Abstract
Sorafenib is a first-line molecular-target drug for advanced hepatocellular carcinoma (HCC), and reducing sorafenib resistance is an important issue to be resolved for the clinical treatment of HCC. In the current study, we identified that ABCC5 is a critical regulator and a promising therapeutic target of acquired sorafenib resistance in human hepatocellular carcinoma cells. The expression of ABCC5 was dramatically induced in sorafenib-resistant HCC cells and was remarkably associated with poor clinical prognoses. The down-regulation of ABCC5 expression could significantly reduce the resistance of sorafenib to HCC cells. Importantly, activation of PI3K/AKT/NRF2 axis was essential for sorafenib to induce ABCC5 expression. ABCC5 increased intracellular glutathione (GSH) and attenuated lipid peroxidation accumulation by stabilizing SLC7A11 protein, which inhibited ferroptosis. Additionally, the inhibition of ABCC5 enhanced the anti-cancer activity of sorafenib in vitro and in vivo. These findings demonstrate a novel molecular mechanism of acquired sorafenib resistance and also suggest that ABCC5 is a new regulator of ferroptosis in HCC cells.
Keywords: Hepatocellular carcinoma, Sorafenib, ABCC5, Ferroptosis, Acquired resistance
Abbreviation: HCC, hepatocellular carcinoma; qRT-PCR, quantitative RT-PCR; IF, immunofluorescence; IHC, immunohistochemistry; GSH, glutathione; ROS, reactive oxygen species; NRF2, nuclear factor erythroid 2-related factor 2; ABCC5, ATP binding cassette subfamily C member 5; SLC7A11, solute carrier family 7 member 11; GPx4, glutathione peroxidase 4; 5-Fu, 5-fluorouracil
Introduction
Hepatocellular carcinoma is a common malignant tumor of the digestive system [1]. For the nonsurgical treatment of patients with advanced hepatocellular carcinoma, sorafenib, a multitarget tyrosine kinase inhibitor (TKI), is currently the first-line chemotherapy drug for hepatocellular carcinoma. Sorafenib is a molecular-target drug that results in an overall survival of 14.7 months for HCC [2], but the therapeutic efficacy is not satisfactory. Multidrug resistance (MDR) is one of the most important reasons for chemotherapy failure. Previous MDR reversal agents, such as PSC833, S9788 and VX-710, have high toxicity and side effects, which have become the main obstacle to their clinical applications [3].
As an important membrane transporter, the ATP-binding cassette (ABC) family can cooperate with many membrane transporters and play a positive role in transporting substances. Members of the ABC family work with other membrane transporters to transport intracellular and extracellular nutrients or toxins, and the intramolecular synergistic effect within the family is also significant [4]. Currently, the ABC-mediated drug efflux is considered to be the main mechanism of MDR, and the multidrug resistance-related transporters in the ABC transporter family include P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs) [5]. The overexpression of ABC transporters in cancer cells is associated with increased efflux of anti-cancer drugs, which can induce tumor resistance [6].
Structurally similar, ABCC1, ABCC2 and ABCC3, which are ATP-dependent glutathione S-conjugate (GSX) transport pumps, are involved in resistance to a variety of natural drugs and folic acid antagonists combining glutathione and glucuronide MTX [7]. In addition, ABCC4 and ABCC5 are mainly resistant to purines and nucleotide mimetics [8]. ABCC5/MRP5, a member of ABC transporter family, is a universal glutamate conjugate that affects the efflux of endogenous metabolites, toxins, drugs, and intracellular ions [9]. For example, ABCC5 excretes 5-fluorouracil(5-Fu) and anti-tumor drugs such as methotrexate and cisplatin, which can cause chemotherapy resistance [10].
Although increasing evidence indicates that ABCC5 expression is a prognostic factor for tumor progression and drug resistance in a variety of malignancies, the role of ABCC5 in the anti-tumor activity of sorafenib in HCC cells remains obscure [11]. In this study, we demonstrated that up-regulation of ABCC5 (but not other ABCCs) contributes to sorafenib resistance through inhibiting ferroptosis. Ferroptosis is a new type of programmed cell death characterized by the overwhelming, iron-dependent accumulation of lethal lipid ROS, and it is significantly different from other forms of cell death in terms of morphology, biology, and genetics [12,13]. It has been reported that sorafenib is a ferroptosis-specific activator, which cause ferroptosis by inhibiting SLC7A11. Our work suggested that the role of ABCC5 is closely related to ferroptosis in mediating sorafenib resistance.
In the present study, we investigated the role of ABCC5 in sorafenib-resistant HCC both in vivo and in vitro, and revealed a potential therapeutic strategy by increasing ferroptosis in HCC.
Materials and methods
Antibodies and reagents
Antibodies against ABCC1 (A11153), ABCC3 (A9849), ABCC5 (A3028), SLC7A11 (A2413, A13685), NRF2 (A11159) and GPx4 (A11243) were obtained from Abclonal (Wuhan, China). PI3K (60225), p-PI3K (55311), AKT (10176), p-AKT (66444) and β-tubulin (66240) were bought from Proteintech (Wuhan, China). β-actin (4970L) was obtained from Cell Signal Technology (Danvers, MA). MK571 (S8126), Sorafenib (S7397), SB216763 (S1075) were purchased from Selleck Chemicals (Houston, USA). Deferoxamine (DFO, 1166003), Erastin (E7781) were obtained from Sigma-Aldrich (Missouri, USA). Brusatol (HY-19543), LY294002 (HY-10108), SC79 (HY-18749), Ferrostatin-1 (Fer-1, HY-100579), RSL3 (HY-100218A), ML385 (HY-100523), MG132 and Chloroquine (CQ) were obtained from MedChemExpress (New Jersey, USA).
Cell lines and treatments
Human hepatocellular carcinoma cell lines HuH7, HepG2 and Sk-Hep-1 were obtained from the ATCC (Manassas, USA). HuH7 cells are well characterized and commonly used for subcutaneous transplantation into nude mice. All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM: Selleck Chemicals, Houston, USA) supplemented with 10% foetal bovine serum (FBS: Gibco, Carlsbad, USA) at 37 °C in a humidified incubator containing 5% CO2.
In terms of treatment with inhibitors or drugs, sorafenib (3 μM or 10 μM), deferoxamine (100 μM), ferrostatin-1 (1 μM), SB216763 (10 μM), SC79 (5 μg/ml), LY294002 (10 μM), ML385 (5 μM), brusatol (50 nM), MK571 (10 μM), erastin (10 μM), RSL3 (3 μM) , MG132 (10 mmol/L) and Chloroquine (10 mmol/L) were first dissolved in DMSO and then added to the cultured HuH7 or Sk-Hep-1 cells or HuH7-SR cells.
Generation of sorafenib-resistant cells
We subjected two HCC cell lines to long-term exposure to sorafenib at a dose of 0.5 μM and escalating through incremental doses to 10 μM, the highest clinically achievable concentration in which the cells survived, thereby establishing HuH7 and Sk-Hep-1 sorafenib-resistant cells (HuH7-SR and Sk-Hep-1-SR). The control cells were named HuH7-WT and Sk-Hep-1-WT. We measured sorafenib sensitivity by CCK-8 assays (24 h), and the results displayed that the sorafenib-resistant cells had lower sorafenib sensitivity than did the WT control cells.
Determination of sorafenib and its metabolites by UPLC8-MS/MS
The UPLC conditions: system, Waters AcquityTM; column, Acquity UPLC HSS T3 column (100 × 2.1 mm, 1.8 μm, Waters,Milford, MA, USA); mobile phase A: 0.1% (v/v) formic acid in water; mobile phase B: 100% acetonitrile; gradient, 0 min to 4 min at 50 to 70% B, 4 min to 5 min at 70 to 50% B; flow rate, 0.3 mL/min; column temperature, 50 °C; and injection volume, 10 μL. The MS/MS detector was a quadrupole tandem mass spectrometer (Waters, USA). Samples were analyzed using electrospray ionization in the positive model. The main working parameters were set as follows: capillary voltage, 3 kV; cone voltage, 38 V; collision voltage, 35 V; source temperature, 120 °C; desolvation temperature, 400 °C; desolvation gas flow, 600 L/H; cone gas flow, 50 L/H; and collision gas glow, 0.20 mL/min. Data were collected and analyzed by Waters Quantify software (Masslynx 4.1, Waters, USA). Sorafenib was monitored at m/z 465.3>252.4. Metabolites including M3, M4 and M5 were monitored at m/z 481.3>268.3, m/z 451.0>406.3 and m/z 467.0>202.0 transitions, respectively. Testosterone was used as an internal standard (IS) and was monitored at m/z 289.5>97.4.
