Abstract
Introduction
Metastatic heart tumors are rare, occurring in 1.5–20% of cancer patient autopsies. Lymphoma, melanoma, leukemia, and carcinomas of the lung, esophagus, and breast are the most prevalent causes of these metastases, although they can originate from any malignant tumor. Here we report a case of triple-negative breast cancer with cardiac metastasis mimicking myxoma.
Presentation of case
A 39-year-old woman presented at the emergency department with shortness of breath. Vital signs were hypotension and tachypnea. There were coarse crackles at the bases of both lungs. Electrocardiography results showed a normal sinus rhythm. Chest X-ray revealed cardiomegaly with signs of pulmonary edema. Echocardiography revealed a large left atrial (LA) mass protruding to the mitral valve and attached to the interatrial septum during diastole. The patient was diagnosed with cardiogenic shock, acute kidney injury, elevated liver enzymes, and an LA mass. Surgical excision through median sternotomy was planned. Intraoperatively, an LA mass was found. The histopathology evaluation showed an LA mass with invasive ductal carcinoma of metastatic breast tumors. Immunohistochemistry (IHC) confirmed the diagnosis of triple-negative breast cancer that had metastasized to the heart. Postoperative echocardiography confirmed complete excision of the tumor.
Discussion
Breast cancer that has metastasized to the heart is uncommon. This patient was referred to the surgical oncology section for the treatment of triple-negative breast cancer with cardiac metastasis.
Conclusion
A heart mass should be suspected of having metastasized if the patient has a history of malignancy, even if it occurred several years earlier.
Keywords: Breast cancer, Cardiac tumor, Case report, Echocardiography, Left atrium, Myxoma
Highlights
-
•
Metastatic heart tumors are rare.
-
•
Melanoma, and lung and breast carcinomas, are the most prevalent causes.
-
•
In this case, cardiac metastasis of triple-negative breast cancer mimicked myxoma.
1. Introduction
Cardiac tumors are uncommon, with an incidence of 0.001% to 0.03% in the general population [1], [2]. More than 70% of tumors are benign. Metastatic cardiac tumors are rare, occurring in 1.5–20% of cancer patient autopsies. Lymphoma, melanoma, leukemia, and carcinomas of the lung, esophagus, and breast, are the most prevalent causes of these metastases, although they can originate from any malignant tumor. The majority of cardiac secondary tumors are clinically asymptomatic (over 90%) and they are commonly found postmortem [3]. Tumor metastases can reach the heart via lymphatic or hematogenous pathways, or by direct extension. Lymphatic spread is the most common route [4]. To our knowledge, this is the first report from Indonesia of a case of triple-negative breast cancer with metastasis to the heart mimicking a myxoma to be published under the 2020 Surgical Case Report guidelines [5].
2. Presentation of case
A 39-year-old woman presented at the emergency department complaining of shortness of breath during the previous 3 days, despite having minimal activity levels and orthopnea; in the previous 2 weeks, the patient had also complained of paroxysmal nocturnal dyspnea. These symptoms were accompanied by a feeling of weakness in the body. There was no chest pain or palpitations. Nausea was present, but there was no vomiting. The patient had experienced shortness of breath 3 months previously. In 2007, she was diagnosed with invasive ductal breast carcinoma and received six cycles of chemotherapy. The patient underwent right-breast mastectomy in 2010. There was no family history of heart disease.
Vital signs were as follows: blood pressure 80/50 mm Hg; pulse rate 94 beats per minute; respiratory rate 24 breaths per minute; and temperature 36.5 °C. There was an increase in central venous pressure R of +4 cmH2O (at 30°), vesicular breath sounds, coarse crackles in the bases of both lungs, no wheezing, regular S1/S2 heart sounds, and no murmur. The lower extremities exhibited cold leg and no edema. Electrocardiography showed a sinus rhythm at 93 beats per minute that was regular with normal cardiac axis and low voltage.
