Abstract
Background
Freezing of gait (FOG) is a highly incapacitating symptom that affects many people with Parkinson's disease (PD). Cueing triggered upon real-time FOG detection (on-demand cueing) shows promise for FOG treatment. Yet, the feasibility of implementation and efficacy in daily life is still unknown. Therefore, this study aims to investigate the effectiveness of DeFOG: a smartphone and sensor-based on-demand cueing solution for FOG.
Methods
Sixty-two PD patients with FOG will be recruited for this single-blind, multi-center, randomized controlled phase II trial. Patients will be randomized into either the intervention group or the active control group. For four weeks, both groups will receive feedback about their physical activity using the wearable DeFOG system in daily life. In addition, the intervention group will also receive on-demand auditory cueing and instructions. Before and after the intervention, home-based assessments will be performed to evaluate the primary outcome, i.e., “percentage time frozen” during a FOG-provoking protocol. Secondary outcomes include the training effects on physical activity monitored over 7 days and the user-friendliness of the technology.
Discussion
The DeFOG trial will investigate the effectiveness of personalized on-demand cueing in a controlled design, delivered for 4 weeks in the patient's home environment. We anticipate that DeFOG will reduce FOG to a greater degree than in the control group and we will explore the impact of the intervention on physical activity levels. We expect to gain in-depth insight into whether and how patients control FOG using cueing methods in their daily lives.
Trial registration
Clinicaltrials.gov NCT03978507.
Keywords: Parkinson's disease, Freezing of gait, Detection of freezing of gait, On-demand cueing, Home environment
1. Background
Freezing of gait (FOG) is a disabling motor disorder in Parkinson's disease (PD), defined as ‘a sudden and brief episode of inability to produce effective forward stepping despite the intention to do so’ [1]. The overall prevalence of people with FOG is 40% [2], and approximately 61–69% of falls are related to FOG [3,4]. The emergence of FOG gives rise to depression and anxiety [5,6] and has a large impact on quality of life [7]. Parkinsonian medication may alleviate FOG, but as the disease progresses, FOG becomes less responsive to medication [8,9]. Alternative treatment options have been shown to be insufficiently efficacious so far [10], underscoring the importance of developing alternative strategies [11].
Patients with PD rely on attentional resources to control their gait due to deficits in automaticity [12,13]. Consequently, FOG is more likely to occur when walking through complex circumstances [14]. Cueing, defined as facilitating movement execution by providing patients with external temporal or spatial stimuli as motor targets [15], is a promising strategy to reduce FOG [16]. Cueing may assist in the shift from automatic to goal-directed motor control [13,17], offering an external sensory reference to re-initiate gait. Considering the high prevalence of non-motor symptoms in PD [18,19], cueing may also facilitate the necessary executive function and attention to overcome FOG. Alternatively, internal strategies, such as prompting oneself to perform mediolateral weight shifting before stepping, may also reduce FOG [[20], [21], [22]]. However, it is unknown if patients remember to employ such rescue strategies without external input.
Although the positive effects of cueing on PD gait are well-known, the effects on FOG are less clear [17,23,24]. Several explanations exist. First, many studies were of insufficient quality and pooling them was difficult due to variable study designs [23,25]. Second, self-reported measures were often used to evaluate the effects, such as the New Freezing of Gait Questionnaire (NFOG-Q) [25]. However, the minimal detectable change of the NFOG-Q precludes a sensitive estimation of small effects [26,27]. Third, cueing was mostly delivered during one session [[28], [29], [30], [31]], disregarding previously shown low retention effects after short cueing periods [15,32]. Finally, cueing is often provided as a one-size-fits-all solution, contradicting the understanding that FOG is a very heterogeneous phenomenon [14,33].
As many unanswered questions remain regarding the feasibility, implementation and efficacy of cues for FOG, the current phase II study will test a novel method to integrate cues in patients’ daily lives. DeFOG is a low-cost, wearable, sensor-based device that provides personalized auditory cues in an on-demand manner, i.e., only when FOG is detected. We hypothesize that using DeFOG will reduce the percentage time frozen (%TF), the primary outcome, in the experimental group to a greater degree than in the control group, who will use the same system configured to give feedback on daily step counts only. Additionally, we will examine the impact of DeFOG on mobility and user-experience. Altogether, this study will significantly further our knowledge on the use of cueing to reduce FOG in the daily lives of patients with PD.
2. Methods/design
2.1. Study design and setting
This randomized controlled trial (RCT) (clinicaltrials.gov NCT03978507) uses a parallel, single-blind, multi-centered design (see Fig. 1), in which the effects of DeFOG will be compared to an active control group that only receives feedback about physical activity. The study will include a power-based sample of 62 patients with Parkinson's disease (PD) with freezing of gait (FOG) and is entirely situated in each patient's home and community setting. Sampling is divided equally over the sites: the Tel Aviv Sourasky Medical Center, Israel (TASMC) and KU Leuven, Belgium (KUL).
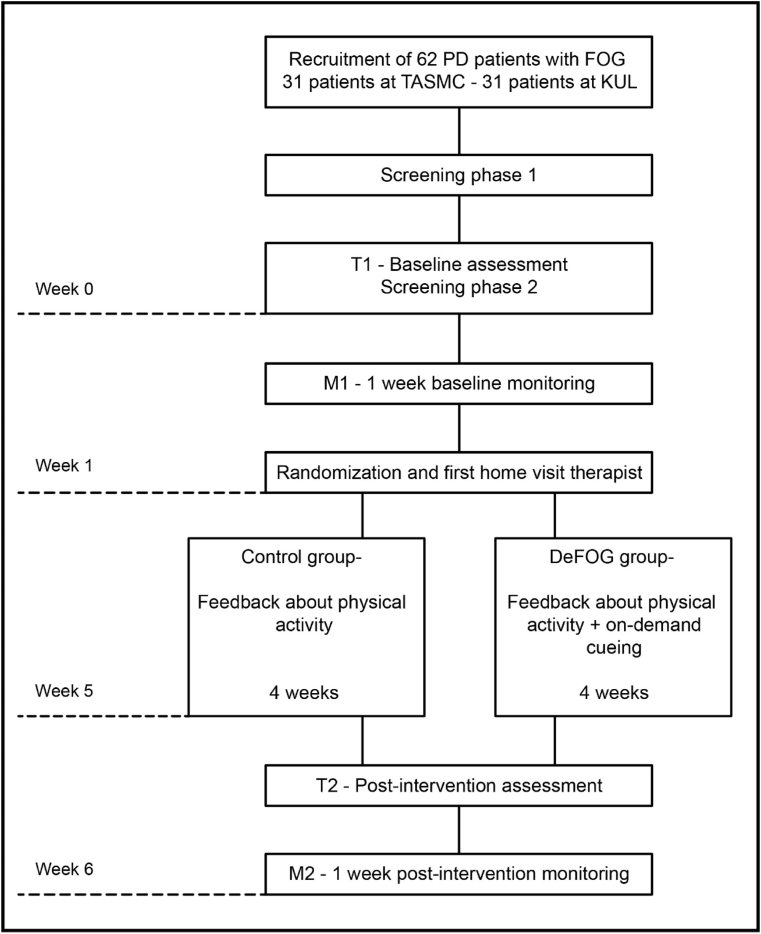
Fig. 1.
Overview of the study design. PD: Parkinson's disease. FOG: freezing of gait. TASMC: Tel Aviv Sourasky Medical Center. KUL: KU Leuven. T1: baseline assessment. M1: baseline gait monitoring week. DeFOG group: intervention group. T2: post-intervention assessment. M2: post-intervention gait monitoring week.
A two-phase screening procedure will be performed by the researchers to establish the eligibility of interested participants (phase 1) and the likelihood to provoke FOG (phase 2). Blinded researchers will administer the first test (T1) in the patient's home, capturing both OFF and ON medication on the same day (without and with anti-Parkinsonian medication), and followed by 1 week of free-living gait monitoring (M1). At the end of M1, participants will be randomly allocated to either the intervention (DeFOG) or the active control group. A home visit of an unblinded therapist will conclude the monitoring week and initiate the 4-week intervention period. In the RESCUE trial [15], intervention effects were found after 3 weeks of supervised at-home cueing training. In the current study, 1 additional intervention week will be implemented for familiarization and personalization of the settings. After the intervention, the assessment and activity monitoring week will be repeated (T2 and M2). In a final home visit, the therapist will administer exit questionnaires and collect the study material. Study procedures are standardized across centers and protocol deviations will be recorded. Regular fidelity checks will be implemented to ensure harmonization across centers whilst avoiding unblinding.