Flow cytometry assay
Changes in intracellular lipid ROS levels were measured by the oxidative conversion of cell permeable 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Sigma) with a fluorescence spectrophotometer. Cells were plated in 6 cm dishes (1 × 106 cells/well) and allowed to attach overnight. The cells were incubated with control medium with or without sorafenib for 24 h. The cells were washed with D-Hank's solution and incubated with DCFH-DA at 37 °C for 20 min. Then, the DCF fluorescence distribution of 20,000 cells was determined by a FACSCalibur flow cytometer (Beckman Coulter, CytoFLEX S, United States) at an excitation wavelength of 488 nm and at an emission wavelength of 535 nm [14].
The mitochondrial membrane potential (MMP) assay is a cytofluorimetric method that is both qualitative and quantitative, and the results are validated by analysing the MMP at the level of a single mitochondrion. JC-1 in an aggregated form emits a red or orange emission (590 ± 17.5 nm) in the matrix of the mitochondria, indicating a normal MMP; with the loss of MMP, JC-1 is converted to the monomeric form and emits green fluorescence with an emission of 530 ± 15 nm. Therefore, a decrease in the red/green fluorescence intensity ratio indicates a reduction in MMP [15].
Construction of lentiviral vectors
To generate cells with ABCC5 knockdown and overexpression, lentiviral vectors were amplified with the insert (full-length human ABCC5; NR_135125.2) by qRT-PCR, compared to human reference cDNA. Lentiviruses were produced by transient transfection of 293T cells with pSPAX2, pMD2G, and pHB-U6-MCS-zsgreen-puro (empty) (Hanbio, Shanghai, China) plasmid DNA (2439 -BamHI and 2456 -EcoRI sites) plus LipoFiter (Hanbio Biotechnology, ShangHai, China), following the manufacturer's protocol.
Quantitative real time polymerase chain reaction
Total RNA isolation and quantitative RT-PCR (qRT-PCR) were carried out according to the manufacturer's protocols. Briefly, first-strand cDNA synthesis was carried out by using a Reverse Transcription System Kit (#11801-025, OriGene Technologies, Rockville, MD, USA). cDNA from various cell samples was amplified with specific primers (Table S2).
Statistical analysis
Data were analyzed using SPSS version 20.0 software (SPSS; Chicago, USA). Student's t-tests and one-way ANOVAs were carried out on the western blot analysis. CCK-8 analyzes were applied to generate the cell growth curve and calculate cell viability. The significance of the correlation between the expression of ABCC5 and various histopathological factors was determined using Pearson's chi square (χ2) test. Multivariate analysis was performed by applying the Cox proportional hazards test. Statistical significance was established at P < 0.05.
Results
Sorafenib induces ABCC5 expression in human HCC cells
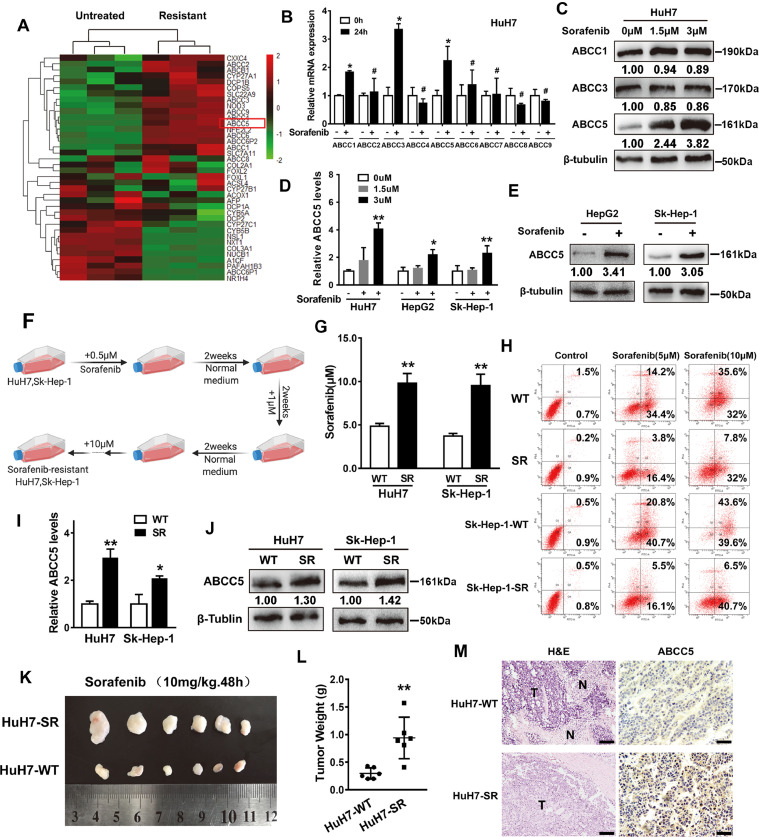
To gain a insight in the possible mechanisms of sorafenib resistanc, a heatmap of differentially expressed genes between sorafenib-resistant HCC cells and the untreated HCC cells was conducted. Compared with the untreated HCC cells, ATP-binding cassette subfamily C (ABCC) members were up-regulated (P < 0.05) in sorafenib-resistant HCC cells (GSE96793; Fig. 1A). To determine whether sorafenib induces ABCC expression, HuH7 (a human HCC cell line) cells were treated with sorafenib for 24 h and the mRNA levels of ABCC family were analyzed via qRT-PCR. As shown in Fig. 1B, the expression levels of ABCC1, ABCC3 and ABCC5 were the highest following sorafenib treatment. In addition, the Western blot analysis revealed that compared with ABCC1 or ABCC3, the protein expression of ABCC5 was significantly increased in HuH7 cells treated with sorafenib (Fig. 1C). Moreover, qRT-PCR and Western blot analysis demonstrated that sorafenib-induced ABCC5 expression was observed in other human HCC cell lines (HepG2 and Sk-Hep-1 cells) (Fig. 1D, E). Intermittent drug induction method was conducted to induce resistance to sorafenib in HuH7 and Sk-Hep-1 cells, named HuH7-SR and Sk-Hep-1-SR, respectively. Cells were cultured in a complete medium containing sorafenib (Fig. 1F). The IC50 values of the two resistant cell lines were calculated as 9.804 ± 0.995 μM and 9.564 ± 1.152 μM, which were significantly higher than HuH7-WT cells (4.851 ± 0.293 μM) and Sk-Hep-1-WT (3.732 ± 0.262 μM) (Fig. 1G). To further verify the reliability of sorafenib-resistant cell lines, flow cytometry assay was performed to detect cell apoptosis. The results displayed that compared with WT cells, SR cells showed significant drug resistance (Fig. 1H). As indicated by qRT-PCR and Western blot analysis, the expression of ABCC5 in both SR cells was higher than that in WT cells (Fig. 1I, J). In vivo, HuH7-WT and HuH7-SR cells were implanted into the subcutaneous space of the left and right flanks of nude mice. Beginning at day seven, these mice were treated with sorafenib. Compared with the control HuH7-WT group, sorafenib treatment did not reduce the size or the weight of tumors in the HuH7-SR group (Fig. 1K, L). Moreover, the histological observation of H&E and immunohistochemical staining indicated that the expression of ABCC5 in the HuH7-SR group was significantly higher than that of the HuH7-WT group (Fig. 1M). These findings indicate that sorafenib remarkably induces ABCC5 expression in human HCC cells.
Fig. 1.