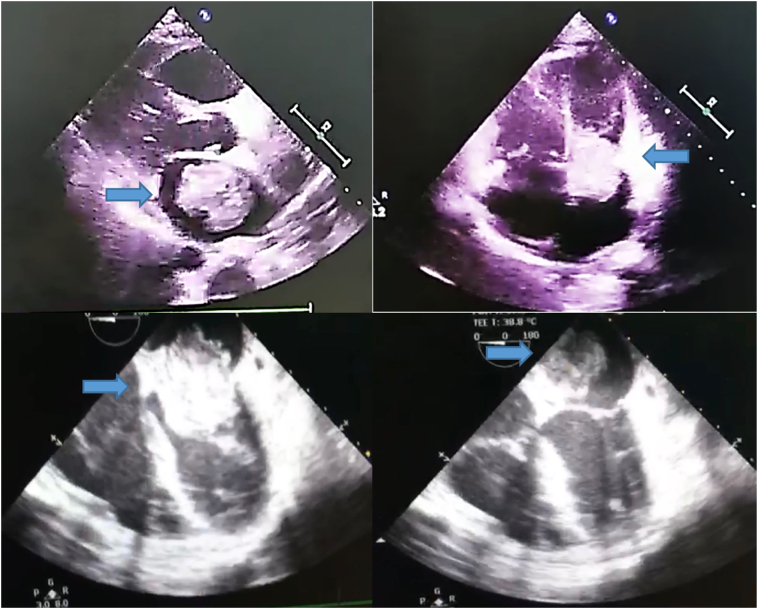
Laboratory results were as follows: leukocytosis 15.200 103/mm3; urea 183 mg/dL; creatinine 5.09 mg/dL; serum glutamic pyruvic transaminase 819 U/L; and serum glutamic oxaloacetic transaminase 663 U/L. A chest X-ray with an erect posture showed results within normal limits. Echocardiography examination revealed the following: a large LA mass mimicking a myxoma protruding to the mitral valve and attached to the interatrial septum during diastole with a significant gradient of 9 mm Hg; moderate tricuspid regurgitation with an intermediate probability of pulmonary hypertension; right atrial (RA), right ventricular, and LA dilatation; normal left ventricular systolic function; an ejection fraction of 60% (according to Simpson's Biplane method); normal right ventricular systolic function; tricuspid annular plane systolic excursion (TAPSE) of 1.9 cm; and mild pericardial effusion (Fig. 1).
Fig. 1.
Transthoracic (above) and transesophageal (below) echocardiography showing a mass mimicking a myxoma in the left atrium (arrows).
The working diagnosis for this patient was cardiogenic shock, acute kidney injury, elevated liver enzymes, and LA mass. She was given an intravenous norepinephrine 0.05 μg/body weight/min. The patient underwent emergency surgery. Thoracic and cardiovascular surgeons removed the LA mass through standard open-heart surgery with a heart–lung machine. The LA mass (Fig. 2A and B) was reached through openings in the RA and interatrial septum. Approximately 1 cm2 of the interatrial septum to which the mass was attached was also excised and removed. The interatrial septum defect was closed by primary suturing without the use of a pericardial patch. The histopathology evaluation obtained from the LA mass was metastatic invasive ductal carcinoma of the breast (Fig. 3). Immunohistochemistry (IHC) showed negative results for the estrogen receptor (ER), progesterone receptor (PR), and human epidermal receptor protein-2 (HER2) (Fig. 4); this confirmed the diagnosis of triple-negative breast cancer that had metastasized to the heart.
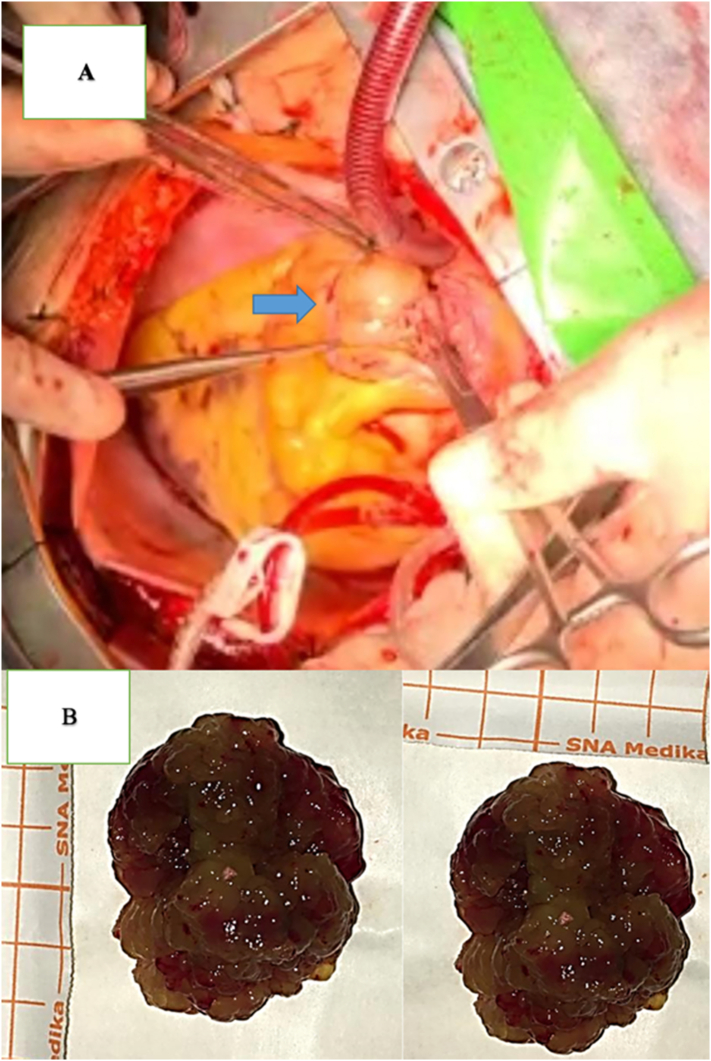
Fig. 2.