2.2. Screening and recruitment
Recruitment will be conducted via existing databases of PD patients, who have given informed consent to save and use their contact information for study participation within the clinical sites, and by referral of clinicians and movement disorders specialists. Potential participants will be informed about the study procedures and goals. If interested, most inclusion and exclusion criteria will be evaluated during the first phase of the screening (see box 1), which includes the 26-item Mini-Mental State Examination (MMSE) [34] and part I and II of the Characterizing Freezing of Gait (C-FOG) questionnaire [33]. The most important criterion is to gauge whether patients will likely show FOG during testing. As this cannot be guaranteed through verbal screening, patients will be informed that the baseline assessment at T1 will be used for exclusion if no observable FOG is determined. This extra screening was implemented for two reasons: 1) to ensure that intervention effects can be detected on the primary outcome (%TF); and 2) to target a clinical population of freezers that is likely to benefit from the DeFOG intervention. Full ethical approval has been granted by the local Ethics committees in Israel: protocol/ID number 0908-18-TLV and in Belgium (EC Research UZ/KU Leuven): S62453. Written informed consent will be obtained prior to study participation.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria.
-
a)
Clinical diagnosis of PD (n = 31 per site) according to the UK PD Society Brain Bank criteria [35];
-
b)
Modified Hoehn & Yahr Stage I to IV in the ON-state [36,37];
-
c)
Age between 40 and 90 years;
-
d)
Ability to walk 5 min while unassisted by another person;
-
e)
Mini-Mental State Examination (MMSE) score of≥21 [38] or > 16 on the 26-item MMSE screening [34];
-
f)
Stable PD medication during the previous month and no medication change foreseen for the next 6 weeks;
-
g)
Self-reported FOG severity of at least 1 FOG episode per day, based on Characterizing of Freezing of Gait Questionnaire (C-FOG) [33], irrespective of FOG occurring ON- or OFF-medication.
Exclusion criteria.
-
a)
Participation in another clinical study;
-
b)
Use of a cueing device as normal practice;
-
c)
A fall frequency of more than once a day;
-
d)
Acute musculoskeletal or other neurological or cardiovascular conditions affecting gait or any other medical condition which, in the opinion of the investigator, may prevent completing the protocol;
-
e)
Hearing problems, precluding use of auditory feedback from the DeFOG system;
-
f)
The occurrence of any of the following within 3 months prior to informed consent: orthopedic surgery of the lower extremity, myocardial infarction, hospitalization for unstable angina, coronary artery bypass graft, percutaneous coronary intervention, implantation of a cardiac resynchronization therapy device, implantation of deep brain stimulation;
-
g)
Substance abuse, major depressive disorder or clinical apathy that affects daily walking activity or may interfere with the patient's compliance;
-
h)
Inability to walk without a rollator indoors;
-
i)
Use of a duodopa pump or apomorphine injections, jeopardizing OFF-medication assessments;
-
j)
Absence of clinically observed FOG during the FOG-provoking assessment at T1.
Alt-text: Box 1
PD: Parkinson's disease. ON-state: optimal medication state. MMSE: Mini-Mental State Examination. FOG: freezing of gait. C-FOG: Characterizing Freezing of Gait Questionnaire. ON-medication: approximately 1 h after PD medication intake. OFF-medication: without intake of Parkinsonian medication in the morning (at least 12 h). T1: baseline assessment.
2.3. Randomization and blinding
At the end of M1, participants will be randomized into either the DeFOG group or the active control group based on computer-generated blocks with a random size of 2, 4 or 6 per center. Randomization procedures are fully concealed and group allocation is conducted automatically within REDCap (http://www.project-redcap.org). All assessments will be performed by a blinded researcher. To avoid performance bias and disappointment when not receiving the on-demand cueing, participants are partially blinded by withholding the exact purpose of the DeFOG trial. Hence, subjects are informed that the study aims to compare the effects of two different types of feedback about levels of physical activity on FOG.
In both arms, the interventions will be supervised by unblinded therapists through home visits and telephone calls. The therapists will remind patients of the risk of unblinding the assessors prior to testing. All cases of unblinding and their corresponding causes will be logged. At T2, the blinded researchers will give an estimation of group allocation of the subject to evaluate the success of the blinding procedure [39].
2.4. Assessment procedures
2.4.1. General assessment procedures
The main outcomes will first be assessed following the overnight withdrawal of anti-parkinsonian medications for at least 12 h (OFF medication). FOG will be evaluated using a specific FOG-provoking protocol (see below and Fig. 2). Additionally, the Movement Disorders Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) [40] part III will be administered. Approximately 1 h after medication intake, when patients are in the ON-medication state as evaluated using a Visual Analogue Scale (VAS), the FOG-provoking protocol and the MDS-UPDRS part III will be repeated together with the Mini Balance Evaluation Systems Test (MiniBESTest) [41]. Between the OFF and ON states, questionnaires will be administered to collect descriptive information.
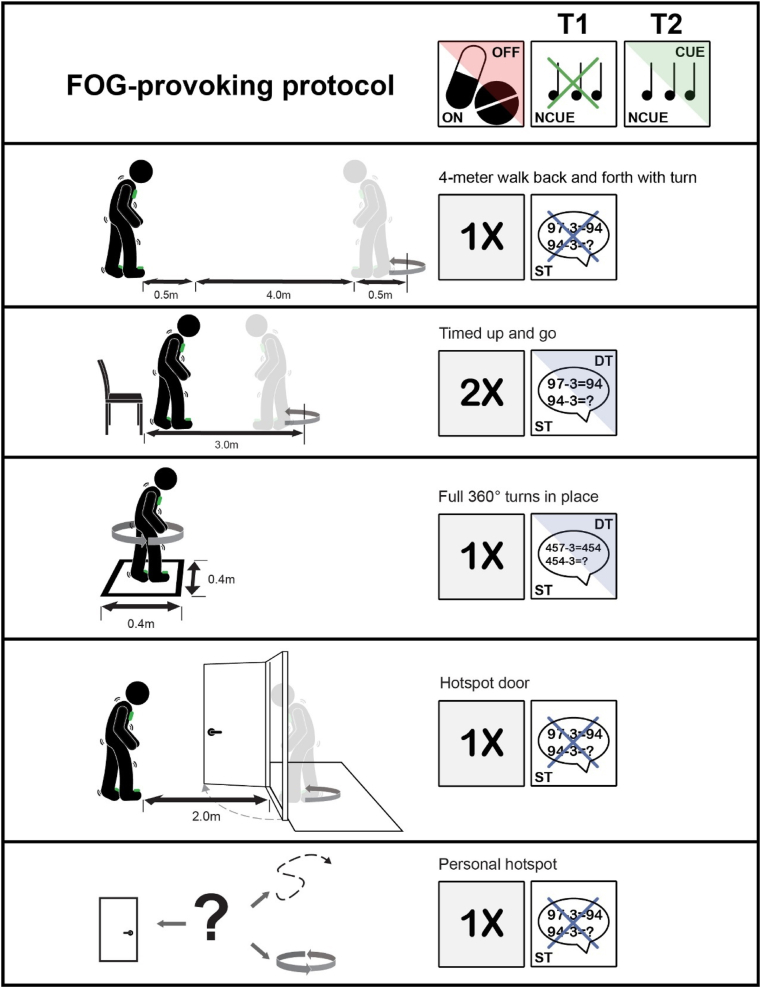
Fig. 2.
An overview of the different tasks included in the FOG-provoking protocol. OFF-medication: without intake of Parkinsonian medication in the morning (at least 12 h). ON-medication: approximately 1 h after PD medication intake. T1: baseline assessment. T2: post-intervention assessment. NCUE: without cueing. CUE: with cueing. ST: single task. DT: dual task (serial-3 subtractions).
2.4.2. FOG-provoking protocol
FOG will be recorded using a video camera during a protocol that consists of five parts, presented in detail in Fig. 2 and adapted from the paper by Ziegler et al. [42]. Inertial measurement units (IMUs) as part of the DeFOG system will also be used to gather data on gait and balance. The following testing conditions will be standardized within patients for repeated measurements within their specific home environment:
-
1)
4-m walk test (4 MW) back and forth with turn: patients will be instructed to perform the 4 MW test back and forth once, including a 180° turn to make the task more FOG provocative. A total space of 5 m is needed, including 0.5 m before and after the 4 m. This task will also enable the determination of straight-line gait outcomes.
