Highlights
-
•
S. cerevisiae growth was differently affected by selected ultrasound frequencies.
-
•
Gompertz and Weibull models showed the best fit to growth and inactivation curves.
-
•
Lag phase was slightly reduced after application of 45 and 130 kHz treatments.
-
•
Frequency of 20 kHz achieved the highest yeast reductions and the longer lag phase.
-
•
Important changes in yeast morphology were shown after 20 kHz treatments.
Keywords: Saccharomyces cerevisiae, Ultrasound, Kinetic models, SEM analysis
Abstract
The yeast Saccharomyces cerevisiae is well known for its application in the food industry for the purpose of developing fermented food. The ultrasound (US) technology offer a wide range of applications for the food industry, including the enhancement of fermentation rates and inactivation of microbial cells. However, a better understanding and standardization of this technology is still required to ensure the scaling-up process. This study investigated the effect of the US technology on the growth of S. cerevisiae using frequencies of 20, 25, 45 and 130 kHz, treatment periods from 2 to 30 min. Furthermore, yeast kinetics subjected to US treatments were evaluated using modelling tools and scanning electron microscopy (SEM) analysis to explore the impact of sonication on yeast cells. Yeast growth was monitored after different US treatments plotting optical density (OD) at 660 nm for 24 h at 30 ⁰C. Growth curves were fitted using models of modified Gompertz and Scale-Free which showed good parameters of the fit. In particular, US frequencies of 45 and 130 kHz did not have a disruptive effect in lag phase and growth rate of the yeast populations, unlike the frequency of 20 kHz. Moreover, inactivation curves of yeast cells obtained after exposure to 20 and 25 kHz also observed the best fit using the Weibull model. US frequency of 20 kHz achieved significant reductions of 1.3 log cfu/mL in yeast concentration and also induced important cell damage on the external structures of S. cerevisiae. In conclusion, the present study demonstrated the significant effect of applying different US frequencies on the yeast growth for potential application in the food industry.
1. Introduction
Yeasts are eukaryotic and unicellular fungi that are naturally found as indigenous microbiota in raw materials. The principal characteristic of these microorganisms is their capacity to transform organic substrates into energy, CO2, and ethanol, a process known as alcoholic fermentation. In terms of food application, yeast fermentation has been employed globally at industrial level for the production of beer, wine, bread, among other products [1]. Specifically, most of the fermentation processes carried out by yeast in the food industry use S. cerevisiae due to its high productivity, greater dominance over other microbial species, and capacity to tolerate high ethanol content, low pH, organic acids, and anaerobic conditions. Currently, the alcoholic fermentation with S. cerevisiae is highly optimised and monitored by a very competitive fermentation industry through a wide range of strategies (e.g. selection of starter cultures, application of technological advances, quality control of raw materials, etc…) [2]. On the contrary, this yeast species has been the focus of the food industry due to its association with food spoilage. High sugar content and/or acidic foods and beverages have demonstrated to facilitate the growth of yeast which can be found in raw materials, air, water, equipment and/or vectored by animals. In this way, spoilage of food and beverages are caused by generation of undesirable fermentations, affecting the quality and sensory properties of these products [3].
The application of novel technologies in food processing has been increasingly studied in the last years for potential implementation in the food chain. The US technology has been considered within these technological advances and has evolved to a wide variety of devices (US alone like water baths and ultrasonic probes or its combination with other technologies) that are currently available in the market for multiple applications. In food industry, a broad spectrum of applications has been considered in specific food products and for several unit operations, such as extraction, foaming, drying, thawing, among others [4], [5]. Specifically, food fermentation could benefit from integrating the US technology into its processing line. High frequency US has been evaluated for monitoring food composition, concentrations, and molecular and physical structure and could be used in monitoring fermentation processes. Additionally, US in low frequencies has been investigated for improving the efficiency of the fermentation step and the final quality and safety of the food product. Thus, ultrasound-assisted fermentation has been previously studied and evaluated in wine, beer and vinegar production and fermented food like milk, yoghurt and sweet whey, among others [5], [6]. Nevertheless, a better understanding and standardization of this technology and its extrinsic (e.g. matrix characteristics, temperature, microbial growth, etc…) and intrinsic (e.g. US frequency, device type, treatment time, etc…) conditions are still required to ensure the scaling-up process at industrial level [6].