A) Opening the left atrium with mass attached to the interatrial septum. B) Post extirpation LA mass. Characteristics included a size of 5 × 4 cm, solid form, dark-red color, soft and irregular borders, and hemorrhagic spots.
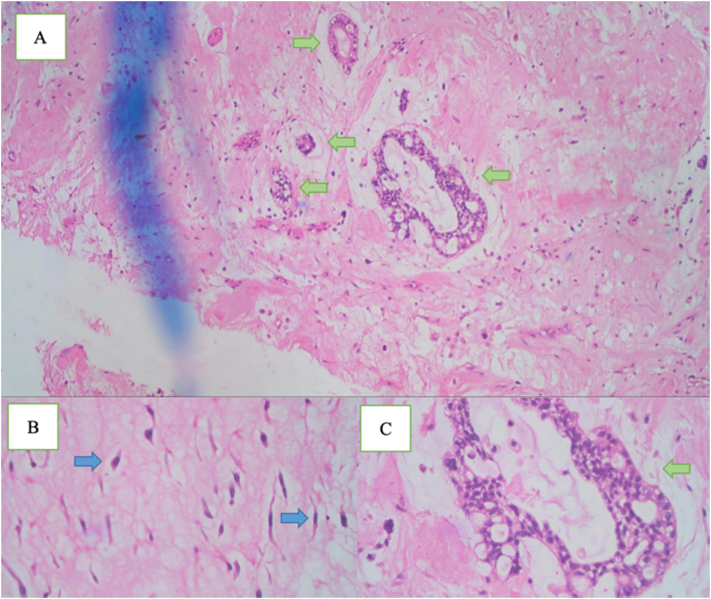
Fig. 3.
Histopathological examination showing pale myoid areas with stellate cells (blue arrows) in between foci of epithelial tumor nests and atypical nuclei forming ductal structures (green arrows). Hematoxylin eosin magnification A) ×4, B) ×100, and C) ×40.
Fig. 4.