-
2)
Timed Up and Go (TUG): patients are instructed to rise from a chair, walk 3 m, turn in place and return to the chair. This task is performed two times with and without a cognitive dual task (DT; i.e., serial-3 subtractions).
-
3)
Full 360° turns in place: a total of four fast turns will be performed, alternating the direction after every turn. This task is the most consistent trigger of FOG [43] and will also be performed with and without the serial-3 subtractions, using different starting numbers as during the TUG.
-
4)
Hotspot door, based on the well known trigger of FOG ‘walking through narrow space passages’ [44]: patients are instructed to walk 2 m (if space allows this), open a door and pass the doorway, turn in place and return to the starting position. If possible, the task is performed at a location where the participant can turn in a small space, such as the bathroom, to enhance the likelihood of FOG occurrence.
-
5)
Personal FOG ‘hotspot’: a particular location in the home indicated by the patients as highly FOG-provoking. The results of part II of the C-FOG questionnaire (about FOG triggers) will be used to help define the hotspot.
The above protocol will be repeated at T2 with and without the on-demand cueing in both groups to ascertain training effects in the DeFOG group and to determine short-term cueing effects in the control group. Cueing will always be applied after the non-cued condition to minimize the likelihood of carryover effects and to ensure that the long-term effects of the interventions can be captured without interference from the device. Patients in the control arm will undergo a familiarization session with the on-demand cueing during the last therapist visit, allowing personalization of the cueing settings.
2.5. Gait monitoring
During week 0 and week 5 (M1 and M2; see Fig. 1), the participant's physical activity will be monitored using the DeFOG system. Participants will not receive cueing or feedback, except for feedback regarding the system's battery life. Additionally, a small activity monitor (AX3, Axivity; Newcastle, UK) will be positioned on the patient's lower back at the level of the fifth lumbar vertebrae using hypoallergenic tape. The activity monitor will record activity for 7 consecutive days at a sampling frequency of 100 Hz. In contrast to the supervised structured home assessments (T1 and T2) which record the participant's gait capacity, the monitoring weeks are unsupervised and aim to record the participant's performance (to what extent the user utilizes his/her capacity).
2.6. Intervention
2.6.1. Both groups
After randomization, the therapist will schedule a home visit for participants in both groups, to set the parameters of the device, both for the DeFOG-mode and the control-mode. All participants will receive the same smartphone and sensor hardware, which will provide a daily step counting function. Participants will be encouraged to activate the system during as many bouts of daily activity as possible to obtain a valid measurement and feedback on their physical activity levels. Each participant's use of the system (compliance) will be reviewed weekly together with the therapists, based on smartphone logs and patient reports using a standardized question. Consultation by telephone or in-person in case of difficulties will also be offered by the therapist and logged in full detail. In the control group, a target will be set for increasing physical activity by 10% over the study period to avoid performance bias and lack of compliance. Considering the fall risk in this population [4], targets will be personalized and not overemphasized. All adverse events and reactions during the full study period will be monitored and logged by the therapists and reviewed by the local ethical committees and a dedicated review board.
2.6.2. DeFOG intervention
Additional to the daily step counts, subjects in the DeFOG group will receive on-demand auditory cueing targeted to reduce FOG, which will be triggered upon FOG detection. Based on pilot work, cues will be released for a fixed duration of 10 s. If FOG persists, a personalized verbal instruction, set by the therapist, will be started to remind the patient to use a personal internal strategy to overcome FOG. Since false positive FOG detections are possible and very short or very long FOG episodes may occur, therapists will personalize the settings to ensure that the system will deliver the appropriate dose of auditory input so as not to overwhelm the patients.
Personalization of the system's settings will be tested at the patients' personal FOG ‘hotspots’ and will include:
-
1.
Adjustment of the cueing frequency to re-initiating walking. This will start from a rhythm that is 10% lower than the patient's preferred cadence determined during the baseline 4 MW gait test. The cueing frequency needed to overcome a FOG episode is expected to be substantially lower than during steady-state walking.
-
2.
Optimization of the verbal instruction setting based on the patient's preferred strategy for overcoming FOG [20]. For instance, consciously shifting the weight mediolaterally to offload the swing leg is one of the most used strategies for FOG [21,22], which can be set as a verbal instruction in the DeFOG system.
-
3.
Adjustment of the FOG detection algorithm (see section on the DeFOG system) to a higher specificity setting in case the patient experiences many false positives. As this may also increase the number of false negatives, the therapists will carefully consider adjustment of the settings in collaboration with the patient. In addition, the therapists will also educate the patient about how to cope with false positives.
2.7. The DeFOG system
2.7.1. Concept of cue-application
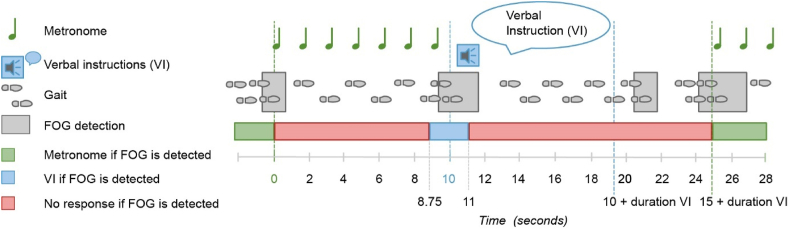
Fig. 3 shows the concept of cue-application in relation to a possible manifestation of FOG as it may occur in real life. When FOG is detected (t = 0), a metronome starts playing for a fixed duration of 10 s (t = 0 until t = 10). If a second FOG episode is detected within the first 8.75 s after FOG detection (t = 0 until t = 8.75), nothing happens after the metronome beats stop. However, if a second FOG episode is detected within the following window of 2.25 s (t = 8.75 until t = 11), the preset verbal instruction will be triggered. The verbal instruction will be triggered immediately after the cueing is stopped (i.e. at t = 10), or at the moment the second FOG episode is detected (i.e. between t = 10 and t = 11 s). When a FOG episode is detected during or in the subsequent 5 s of the verbal instruction, no cueing or verbal instruction will be triggered to avoid overburdening the patient and the technology. The same cycle of detection and response will start again if FOG re-occurs.
Fig. 3.
Concept of cue application in response to detection of a FOG episode. VI: verbal instruction. FOG: freezing of gait.
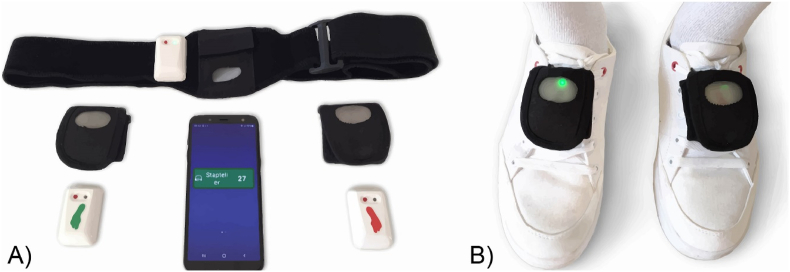
2.7.2. Device hardware
The DeFOG device represents a development of the Gait Tutor system (mHealth Technologies; Bologna, Italy), which is certified as a Medical Device. Fig. 4A illustrates its components and patient-friendly technology. Two IMUs will be placed on the patient's shoes with Velcro straps, connected to a smartphone via Bluetooth (Fig. 4B). Auditory cueing is delivered through earphones. Patients will be advised to use only one earphone for safety and social reasons. The smartphone-sensor Bluetooth connection is automated, requiring no patient interaction. Connection status is indicated via the color of the DeFOG widget on the smartphone. Social acceptance in daily life is facilitated by camouflaging the sensors using black Velcro straps and turning off the sensor lights upon Bluetooth connection. The system set-up including the battery recharging will be demonstrated by the assessor at the end of T1 and by the provision of a detailed user manual and instruction videos on the smartphone.
Fig. 4.
Device components of the DeFOG system. A: Smartphone showing the step count and the three sensors with Velcro straps to attach them to the feet and chest. B: The feet sensors attached to the shoes.
The IMUs consist of a tri-axial accelerometer (full scale at ±8 g) and a tri-axial gyroscope (full scale at ± 1000 deg/s). Raw IMU data are transmitted to the smartphone with a sampling frequency of 200 Hz. This results in a battery capacity of approximately 3–4 h, exceeding the average daily walking duration in PD [45]. The smartphone is used for real-time calculations and for data storage of the raw and processed data. Overnight, all data is uploaded to the cloud for backup purposes. Daily performance reports are sent automatically to the therapists, enabling remote follow-up and adjustment of therapeutic settings.