Sorafenib induces ABCC5 expression in human HCC cells. (A) Data from GSE96793 showed that the expression of ABCC members were markedly increased in sorafenib-resistant cells compared with untreated cells. (B) HuH7 cells were treated with sorafenib (3 µM) for 24 h, and the mRNA expression levels of ABCC members were examined by qRT-PCR (n = 3, *P < 0.05 versus the untreated group). (C) HuH7 cells were treated with sorafenib (1.5 μM and 3 µM) for 24 h, and Western blot analysis evaluated the protein expression of ABCC1, ABCC3 and ABCC5. (D) Human HCC cell lines (HuH7, HepG2 and Sk-Hep-1 cells) were treated with sorafenib (1.5 μM and 3 µM) for 24 h, and the mRNA expression of ABCC5 was examined by qRT-PCR (n = 3, *P < 0.05 versus the untreated group). (E) HepG2 and Sk-Hep-1 cells were treated with sorafenib (1.5 μM and 3 µM) for 24 h, and Western blot analysis detected the expression of ABCC5. (F) The generation of acquired resistance to sorafenib in HuH7 and Sk-Hep-1 HCC cell lines. (G) CCK-8 assays examined the effect of sorafenib on HCC cell activity, and SPSS was used to calculate the drug concentration required to cause death of one-half of HCC cells. (H) Flow cytometry detected the effects of sorafenib (5 μM and 10 μM) on the apoptosis of HCC cells. (I, J) The expression of ABCC5 in HuH7-SR and Sk-Hep-1-SR cell lines was determined by qRT-PCR and Western blot assays. (K) HuH7-SR cells enhanced the anti-cancer activity of sorafenib in vivo. Nude mice were injected with HuH7-SR and HuH7 cells (2 × 106 cells/mouse) into the subcutaneous space on their left and right flanks and were treated with sorafenib (10 mg/kg/i.p., once every other day) at day seven for two weeks (n = 6 mice/group). (L) The tumor weight of each mice was calculated. (M) H&E staining and immunohistochemical staining (expression of ABCC5) were used to visualize the tumor tissue of mice. Representative figures were shown. Scale bars, 50 μm. *P < 0.05, ** P < 0.01, # P > 0.05.
PI3K/AKT/NRF2 pathway activation is required for sorafenib-induced ABCC5 expression
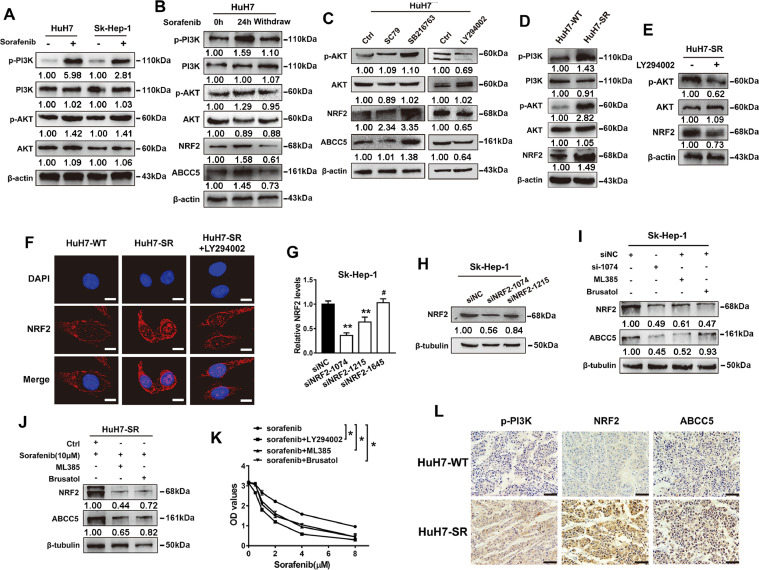
Long-term treatment with sorafenib can activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway in HCC [16,17]. Consistently, our data revealed that sorafenib specifically induced PI3K/AKT pathway activation in HuH7 cells and Sk-Hep-1 cells (Fig. 2A). The nuclear factor erythroid 2-related factor 2 (NRF2), a downstream target of the PI3K/AKT pathway, can protect cells against multiple injuries and mediate antioxidation [18]. Additionally, it's reported that sorafenib can regulate the activity of NRF2 to affect the death and survival of HCC cells [19,20]. To determine if short-term sorafenib treatment could activate PI3K/AKT or increase NRF2 expression in HuH7 cells, the levels of them were recovered when the drug was withdrawn (Fig. 2B). The Western blot analysis showed that NRF2 and ABCC5 were significantly up-regulated by the PI3K/AKT pathway agonists SC79 and SB216763. After treated with the PI3K/AKT pathway inhibitor LY294002, the expression of NRF2 was reduced, indicating that the AKT pathway positively regulated NRF2 in HuH7 cells (Fig. 2C). Furthermore, the Western blot analysis revealed that compared with HuH7-WT cells, the expression of p-PI3K, p-AKT, and NRF2 was increased in HuH7-SR cells. However, LY294002 treatment decreased the expression of NRF2 in HuH7-SR cells, suggesting that the AKT pathway positively regulated NRF2 in sorafenib-resistant HCC cells (Fig. 2D, E). Consistently, immunofluorescence staining showed that the fluorescence intensity of NRF2 was enhanced in sorafenib-resistant HCC cells (HuH7-SR), especially in the nucleus, but LY294002 significantly decreased the expression of NRF2, further indicating that long-term sorafenib exposure activated the PI3K/AKT/NRF2 pathway (Fig. 2F). qRT-PCR analysis and bioinformatics data (GEPIA2 websites) showed that the correlation between ABCC5 and NRF2 was significant in HCC (Fig. S1). Moreover, ABCC5 expression was significantly reduced in WT and SR cells after treated with target-specific siRNAs against NRF2 (Fig. 2G, H) or NRF2 inhibitors (ML385 and Brusatol) (Fig. 2I, J), suggesting that NRF2 was required for the sorafenib-induced ABCC5 expression. The inhibitors of the AKT/NRF2 pathway as previously mentioned significantly enhances sorafenib-induced cell death at 24 h as shown by cell viability assay (Fig. 2K). Besides, the expression of p-PI3K, NRF2 and ABCC5 were evaluated by immunohistochemistry (IHC) in the hepatocellular carcinoma tissue of each group. Immunohistochemical staining showed that compared with the control HuH7-WT group, the p-PI3K, NRF2 and ABCC5 expression levels were significantly increased in HuH7-SR (Fig. 2L). Taken together, these data indicate that activation of PI3K/AKT/NRF2 pathway is required for sorafenib-induced ABCC5 expression.
Fig. 2.
PI3K/AKT/NRF2 pathway activation is required for sorafenib-induced ABCC5 expression. (A) Western blot analysis demonstrated the expression of PI3K, p-PI3K, AKT, and p-AKT in HuH7 and Sk-Hep-1 cells treated with sorafenib (3 μM) for 24 h. (B) HuH7 cells were treated with sorafenib (3 μM) for 24 h, and the protein expression of p-PI3K, p-AKT, NRF2 and ABCC5 were detected by Western blot assays after drug withdrawal. (C) HuH7 cells were treated with SC79 (5 μg/ml), SB216763 (10 μM) and LY294002 (10 μM) for 24 h, and the protein expression of p-AKT, NRF2 and ABCC5 were determined by Western blot assays. (D) Western blot analysis examined the protein expression of PI3K, p-PI3K, AKT, and p-AKT in HuH7-SR cells. (E) HuH7-SR cells were treated with LY294002 (10 μM) for 24 h, and the expression of p-AKT, NRF2 and ABCC5 were detected by Western blot assays. (F) HuH7-SR cells were treated with LY294002 (10 μM) for 24 h, and the subcellular localization of NRF2 in indicated cells was assessed by confocal microscopy (original magnification, × 400). Scale bars, 50 μm. (G, H) After transfection with siRNA, the protein expression of NRF2 in Sk-Hep-1 cells was determined by qRT-PCR and Western blot assays. (I) NRF2 expression was down-regulated by siRNA, ML385 (5 μM) and Brusatol (50 nM) in Sk-Hep-1 cells. The protein expression of NRF2 and ABCC5 were determined by Western blot assays. (J) HuH7-SR cells were treated with sorafenib (10 μM) with or without NRF2 inhibitors (Brusatol, 50nM; MK571, 10 µM). Western blot analysis detected the protein expression of NRF2 and ABCC5. (K) After treatment with LY294002 (10 μM), ML385 (5 μM) and brusatol (50 nM) for 24 h, the cell activity of Sk-Hep-1 cells was evaluated by CCK-8 assays. (L) IHC staining detected the expression of p-PI3K, NRF2 and ABCC5 in tumor tissue formed by HuH7 cells and HuH7-SR cells. Representative figures were shown. Scale bars, 50 μm. *p < 0.05, ** P < 0.01, # P > 0.05.