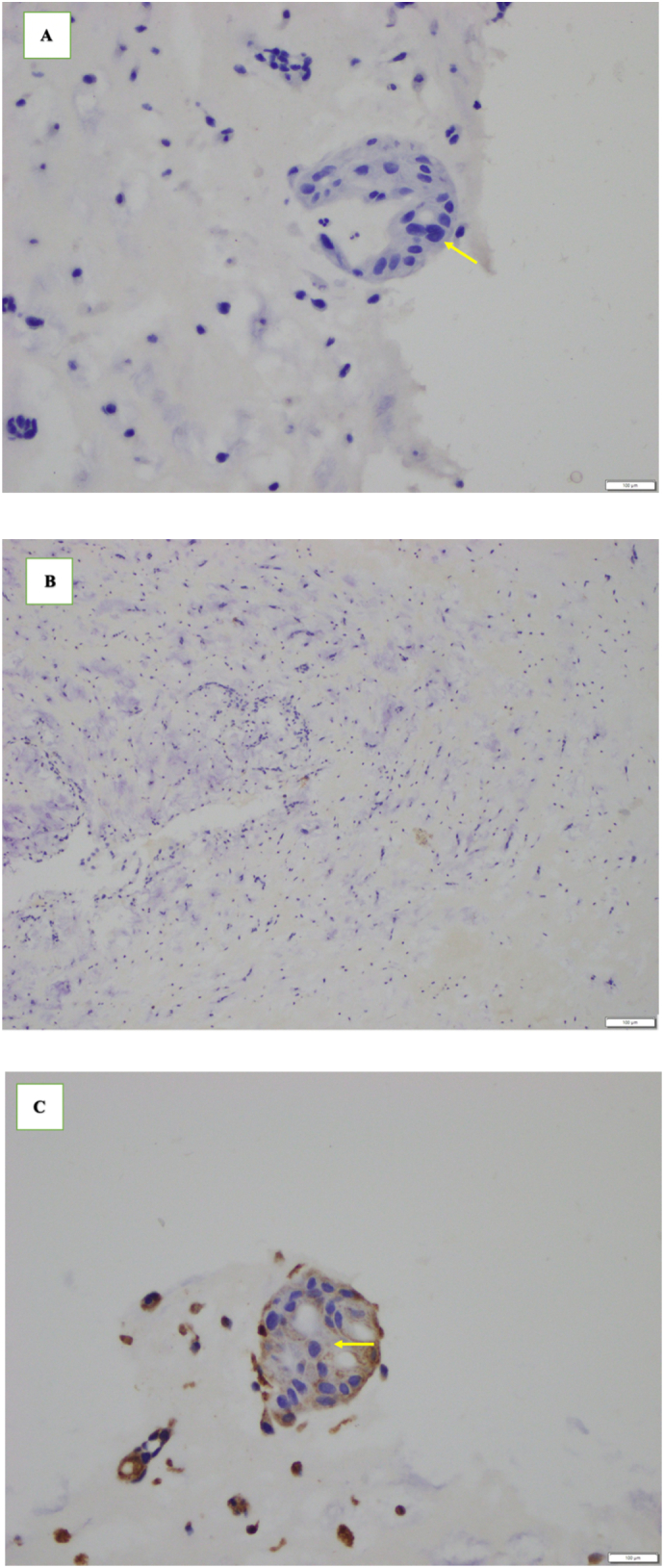
IHC findings. A) Negative staining of ER protein in tumor cell nuclei (arrow) (magnification ×40). B) Negative staining of PR protein in tumor-cell nuclei (magnification ×10). C) Negative staining of HER2 protein expression in the cytoplasmic membrane of tumor cells (arrow) (magnification ×40).
Postoperative echocardiography confirmed the complete excision of the mass (Fig. 5). An outpatient consultation on the 10th postoperative day determined that the recovery was progressing well with no complaints of shortness of breath or orthopnea. The renal function test and liver function test results were normal. The patient was then referred to the oncology division for further management of the metastatic breast cancer.
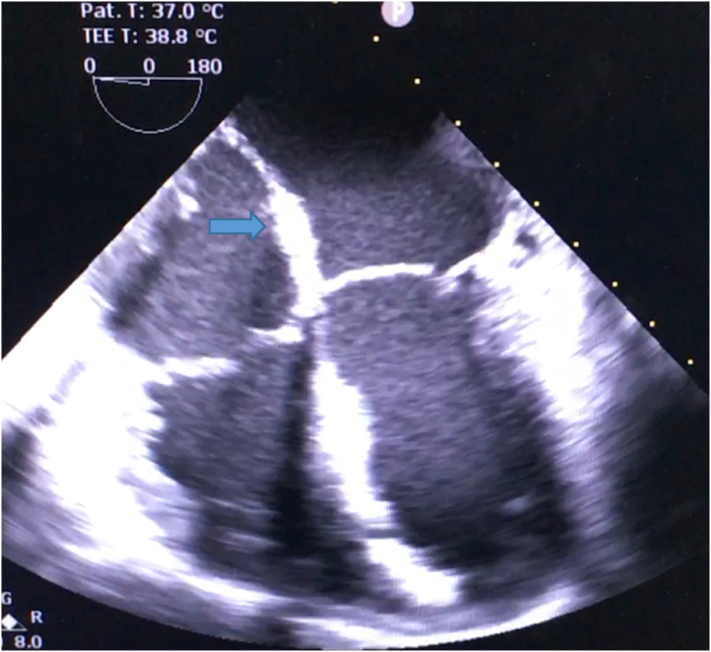
Fig. 5.