2.7.3. Device operating modes
The DeFOG system has three operating modes:
-
(1)
Assessment mode, used with an additional chest sensor to gather detailed information on performance during the FOG-provoking protocol. The on-demand cueing can be turned ‘off’ (T1 and T2) or ‘on’ (only T2).
-
(2)
Intervention mode, employed during daily activity to provide the number of steps taken (both groups) and the on-demand cueing for the DeFOG group. DeFOG intervention settings will be hidden on the smartphone to prevent unblinding of the assessor when switching the settings to the Assessment mode during T2.
-
(3)
Monitoring mode, used for activity monitoring in week M1 and M2. Participants will only receive information regarding battery life during these weeks.
Table 1 provides an overview of the functions of the different devices throughout the study, including the different operating modes of the DeFOG system.
Table 1.
Use of the technology in the different tests and phases of the study.
| T1 & T2 | M1 & M2 | Intervention period control group | Intervention period DeFOG group | ||
|---|---|---|---|---|---|
| DeFOG system | Gait monitoring | x | x | x | x |
| Gait monitoring chest sensor | x | ||||
| FOG detection | x | x | x | x | |
| Provision of a step count | x | x | |||
| Provision of on-demand cueing and verbal instructions | x | ||||
| Axivity | Gait monitoring | x | x | ||
| Video camera | FOG annotations | x |
T1: baseline assessment. M1: baseline 1-week free-living gait monitoring. T2: post-intervention assessment. M2: post-intervention 1-week free-living gait monitoring.
2.7.4. FOG detection algorithm and real-time step count
The DeFOG algorithm, provided by mHealth Technologies, enables the automatic and on-line detection of FOG events based on the Gait Tutor system. The algorithm was refined using data from 31 patients suffering from FOG: eighteen from the CuPiD dataset [46], nine from the DeFOG Leuven cohort, and four from the Tel Aviv DeFOG cohort. These patients produced over 300 FOG events during FOG-provoking protocols in the laboratory setting. The presence of FOG is evaluated every 0.5 s using a sliding window of 3.2 s. The latency between the presence of a FOG episode and the actual detection is approximately 1 s. The real-time step count calculations are based on the processing of the angular velocity signal along the medio-lateral axis of the foot [47].
2.8. Outcome measures
The primary outcome measure is the percentage time frozen (%TF) during the FOG-provoking protocol in the OFF and ON medication states combined, as determined by video ratings using the annotation software Elan [48,49]. %TF is a reliable and responsive outcome when applied to the TUG test in the OFF state (intraclass coefficient of 0.73) and is currently the gold standard when based on expert ratings of video recordings [50,51]. Here, we will build on this knowledge by determining %TF in the home situation during several FOG-provoking tests in both OFF and ON medication to enhance ecological validity. %TF will be calculated as the total duration of FOG divided by the total duration of the motor tasks multiplied by 100%. Blinded researchers will perform the video annotations based on definitions (see Table 2). Several fidelity checks will be performed between centers to ensure a consistent approach. Furthermore, the inter-rater reliability will be calculated between centers on a sample of approximately 10% of the videos.
Table 2.
Definitions for the annotation of FOG and the tasks during the FOG-provoking protocol.
| Definitions video annotations | ||
|---|---|---|
| Freezing of gait (FOG) | ||
| Akinetic FOG | Onset | The moment the intention to move is first observeda and the participant is unable to do so, showing ‘clear sticking of the feet’ without considerable trembling movements in the legs. |
| Termination | The moment of initial toe-off after the FOG when the participant is again able to perform at least two effective alternating steps with both legs showing no FOG-related features. | |
| Trembling FOG | Onset | The moment when the foot of the participant is suddenly no longer producing an effective step forward and is displaying trembling in the legs, despite the participant's intention to continue walking, or the moment the intention to move is first observed and the participant is unable to do so, showing clear trembling in the legs. |
| Termination | The moment of initial toe-off after the FOG when the participant is again able to perform at least two effective alternating steps with both legs showing no FOG-related features. | |
| Festination | Onset | The first moment of toe-off when an abnormal and high-pace oscillatory stepping behavior is observed without considerable FOG-related features. |
| Termination | The moment of initial toe-off when the participant is again able to perform at least two effective alternating steps with both legs showing no FOG- or festination related features. | |
| Movement interruptionsb |
Onset | The moment a movement interruption is first observed - when the foot of the participant is not or is suddenly no longer producing an effective step forward (despite the task instruction to do so), without a definite indication to consider it either as a FOG subtype or as voluntary stopping. |
| Termination | The moment of initial toe-off after the movement interruption when the participant is again able to perform at least two effective alternating steps with both legs showing no FOG-related features or the first moment a definite indication is observed to annotate it as FOG or voluntary stopping. | |
| Task duration | ||
| As outcomec | Onset | The moment the researcher gives the ‘Go’ signal to start the task. |
| Termination | The moment the participant has finished the task. | |
| For calculation %TFc | Onset | The moment the first intention to start the task is observed. |
| Termination | The moment the participant has finished the task. | |
FOG: freezing of gait. %TF: percentage time frozen.
An all-encompassing definition for the ‘first intention to move’ cannot be provided as this varies between situations and is therefore left for the interpretation of the expert rater. Examples are movements observed in the upper body, arms and/or clothing indicating an attempt to initiate the intended movement, multiple APA's during gait initiation, etc.).
Not included in the calculation of %TF.
The start of the Task is defined differently for task duration as an outcome on its own and for calculation of the %TF, as the duration for the patient to respond to the ‘Go’ signal can be indicative for impairment but would add noise to the calculation of %TF.
An overview of all the primary and secondary outcome measures and all descriptors are shown in Table 3, Table 4, respectively. In addition, exploratory outcomes are shown in Table A1. All data, except for the raw video and sensor data, will be saved electronically in RedCAP (www.project-redcap.org). Data processing of the digitized data and the extraction of the dependent variables will be executed using custom Matlab software. The analysis of the Axivity gait monitoring data will focus on: (1) the identification of bouts of walking activity; (2) the calculation of quantitative accelerometer-derived gait measures, as previously described [52].
Table 3.
Primary and secondary outcome measures.
| Outcome measures | T1 & T2 | M1 & M2 | |
|---|---|---|---|
| Primary outcome measures | |||
| FOG severity | %TF OFF + ON (video annotationsa) | x | |
| Secondary outcome measures | |||
| FOG severity | %TF OFF (video annotationsa) | x | |
| %TF ON (video annotationsa) | x | ||
| Number and duration of FOG episodes, OFF and ON (video annotationsa) | x | ||
| NFOG-Q | x | ||
| Physical activity | Number of steps per dayb | x | |
| Duration of bouts of activityb | x | ||
| Number of bouts of daily activityb | x | ||
| Gait performance | Task duration: TUG, ST and DTa, OFF and ON | x | |
| Duration 4 m: 4 MWa, OFF and ON | x | ||
| Motor function | MDS-UPDRS III, OFF and ON | x | |
| Balance | MiniBESTest score | x | |
T1: baseline assessment. M1: baseline 1-week free-living gait monitoring. T2: post-intervention assessment. M2: post-intervention 1-week free-living gait monitoring. FOG: freezing of gait. %TF: percentage time frozen. OFF-medication: without intake of Parkinsonian medication in the morning (at least 12 h). ON-medication: approximately 1 h after PD medication intake. NFOG-Q: New Freezing of Gait Questionnaire [26]. TUG: Timed Up and Go. ST: single task. DT: dual task. 4 MW: 4-m walk test. MDS-UPDRS: Movement Disorder Society-Unified Parkinson's Disease Rating Scale [40]. MiniBESTest: Mini-Balance Evaluation Systems Test [41].
Recorded with a video camera.
Recorded using an Axivity sensor.
Table 4.