ABCC5 mediates acquired resistance to sorafenib in vitro
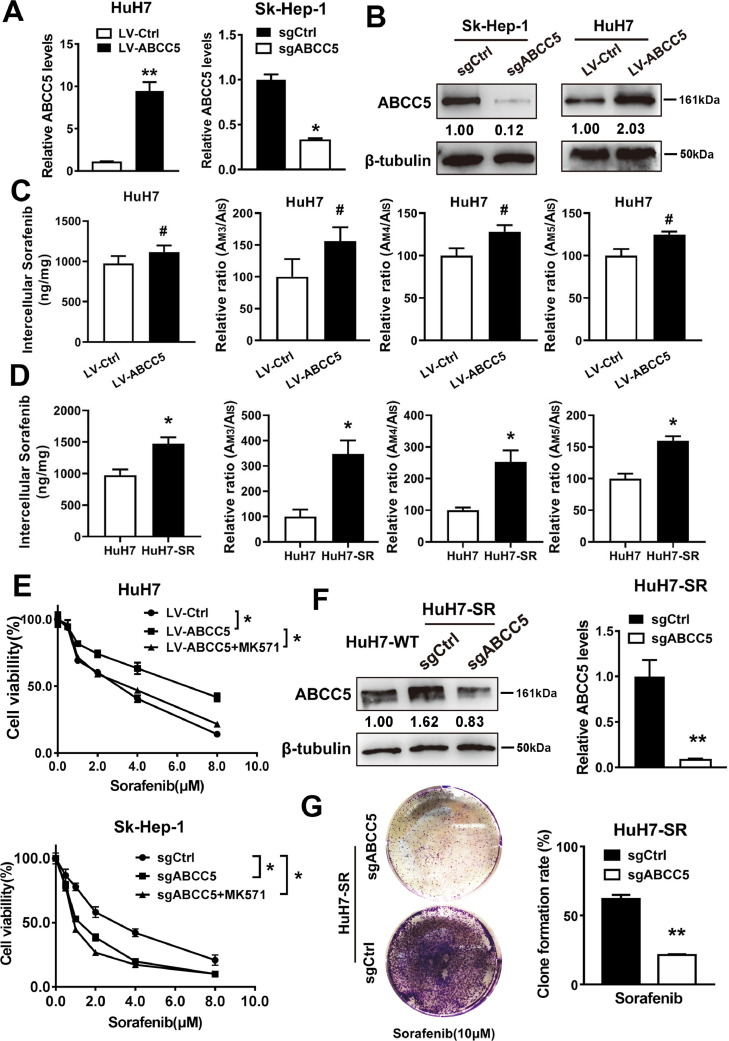
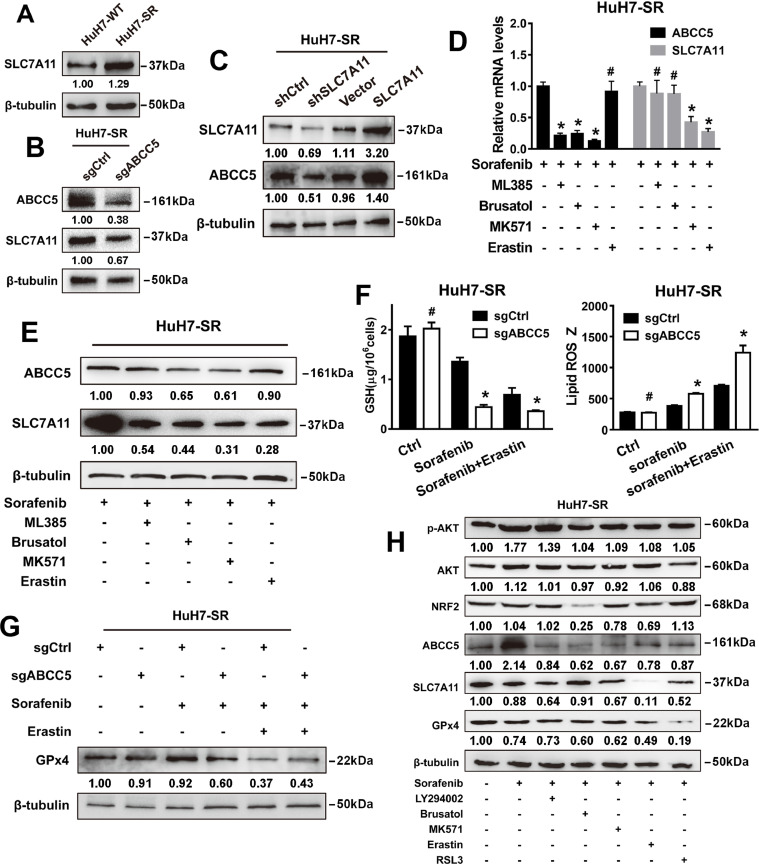
To determine whether ABCC5 expression regulates the anti-cancer activity of sorafenib, sgRNA targeting ABCC5 and a lentivirus vector of ABCC5 were transfected into Sk-Hep-1 and HuH7 cells (Fig. 3A, B). First, to evaluate the effect of ABCC5 on the transport of sorafenib in cells, HuH7-SR and ABCC5-overexpressing HuH7 cells were treated with sorafenib and detected by LC-MS. We found that overexpression of ABCC5 had no significant effect on the intercellular concentration of sorafenib or the intracellular concentration of its metabolites M3, M4 and M5 in HuH7 cells (P > 0.05) (Fig.3C). However, intercullar sorafenib and its metabolites were increased in drug-resistant strain HuH-SR compared with HuH-WT (Fig.3D). The data suggested that the efflux effect of ABCC5 on sorafenib was not the main mechanism causing sorafenib resistance. Overexpression of ABCC5 in HuH7 cells significantly inhibited sorafenib-induced cytotoxicity, which was effectively recovered by the ABCC inhibitor MK571. Additionally, knockdown and inhibition of ABCC5 expression can further intensify sorafenib-induced cytotoxicity in Sk-Hep-1cells (Fig. 3E). Furthermore, sgRNA targeting ABCC5 was used to neutralize the increased expression of ABCC5 in HuH7-SR cells (Fig. 3F). Colony formation assays also indicated that the suppression of ABCC5 expression inhibited long-term HuH7-SR cell proliferation following sorafenib treatment (Fig. 3G). Thus, these findings suggest that ABCC5 mediates acquired resistance to sorafenib in HCC cells in vitro.
Fig. 3.
ABCC5 mediates the acquired resistance to sorafenib in vitro. (A, B) qRT-PCR and Western blot analysis detected the expression of ABCC5 in HCC cells after knockdown and overexpression of ABCC5. (C) HuH7 cells were transfected with LV-ABCC5 and were treated with 3 μM sorafenib for 24 h. UPLC8-MS/MS was used to examine the intercellular concentration of sorafenib. The formation rates of M3, 4, 5 were expressed as relative ratios (Mean ± SD). (D) HuH7-SR cells were treated with 3 μM sorafenib for 24 h, and the intercellular concentration of sorafenib was determined by UPLC8-MS/MS. The formation rates of M3, 4, 5 were expressed as relative ratios (Mean ± SD). (E) The effect of the ABCC inhibitor MK571 (10 μM) on sorafenib resistance was evaluated in HuH7 and Sk-Hep-1 cells by CCK-8 assays. (F) qRT-PCR and Western blot analysis detected the expression of ABCC5 in HuH7 cells and ABCC5-knockdown HuH7-SR cells. (G) Clonogenic cell survival assay. HuH7-SR cells were treated with sorafenib (10 µM) for 24 h, then 1000 cells were plated into 6-well plates. Colonies were visualized by crystal violet staining two weeks later. # P > 0.05, * P < 0.05, ** P < 0.01.
