diversifolia, and Streblus asper [49, 52, 53, 54].
In Figure 4, both the treatment groups produced a reversal of alloxan mediated rise in the SGOT level, exhibiting a gradual decline at low, medium, and high doses of metformin and plant extract individually. The rest of the groups treated individually with only metformin or only plant extract demonstrated no significant fluctuation with the negative control group in SGOT values. Therefore, it could be concluded that metformin or the plant appeared to be free from any significant detrimental effects. Similar results were also obtained after the administration of Anacardium occidentale L, Sargassum longiotom, Tithonia diversifolia and Streblus asper [49, 52, 53, 54].
In Figure 5, upon alloxan administration, the creatinine level was increased to an elevated position than that of the negative control. The introduction of metformin in low, medium, and high doses to alloxan-induced rats effectively lowered creatinine levels gradually. Though groups treated with plant extract exhibited a similar trend in declining the creatinine level, the effects were slightly weaker than metformin. Similar effects were obtained in the case of Zizyphusmauritiana, Anacardiumoccidentale L., Ricinuscommunis, Chloroxylonswietenia, Perseaamericana, Catharentusroseus, Eucalyptus globulus, and Sedum adenotrichum [3, 47, 48, 52, 55, 56].
In Figure 6, treatment with various doses of metformin following alloxan administration culminated in a huge drop in urea level. The other three treatment groups who received plant extract in three different doses also opposed the alloxan-mediated elevation of urea level. However, the reversal caused by plant extract appeared to be slightly less potent than metformin. The urea level values noted from the rest of the groups fed with only metformin or only plant extract were much closer to that of the negative control group, evidencing no severe effects when metformin or plant extract is administered. Similar effects were observed in the case of Zizyphus mauritiana, Anacardium occidentale L, Richinus communis, Persea americana, Catharentus roseus, Eucalyptus globulus, and Sedum adenotrichum [45, 46, 47].
In Figure 7, metformin and plant extract treatment groups exerted almost the same pattern of response. Still, if a comparison is drawn between the two groups, a slightly better outcome was found in groups fed with metformin. Besides, better result was observed in the high dose of drugs in case of both treatment groups. The total cholesterol values yielded by the remaining non-alloxan induced groups were reported to be lying close to that shown by the negative control group nullifying the possibility of occurrence of prominent side effects upon administration of either the drug or the plant. Similar effects were observed in the case of Zizyphusmauritiana, Sigesbeckiaorientalis, Anacardium occidentale L, Chloroxylonswietenia, Perseaamericana, Catharentusroseus, Eucalyptus globulus, Sargassumlongiotom, Streblusasper, and Sedum adenotrichum [3, 45, 47, 48, 50, 53, 55, 56] but, T. arjuna extract was observed to be more potent than Calpurnia aurea leaves [49].
In Figure 8, the lowering of LDL was slightly more pronounced in metformin-fed groups compared to groups receiving plant extract. The rest of the groups receiving only metformin or only plant extract exhibited LDL values closer to the negative control group discarding any significant possibility of lethal effects to occur following the ingestion of metformin or plant. Similar effects were observed in the case of Zizyphusmauritiana, Sigesbeckiaorientalis, Anacardium occidentale L., Chloroxylonswietenia, Streblusasper, and Sargassumlongiotom [44, 47, 49, 52, 54, 55]. A similar effect was obtained from Calpurnia aurea leaves, Aloe megalacantha extract, Forsythia suspense, and Cynodon dactylon, etc [44, 50, 51, 57]. In Figure 10, a comparison between the positive control group and the groups fed with metformin or the plant extract displayed a contrasting behavior as both treatment groups could effectively reverse the rise of triglyceride level upon alloxan induction towards normal values. The triglyceride level demonstrated by the rest of the non-alloxan fed groups didn't fluctuate much from the triglyceride value of the negative control group. Therefore, it could be discerned that neither metformin nor the plant has any massive association with lethality. Similar effects were observed in the case of Ziziphus mauritiana, Aloe megalacantha, Sigesbeckia orientalis, Anacardium occidentale L, Chloroxylon swietenia, Persea Americana, Sargassum longbottom and Sedum adenotrichum [39, 41, 47, 48, 49, 50, 51].
In the assessment of insulin level, it has been evidenced that the plant extract may significantly increase the insulin level. Consequently, the liver glycogen content was also increased significantly in the diabetic groups after treating with plant extract. Both these phenomena may conclude that the treatment of plant extract may increase the secretion of insulin.
In our study, it has been observed that blood sugar levels were not declined due to diminished intake of food. The destructive effects of alloxan cause cellular atrophy and initially reduce the body weight in all alloxan-treated rats. On the contrary, after treating the rat with extract (both diabetic and non-diabetic), the body weight of rats was increased as like as rats belong to other groups. So the mechanism of action of anti-diabetic activity may not associate with loss of appetite. Apart from that, serum insulin level was significantly increased in groups 6,7, and 8, who were alloxan-induced diabetic rats and treated with T. Arjuna extract. In contrast, the level of insulin was increased and decreased non-significantly in the rest of the other groups. This interpretation confers that the extract may affect the pancreatic beta-cell for the secretion of insulin. Also, the hepatic glycogen content increased significantly in rats that belonged to groups 6, 7, 8, and possibly increase hepatic glycogen content is a phenomenon of increased insulin level. Conversely, in another study, it has been found that the plant extract did not increase insulin secretion [58]. In another study, it has been observed that T. arjuna helps to increase glucose uptake and carbohydrate breakdown. T. arjuna possesses different types of compounds like flavonoids, tri-terpenoids that possess profound affinity toward alpha-amylase and alpha-glucosidase. This compound has synergistic binding effects toward those receptors. Again, Insulin sensitivity, increased rate of glucose digestion, stimulation of glucose uptake by peripheral tissue could also be possible mechanisms. We could not evaluate the effect of the extract in glucose absorption in the intestine, which can be assessed by using Rat Everted Jejunal sacs. As unfortunately, we were not provided required technical support required for this test. Furthermore, diabetogenic alloxan causes the generation of free radicals that leads to tissue injury, and anti-oxidant constituents may work as free radical scavenging agents and impart its activity against lipid peroxidation, OH•, and O2•−. By doing so, it may cause the decline of concentration of free radical and may diminish the rate of damage. According to our findings and assessment, it may confer that the possible mechanism of action by which our extract imparts its anti-diabetic activity is the increase of insulin secretion. But the further meticulous study is needed to justify all possible MoA so that the authenticity will be established [58, 59, 60]. There are two most common tests - sucrose preference test and tail suspension test were implemented to assess the anti-depressant activity of T. arjuna.