Transesophageal echocardiography post-excision of LA mass (arrow).
3. Discussion
One of the least studied phenomena in clinical and experimental oncology is malignant tumors that metastasize to the heart. The low incidence of cardiac cancer was formerly attributed to the fact that the heart was not susceptible to malignant cells. Although no malignant tumors are known to preferentially spread to the heart, this condition has been linked to breast cancer, esophageal and lung cancer, lymphoproliferative malignancies, and melanoma [4], [6].
Although primary cardiac tumors are uncommon, secondary tumors are more prevalent, and any type of cancer can spread to the heart. So far, only central nervous system tumors have been shown not to spread to the heart. Cardiac metastases have an unknown incidence. Secondary deposits are rare; however when they are found, they frequently occur in the context of a widespread disease. According to the ‘seed and soil’ theory, it is difficult for cancer cells to survive outside of their original territory, so they require a location with similar features for metastasis [6].
There are four ways for tumors to spread to the heart: 1) through the bloodstream; 2) through the lymphatic system; 3) through intracavitary diffusion via the inferior vena cava or pulmonary veins; and 4) through direct extension.
Clinical distinctions should be made based on which heart structure is affected first. Pericardial involvement can occur as a result of direct invasion by a mediastinal or intrathoracic tumor, retrograde bronchomediastinal lymphatic channels or lymphatic dissemination through tracheal, or secondary involvement by epicardial or myocardial metastases [4].
Involvement of the heart chambers is uncommon, and the symptoms of intracardiac and venous involvement are frequently minimal compared to the extensive involvement seen. Tumor emboli that reach the chambers can cause valvular insufficiency, valve stenosis, or heart failure by direct implantation on the endocardium. Metastatic deposits may generate stenosis-related murmurs that alter with posture. Syncope and sudden death have also been reported [4], [6], [7].
Tumor metastatic spread is a well-coordinated series of events in which cells that are shed from primary tumors enter the bloodstream and move to distant organs. However, this method is inefficient, as the majority of cells are expected to die during dispersion. Some disseminated tumor cells (DTCs) will begin to proliferate and colonize the new environment right away, while others may enter a growth-arrested state while they are still viable [8]. Dormant cells suspend their growth and can remain alive, and clinically undetectable, for long periods of time. Even after an apparently successful treatment of the main tumor, latent cells can reawaken, and resume proliferation and colonization many years later [8], [9]. Tumor metastasis can occur years after an apparent cure due to a phenomenon known as metastatic tumor dormancy, in which tumor masses or individual tumor cells are growth restricted for extended periods of time. Several factors, including cancer therapies, metastasis suppressor gene activity, and tumor microenvironment factors like cytokine production, angiogenesis, and immunosurveillance, all contribute to this period of dormancy.
Clinical manifestations are often asymptomatic and manifest only when the mass size is large enough to interfere with the function of the mitral valve causing obstruction or regurgitation, which can predispose the patient to atrial fibrillation [10], [11]. Often this cardiac tumor is incidentally identified upon transthoracic echocardiography [11]. Shortness of breath is the most common symptom, and can worsen when lying on the left-hand side, further increasing the suspicion of an LA tumor. In addition, the signs and symptoms that arise can be caused by obstruction or regurgitation of the mitral valve, such as shortness of breath and pulmonary edema [12]. Patients can also complain of non-specific systemic symptoms such as fatigue, cough, fever, arthralgia, and myalgia [13]. The degree of tumor metastases to the heart and clinical symptoms had no strong relationship. Even with extensive heart metastases, some cancers are asymptomatic and are only found accidentally. Typically, cardiac involvement is not discovered until the patient has passed away. Only around 10% of patients with metastatic illness who died had symptoms or signs of heart failure, according to several retrospective studies [14].
On physical examination, in 64–67% of cases, a diastolic murmur was detectable. A tumor plop sound was more specific for diagnosis; however, it was detected in only 15% of cases [15]. On examination of the lungs, fine wet crackles suggested pulmonary congestion leading to pulmonary edema [16]. In the present case, the patient's symptoms were shortness of breath, especially during activity, and minimal crackles at the bases of both lungs, with no murmurs or additional heart sounds.