ABCC5 is a negative regulator of ferroptosis and contributes to sorafenib resistance
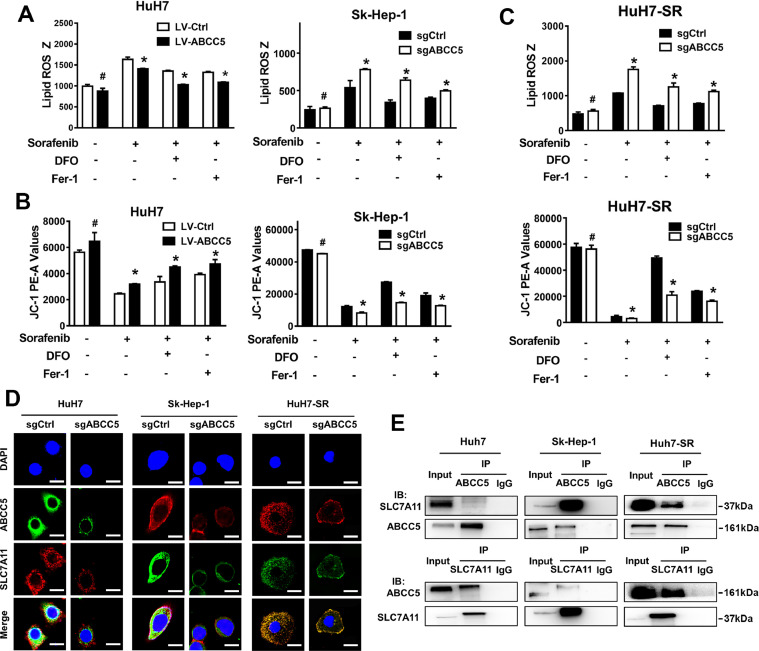
Sorafenib is a strong inducer of ferroptosis but not of apoptosis of HCC cells [21]. Sorafenib induced lipid peroxidation accumulation and mitochondrial damage (Fig. S2A, B) and suppressed the expression of SLC7A11 and GPx4 (Fig. S2C) in HCC cells. Ferroptosis inhibitors Fer-1 and DFO significantly restored HCC cell viability inhibited by sorafenib (Fig. S2D). Ferroptosis is a new type of regulated cell death, which is mainly mediated by iron-dependent lipid peroxidation [22]. Thus, we further investigated whether ABCC5 is involved in the regulation of lipid peroxidation and mitochondrial damage contributing to sorafenib resistance. Overexpression of ABCC5 significantly offset the lipid peroxidation accumulation and the mitochondrial membrane potential (MMP) reduction induced by sorafenib in HuH7 cells. Consistently, ferroptosis inhibitors Fer-1 and DFO partially ameliorated lipid peroxidation and mitochondrial damage in HuH7 cells treated with sorafenib (Fig. 4A). Moreover, suppression of ABCC5 expression by sgRNA attenuated lipid peroxidation and enhanced MMP in Sk-Hep-1 cells and HuH7-SR cells treated with sorafenib. As expected, ferroptosis inhibitors Fer-1 and DFO partially attenuated lipid peroxidation and mitochondrial damage in HuH7-SR cells and ABCC5-knockdown Sk-Hep-1 cells treated with sorafenib (Fig. 4B, C). In summary, these results suggest that ABCC5 negatively regulates ferroptosis via suppressing lipid peroxidation and increasing MMP, which contributes to sorafenib resistance in HCC cells.
Fig. 4.
ABCC5 is a negative regulator of ferroptosis and contributes to sorafenib resistance. (A–C) The indicated HCC cells and sorafenib-resistant cells were treated with sorafenib (3 µM and 10 μM) with or without ferroptosis inhibitors (Fer-1, 1 µM; DFO, 100 µM) for 24 h. Flow cytometry assays determined the levels of lipid ROS and the MMP (n = 3, *P < 0.05 versus sorafenib treatment group). *P < 0.05, ** P < 0.01, # P > 0.05. (D) Subcellular localization of ABCC5 and SLC7A11 in indicated HCC cells and sorafenib-resistant cells was observed by confocal microscopy (original magnification, × 400). Representative figures were shown. Scale bars, 10 μm. (E) Total lysates from WT and SR cells were subjected to immunoprecipitation with ABCC5 antibody, followed by Western blot assays using SLC7A11 antibody, and the total lysates from WT and SR cells were subjected to immunoprecipitation with SLC7A11 antibody, followed by Western blot assays using ABCC5 antibody.
ABCC5 negatively regulates ferroptosis by stabilizing SLC7A11
Sorafenib increases intracellular lipid peroxidation levels by inhibiting SLC7A11, a specific light-chain subunit of the cystine/glutamate antiporter [23]. Next, we explored whether ABCC5 could interact with SCL7A11 to regulate ferroptosis. The bioinformatics analysis based on the starBase database showed that ABCC5 was significantly correlated with SLC7A11 (Fig. S3). IF assays showed that ABCC5 colocalized with SLC7A11, and knockdown of ABCC5 decreased the expression of SLC7A11 in HuH7, Sk-Hep-1 and HuH7-SR cells (Fig. 4D). Furthermore, the interaction of ABCC5 with SLC7A11 was detected by co-IP assays in WT and SR cells (Fig. 4E). Western blot analysis revealed that SLC7A11 was highly expressed in sorafenib-resistant cells (Fig. 5A). In addition, knockdown of ABCC5 suppressed SLC7A11 protein expression in HuH7-SR cells (Fig.5B). Conversely, the suppression or overexpression of SLC7A11 decreased or increased the expression of ABCC5 (Fig. 5C). In addition, we used the autophagy inhibitor chloroquine (CQ) and the proteasome degradation inhibitor MG132 to confirm that ABCC5 protected SLC7A11 from being degraded by autophagy (Fig. S4). NRF2 negatively regulates ferroptosis by limiting ROS production and reducing cellular iron uptake [24]. Analysis using the STRING database indicated that NRF2 may interact with ABCC5 and SLC7A11, possibly as the intermediary (Fig. S5). Treatment with sorafenib in combination with ML385, brusatol, or MK571, but not with the SLC7A11 inhibitor erastin [25], reversed the overexpression of ABCC5 and SLC7A11 induced by sorafenib in HuH7-SR cells (Fig. 5D,E). Similarly, knockdown of ABCC5 increased GSH depletion, mitigated lipid peroxidation and GPx4 expression in HuH7-SR cells treated with sorafenib and/or erastin (Fig. 5F,G). In addition, intracellular cystine content indicated that ABCC5 could further enhance the uptake of Cystine by SLC7A11 protein and generation of GSH (Fig. S6). To test whether sonifenib resistance is mediated through NRF2/ABCC5/SLC7A11 axis, the expression of ABCC5 and ferroptosis-related proteins in HuH7-SR cells were examined by Western blot assays. Our data demonstrated that NRF2/ABCC5/SLC7A11 axis was inhibited in HuH7-SR cells treated with inhibitors LY294002, Brusatol, MK571, Erastin or RSL3, respectively (Fig. 5H). Thus, after being transcriptionally activated by NRF2, ABCC5 interacts with SCL7A11 to negatively regulate ferroptosis.
Fig. 5.
ABCC5 negatively regulates ferroptosis by stabilizing SLC7A11. (A) Western blot analysis determined the expression of SLC7A11 in HuH7-SR cells. (B,C) Western blot analysis detected the expression of ABCC5 and SLC7A11 in HuH7-SR cells after knockdown and overexpression of ABCC5. (D,E) HuH7-SR cells were treated with ML385 (5 μM), brusatol (50 nM),MK571 (10 μM), Erastin (10 μM) and RSL3 (3 μM) for 24 h, and the protein expression of ABCC5 and SLC7A11 in HuH7-SR cell lines were determined by qRT-PCR and Western blot assays. (F) The ABCC5-knockdown HuH7-SR cells were treated with sorafenib (10 µM) with or without Erastin(10 μM) for 24 h, and the levels of glutathione (GSH) and lipid ROS were assayed (n = 3, * P < 0.05 relative to the control group). (G) Western blot assays detected the protein expression of GPx4 in HuH7-SR cells . (H) HuH7-SR cells were treated with sorafenib (10 µM) with or without inhibitors (LY294002, 10 µM; Brusatol, 50 nM; MK571, 10 µM; Erastin, 10 µM; RSL3 3 µM) for 24 h. Western blot evaluated the protein expression of p-AKT, AKT, NRF2, ABCC5, SLC7A11 and GPx4. *P < 0.05, ** P < 0.01, # P > 0.05.