4.2. Anti-depressant activity
In Figure 11, in the sucrose preference tests, the treatment groups exhibit a similar pattern of sucrose intake as the positive control group in the pre-treatment condition, but a statistically significant improvement was identified during treatment. The treatment groups have demonstrated an improved preference for sucrose in the post-treatment state, suggesting the extract's effectiveness to treat the disease condition. A difference was observed between the effects of treatment groups of T. arjuna extract and standard drug, but that was statistically nonsignificant. T. arjuna extracts have therefore demonstrated a positive effect in reducing anhedonia-like symptoms in the test. Taurine and Angelica sinensis were observed to exert a similar effect as the test extract [57, 61].
Figure 12, in the tail suspension test, in the time of immobility between the negative and the positive control groups, a dramatic differential was found, indicating the development of depression in the positive control group. But the standard drug exerted better results to reduce immobility than the test extract though the difference was nonsignificant. We found that the treatment groups exerted a shortened time of immobility closer to the negative control group but in a dose-dependent manner. If we equate the treatment group's pre-treatment and post-treatment status, the previous data would still be upheld. From the above information, we can therefore conclude that the test extract was found to reduce the depression level of rodents in the TST efficiently. A similar result was obtained in the case of Eclipta alba, Cyperusrotundus L, Channastriatus, methanol extract of Micromeriamyrtifolia, and Glycyrrhizin in TST [6, 62, 63, 64].
It's very difficult to explain the mechanism of the antidepressant activity of the plant. Because it is not well identified that whether a single compound or a synergism of compounds are exerting anti-depressant activity. But it may confer that one or more constituents of extract may induce anti-depressant activity by working as a serotonin reuptake inhibitor. Also, MAO-A inhibition can be considered as a potential mechanism of the anti-depressant activity of Terminalia arjuna [65].
4.3. Anti-inflammatory response
Several methods are available for measuring inflammation, but the most common method relies on the edema formation in rat paws [66]. Paw edema in rats induced by carrageenan is a standard experimental measurement (shown in Eq. (2)) that develops in only a few hours and is related directly to the release of kinins, prostaglandins, histamine, and serotonin [63, 64, 65, 66]. In Table 4, the standard drug, T. arjuna extract, significantly reduces edema formation in low, medium, and high doses. This reduction indicates the effectiveness of T. arjuna extracts to treat inflammation in the rat model. T. arjuna exerted better results than Caesalpiniabonducella [37]. On the contrary, Astragalushamosus, Arum palaestinum, and Inula cuspidate, Murrayakoenigii Linn gave a similar effect as T. arjuna [67, 68, 69, 70].
Table 4.
An Evaluation of Insulin level (ng/ml) and Hepatic Glucose (mg/gm) Content are displayed below.
| Group | Insulin Level (ng/ml) | Hepatic Glucose Content (mg/gm) |
|---|---|---|
| N | 0.728 ± 0.034 | 1.371 ± 0.48 |
| A | 0.564 ± 0.031 | 1.12 ± 0.050 |
| A + M100 | 0.586 ± 0.025 | 1.151 ± 0.042 |
| A + M250 | 0.564 ± 0.030 | 1.177 ± 0.055 |
| A + M500 | 0.582 ± 0.017 | 1.193 ± 0.040 |
| A + TA500 | 0.646 ± 0.036∗ | 1.24 ± 0.044∗ |
| A + TA750 | 0.674 ± 0.038∗ | 1.28 ± 0.033∗ |
| A + TA1000 | 0.695 ± 0.047∗ | 1.328 ± 0.020∗ |
| M100 | 0.737 ± 0.031 | 1.362 ± 0.060 |
| M250 | 0.727 ± 0.049 | 1.38 ± 0.033 |
| M500 | 0.731 ± 0.047 | 1.415 ± 0.028 |
| TA500 | 0.732 ± 0.042 | 1.382 ± 0.0399 |
| TA750 | 0.726 ± 0.041 | 1.374 ± 0.045 |
| TA1000 | 0.73 ± 0.037 | 1.378 ± 0.050 |
Accordingly, in our study, it has been found that Terminalia arjuna has started imparting its anti-inflammatory activity within 1 h after injecting Carrageenan and Carrageenan edema is a multimedia phenomenon that liberates diverse types of mediators. The first phase (1 h) is associated with the release of histamine and serotonin. Besides, the second phase (over 1 h) is mediated through prostaglandins, the cyclooxygenase products. Also, the continuity between the two phases is provided by kinins. Also, the mechanisms of anti-inflammatory activity would be associated with the antiphlogistic activity imparted by tannins.