Descriptors.
| Descriptors | |
|---|---|
| PD severity | MDS-UPDRS (full) |
| FOG severity | C-FOG |
| Cognitive function | Montreal Cognitive Assessment (MoCA) |
| Interference (Stroop test) | |
| Alternating fluency (ANT) | |
| Anxiety | PAS |
| Medication | Levodopa equivalent daily dose (LEDD) |
| Technology skills | Mobile Device Proficiency (MDPQ) |
| Functional mobility | Life-Space Assessment (LSA) |
| Other | Demographics |
PD: Parkinson's disease. MDS-UPDRS: Movement Disorder Society-Unified Parkinson's Disease Rating Scale [40]. FOG: freezing of gait. C-FOG: Characterizing Freezing of Gait Questionnaire [33]. MoCA: Montreal Cognitive Assessment [53]. ANT: Alternating Names Test [54]. PAS: Parkinson Anxiety Scale [55]. LEDD: Levodopa equivalent daily dose [56]. MDPQ: Mobile Device Proficiency Questionnaire: only parts 1, 2 and 8 are used. LSA: Life-Space Assessment.
2.9. Statistics
2.9.1. Power analysis
Based on a single group pilot study on visual cueing [30], the estimated %TF in the ON-medication state is 8.8% ± 4.1% in the control group and 6.0% ± 3.1% in the intervention group (Cohen's D: 0.77). With alpha set at 0.05, power set at 80% and considering a potential dropout rate of 10%, this implies that the estimated sample size requires 31 patients per group to find a group difference in ON-medication state. As the effect size was much larger in OFF, we relied on the most conservative estimate (in ON) for our power analysis.
2.9.2. Planned analysis
Descriptive statistics will be presented by means and standard deviations, or if data are skewed, by medians and quartiles for numerical data. The statistical analysis plan will be finalized before blinding is undone. The main analysis will rely on the intention-to-treat technique. %TF will be evaluated using generalized mixed models controlling for baseline values and with the clinical site (TASMC/KUL) as a random factor. We will evaluate between-group clinical differences at baseline and test the influence of possible confounders such as medication dose, cognitive capacity and FOG severity. Accordingly, we will add an interaction term to the model or if necessary control for confounders in an analysis of covariance. Secondary endpoints will be analyzed using the same approach. As a secondary analysis, a per protocol analysis will be performed, including all patients that completed both the intervention and the measurements (excluding drop-outs). Furthermore, an exploratory sensitivity analysis will be conducted excluding patients within the lowest quartile of compliance (total duration of system use during the intervention period), as recorded by the DeFOG system. Alpha will be set at 0.05 (two-sided).
3. Discussion
The aim of this two-center single-blind randomized controlled trial is to evaluate the effectiveness of on-demand cueing delivered with wearable technology to ameliorate FOG and this in contrast to a control device aimed to give feedback on step counts in general. The novelty of the study is threefold. It addresses 1) on-demand personalized cueing in conjunction with verbal instructions to overcome FOG, 2) automatic FOG detection outside a laboratory setting and 3) a user-tool to prevent FOG at home in the daily lives of people with PD. These three novel aspects will be discussed in detail in the next sections.
First, the current study tests the efficacy of a personalized cueing method, thereby responding to the notoriously heterogeneous expression of FOG. Freezing episodes have been stratified by their predominant triggers, which can be motor, cognitive or limbic [33], as well as by their kinematic manifestation, e.g., festinating, trembling and akinetic FOG [11]. To date, the pathophysiological basis for this heterogeneity is not well understood [57]. Prospective studies have identified a range of risk factors that predict the onset of FOG, including early lower limb or gait abnormalities, more axial symptoms, more cognitive disturbances, poorer balance, depression, anxiety and levodopa response [[58], [59], [60], [61]]. Irrespective of the exact origins of FOG, this trial will likely make further progress in finding a low-cost, non-invasive therapeutic solution for this complex clinical problem as it plays into some of its diversity and provides personalized behavioral solutions. The DeFOG system gives feedback only when and where FOG occurs, which is considered as an innovative form of cueing. Although FOG may occur frequently, patients do not necessarily undergo the experience consciously. Recently, this notion came to the fore during an analysis of repeated measures of self-reported FOG using the NFOG-Q, showing high variability and poor alertness of this symptom [27]. We anticipate that using the DeFOG system may increase the patient's awareness about their personal pattern of FOG, teaching the patient inadvertently to anticipate an upcoming episode. Thus, patients may become more confident knowing that the device will respond and assist them through difficult situations, putatively resulting in enhanced physical activity and a reduction of FOG-related anxiety.
So far, the evidence about the effects of cueing on FOG is inconsistent [17,23,25,62] and few studies exist that address on-demand cueing upon automatic FOG detection [63,64]. Part of the lack of success of cueing for FOG may be ascribed to an impaired cognitive ability, which is more prevalent in freezers than in non-freezers [65]. In addition, during an actual FOG episode, patients may also experience cognitive overload and anxiety-related distraction [66], making it more difficult to respond appropriately to cueing in contrast to when it is applied during normal gait. To overcome these problems, the DeFOG system will provide a ‘paired strategy’ consisting of cueing plus a verbal instruction to use as an attentional strategy administered via headphones. This verbal instruction is only triggered in case FOG persists, and could include asking a patient to stop first and subsequently perform a movement, promoting gait initiation. Verbal rescue strategies may prompt a ‘mental resetting’ and thus have a greater impact than a simple auditory signal, especially when FOG is persistent. Previous work from our group also showed that patients with FOG seemed to prefer the use of verbal instructions over auditory cueing, albeit not pertaining to FOG but to ongoing gait [67]. Furthermore, DeFOG makes it possible to personalize the cue settings and verbal instructions, taking into account FOG-provoking factors, fall risk and cognitive capacity. As such, DeFOG is also likely to reduce the risk of cue-induced habituation and fatigue [68,69]. In summary, this study will collect novel data on the experience of FOG and the remaining therapeutic reserve during these episodes in people with different disease profiles.
The second novel aspect of this study is that it will rely on online automated FOG detection in an uncontrolled setting. Real-time detection requires high computational speed whilst safeguarding against energy costs to avoid battery drainage. Also, an optimal trade-off between sensitivity and specificity should be achieved using the limited information within a sliding window. Over the past years, many studies have been conducted on automatic sensor-based FOG detection with various sensor configurations and FOG detection algorithms [23,70]. These studies sometimes lacked clinical validation against a gold standard and were often not tested in daily life. Bearing these limitations in mind, FOG detection algorithms have shown high values for sensitivity and specificity. However, as daily life is less structured, higher rates of false positives can be expected, depending on the patient's disease phenotype (degree of tremor, the presence of dyskinesia and the different types of FOG). Bächlin et al. demonstrated a relatively high average sensitivity and specificity for their FOG detection algorithms, but with considerable variability between patients, underscoring the importance of personalized settings [63]. Therefore, DeFOG allows therapists to select a FOG detection option with a lower sensitivity and higher specificity if needed for a specific patient to enhance the face validity of the tool and reduce the risk of low compliance. Of note, false positives may not always be detrimental, as it may reflect an upcoming breakdown of gait, creating an opportunity to learn how to prevent FOG. In the present study, it will be particularly informative to evaluate how the FOG detection algorithm will perform in an uncontrolled setting for a therapeutic purpose. As the day-to-day FOG detection will not be compared against a gold standard, the subjective experience of patients will be used for this purpose. However, the home-based structured protocol of using FOG-triggers in patients' daily environment to test FOG at T1 and T2 will be validated against video annotations. By carrying out these standardized procedures both OFF and ON medication, a robust and ecologically valid picture of daily life FOG will emerge, contributing to the refinement of digitized outcomes against video ratings [62]. In short, the DeFOG study has the potential to improve real-time FOG detection algorithms using a wearable device and advance the development of digitized FOG outcomes.
The third novel feature of DeFOG-trial is the opportunity to assess the feasibility of relatively long-term use of the technology in the home in a population with FOG, who usually are more severely affected by non-motor symptoms than those without. A recent study in the Netherlands showed that in a large cohort of mild to moderately affected PD patients wearing a smartwatch for 3 months was highly acceptable [71]. However, when devices have more complex features, such as those used in a FOG-preventing therapeutic context, people give high scores for usability in a laboratory, but acceptance rates tend to be lower in daily life [64]. For daily use, wearable systems should be low-cost, unobtrusive and have a simple interface. During the development of DeFOG, the user-feedback from the GaitTutor was considered, a certified Medical Device on which DeFOG was based [72]. DeFOG requires minimal user interaction, as it establishes Bluetooth connection automatically, and voice messages indicate when the sensors or smartphone need to be charged. DeFOG requires input from a therapist in selecting appropriate settings, in troubleshooting, and in helping the patient cope with false positives. Nonetheless, we anticipate that once the patient is familiar with the system, it can be used with minimal assistance. Based on the results of this study, we will be able to characterize the profiles of patients who can achieve independent use and who will comply with the DeFOG method. This will help clinicians to provide more targeted treatment with less in-person assistance and help engineers with the development of updated systems. In conclusion, by teaching patients to use cueing in the very circumstances in which they are likely to experience FOG, the DeFOG trial will reveal whether the on-demand cueing concept can decrease the burden of FOG and enhance physical activity and quality of life for patients experiencing this symptom.