Knockdown of ABCC5 enhances the anti-cancer activity of sorafenib in resistant HCC cells in vivo
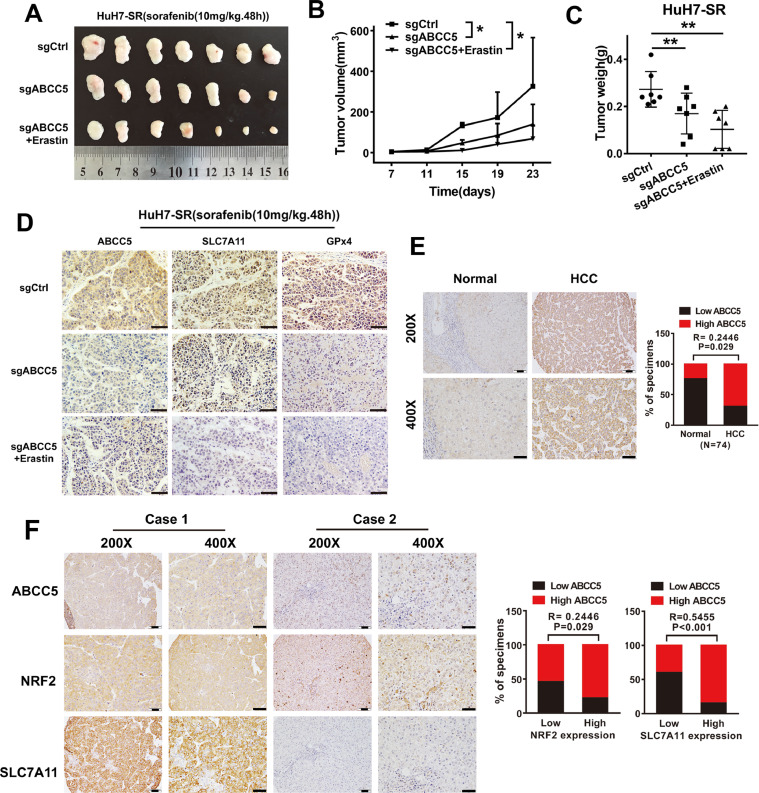
To determine whether inhibition of ABCC5 expression led to the resistance to sorafenib in vivo, the control Huh7-SR cells (i) and ABCC5-knockdown Huh7-SR cells (ii and iii) were implanted into the subcutaneous space of the right flank of nude mice in different groups. Beginning at day seven, the three groups were treated with sorafenib, and the iii group was also treated with erastin. Compared with the control group, sorafenib treatment effectively reduced the size and weight of tumors formed by ABCC5-knockdown cells in the ii group. Moreover, compared with the ii group, both the size and weight of tumors were reduced in iii group, which was additionally treated with erastin (Fig. 6A, B, C). IHC assays showed decreased expression of SLC7A11 and GPx4 in tumors of the ii and iii group mice (Fig. 6D).
Fig. 6.
Knocking down targeted ABCC5 enhances the anticancer activity of sorafenib in the resistant HCC cells in vivo. (A) Nude mice were injected subcutaneously with HuH7-SR cells (2 × 106 cells/mouse) and treated with sorafenib (10 mg/kg/i.p., once every other day) at day seven for two weeks (n = 7 mice/group). (B) Tumor volume of each mice was calculated every two days. (C) In addition, the tumor weight was measured at day 22 (*P < 0.05, **P < .01). (D) IHC staining detected the expression of ABCC5, SLC7A11, and GPx4 in tumor tissue formed by ABCC5-knockdown HuH7-SR cells treated with sorafenib with or without erastin. (E) IHC staining evaluated the expression of ABCC5 in normal liver and HCC tissues. (F) IHC staining examined the expression of NRF2, ABCC5 and SLC7A11 in the tissues of patients with primary HCC. Representative figures were shown. Scale bars, 50μm. ** P < 0.01, # P > 0.05.
Clinically, the TCGA analysis showed that ABCC5 expression was higher in HCC tissues than in normal liver tissues (Fig. S7, P < 0.05). Consistently, IHC assays confirmed higher ABCC5 expression in HCC tissues compared to normal liver tissues (Fig. 6E, P < 0.05). In addition, IHC analysis indicated that NRF2, ABCC5 and SLC7A11 expression were significantly correlated in HCC tissues (Fig. 6F, P < 0.05). Furthermore, Kaplan–Meier survival curves based on the TCGA data demonstrated that the OS of HCC patients with low ABCC5 expression was better than that of HCC patients with high ABCC5 expression (Fig. S8).
Discussion
Acquired resistance to sorafenib hinders the survival in patients with advanced HCC. In this study, we demonstrated that up-regulation of ABCC5 through PI3K/AKT/NRF2 pathway activation promoted acquired sorafenib resistance in human HCC cells, while cotreatment of LY294002 decreased sorafenib-induced ABCC5 expression. ABCC5 inhibited lipid peroxidation by stabilizing SLC7A11 protein and decreasing GPx4 depletion in the sorafenib-resistant HCC cells. Thus, blocking ABCC5 expression can enhance the anti-cancer activity of sorafenib by induction of ferroptosis in vitro and in vivo. In the present study, we explored the underlying specific mechanisms of increased ABCC5 expression in sorafenib-acquired resistant HCC cells.
Acquired resistance is mostly mediated by cytogenetic material. When exposed to anti-cancer drugs, cancer cells can change their metabolic pathways and induce gene mutations to prevent them from being suppressed or killed by drugs. The mechanisms of sorafenib resistance include: up-regulation of drug efflux transporters, inhibition of drug-metabolizing enzymes, down-regulation of cellular uptake transporters, target mutations, autophagy, the epithelial-mesenchymal transition (EMT), inhibition of ferroptosis, and tumor-initiating cell (TIC) and blood vessel selection [26], [27], [28], [29]. The signaling pathways involved in acquired sorafenib resistance generally include PI3K/AKT, JAK/STAT, and Ras/Raf/MEK/ERK [30,31]. Our data strongly suggested that sustained treatment with sorafenib at low concentration dramatically activated the PI3K/AKT/NRF2 pathway. Whether sorafenib might cause activation of other pathways needs to be further explored in future experiments. In addition, compared with acquired resistance to single drug, acquired multidrug resistance is one of the most important causes for chemotherapy failure. Drug efflux mediated by ABC family is the main cause for multi-drug resistance (MDR). It has been reported that the multidrug resistance gene 1 (MDR1, also known as ABCB1) and ABCG2 (a P-glycoprotein, P-gp) in the ABC family are closely related to chemotherapy resistance in liver cancer [32]. ABCC2 may be involved in the intrinsic resistance of liver cancer, ABCC3 and ABCC5 are associated with acquired drug resistance in HCC cells [33]. In addition, previous studies have suggested that MRPs, such as ABCC1 [34], ABCC2 [35], and ABCC3 [36], are closely correlated with sorafenib resistance. Furthermore, ABCC3, ABCC4 and ABCC5 form protein multimers and participate in drug efflux. It has been reported that ABCC5 is a drug efflux transporter that induces drug resistance [37]. However, our results showed that ABCC5 had a slight effect on sorafenib efflux, but metabolized sorafenib into M3, M4 and M5, thereby reducing the drug's intracellular toxicity in sorafenib-resistant HCC cells. Furthermore, ABCC5 was activated by intracellular transcription factor NRF2 and inhibited ferroptosis through interacting with SLC7A11. Multidrug resistance has always been a difficult problem for clinical treatment. Our study indicates that inhibition of ferroptosis is a new potential mechanism of multidrug resistance. We report for the first time that multidrug resistant molecules can further induce drug resistance by inhibiting ferroptosis, providing new ideas for clinical combination anti-tumor.