So our extract may start exerting its anti-inflammatory activity by inhibiting the serotonin and histamine. However, if the extract reduces the level of serotonin, then the rats may exhibit depressant activity. Instead, the extract showed anti-depressant activity. So it may confer that the extract may induce anti-inflammatory activity by inhibiting the pharmacological activity of histamine. Furthermore, the extract contains flavonoids, and flavonoids can inhibit the production of prostaglandin. So the extract of Terminalia arjuna may induce anti-inflammatory activity both by blocking the histamine and inhibiting the production of prostaglandin [17, 71].
4.4. Analgesic activity of Terminalia arjuna
The analgesic activity of T arjuna was assayed by a tail-flick test, as well as a writhing test by acetic acid because these tests are considered standard pharmacological models that use a natural product to evaluate analgesia [72]. The writhing test induced by acetic acid is implemented mainly for the drugs acting peripherally (shown in Eq. (3)) [73].
The cause of the difference between the effects of Aspirin and test extract is assumed to be the low content of active compounds present in the test extract. A significant pain reduction in the treatment groups than the positive control group indicates the analgesic activity of T arjuna extract. A similar result was observed in Caesalpiniabonducella, Astragalushamosus, Arum palaestinum, Inula cuspidate, Murrayakoenigii Linn., Rhynchosiacapitata DC., Glycine tomentella, Clutiaabyssinica, Scoparia dulcis L., Ficusracemosa Linn., and Limoniastrumfeei [35, 69, 70, 71, 73, 74, 75, 76, 77].
In the tail-flick test, T arjuna exerted significant antinociceptive activity in a high dose (1000 mg). There is a drastic difference observed between the treatment groups containing different doses and time duration. Possible causes behind this phenomenon may include the smaller number of active compounds present in the extract. Overall improvisation of disease condition indicates the effectivity of T arjuna in the tail-flick test. T. arjuna gave a better result than Caesalpiniabonducella [37], but a similar result was obtained in the case of Rhynchosiacapitata DC [78, 79, 80].
In a quantitative study regarding the screening of phytochemical constituents, the presence of tannin, flavonoids, gums, alkaloids, and carbohydrates was confirmed through an extensive literature study. In a previous study, it has been evidenced that flavonoids may impart analgesic activity via targeting prostaglandins (inflammation 1). Besides, alkaloids are well known for their ability to inhibit pain perception. Tannins are important compounds known to be potent cyclooxygenase-1 inhibitors and with anti-phlogistic activity [17]. The flavonoids isolated from Caesalpinia pulcherrima are evidenced with potent inhibitory effects on TNF-_ and IL- 12. Since TNF-_ and IL-12 are known as the chief proinflammatory cytokines secreted during the early phase of acute and chronic inflammatory diseases. Being an enormous source of flavonoid Terminalia arjuna may impart its anti-inflammatory in the same manner [81].
4.5. Docking studies to characterize different pharmacological activity obtained from T. Arjuna's phytochemicals
4.5.1. Materials and methods
After a wide range of literature studies on T. Arjuna, it was observed that several enzymes are involved in possessing anti-diabetic, analgesic, anti-hypertensive, and anti-oxidants activity in T. Arjuna. In the present study, six different enzymes of our interest [Table 5] were selected to analyze potential interaction studies with natural ligands. To perform an in-silico molecular docking study against these six endogenous peptides, a list of 22 compounds of different chemical classes was identified from T. Arjuna and also shorted through an extensive literature review [82, 83]. The 3D structures of these compounds were downloaded from Pubchem and later optimized through PyRx. All the proteins possessed x-ray crystallographic structure and were retrieved from the protein data bank (RCSB-PDB). This server was also used to determine the binding site of the reference drug for the respective enzymes, which was later specified in the docking process. For docking purposes, all targets were made free from unnecessary water molecules, heteroatoms by using PyMol software. Free energy was also minimized with the help of SwissDock. All protein targets and ligands were prepared as Pdbqt form with Autodock Vina and Open babel version 2.2.3 software, respectively. Next, molecular docking was performed using AutodockVina, and the results were sorted using Microsoft Excel. Moreover, an interaction study was conducted using the Biovia Discovery Studio Visualizer.
Table 5.
List of targets with reference ligands for in-silico study.
| Target Protein & PDB ID | Protein Function | Organism | Resolution | Reference Ligand |
|---|---|---|---|---|
| Alpha-Glucosidase, 2QMJ | Catalyze the final glucose releasing step in starch digestion | Homo Sapiens | 1.90 Å | Acarbose |
| Alpha-Amylase, 1B2Y | Catalyzes the hydrolyses of Polysaccharides | Homo Sapiens | 3.20 Å | Acarbose |
| Xanthine Oxidase, 3NVY | Generate reactive oxygen species | Bos Taurus | 2.00 Å | Quercetin |
| Cyclooxygenase 1, 1EQG | Produce prostaglandins | Homo Sapiens | 2.61 Å | Ibuprofen |
| Thrombin Enzyme, 2A2X | Catalyze blood coagulation cascade | Homo Sapiens | 2.44 Å | NA9 |
| Angiotensin Receptor, 4ZUD | Involved in controlling blood pressure | Homo Sapiens | 2.80 Å | Olmesartan |
4.6. Virtual screening of compounds by molecular docking and their interaction study
After successful docking, the compound with better binding affinity than the standard or reference resulting from the simulation was initially selected for further analysis. The various ligands were imported onto the Biovia Discovery studio platform, and the interaction between the target site and ligand was visualized.