The diagnostic significance of electrocardiography and chest X-ray is limited. Electrocardiography may reveal atrial fibrillation and bundle branch block. Chest X-ray can identify cardiomegaly caused by atrial dilation. Currently, echocardiography is the main diagnostic modality of atrial tumor, which can determine its location, shape, size, and relationship to the structure of the heart [13]. Transthoracic echocardiography is the most widely used modality; however, transesophageal echocardiography is more sensitive and specific because it can detect tumors of only 1–3 mm in size. Transthoracic echocardiography examination of this patient found a mass in the left atrium, which was pedunculated and mobile; there was also dilatation of the left atrium, right ventricle, and RA, suggesting obstruction of an LA tumor leading to functional mitral stenosis. After the diagnosis of cardiac tumor has been confirmed by imaging studies, resection of the tumor is necessary because of the risk of embolization or cardiovascular complications including sudden death [12].
The definitive treatment for metastatic breast cancer is systemic therapy (endocrine therapy, chemotherapy, targeted drugs, immunotherapy, or a combination of surgery and/or radiation therapy) [17], [18], [19]. In this patient, surgery was planned because the mass mimicked a myxoma on echocardiography examination, and clinical manifestations of shortness of breath and decreased cardiac output, which impacted functional mitral stenosis. However, histopathologic examination revealed an incidental finding of triple-negative breast cancer that had metastasized to the heart.
In this case, echocardiography after surgery showed no functional mitral jet stenosis, although mild mitral regurgitation was seen. The patient was recommended to undergo transthoracic echocardiography control annually to monitor the possibility of recurrence. Surgical removal of the tumor was performed to alleviate the patient's symptoms, although this did not guarantee survival due to distant-metastatic breast cancer.
According to the IHC study, this patient's breast cancer was classified as the triple-negative subtype. Modern genetic and immunohistochemical approaches have increased understanding of breast cancer biology and the ability to classify breast tumors into discrete subgroups, such as hormone-receptor-positive kinds (luminal A and luminal B), HER2-positive, and normal-like or basal-like. This last group is classified as ‘triple-negative’ breast cancers because the tumors do not stain for ER, PR, or HER2 [20].
Molecular studies of basal breast or triple-negative cancers have revealed proteins that are specific to the phenotype, in addition to ER, PR, and HER2. Some of these or other proteins are likely to play a role in developing visceral metastases. Triple-negative cancers have many of the same characteristics as rapidly dividing malignancies, such as a high mitotic index, the expression of proteins linked to cellular proliferation, and high histologic grade [20]. However, it is still unclear whether there is a relationship between the triple negative subtype and an affinity for cardiac metastases.
4. Conclusion
Cardiac metastasis of breast cancer is rare and has not previously been reported in Indonesia. Patients can be asymptomatic for years. Surgical resection may be required in symptomatic cases. All cardiac masses should be suspected as metastatic if the patient has a history of malignancy, even if it occurred several years earlier. Currently, there is no known direct relationship between triple-negative breast cancer and the incidence of cardiac metastases. Triple-negative breast cancer, by contrast, has the worst prognosis and a larger risk of distant metastases than other kinds of breast cancer.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
The study is exempt from ethical approval in our institution.
Funding
No funding or sponsorship.
Guarantor
Muhammad Nuralim Mallapasi and Jayarasti Kusumanegara.
Research registration number
Not applicable – single case report.
CRediT authorship contribution statement
Jayarasti Kusumanegara, Muhammad Nuralim Mallapasi, Umar Usman, and Peter Kabo: study concept and surgical therapy for this patient. Jayarasti Kusumanegara and Mario Tri Mulyono: Data collection and Writing-Original draft preparation. Muhammad Nuralim Mallapasi and Peter Kabo: senior author and the manuscript reviewer. Jayarasti Kusumanegara and Muhammad Faruk: Editing and Writing. All authors read and approved the final manuscript.
Declaration of competing interest
Nothing to declare.
Acknowledgment
We would like to express our appreciation to Agung Sindu Pranoto MD and Robert Christeven MD, for their contribution in reviewing this case report, Imeldy Prihatni Purnama MD and Futriani MD, for their contribution in immunohistochemistry image in this case report, Bayu Satria MD for his help in providing us with the linguistic assistance for this case report, and Manwan Basra, Ahmad, Amran Nurfataillah, Nurkamar, Fitriyana, and Ernawati Abbas, as the operating theater staff at Wahidin Sudirohusodo Hospital, Makassar, Indonesia.