Ethics approval and consent to participate
Full ethical approval has been granted by the local Ethics committees in Israel protocol/ID number: 0908-18-TLV and in Belgium (EC Research UZ/KU Leuven): S62453. Written informed consent will be obtained from each participant prior to study participation.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This research is funded by the Michael J. Fox Foundation (https://www.michaeljfox.org/; Grant ID = 16347). The Michael J. Fox Foundation did not have a role in the study design and will not be involved in the collection, analyses and interpretation of the data, or in the decision to submit results.
Authors’ contributions
Demi Zoetewei: Methodology, Resources, Writing – Original draft. Talia Herman: Methodology, Resources, Writing – Review and editing. Marina Brozgol: Methodology, Resources. Pieter Ginis: Conceptualization, Methodology, Resources, Project administration, Writing – Review and editing. Pablo Cornejo Thumm: Methodology, Resources. Eva Ceulemans: Methodology, Resources. Eva Decaluwé: Resources, Writing – Review and editing. Luca Palmerini: Methodology, Software, Writing – Review and editing. Alberto Ferrari: Methodology, Software, Writing – Review and editing. Alice Nieuwboer: Conceptualization, Funding acquisition, Methodology, Writing – Review and editing, Supervision. Jeffrey M. Hausdorff: Conceptualization, Funding acquisition, Methodology, Writing – Review and editing, Supervision. All authors have approved the final manuscript.
Declaration of competing interest
Luca Palmerini and Alberto Ferrari are co-founders and own shares of mHealth Technologies. All other authors declare that they have no competing interests.
Acknowledgements
We would like to thank Robert Marinus Francis van Dijk for his considerable contribution to the design and editing of the figures.
Contributor Information
Demi Zoetewei, Email: demi.zoetewei@kuleuven.be.
Talia Herman, Email: talih@tlvmc.gov.il.
Marina Brozgol, Email: marinab@tlvmc.gov.il.
Pieter Ginis, Email: pieter.ginis@kuleuven.be.
Pablo Cornejo Thumm, Email: pablob@tlvmc.gov.il.
Eva Ceulemans, Email: eva.ceulemans1@kuleuven.be.
Eva Decaluwé, Email: eva.decaluwe@kuleuven.be.
Luca Palmerini, Email: luca.palmerini@unibo.it.
Alberto Ferrari, Email: alberto.ferrari@unibo.it.
Alice Nieuwboer, Email: alice.nieuwboer@kuleuven.be.
Jeffrey M. Hausdorff, Email: jhausdor@tlvmc.gov.il.
Appendices.
Table A.1.
Exploratory outcome measures
| Exploratory outcome measures | T1&T2 | M1&M2 | Intervention | Exit questionnaire | |
|---|---|---|---|---|---|
| FOG severity, OFF and ON | %TF and total duration and number of FOG episodes (DeFOG algorithma), OFF and ON | x | |||
| %TF, as detected using the algorithm of Mancini et al. (2021) [73]a, OFF and ON | x | ||||
| FOG severity, free-living | %TF and total duration and number of FOG episodes during free-living (DeFOG algorithma) | x | |||
| %TF during free-living, as detected using the algorithm of Mancini et al. (2021) [73]a | x | ||||
| FOG manifestations | %TF: Akinetic FOG only, OFF and ON (video annotationsb) | x | |||
| %TF: Trembling FOG only, OFF and ON (video annotationsb) | x | ||||
| %TF: Festination only, OFF and ON (video annotationsb) | x | ||||
| Turning | Turning speed during Full 360° turns in placea, OFF and ON | x | |||
| Gait and balance | IMU-based measures during 30s standinga, OFF and ON | x | |||
| IMU-based measures during 4 MW, TUGa, OFF and ON | x | ||||
| Falls and fear | Fall frequency | x | x | ||
| Fear of falling (FES-I) | x | ||||
| Depression and QoL | Depression (GDS-15) | x | |||
| Quality of life (PDQ-8) | x | ||||
| Patient experience | Perceived effects of intervention | x | |||
| User satisfaction | x | ||||
T1: baseline assessment. M1: baseline 1-week free-living gait monitoring. T2: post-intervention assessment. M2: post-intervention 1-week free-living gait monitoring. FOG: freezing of gait. OFF-medication: without intake of Parkinsonian medication in the morning (at least 12 h). ON-medication: approximately 1 h after PD medication intake. %TF: percentage time frozen. IMU: inertial measurement unit. 4 MW: 4-m walk test. TUG: Timed Up and Go. FES-I: Falls Efficacy Scale-International [74]. QoL: quality of life. GDS-15: Geriatric Depression Scale-15 items. PDQ-8: Parkinson's Disease Questionnaire-8 items [75].
Recorded using the DeFOG system.
Recorded with a video camera.
References
- 1.Giladi N., Nieuwboer A. Understanding and treating freezing of gait in Parkinsonism, proposed working definition, and setting the stage. Mov. Disord. 2008;23(Suppl 2):423–425. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 2.Ge H.L., Chen X.Y., Lin Y.X., Ge T.J., Yu L.H., Lin Z.Y., Wu X.Y., Kang D.Z., Ding C.Y. The prevalence of freezing of gait in Parkinson's disease and in patients with different disease durations and severities, Chinese Neurosurg. J. 2020;6:1–11. doi: 10.1186/s41016-020-00197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuma Y., Silva de Lima A.L., Fukae J., Bloem B.R., Snijders A.H. A prospective study of falls in relation to freezing of gait and response fluctuations in Parkinson's disease. Park. Relat. Disord. 2018;46:30–35. doi: 10.1016/j.parkreldis.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Pelicioni P.H.S., Menant J.C., Latt M.D., Lord S.R. Falls in Parkinson's disease subtypes: risk factors, locations and circumstances. Int. J. Environ. Res. Publ. Health. 2019;16:2216. doi: 10.3390/ijerph16122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehgoetz Martens K.A., Hall J.M., Gilat M., Georgiades M.J., Walton C.C., Lewis S.J.G. Anxiety is associated with freezing of gait and attentional set-shifting in Parkinson's disease: a new perspective for early intervention. Gait Posture. 2016;49:431–436. doi: 10.1016/j.gaitpost.2016.07.182. [DOI] [PubMed] [Google Scholar]
- 6.Lord S.R., Bindels H., Ketheeswaran M., Brodie M.A., Lawrence A.D., Close J.C.T., Whone A.L., Ben-Shlomo Y., Henderson E.J. Freezing of gait in people with Parkinson's disease: nature, occurrence, and risk factors. J. Parkinsons Dis. 2020;10:631–640. doi: 10.3233/JPD-191813. [DOI] [PubMed] [Google Scholar]
- 7.Moore O., Peretz C., Giladi N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Mov. Disord. 2007;22:2192–2195. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Lloret S., Negre-Pages L., Damier P., Delval A., Derkinderen P., Destée A., Meissner W.G., Schelosky L., Tison F., Rascol O. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 2014;71:884–890. doi: 10.1001/jamaneurol.2014.753. [DOI] [PubMed] [Google Scholar]
- 9.Lucas McKay J., Goldstein F.C., Sommerfeld B., Bernhard D., Perez Parra S., Factor S.A. Freezing of Gait can persist after an acute levodopa challenge in Parkinson's disease. NPJ Park. Dis. 2019;5:25. doi: 10.1038/s41531-019-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilat M., Lígia Silva de Lima A., Bloem B.R., Shine J.M., Nonnekes J., Lewis S.J.G. Freezing of gait: promising avenues for future treatment. Park. Relat. Disord. 2018;52:7–16. doi: 10.1016/j.parkreldis.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Nutt J.G., Bloem B.R., Giladi N., Hallett M., Horak F.B., Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T., Hallett M., Chan P. Motor automaticity in Parkinson's disease. Neurobiol. Dis. 2015;82:226–234. doi: 10.1016/j.nbd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redgrave P., Rodriguez M., Smith Y., Rodriguez-Oroz M.C., Lehericy S., Bergman H., Agid Y., Delong M.R., Obeso J.A. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat. Rev. Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehgoetz Martens K.A., Hall J.M., Georgiades M.J., Gilat M., Walton C.C., Matar E., Lewis S.J.G., Shine J.M. The functional network signature of heterogeneity in freezing of gait. Brain. 2018;141:1145–1160. doi: 10.1093/brain/awy019. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwboer A., Kwakkel G., Rochester L., Jones D., Van Wegen E., Willems A.M., Chavret F., Hetherington V., Baker K., Lim I. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J. Neurol. Neurosurg. Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein T.C., Giladi N., Hausdorff J.M. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson's disease. Mov. Disord. 2002;17:1148–1160. doi: 10.1002/mds.10259. [DOI] [PubMed] [Google Scholar]