4.6.1. Anti-diabetic activity
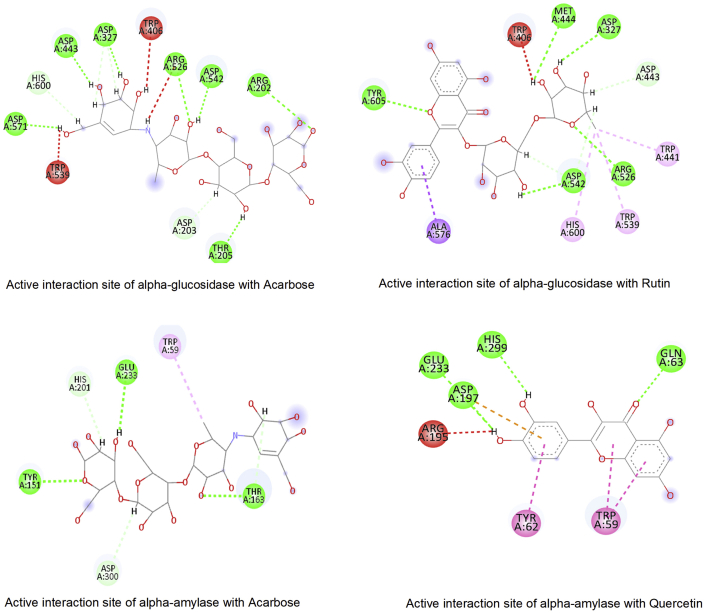
The results have shown that flavonoids from T. Arjuna exert better binding affinity towards targeted protein compared tothe standard drug for respected diseases. Alpha-amylase and alpha-glucosidase are the enzymes responsible for carbohydrates breakdown and elevate sugar levels in type-II diabetes. Acarbose acts asa standard inhibitor to these enzymes and is used to treat type-II diabetes. The binding interaction study shows that acarbose and rutin bind in similar target residues, including mandatory binding sites Asp327, Asp542, Arg526, and His600 [84], and rutin havea better binding affinity -9.2Kcal/mol compared with acarbose, -7.0Kcal/mol. Similarly, alpha-amylase is also inhibited better by flavonoids and represents a similar binding pattern with acarbose [Figure 13].
Figure 13.
Active site interaction study of alpha-glucosidase and alpha-amylase complex with flavonoids and reference compound.
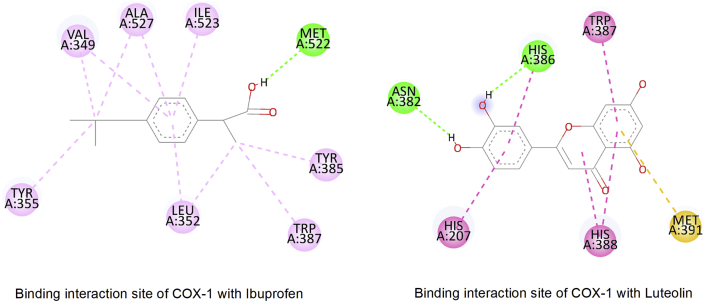
4.6.2. Analgesic activity
Cyclooxygenase enzyme inhibitory activity of flavonoids was first time founded in 1980 [85]. Active residues Arg120, Val349, Tyr355, Tyr385, Trp387, Ser530, Ile523, Phe209, and Met522 are considered the important binding sites for protein inhibition. In our study, all the natural flavonoids from T. Arjuna were docked in the Cox-1 enzyme. Ibuprofen was also docked in this enzyme as the positive control. Molecular docking showed that the most common flavonoids Luteolin and Quercetin exhibit better binding affinity -8.3Kcal/mol, -8.8Kcal/mol, respectively, than Ibuprofen, -7.6Kcal/mol. In addition,a similar pattern of interaction between Arg120-Ser530 with heteroatoms of ligands was summarized to be the way that these flavonoids interact and inhibit the activity of COX-1 [Figure 14].
Figure 14.
Active site interaction study of COX-1 complex with flavonoids and reference compound (Ibuprofen).
4.6.3. Anti-hypertensive activity
T. Arjuna showed significant improvement in endothelial dysfunction, hypertension, and other heart diseases [14]. Triterpenoids from T. arjuna acts as cardioprotective drugs [86]. In our study, we observed that triterpenoids could inhibit angiotensin and thrombin enzymes with higher affinity than their standard drugs [Table 6a,b].
Table 6.
a: Angiotensin receptor binding affinity with triterpenoids & Olmesartan. b: Thrombin enzyme binding affinity with triterpenoids & reference drugs.
| Beta-Sitosterol-D-glucoside | -10.4 |
| Arjunetin | -10.3 |
| Oleanolic acid | -10 |
| Lupeol | -10 |
| Arjunic acid | -9.8 |
| B-Sitosterol | -9.7 |
| Arjungenin | -9.4 |
| Olmesartan | -9 |
| Arjunetin | -9.3 |
| Arjunoglycoside 1 | -8.7 |
| Beta-Sitosterol-D-glucoside | -8.5 |
| Lupeol | -8.4 |
| Oleanolic acid | -8.3 |
| Inogatran | -8.1 |
∗All units are in Kcal/mol.
4.6.4. Anti-oxidant activity
Bark extract of Terminalia Arjuna has scavenging effects on reactive oxygen species [87]. Natural flavonoids such as Quercetin act on the Xanthine Oxidase enzyme and reduce the ROS concentration. Our molecular docking study showed that Luteolin, other flavonoids from T. arjuna showed better affinity on inhibition of xanthine oxidase complex than synthetic drugs, Allopurinol, Oxypurinol [Table 7].
Table 7.