Contributor Information
Muhammad Nuralim Mallapasi, Email: nuralim811@gmail.com.
Jayarasti Kusumanegara, Email: jayarasti@gmail.com.
Peter Kabo, Email: drpeterkabo@gmail.com.
Umar Usman, Email: justumarusman@gmail.com.
Mario Tri Mulyono, Email: mariotrimulyono@gmail.com.
Muhammad Faruk, Email: faroex8283@gmail.com.
References
- 1.Kataoka S., Otsuka M., Goto M., Kahata M., Kumagai A., Inoue K., et al. Primary multiple cardiac myxomas in a patient without the carney complex. J. Cardiovasc. Ultrasound. 2016;24(1):71. doi: 10.4250/jcu.2016.24.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossert T. Surgical experience with 77 primary cardiac tumors. Interact. Cardiovasc. Thorac. Surg. 2005;4(4):311–315. doi: 10.1510/icvts.2004.103044. [DOI] [PubMed] [Google Scholar]
- 3.Burazor I., Aviel-Ronen S., Imazio M., Goitein O., Perelman M., Shelestovich N., et al. Metastatic cardiac tumors: from clinical presentation through diagnosis to treatment. BMC Cancer. 2018;18:202. doi: 10.1186/s12885-018-4070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Katalinic D., Stern-Padovan R., Ivanac I., Aleric I., Tentor D., Nikolac N., et al. Symptomatic cardiac metastases of breast cancer 27 years after mastectomy: a case report with literature review - pathophysiology of molecular mechanisms and metastatic pathways, clinical aspects, diagnostic procedures and treatment modalities. World J. Surg. Oncol. 2013;11:1–10. doi: 10.1186/1477-7819-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussani R., Abbate A., Silvestri F. Cardiac metastases. J. Clin. Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osisami M., Keller E. Mechanisms of metastatic tumor dormancy. J. Clin. Med. 2013;2:136–150. doi: 10.3390/jcm2030136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 10.Fakhari S., Bilehjani E. A large left ventricle myxoma: presenting with epigastric pain and weight loss. Case Rep. Cardiol. 2016;2016:1–4. doi: 10.1155/2016/9018249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazir A., Douthwaite H. Rapidly growing left atrial myxoma: a case report. J. Med. Case Rep. 2011;5:417. doi: 10.1186/1752-1947-5-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Türkmen N., Eren B., Fedakar R., Çomunoglu N. An unusual cause of sudden death: cardiac myxoma. Adv. Ther. 2007;24:529–532. doi: 10.1007/BF02848775. [DOI] [PubMed] [Google Scholar]
- 13.Shabab S., Erfanzadeh M., Ahmadian S., Mahmoudabady M., Mazloum N. A case report of left atrial myxoma presenting with amnesia. BMC Cardiovasc. Disord. 2021;21:225. doi: 10.1186/s12872-021-02036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gošev I., Paić F., Đurić Ž., Gošev M., Ivčević S., Jakuš F.B., et al. Cardiac myxoma the great imitators: comprehensive histopathological and molecular approach. Int. J. Cardiol. 2013;164:7–20. doi: 10.1016/j.ijcard.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Leo S., Yang K., Weng C., Liang Z. Large atrial myxoma mimicking severe mitral stenosis associated with right heart enlargement and severe pulmonary hypertension. Cardiovasc. Diagn. Ther. 2013;3:52–54. doi: 10.3978/j.issn.2223-3652.2013.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramasamy K.A., Onal F., Pell J., Kumar P., Vassallo M. Left atrial myxoma presenting with acute pulmonary oedema in an elderly woman. Eur. J. Intern. Med. 2002;13:206–209. doi: 10.1016/s0953-6205(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 17.Liedtke C., Kolberg H.-C. Systemic therapy of Advanced/Metastatic breast cancer - current evidence and future concepts. Breast Care. 2016;11:275–281. doi: 10.1159/000447549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prihantono M. Faruk, breast cancer resistance to chemotherapy: when should we suspect it and how can we prevent it? Ann. Med. Surg. 2021;70 doi: 10.1016/j.amsu.2021.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gradishar W.J., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 20.Dent R., Hanna W.M., Trudeau M., Rawlinson E., Sun P., Narod S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]