- 17.Ginis P., Nackaerts E., Nieuwboer A., Heremans E. Cueing for people with Parkinson's disease with freezing of gait: a narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018;61:407–413. doi: 10.1016/j.rehab.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Banks S.J., Bayram E., Shan G., Labelle D.R., Bluett B., Diego S., Clinic C., Vegas L., Program B., Vegas L. Non-motor predictors of freezing of gait in Parkinson's disease. Gait Posture. 2019;68:311–316. doi: 10.1016/j.gaitpost.2018.12.009.Non-motor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortelli P., Ferrazzoli D., Cian V., Zarucchi M., Palamara G., Giobbia A., Frazzitta G., Maestri R., Canesi M. How cognition and motivation “freeze” the motor behavior in Parkinson's disease. Front. Neurosci. 2019;13:1–9. doi: 10.3389/fnins.2019.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonnekes J., Růžička E., Nieuwboer A., Hallett M., Fasano A., Bloem B.R. Compensation strategies for gait impairments in Parkinson disease: a review. JAMA Neurol. 2019;76:718–725. doi: 10.1001/jamaneurol.2019.0033. [DOI] [PubMed] [Google Scholar]
- 21.Maslivec A., Fielding A., Wilson M., Norris M., Young W. ‘Recoupling’ the attentional and motor control of preparatory postural adjustments to overcome freezing of gait in Parkinson's. J. NeuroEng. Rehabil. 2020;17 doi: 10.1186/s12984-020-00776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman S., Griffin H.J., Quinn N.P., Jahanshahi M. The factors that induce or overcome freezing of gait in Parkinson's disease. Behav. Neurol. 2008;19:127–136. doi: 10.1155/2008/456298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney D., Quinlan L.R., Browne P., Richardson M., Meskell P., Ólaighin G. A technological review of wearable cueing devices addressing freezing of gait in Parkinson's disease. Sensors. 2019;19:1277. doi: 10.3390/s19061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim I., van Wegen E., de Goede C., Deutekom M., Nieuwboer A., Willems A., Jones D., Rochester L., Kwakkel G. Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. Clin. Rehabil. 2005;19:695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- 25.Cosentino C., Baccini M., Putzolu M., Ristori D., Avanzino L., Pelosin E. Effectiveness of physiotherapy on freezing of gait in Parkinson's Disease : a systematic review and meta-analyses. Mov. Disord. 2020;35:523–536. doi: 10.1002/mds.27936. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwboer A., Rochester L., Herman T., Vandenberghe W., Emil G.E., Thomaes T., Giladi N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 27.Hulzinga F., Nieuwboer A., Dijkstra B.W., Mancini M., Strouwen C., Bloem B.R., Ginis P. The new freezing of gait questionnaire: unsuitable as an outcome in clinical trials? Mov. Disord. Clin. Pract. 2020;7:199–205. doi: 10.1002/mdc3.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraye M.U., Fraix V., Pollak P., Bloem B.R., Debû B. The laser-shoe: a new form of continuous ambulatory cueing for patients with Parkinson's disease. Park. Relat. Disord. 2016;29:127–128. doi: 10.1016/j.parkreldis.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Heremans E., Nieuwboer A., Vercruysse S. Freezing of gait in Parkinson's disease: where are we now? Curr. Neurol. Neurosci. Rep. 2013;13 doi: 10.1007/s11910-013-0350-7. [DOI] [PubMed] [Google Scholar]
- 30.Barthel C., Nonnekes J., Van Helvert M., Haan R., Janssen A., Delval A., Weerdesteyn V., Debû B., Van Wezel R., Bloem B.R., Ferraye M.U. The laser shoes: a new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology. 2018;90:e164. doi: 10.1212/WNL.0000000000004795. –e171. [DOI] [PubMed] [Google Scholar]
- 31.Barthel C., van Helvert M., Haan R., Janssen A.M., Delval A., de Vries N.M., Weerdesteyn V., Debû B., van Wezel R., Bloem B.R., Ferraye M.U. Visual cueing using laser shoes reduces freezing of gait in Parkinson's patients at home. Mov. Disord. 2018;33:1664–1665. doi: 10.1002/mds.27455. [DOI] [PubMed] [Google Scholar]
- 32.Spildooren J., Vercruysse S., Meyns P., Vandenbossche J., Heremans E., Desloovere K., Vandenberghe W., Nieuwboer A. Turning and unilateral cueing in Parkinson's disease patients with and without freezing of gait. Neuroscience. 2012;207:298–306. doi: 10.1016/j.neuroscience.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Ehgoetz Martens K.A., Shine J.M., Walton C.C., Georgiades M.J., Gilat M., Hall J.M., Muller A.J., Szeto J.Y.Y., Lewis S.J.G. Evidence for subtypes of freezing of gait in Parkinson's disease. Mov. Disord. 2018;33:1174–1178. doi: 10.1002/mds.27417. [DOI] [PubMed] [Google Scholar]
- 34.Newkirk L.A., Kim J.M., Thompson J.M., Tinklenberg J.R., Yesavage J.A., Taylor J.L. Validation of a 26-point telephone version of the mini-mental state examination. J. Geriatr. Psychiatr. Neurol. 2004;17:81–87. doi: 10.1177/0891988704264534. [DOI] [PubMed] [Google Scholar]
- 35.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 37.Fahn S., Elton R.L. Members of the UPDRS Development Committee, Recent developments in Parkinson's disease. Macmillan Heal. Care Inf. 1987;2:153–163. [Google Scholar]
- 38.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Kolahi J., Bang H., Park J. Towards a proposal for assessment of blinding success in clinical, Community Dent. Oral Epidemiol. 2009;37:477–484. doi: 10.1111/j.1600-0528.2009.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N., Agarwal P., Athar S., Bordelan Y., Bronte-Stewart H.M., Camicioli R., Chou K., Cole W., Dalvi A., Delgado H., Diamond A., Dick J.P., Duda J., Elble R.J., Evans C., Evidente V.G., Fernandez H.H., Fox S., Friedman J.H., Fross R.D., Gallagher D., Goetz C.G., Hall D., Hermanowicz N., Hinson V., Horn S., Hurtig H., Kang U.J., Kleiner-Fisman G., Klepitskaya O., Kompoliti K., Lai E.C., Leehey M.L., Leroi I., Lyons K.E., McClain T., Metzer S.W., Miyasaki J., Morgan J.C., Nance M., Nemeth J., Pahwa R., Parashos S.A., Schneider J.S.J.S., Schrag A., Sethi K., Shulman L.M., Siderowf A., Silverdale M., Simuni T., Stacy M., Stern M.B., Stewart R.M., Sullivan K., Swope D.M., Wadia P.M., Walker R.W., Walker R., Weiner W.J., Wiener J., Wilkinson J., Wojcieszek J.M., Wolfrath S., Wooten F., Wu A., Zesiewicz T.A., Zweig R.M. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 41.Franchignoni F., Horak F., Godi M., Nardone A., Giordano A. Vol. 42. 2011. Using Psychometric Techniques to Improve the Balance Evaluation System’s Test: the mini-BESTest; pp. 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziegler K., Schroeteler F., Ceballos-Baumann A.O., Fietzek U.M. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov. Disord. 2010;25:1012–1018. doi: 10.1002/mds.22993. [DOI] [PubMed] [Google Scholar]
- 43.Van Dijsseldonk K., Wang Y., Van Wezel R., Bloem B.R., Nonnekes J. Provoking freezing of gait in clinical practice: turning in place is more effective than stepping in place. J. Parkinsons Dis. 2018;8:363–365. doi: 10.3233/JPD-181332. [DOI] [PubMed] [Google Scholar]
- 44.Matar E., Shine J.M., Gilat M., Ehgoetz Martens K.A., Ward P.B., Frank M.J., Moustafa A.A., Naismith S.L., Lewis S.J.G. Identifying the neural correlates of doorway freezing in Parkinson's disease. Hum. Brain Mapp. 2019;40:2055–2064. doi: 10.1002/hbm.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss A., Herman T., Giladi N., Hausdorff J.M. New evidence for gait abnormalities among Parkinson's disease patients who suffer from freezing of gait: insights using a body-fixed sensor worn for 3 days. J. Neural. Transm. 2015;122:403–410. doi: 10.1007/s00702-014-1279-y. [DOI] [PubMed] [Google Scholar]