Xanthine oxidase enzyme binding affinity with flavonoids & reference drugs.
| Luteolin | -9.2 Kcal/mol |
| Quercetin | -7.9Kcal/mol |
| Leucocyanidin | -7.8Kcal/mol |
| Kaempferol | -7.6Kcal/mol |
| Oxipurinol | -7.3 Kcal/mol |
| Allopurinol | -6.5 Kcal/mol |
5. Conclusion
Our findings concerning the responses of rat models under diverse test batteries impart a series of indications that the ethanolic extract of the bark of Terminalia arjuna may be capable of reversing several disturbed pathophysiological states towards the healthy status. The dose-dependent improvements in responses also indicated that proper and precise dosing of extract via justified isolation of target therapeutic specimen from the whole extract might amplify the therapeutic effect to a decent degree. In-silico study on T. arjuna's natural compounds also validates our findings to some extent and brings prospects to study the medicinal plant in molecular depth. So, it can be concluded that the further meticulous investigation of T. arjuna regarding pharmacological response and phytochemical analysis may bring about new doorways in the disease management system.
Declarations
Author contribution statement
Md. Rafat Tahsin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Arifa Sultana: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Muhammad Shah Mohtasim Khan: Conceived and designed the experiments; Analyzed and interpreted the data.
Ishrat Jahan: Analyzed and interpreted the data; Wrote the paper.
Sabiha Rahman Mim: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Tanzia Islam Tithi; Mokaddas Flora Ananta; Sadia Afrin: Performed the experiments; Wrote the paper.
Mehnaz Ali: Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Sajjad Hussai; Shaila Kabir; Abu Asad Choudhury; Md. Shah Amran; Fahima Aktar; Jakir Ahmed Chowdhury: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Boyd K.M. Disease, illness, sickness, health, healing, and wholeness: exploring some elusive concepts. Med. Humanit. Jun. 2000;26(1):9–17. doi: 10.1136/mh.26.1.9. [DOI] [PubMed] [Google Scholar]
- 2.Marinker M. Why make people patients? J. Med. Ethics. Jul. 1975;1(2):81–84. doi: 10.1136/jme.1.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gad-Elkareem M.A.M., Abdelgadir E.H., Badawy O.M., Kadri A. Potential anti-diabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ. Feb. 2019;7 doi: 10.7717/peerj.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agoramoorthy G., Chen F.-A., Venkatesalu V., Kuo D.-H., Shea P.-C. Evaluation of anti-oxidant polyphenols from selected mangrove plants of India. Asian J. Chem. 2008:12. [Google Scholar]
- 5.Singab A.N., Youssef F., Ashour M. Medicinal & aromatic plants medicinal plants with potential anti-diabetic activity and their assessment. Med. Aromatic Plants. Feb. 2014;3:151. [Google Scholar]
- 6.Küpeli Akkol E., Gürağaç Dereli F.T., Ilhan M. Assessment of antidepressant effect of the aerial parts of micromeria myrtifolia boiss. & hohen on mice. Mol. Basel Switz. May 2019;24(10) doi: 10.3390/molecules24101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.factsheet.pdf. https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/20042005RighttoPainRelief/factsheet.pdf [Online]. Available:
- 8.Oguntibeju O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018;11:307–317. doi: 10.2147/JIR.S167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A. An overview of inflammation: mechanism and consequences. Front. Biol. 2011;6(4):274–281. [Google Scholar]
- 10.Chronic Diseases Claim at Least 75% of Health Care Spending in Most Developed Countries; Pose a Growing Economic burden on Low-And Middle-Income Countries | Ward Health. http://www.wardhealth.com/articles/chronic-diseases-claim-least-75-health-care-spending-most-developed-countries-pose-growing-
- 11.Biswas1 M., Biswas2 K., Karan3 T.K., Bhattacharya4 S., Ghosh5 A.K., Haldar3∗ P.K. Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J. Phytol. Jan. 2011 https://updatepublishing.com/journal/index.php/jp/article/view/2218 [Online]. Available: [Google Scholar]
- 12.Twenty-five years of research on medicinal plants in Latin America: a personal view - PubMed. https://pubmed.ncbi.nlm.nih.gov/16006081/ [DOI] [PubMed]
- 13.Pothuraju R., Sharma R.K., Onteru S.K., Singh S., Hussain S.A. Hypoglycemic and hypolipidemic effects of Aloe vera extract preparations: a review. Phytother. Res. PTR. Feb. 2016;30(2):200–207. doi: 10.1002/ptr.5532. [DOI] [PubMed] [Google Scholar]
- 14.Amalraj A., Gopi S. Medicinal properties of Terminalia arjuna (Roxb.) wight & arn.: a review. J. Tradit. Complement. Med. Jan. 2017;7(1):65–78. doi: 10.1016/j.jtcme.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramesh B., et al. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and anti-oxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. Nov. 2012;2(11):895–900. doi: 10.1016/S2221-1691(12)60249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaliq F., Fahim M. Role of Terminalia Arjuna in improving cardiovascular functions : a review. Indian J. Physiol. Pharmacol. Jan. 2018;62:8–19. [Google Scholar]
- 17.Shahriar M., Aich R., Haque A., Sayeed M., Kadir M. Evaluation of the analgesic and neuropharmacological activities of the bark of Terminalia arjuna in mice. Int. J. Recent Sci. Res. Mar. 2013;4:285–289. [Google Scholar]