The ABC family are closely related to the SLC family, and they both assist and cotransport substances of cells. Evidence shows that sorafenib reduces the expression of GPx4 and resultes in ferroptosis. GPx4 is an enzyme that prevents detrimental phospholipid oxidation [38].The inactivation of GPx4 impedes the metabolism of lipid oxides in cells and causes reactive oxygen radical accumulation, which results in cell death and the release of pro-inflammatory mediators [39,40]. GSH, a cofactor for GPx4 activation, is a key substance in the clearance of clear intracellular ROS. SLC7A11 plays an important negative role in the process of ferroptosis. Down-regulation of SLC7A11 ultimately contributes to loss of intracellular cystine levels and subsequent depletion of glutathione biosynthesis, which indirectly inhibits GPx4 activity and activates ferroptosis [41].
Our current data indicated that ABCC5 is a transcriptional target of NRF2. Furthermore, during oxidative stress, NRF2 becomes unleashed from the Keap1 binding and transferred into the nucleus. There, NRF2 transcripts antioxidant response element ARE-dependent genes which encode antioxidant proteins and phase II detoxification enzymes. These protective proteins can scavenge oxygen free radicals, reduce peroxide levels, and protect against endothelial damage caused by oxidative stress [24,42]. Recent studies have found that NRF2 activation can inhibit apoptosis and thus participates in sorafenib resistance in various tumors [43]. Sorafenib is a specific inhibitor of SLC7A11 but increases the expression of NRF2. However, NRF2 can transcribe and activate the expression of SLC7A11 [43]. Our results suggested that NRF2 could not increase the expression of SLC7A11 at the mRNA level after sorafenib administration, but in sorafenib-resistant HCC cells, NRF2 enhanced the protein expression of SLC7A11, which was stabilized by ABCC5. Although our data preliminarily confirmed that ABCC5 bound to SLC7A11 and regulated its protein levels, additional research is needed to further explainhow ABCC5 stabilizes the SLC7A11 protein level in the future. In addition, our results demonstrated that sorafenib increased NRF2 expression levels and stabilized the overexpression of ABCC5. In addition to ABCC5, other NRF2 target genes, including MT-1G, quinone oxidoreductase 1, haem oxygenase-1, and FTH1, also inhibit sorafenib-induced ferroptosis [21,43]. Second, ABCC1, a molecule in the same family as ABCC5, has been shown to promote the occurrence of ferroptosis [44]. ABCC1 is a critical transporter for GSH efflux to promote ferroptosis, while ABCC5 does not transport GSH and is more likely to play a role in intracellular toxic molecule and drug efflux, which was confirmed in our study. Ferroptosis is a novel type of cell death that is characterized by iron-dependent lipid peroxidation. However, ABCC5 is an anion transporter associated with intracellular GSH metabolism, and iron metabolism is not involved in liver cancer cell death. Therefore, we did not evaluated iron metabolism specifically, such as iron metabolism genes (e.g., FTH1, TFR1, and DMT1) [25].
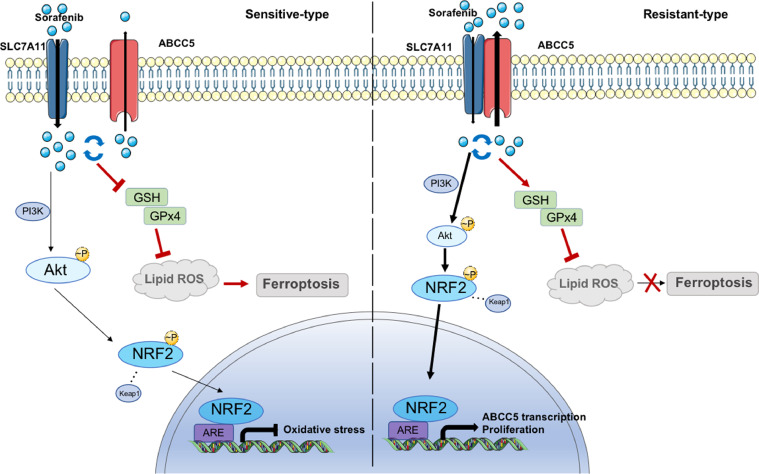
In conclusion, the regulation of cell death is an important factor in tumor development and anti-cancer efficacy. We demonstrated that ABCC5 accumulation protected HCC cells against sorafenib, and facilitated cancer progression by inhibiting lipid peroxidation-mediated ferroptosis (Fig. 7). Importantly, inhibition of ABCC5 expression and promotion of ferroptosis significantly enhanced the anti-cancer activity of sorafenib in vivo. Thus, regulation of ABCC5 expression to induce ferroptosis is a potential therapeutic strategy to overcome the acquired sorafenib resistance in HCC cells.
Fig. 7.
Sketch map illustrated the mechanism of ABCC5 in Sorafenib Resistance of HCC. Sorafenib induced ABCC5 expression via activating PI3K/AKT/NRF2 axis. Accumulation of ABCC5 increased intracellular glutathione (GSH) by interacting with and stabilizing SLC7A11, which attenuated lipid peroxidation and inhibited ferroptosis.
Declarations
Ethics approval and consent to participate
All experiments performed are endorsed by the Ethics Committee of Southern Medical University and complied with the Declaration of Helsinki. All animal experiments were carried out with the approval of the Southern Medical University Animal Care and Use Committee in accordance with the guidelines for the ethical treatment of animals. All animal experiments involved ethical and humane treatment under a license from the Guangdong Provincial Bureau of Science.
Consent for publication
No informed consent was required because data were analyzed anonymously.
Availability of data and material
Not applicable.
CRediT authorship contribution statement
Wenbin Huang: Investigation. Kunling Chen: Investigation. Yishi Lu: Investigation. Donghui Zhang: Resources, Visualization. Yuan Cheng: Resources, Visualization. Liuran Li: Validation, Formal analysis. Weimei Huang: Validation, Formal analysis. Guolin He: Resources, Visualization. Hangyu Liao: Validation, Formal analysis. Lei Cai: Data curation. Yujun Tang: Data curation. Liang Zhao: Conceptualization, Project administration, Funding acquisition, Writing – review & editing. Mingxin Pan: Methodology, Funding acquisition, Writing – original draft.
Declaration of Competing Interest
The authors have declared that no conflicts of interest exist.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82072627, 81972813) and Fork Ying Tung Education Foundation (161035).
Acknowledgments
We would like to thank AJE [aje.com] for English language editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.11.002.
Contributor Information
Wenbin Huang, Email: hwb1298971713@foxmail.com.
Kunling Chen, Email: 1987454446@qq.com.
Yishi Lu, Email: louis_ann@163.com.
Donghui Zhang, Email: 18819129896@163.com.
Yuan Cheng, Email: chengyuan9226@sohu.com.
Liuran Li, Email: 1012139428@qq.com.
Weimei Huang, Email: weimei135gx@163.com.
Guolin He, Email: dwtou@126.com.
Hangyu Liao, Email: Lhymarc@163.com.
Lei Cai, Email: cailei_427@163.com.
Yujun Tang, Email: tangyujun1994@sohu.com.
Liang Zhao, Email: liangsmu@foxmail.com.
Mingxin Pan, Email: pmxwxy@sohu.com.