- 46.Palmerini L., Rocchi L., Mazilu S., Gazit E., Hausdorff J.M., Chiari L. Identification of characteristic motor patterns preceding freezing of gait in Parkinson's disease using wearable sensors. Front. Neurol. 2017;8:1–12. doi: 10.3389/fneur.2017.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrari A., Ginis P., Hardegger M., Casamassima F., Rocchi L., Chiari L. A mobile Kalman-filter based solution for the real-time estimation of spatio-temporal gait parameters. IEEE Trans. Neural Syst. Rehabil. Eng. 2016;24:764–773. doi: 10.1109/TNSRE.2015.2457511. [DOI] [PubMed] [Google Scholar]
- 48.Gilat M. How to annotate freezing of gait from video: a standardized method using open-source software. J. Parkinsons Dis. 2019;9:821–824. doi: 10.3233/JPD-191700. [DOI] [PubMed] [Google Scholar]
- 49.ELAN (version 5.5) [computer software] 2019. Nijmegen: Max Planck Institute for Psycholinguistics, the Language Archive.https://archive.mpi.nl/tla/elan Retrieved from. [Google Scholar]
- 50.Morris T.R., Cho C., Dilda V., Shine J.M., Naismith S.L., Lewis S.J.G., Moore S.T. A comparison of clinical and objective measures of freezing of gait in Parkinson's disease. Park. Relat. Disord. 2012;18:572–577. doi: 10.1016/j.parkreldis.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Barthel C., Mallia E., Debû B., Bloem B.R., Ferraye M.U. The practicalities of assessing freezing of gait. J. Parkinsons Dis. 2016;6:667–674. doi: 10.3233/JPD-160927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss A., Herman T., Giladi N., Hausdorff J.M. Objective assessment of fall risk in Parkinson's disease using a body-fixed sensor worn for 3 days. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 54.Hyde T., Fritsch T. Assessing executive function in Parkinson disease: the Alternating Names Test. Part I. Reliability, validity, and normative data. Park. Relat. Disord. 2011;17:100–105. doi: 10.1016/j.parkreldis.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 55.Leentjens A.F.G., Dujardin K., Pontone G.M., Starkstein S.E., Weintraub D., Martinez-Martin P. The Parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov. Disord. 2014;29:1035–1043. doi: 10.1002/mds.25919. [DOI] [PubMed] [Google Scholar]
- 56.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 57.Weiss D., Schoellmann A., Fox M.D., Bohnen N.I., Factor S.A., Nieuwboer A., Hallett M., Lewis S.J.G. Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain. 2020;143:14–30. doi: 10.1093/brain/awz314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehgoetz Martens K.A., Lukasik E.L., Georgiades M.J., Gilat M., Hall J.M., Walton C.C., Lewis S.J.G. Predicting the onset of freezing of gait: a longitudinal study. Mov. Disord. 2018;33:128–135. doi: 10.1002/mds.27208. [DOI] [PubMed] [Google Scholar]
- 59.Herman T., Shema-Shiratzky S., Arie L., Giladi N., Hausdorff J.M. Depressive symptoms may increase the risk of the future development of freezing of gait in patients with Parkinson's disease: findings from a 5-year prospective study. Park. Relat. Disord. 2019;60:98–104. doi: 10.1016/j.parkreldis.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 60.D'Cruz N., Vervoort G., Fieuws S., Moreau C., Vandenberghe W., Nieuwboer A. Repetitive motor control deficits most consistent predictors of conversion to freezing of gait in Parkinson's disease: a prospective cohort study. J. Parkinsons Dis. 2020;10:559–571. doi: 10.3233/jpd-191759. [DOI] [PubMed] [Google Scholar]
- 61.Forsaa E.B., Larsen J.P., Wentzel-Larsen T., Alves G. A 12-year population-based study of freezing of gait in Parkinson's disease. Park. Relat. Disord. 2015;21:254–258. doi: 10.1016/j.parkreldis.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Mancini M., Bloem B.R., Horak F.B., Lewis S.J.G., Nieuwboer A., Nonnekes J. Clinical and methodological challenges for assessing freezing of gait: future perspectives. Mov. Disord. 2019;34:783–790. doi: 10.1002/mds.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bächlin M., Plotnik M., Roggen D., Giladi N., Hausdorff J.M., Tröster G. A wearable system to assist walking of parkinsońs disease patients benefits and challenges of context-triggered acoustic cueing. Methods Inf. Med. 2010;49:88–95. doi: 10.3414/ME09-02-0003. [DOI] [PubMed] [Google Scholar]
- 64.Mazilu S., Blanke U., Dorfman M., Gazit E., Mirelman A., Hausdorff J.M., Tröster G. A wearable assistant for gait training for Parkinson's disease with freezing of gait in out-of-the-lab environments. ACM Trans. Interact. Intell. Syst. 2015;5:1–31. doi: 10.1145/2701431. [DOI] [Google Scholar]
- 65.Peterson D.S., King L.A., Cohen R.G., Horak F.B. Cognitive contributions to freezing of gait in Parkinson disease: implications for physical rehabilitation. Phys. Ther. 2016;96:659–670. doi: 10.2522/ptj.20140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehgoetz Martens K.A., Peterson D.S., Almeida Q.J., Lewis S.J.G., Hausdorff J.M., Nieuwboer A. Behavioural manifestations and associated non-motor features of freezing of gait: a narrative review and theoretical framework. Neurosci. Biobehav. Rev. 2020;116:350–364. doi: 10.1016/j.neubiorev.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Ginis P., Heremans E., Ferrari A., Bekkers E.M.J., Canning C.G., Nieuwboer A. External input for gait in people with Parkinson's disease with and without freezing of gait: one size does not fit all. J. Neurol. 2017;264:1488–1496. doi: 10.1007/s00415-017-8552-6. [DOI] [PubMed] [Google Scholar]
- 68.Gallo P.M., McIsaac T.L., Garber C.E. Walking economy during cued versus non-cued treadmill walking in persons with Parkinson's disease. J. Parkinsons Dis. 2014;4:705–716. doi: 10.3233/JPD-130217. [DOI] [PubMed] [Google Scholar]
- 69.Ekker M.S., Janssen S., Nonnekes J., Bloem B.R., De Vries N.M. Neurorehabilitation for Parkinson's disease: future perspectives for behavioural adaptation. Park. Relat. Disord. 2016;22(Suppl 1):S73–S77. doi: 10.1016/j.parkreldis.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 70.Pardoel S., Kofman J., Nantel J., Lemaire E.D. Wearable-sensor-based detection and prediction of freezing of gait in Parkinson's disease: a review. Sensors. 2019;19:5141. doi: 10.3390/s19235141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Lima A.L.S., Hahn T., Evers L.J.W., De Vries N.M., Cohen E., Afek M., Bataille L., Daeschler M., Claes K., Boroojerdi B., Terricabras D., Little M.A., Baldus H., Bloem B.R., Faber M.J. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson's disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ginis P., Nieuwboer A., Dorfman M., Ferrari A., Gazit E., Canning C.G., Rocchi L., Chiari L., Hausdorff J.M., Mirelman A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson's disease: a pilot randomized controlled trial. Park. Relat. Disord. 2016;22:28–34. doi: 10.1016/j.parkreldis.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Mancini M., Shah V.V., Stuart S., Curtze C., Horak F.B., Safarpour D., Nutt J.G. Measuring freezing of gait during daily-life: an open-source, wearable sensors approach. J. NeuroEng. Rehabil. 2021;18:1–13. doi: 10.1186/s12984-020-00774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kempen G.I.J.M., Yardley L., Van Haastregt J.C.M., Zijlstra G.A.R., Beyer N., Hauer K., Todd C. The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37:45–50. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 75.Jenkinson C., Fitzpatrick R., Peto V., Greenhall R., Hyman N. The PDQ-8: development and validation of a short-form Parkinson's disease questionnaire. Psychol. Health. 1997;12:805–814. doi: 10.1080/08870449708406741. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.