- 18.Haq A.M.M., Huque M.M., Chaudhury S.A.R., Haque M.N. Cardiotonic effects of Terminalia arjuna extracts on Guinea pig heart in vitro. Bangladesh J. Pharmacol. Aug. 2012;7(3) Art. no. 3. [Google Scholar]
- 19.N C.R., Chopra I.C. Academic Publishers; 1994. Indigenous Drugs of India. [Google Scholar]
- 20.Khalil M., et al. Preclinical lipid profile of a classical ayruvedic preparation "Arjunarista" after chronic administration to male sprague-dawley rats. Int. J. Pharm. Oct. 2014;4:146–150. [Google Scholar]
- 21.Anesini C., Ferraro G.E., Filip R. Total polyphenol content and anti-oxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. Oct. 2008;56(19):9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- 22.Shahriar M., Akhter S., Hossain M., Haque A., Bhuiyan M. Evaluation of in vitro anti-oxidant activity of bark extracts of Terminalia arjuna. J. Med. Plants Res. Oct. 2012;6:5286–5298. [Google Scholar]
- 23.Debnath S., Dey D., Hazra S., Ghosh (Basu) S., Ray R., Hazra B. Antibacterial and antifungal activity of Terminalia arjuna Wight & Arn. bark against ATCC strains and multi-drug resistant clinical isolates. J. Coast. Life Med. Nov. 2013;4:312–318. [Google Scholar]
- 24.Aneja K.R., Sharma C., Joshi R. Antimicrobial activity of Terminalia arjuna Wight & Arn.: an ethnomedicinal plant against pathogens causing ear infection. Braz. J. Otorhinolaryngol. Jan. 2012;78(1):68–74. doi: 10.1590/S1808-86942012000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doorika P., Ananthi T. Anti-oxidant and hepatoprotective properties of Terminalia arjuna bark on isoniazid induced toxicity in albino rats. Asian J. Pharm. Technol. Mar. 2012;2(1):15–18. [Google Scholar]
- 26.Sivová V., Bera K., Ray B., Nosáľ S., Nosáľová G. In: Pulmonary Infection and Inflammation. Pokorski M., editor. Cham: Springer International Publishing; 2016. Cough and arabinogalactan polysaccharide from the bark of Terminalia arjuna; pp. 43–52. [DOI] [PubMed] [Google Scholar]
- 27.Alawa J.P., Jokthan G.E., Akut K. Ethnoveterinary medical practice for ruminants in the subhumid zone of northern Nigeria. Prev. Vet. Med. May 2002;54(1):79–90. doi: 10.1016/s0167-5877(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 28.Priya N., Mathur K., Sharma A., Agrawal R., Agarwal V., Acharya J. Effect of Terminalia Arjuna on total platelet count and lipid profile in patients of coronary artery disease. Adv. Hum. Biol. Jan. 2019;9:98. [Google Scholar]
- 29.Dwivedi S. Terminalia arjuna Wight & Arn.—a useful drug for cardiovascular disorders. J. Ethnopharmacol. Nov. 2007;114(2):114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Maulik S.K., Talwar K.K. Therapeutic potential of Terminalia arjuna in cardiovascular disorders. Am. J. Cardiovasc. Drugs. Jun. 2012;12(3):157–163. doi: 10.2165/11598990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor D., Vijayvergiya R., Dhawan V. Terminalia arjuna in coronary artery disease: ethnopharmacology, pre-clinical, clinical & safety evaluation. J. Ethnopharmacol. Sep. 2014;155(2):1029–1045. doi: 10.1016/j.jep.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 32.Charan J., Kantharia N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. Oct. 2013;4(4):303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin P., Wang Y., Yang L., Sui J., Liu Y. Hypoglycemic effects in alloxan-induced diabetic rats of the phenolic extract from Mongolian oak cups enriched in Ellagic acid, Kaempferol and their derivatives. Mol. Basel Switz. Apr. 2018;23(5) doi: 10.3390/molecules23051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thierry B., Stéru L., Simon P., Porsolt R.D. The tail suspension test: ethical considerations. Psychopharmacology (Berl.) Sep. 1986;90(2):284–285. doi: 10.1007/BF00181261. [DOI] [PubMed] [Google Scholar]
- 35.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. Dec. 1962;111(3):544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 36.Adeyemi O.O., Okpo S.O., Ogunti O.O. Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana Mill (Lauraceae) Fitoterapia. Aug. 2002;73(5):375–380. doi: 10.1016/s0367-326x(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 37.Devi R.A., Tandan S.K., Kumar D., Dudhgaonkar S.P., Lal J. Analgesic activity of Caesalpinia bonducella flower extract. Pharm. Biol. Jan. 2008;46(10–11):668–672. [Google Scholar]
- 38.Ahmed S., Naved A., Khan R.A., Siddiqui S. Analgesic activities of methanol extract of Terminalia chebula fruit. Pharmacol. Amp Pharm. Dec. 2015;6(12) Art. no. 12. [Google Scholar]
- 39.D'amour F.E., Smith D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Therapeut. May 1941;72(1):74–79. [Google Scholar]
- 40.Luo Y., et al. Plasma periostin levels are increased in Chinese subjects with obesity and type 2 diabetes and are positively correlated with glucose and lipid parameters. Mediat. Inflamm. 2016;2016:6423637. doi: 10.1155/2016/6423637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Federiuk I.F., Casey H.M., Quinn M.J., Wood M.D., Ward W.K. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp. Med. Jun. 2004;54(3):252–257. [PubMed] [Google Scholar]
- 42.Goldner M.G., Gomori G. Studies on the mechanism of alloxan diabetes. Endocrinology. Oct. 1944;35(4):241–248. [Google Scholar]