Appendix. Supplementary materials
References
- 1.White D.L., Thrift A.P., Kanwal F., Davila J., El-Serag H.B. Incidence of hepatocellular carcinoma in All 50 United States, from 2000 through 2012. Gastroenterology. 2017;152:812–820. doi: 10.1053/j.gastro.2016.11.020. e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reig M., Torres F., Rodriguez-Lope C., Forner A., N L.L., Rimola J., Darnell A., Rios J., Ayuso C., Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–324. doi: 10.1016/j.jhep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Zhai B., Sun X.Y. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeley R.G., Cole S.P. Function, evolution and structure of multidrug resistance protein (MRP) Semin Cancer Biol. 1997;8:193–204. doi: 10.1006/scbi.1997.0070. [DOI] [PubMed] [Google Scholar]
- 5.Leandro K., Bicker J., Alves G., Falcao A., Fortuna A. ABC transporters in drug-resistant epilepsy: mechanisms of upregulation and therapeutic approaches. Pharmacol Res. 2019 doi: 10.1016/j.phrs.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Kathawala R.J., Gupta P., Ashby C.R., Chen Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Assaraf Y.G. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat. 2006;9:227–246. doi: 10.1016/j.drup.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Adema A.D., Floor K., Smid K., Honeywell R.J., Scheffer G.L., Jansen G., Peters G.J. Overexpression of MRP4 (ABCC4) and MRP5 (ABCC5) confer resistance to the nucleoside analogs cytarabine and troxacitabine, but not gemcitabine. Springerplus. 2014;3:732. doi: 10.1186/2193-1801-3-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen R.S., Mahakena S., de Haas M., Borst P., van de Wetering K. ATP-binding cassette subfamily C member 5 (ABCC5) functions as an efflux transporter of glutamate conjugates and analogs. J Biol Chem. 2015;290:30429–30440. doi: 10.1074/jbc.M115.692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt S., Shepard R.L., Kandasamy R.A., Johnston P.A., Perry W., 3rd A.H.Dantzig. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 11.Gillet J.P., Andersen J.B., Madigan J.P., Varma S., Bagni R.K., Powell K., Burgan W.E., Wu C.P., Calcagno A.M., Ambudkar S.V., Thorgeirsson S.S., Gottesman M.M. A gene expression signature associated with overall survival in patients with hepatocellular carcinoma suggests a new treatment strategy. Mol Pharmacol. 2016;89:263–272. doi: 10.1124/mol.115.101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd B.R.Stockwell. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badgley M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M., Decker A.R., Sastra S.A., Palermo C.F., Andrade L.R., Sajjakulnukit P., Zhang L., Tolstyka Z.P., Hirschhorn T., Lamb C., Liu T., Gu W., Seeley E.S., Stone E., Georgiou G., Manor U., Iuga A., Wahl G.M., Stockwell B.R., Lyssiotis C.A., Olive K.P. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sui X., Zhang R., Liu S., Duan T., Zhai L., Zhang M., Han X., Xiang Y., Huang X., Lin H., Xie T. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Yang Y., Xu C., Gao S. A flow cytometry-based assay for measuring mitochondrial membrane potential in cardiac myocytes after hypoxia/reoxygenation. J Vis Exp. 2018;137 doi: 10.3791/57725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Wang Q., Liu J., Cao H. Inhibition of the PI3K/Akt signaling pathway reverses sorafenib-derived chemo-resistance in hepatocellular carcinoma. Oncol Lett. 2018;15:9377–9384. doi: 10.3892/ol.2018.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahraman D.C., Kahraman T., Cetin-Atalay R. Targeting PI3K/Akt/mTOR pathway identifies differential expression and functional role of IL8 in liver cancer stem cell enrichment. Mol Cancer Ther. 2019;18:2146–2157. doi: 10.1158/1535-7163.MCT-19-0004. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Pan W., Miao L., Lin Y., Huang X., Ge X., Moosa S.L., Liu B., Ren M., Zhou Q., Liang H., Zhang W., Pan L. Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala) Fish Shellfish Immunol. 2017;70:66–75. doi: 10.1016/j.fsi.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Li S.T., Dai Q., Zhang S.X., Liu Y.J., Yu Q.Q., Tan F., Lu S.H., Wang Q., Chen J.W., Huang H.Q., Liu P.Q., Li M. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018;39:1294–1304. doi: 10.1038/aps.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai T., Li M., Liu Y., Qiao Z., Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23 doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Y., Zhao B., Zhou L., Zhang Z., Shen Y., Lv H., AlQudsy L.H.H., Shang P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–136. doi: 10.1016/j.canlet.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoccoli G., Miele L., Oben J., Grieco A., Vinciguerra M. Biology, epidemiology, clinical aspects of hepatocellular carcinoma and the role of sorafenib. Curr Drug Targets. 2016;17:783–799. doi: 10.2174/1389450117666151209120831. [DOI] [PubMed] [Google Scholar]
- 27.Louandre C., Ezzoukhry Z., Godin C., Barbare J.C., Maziere J.C., Chauffert B., Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 28.Luo T., Fu J., Xu A., Su B., Ren Y., Li N., Zhu J., Zhao X., Dai R., Cao J., Wang B., Qin W., Jiang J., Li J., Wu M., Feng G., Chen Y., Wang H. PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy. 2016;12:1355–1371. doi: 10.1080/15548627.2015.1034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han T., Zhang Y., Yang X., Han L., Li H., Chen T., Zheng Z. miR-552 regulates liver tumor-initiating cell expansion and sorafenib resistance. Mol Ther Nucleic Acids. 2020;19:1073–1085. doi: 10.1016/j.omtn.2019.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K.F., Tai W.T., Hsu C.Y., Huang J.W., Liu C.Y., Chen P.J., Kim I., Shiau C.W. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur J Med Chem. 2012;55:220–227. doi: 10.1016/j.ejmech.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Zhou X., Shen H., Wang D., Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med. 2009;7:41. doi: 10.1186/1741-7015-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W., Li J., Wang R., Xie H., Jia Z. MDR1 will play a key role in pharmacokinetic changes under hypoxia at high altitude and its potential regulatory networks. Drug Metab Rev. 2015;47:191–198. doi: 10.3109/03602532.2015.1007012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Fang C.H., Fan Y.F. Detection of multidrug resistance-associated proteins MRP2, MRP3, and MRP5 mRNA expressions in hepatocarcinoma cells using SYBR real-time PCR. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:219–221. [PubMed] [Google Scholar]
- 34.Ding J., Zhou X.T., Zou H.Y., Wu J. Hedgehog signaling pathway affects the sensitivity of hepatoma cells to drug therapy through the ABCC1 transporter. Lab Invest. 2017;97:819–832. doi: 10.1038/labinvest.2017.34. [DOI] [PubMed] [Google Scholar]
- 35.Shibayama Y., Nakano K., Maeda H., Taguchi M., Ikeda R., Sugawara M., Iseki K., Takeda Y., Yamada K. Multidrug resistance protein 2 implicates anticancer drug-resistance to sorafenib. Biol Pharm Bull. 2011;34:433–435. doi: 10.1248/bpb.34.433. [DOI] [PubMed] [Google Scholar]
- 36.Tomonari T., Takeishi S., Taniguchi T., Tanaka T., Tanaka H., Fujimoto S., Kimura T., Okamoto K., Miyamoto H., Muguruma N., Takayama T. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget. 2016;7:7207–7215. doi: 10.18632/oncotarget.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Wang Z., Gao S., Wu K., Bai F., Zhang Q., Wang H., Ye Q., Xu F., Sun H., Lu Y., Liu Y. Human drug efflux transporter ABCC5 confers acquired resistance to pemetrexed in breast cancer. Cancer Cell Int. 2021;21:136. doi: 10.1186/s12935-021-01842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mou Y., Wang J., Wu J., He D., Zhang C., Duan C., Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., Thomas A.G., Gleason C.E., Tatonetti N.P., Slusher B.S., Stockwell B.R. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T., Jiang L., Tavana O., Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79:1913–1924. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H., Zhang Q., Li W., Li H., Bao J., Yang C., Wang A., Wei J., Chen S., Jin H. Role of Nrf2 in the antioxidation and oxidative stress induced developmental toxicity of honokiol in zebrafish. Toxicol Appl Pharmacol. 2019;373:48–61. doi: 10.1016/j.taap.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Sun X., Niu X., Chen R., He W., Chen D., Kang R., Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao J.Y., Poddar A., Magtanong L., Lumb J.H., Mileur T.R., Reid M.A., Dovey C.M., Wang J., Locasale J.W., Stone E., Cole S.P.C., Carette J.E., Dixon S.J. A genome-wide haploid genetic screen identifies regulators of glutathione abundance and ferroptosis sensitivity. Cell Rep. 2019;26:1544–1556. doi: 10.1016/j.celrep.2019.01.043. e1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.