- 43.Cruz A.B., Amatuzio D.S., Grande F., Hay L.J., ' Effect of intra-arterial insulin on tissue cholesterol and fatty acids in alloxan-diabetic dogs. Circ. Res. Jan. 1961;9(1):39–43. doi: 10.1161/01.res.9.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Feng F., Chen T., Li Z., Shen Q.W. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J. Ethnopharmacol. Nov. 2016;192:256–263. doi: 10.1016/j.jep.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Asif M., et al. Anti-diabetic activity of aqueous extract of Sigesbeckia orientalis (St. Paul's Wort) in alloxan-induced diabetes model. Braz. J. Pharm. Sci. 2019;55 [Google Scholar]
- 46.Okokon J.E., Antia B.S., Udobang J.A. Anti-diabetic activities of ethanolic extract and fraction of Anthocleista djalonensis. Asian Pac. J. Trop. Biomed. Jun. 2012;2(6):461–464. doi: 10.1016/S2221-1691(12)60076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadali S.L.D.V.R.M., Das M.C., Vijayaraghavan R., Kumar M.V. Evaluation of anti-diabetic activity of aqueous and ethanolic extracts of leaves of Chloroxylon swietenia in streptozotocin (Stz) induced diabetes in Albino rats. Biomed. Pharmacol. J. Sep. 2017;10(3):1347–1353. [Google Scholar]
- 48.Yetendje L., et al. Aug. 2019. In vivo antidiabetic activity and mechanism of action of three cameroonian medicinal plant extracts. [Google Scholar]
- 49.Yuneldi R.F., Saraswati T.R., Yuniwarti E.Y.W. Profile of SGPT and SGOT on male rats (Rattus norvegicus) hyperglycemic after giving insulin leaf extract (Tithonia diversifolia) Biosaintifika J. Biol. Biol. Educ. Dec. 2018;10(3) Art. no. 3. [Google Scholar]
- 50.Belayneh Y.M., Birru E.M. Antidiabetic activities of hydromethanolic leaf extract of Calpurnia aurea (Ait.) Benth. Subspecies aurea (Fabaceae) in mice. Evid. Based Complement. Alternat. Med. Sep. 2018;2018:e3509073. doi: 10.1155/2018/3509073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammeso W.W., Emiru Y.K., Ayalew Getahun K., Kahaliw W. Anti-diabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evid. Based Complement. Alternat. Med. Apr. 2019;2019:e8263786. doi: 10.1155/2019/8263786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaiswal Y.S., Tatke P.A., Gabhe S.Y., Vaidya A.B. Anti-diabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J. Tradit. Complement. Med. Oct. 2017;7(4):421–427. doi: 10.1016/j.jtcme.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selvaraj S., Palanisamy S. Investigations on the anti-diabetic potential of novel marine seaweed Sargassum longiotom against alloxan-induced diabetes mellitus: a pilot study. Bangladesh J. Pharmacol. Apr. 2014;9(2) Art. no. 2. [Google Scholar]
- 54.Karan S.K., Mondal A., Mishra S.K., Pal D., Rout K.K. Anti-diabetic effect of Streblus asper in streptozotocin-induced diabetic rats. Pharm. Biol. Mar. 2013;51(3):369–375. doi: 10.3109/13880209.2012.730531. [DOI] [PubMed] [Google Scholar]
- 55.Jarald E.E., Joshi S.B., Jain D.C. Anti-diabetic activity of extracts and fraction of Zizyphus mauritiana. Pharm. Biol. Apr. 2009;47(4):328–334. [Google Scholar]
- 56.Naz D., Muhamad A., Zeb A., Shah I. In vitro and in vivo antidiabetic properties of phenolic antioxidants from Sedum adenotrichum. Front. Nutr. 2019;6 doi: 10.3389/fnut.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhuiyan A., et al. An in vivo assessment of diabetes ameliorating potentiality of ethanolic extract of Cynodon dactylon on alloxan- induced diabetic rat model. Health Sci. J. Feb. 2021;15:783. [Google Scholar]
- 58.Alam Md.M., Haque A., Begum R. Anti-hyperglycemic and lipid lowering effect of Terminalia arjuna bark extract on streptozotocin induced type-2 diabetic model rats. Int. J. Pharm. Pharmaceut. Sci. Oct. 2011;3:449–453. [Google Scholar]
- 59.Thomson H.A.J., Ojo O.O., Flatt P.R., Abdel-Wahab Y.H.A. Aqueous bark extracts of Terminalia arjuna stimulates insulin release, enhances insulin action and inhibits starch digestion and protein glycation in vitro. Austin J. Endocrinol. Diabetes. 2014;1(1):1001. https://www.bing.com/search?q=Thomson+HAJ/C+Ojo+OO/C+Flatt+PR/C+Abdel-Wahab+YHA.+Aqueous+Bark+Extracts+of+Terminalia+Arjuna+Stimulates+Insulin+Release%2C+Enhances+Insulin+Action+and+Inhibits+Starch+Digestion+and+Protein+Glycation+in+Vitro.+Austin+J+Endocrinol+Diabetes.+2014%3B1(1)%3A+1001.+ISSN%3A+2381-9200.&cvid=bde6bcc1cc674c618af7cf39645f8be2&aqs=edge..69i57.562j0j1&pglt=2083&FORM=ANNTA1&PC=HCTS Bing. [Google Scholar]
- 60.Miaffo D., Guessom Kamgue O., Ledang Tebou N., Maa Maa Temhoul C., Kamanyi A. Anti-diabetic and anti-oxidant potentials of Vitellaria paradoxa barks in alloxan-induced diabetic rats. Clin. Phytosci. Dec. 2019;5(1):44. [Google Scholar]
- 61.Shen J., Zhang J., Deng M., Liu Y., Hu Y., Zhang L. The antidepressant effect of Angelica sinensis extracts on chronic unpredictable mild stress-induced depression is mediated via the upregulation of the BDNF signaling pathway in rats. Evid. Based Complement. Alternat. Med. Aug. 2016;2016:e7434692. doi: 10.1155/2016/7434692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu G.-F., et al. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci. Rep. Jul. 2017;7(1) doi: 10.1038/s41598-017-05051-3. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhingra D., Sharma A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J. Pharmacol. Jan. 2005;37(6):390. [Google Scholar]
- 64.Hao G., Tang M., Wei Y., Che F.Q. Determination of antidepressant activity of Cyperus rotundus L extract in rats. Trop. J. Pharmaceut. Res. 2017;16(4):867–871. [Google Scholar]
- 65.Santos A.R.S., Calixto J.B. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides. Aug. 1997;31(4):381–389. doi: 10.1016/s0143-4179(97)90075-5. [DOI] [PubMed] [Google Scholar]
- 66.Fereidoni M., Ahmadiani A., Semnanian S., Javan M. An accurate and simple method for measurement of paw edema. J. Pharmacol. Toxicol. Methods. Feb. 2000;43(1):11–14. doi: 10.1016/s1056-8719(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 67.Singh A., Singh A., Chouhan O., Tandi G.P., Dua M., Gehlot A. Anti-inflammatory and analgesic activity of aqueous extracts of dried leaves of Murraya koenigii Linn. Natl. J. Physiol. Pharm. Pharmacol. 2016;6(4):286–290. [Google Scholar]
- 68.Shojaii A., Motaghinejad M., Norouzi S., Motevalian M. Evaluation of anti-inflammatory and analgesic activity of the extract and fractions of Astragalus hamosus in animal models. Iran. J. Pharm. Res. IJPR. 2015;14(1):263–269. [PMC free article] [PubMed] [Google Scholar]
- 69.Qnais E., Bseiso Y., Wedyan M., Alkhateeb H. Evaluation of analgesic activity of the methanol extract from the leaves of Arum palaestinum in mice and rats. Biomed. Pharmacol. J. Sep. 2017;10(3):1159–1166. [Google Scholar]
- 70.Kumar Paliwal S., Sati B., Faujdar S., Sharma S. Studies on analgesic, anti-inflammatory activities of stem and roots of Inula cuspidata C.B Clarke. J. Tradit. Complement. Med. Oct. 2017;7(4):532–537. doi: 10.1016/j.jtcme.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perianayagam J.B., Sharma S.K., Pillai K.K. Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J. Ethnopharmacol. Apr. 2006;104(3):410–414. doi: 10.1016/j.jep.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 72.Hiruma-Lima C.A., Gracioso J.S., Bighetti E.J.B., Germonsén Robineou L., Souza Brito A.R.M. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J. Ethnopharmacol. Jul. 2000;71(1):267–274. doi: 10.1016/s0378-8741(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 73.Lu T.-C., Ko Y.-Z., Huang H.-W., Hung Y.-C., Lin Y.-C., Peng W.-H. Analgesic and anti-inflammatory activities of aqueous extract from Glycine tomentella root in mice. J. Ethnopharmacol. Aug. 2007;113(1):142–148. doi: 10.1016/j.jep.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 74.Doak G.J., Sawynok J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. Jul. 1997;80(3):939–949. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 75.Coruzzi G., Adami M., Guaita E., de Esch I.J.P., Leurs R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur. J. Pharmacol. Jun. 2007;563(1):240–244. doi: 10.1016/j.ejphar.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto T., Nozaki-Taguchi N. The role of Cyclooxygenase1 and -2 in the rat formalin test. Anesth. Analg. Apr. 2002;94:962–967. doi: 10.1097/00000539-200204000-00035. [DOI] [PubMed] [Google Scholar]
- 77.Sc K., Odhiambo R., Nm M., Mm I., Ngugi M., Nm N. Analgesic activity of dichloromethanolic root extract of clutia abyssinica in Swiss albino mice. Nat. Prod. Chem. Res. Jan. 2017;5 [Google Scholar]
- 78.Varshney R.K., Saxena R.K., Jackson S.A. Springer; 2017. The Pigeonpea Genome. [Google Scholar]
- 79.Zulfiker A., Rahman Md.M., Hossain M., Hamid K., Mazumder M., Rana M. In vivo analgesic activity of ethanolic extracts of two medicinal plants - Scoparia dulcis L. And Ficus racemosa Linn. Biol. Med. Oct. 2010;2:42–48. [Google Scholar]
- 80.In vivo analgesic activity of aqueous extract from leaves of Limoniastrum feei. MOJ Bioequivalence Bioavailab. Dec. 2017;4(Issue 3) [Google Scholar]
- 81.Rao Y., Fang S.-H., Tzeng Y.-M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J. Ethnopharmacol. Oct. 2005;100:249–253. doi: 10.1016/j.jep.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 82.Kumar V., Sharma N., Sourirajan A., Khosla P.K., Dev K. Docking studies in targeting proteins involved in cardiovascular disorders using phytocompounds from Terminalia arjuna. Bioinformatics. Jun. 2020 preprint. [Google Scholar]
- 83.Terminalia arjuna a sacred medicinal plant: phytochemical and pharmacological profile | Request PDF. https://www.researchgate.net/publication/226169012_Terminalia_arjuna_a_sacred_medicinal_plant_Phytochemical_and_pharmacological_profile
- 84.Sim L., Quezada-Calvillo R., Sterchi E.E., Nichols B.L., Rose D.R. Human intestinal maltase–glucoamylase: crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. J. Mol. Biol. Jan. 2008;375(3):782–792. doi: 10.1016/j.jmb.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 85.Baumann J., von Bruchhausen F., Wurm G. Flavonoids and related compounds as inhibition of arachidonic acid peroxidation. Prostaglandins. Oct. 1980;20(4):627–639. doi: 10.1016/0090-6980(80)90103-3. [DOI] [PubMed] [Google Scholar]
- 86.Pawar R.S., Bhutani K.K. Effect of oleanane triterpenoids from Terminalia arjuna — a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine. May 2005;12(5):391–393. doi: 10.1016/j.phymed.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Sivalokanathan S., Ilayaraja M., Balasubramanian M.P. Anti-oxidant activity of Terminalia arjuna bark extract on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Mol. Cell. Biochem. Jan. 2006;281(1–2):87–93. doi: 10.1007/s11010-006-0433-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.