The effect of low frequencies (20–28 kHz) on the growth, viability, and fermentation performance of S. cerevisiae has been studied by several authors [7], [8], [9], [10]. For instance, Zhang et al. [8] observed an increase in the fermentation rate of S. cerevisiae when US was applied at a frequency of 23 kHz, however, frequencies higher than 33 kHz caused greater mortality rate of yeast cells. In contrast, Huezo et al. [9] found that the application of US at 20 kHz during the first 12 h of fermentation decreased the performance and growth of S. cerevisiae. Moreover, it should be noted that an optimization of the US parameters previous to the fermentation process could be advantageous in enhancing the fermentation rates of the yeast cells. Furthermore, other authors have considered the US technology as a potential inactivation strategy towards S. cerevisiae contamination of beverages [11], [12], [13], [14], [15]. Within these studies, US at a frequency of 20 kHz achieved reductions of around 1 log cfu/mL, although different kinetics of inactivation were found as a result of variations in the surrounding media or food matrix, and US parameters like device, treatment time and frequency. For instance, the technology of pulsed-thermosonication was evaluated as a potential disinfection strategy using different predictive models for the inactivation of Listeria monocytogenes, Shigella sonnei, Byssochlamys fulva and S. cerevisiae. Among the studied models, the Weibull model showed the best fit for all the inactivation curves and helped to predict the effect of the pulsed-thermosonication technology on the studied microorganisms [15]. Paniagua-Martínez et al. [16] suggested that kinetic models could be used to validate novel technologies like the US method and thereby, facilitate its scaling-up process and implementation in the food industry. Mathematical models are key to predict and evaluate the growth and/or inactivation kinetics of a technology and are commonly developed with previous experimental data and subjected to multiple factors (e.g. biological variability, time, temperature, etc…) [17]. Studies using modelling tools for evaluation of the US technology on the kinetics (growth or inactivation) of S. cerevisiae are scarce and a particular focus on its treatment conditions is required for potential implementation in the food industry. As an example, Yang et al. [10] investigated the effect of low intensity ultrasound on the fermentation kinetics of S. cerevisiae. However, the established model was developed considering a single US frequency and treatment time [10]. These parameters could have an influence on the yeast kinetics and therefore, further information is needed to understand their importance for the optimization of this technology. To the best of our knowledge, our study is the first to evaluate the effect of US frequency and treatment time on the S. cerevisiae growth through modelling tools.
The objective of the present study consisted of investigating the effect of the US technology on the growth of S. cerevisiae using different frequencies, treatment periods, and device types. For this purpose, the kinetics of the yeast cells were evaluated towards US treatments and compared using modelling tools. Moreover, the impact of sonication was explored through electron microscopy to observe potential cell damage on the external structures of the yeast cells.
2. Materials and methods
2.1. Preparation of yeast cultures
Saccharomyces cerevisiae DSM 70,449 obtained from the microbiology stock cultures at Teagasc Food Research Centre (Ashtown, Dublin) was selected to conduct the present study. Yeast cells were cultured in Yeast Extract Peptone Dextrose broth medium (YPD, 3 g/L yeast extract, 5 g/L peptone, 10 g/L glucose, Oxoid, UK) and incubated overnight at 30 ⁰C with continuous shaking at 150 rpm. The suspension of S. cerevisiae cells was harvested by centrifugation at 4000 × g for 10 min and washed by resuspending the pellet in Maximum Recovery Diluent (MRD, Oxoid, UK). To determine the concentration of overnight cultures, 10-fold serial dilutions were made and transferred into YPD agar medium (3 g/L yeast extract, 5 g/L peptone, 10 g/L glucose, 15 g/L agar, Oxoid, UK) by plating the suspension followed by incubation at 30 ⁰C for 48 h. Once the concentration of overnight cultures was determined, a volume of the yeast suspension was inoculated in YPD broth medium to obtain a final concentration of ∼ 6 log cfu/mL which was used to conduct US treatments.
2.2. Ultrasound treatment
The effect of US treatment was evaluated taking in account different variables, such as frequency (20, 25, 45, 130 kHz), treatment time (2 to 30 min), and type of US system (bath or probe). The ultrasonic power (W) was measured using calorimetric method outlined previously by Tiwari et al. [18]. The ultrasound power level measured using calorimetric method was 155.3, 4.7, 5.5, and 7.2 W respectively. Samples were placed into flat bottom glass tubes (containing 20 mL of yeast suspension) into ultrasonic baths of 25, 45, and 130 kHz (Elma Schmidbauer GmbH, Singen, Germany) for 10, 20, and 30 min. Test tubes were maintained in the centre of the US bath by a fixed floating appliance to avoid the movement of the samples and a corresponding treatment variation between replicas. The temperature in the US bath was maintained below 15 ⁰C using a refrigerated circulator (LTD20G, Grant Instrument, UK) through which glycol coolant (-0.5 ⁰C) was circulated using a heat exchanger and a pump with a flow rate of about 1 L/min. Furthermore, sonication with a 20 kHz probe system (VCX 750 Ultrasonic Microprocessor, Sonics Materials, Newton, CT, USA) at an amplitude of 76 µm was applied for 2, 5, 10, 20, and 30 min by submerging the probe tip (ø, 1.3 cm) into the sample (100 mL). Thus, the yeast suspension was placed into a sterile jacket beaker (250 mL) using the refrigeration system previously mentioned to avoid any inactivating effects due to the increase of temperature inside the container. Samples no subjected to US treatment were considered as controls. Treatments were performed in biological triplicates and samples were conducted in duplicates for each frequency and time point.
2.3. Microbiological analysis and yeast growth curves
Recovery of S. cerevisiae population of control and US treated samples was performed immediately after treatment and consisted of taking 1 mL aliquot of each sample at specified treatment time points and diluting in 9 mL of MRD. Afterwards, suspensions were spread plating on YPD agar as described previously and yeast colonies were enumerated by colony counting. Then, counts were plotted as log cfu/mL and the mean and standard deviation of samples were calculated.
In parallel, yeast growth of control and US treated samples was monitored by optical density (OD) measurements at 660 nm at a regular interval of 30 min using a 96 well plate in a temperature-controlled microplate reader spectrophotometer (Epoch 2, BioTek, Swindon, UK) during 24 h at 30 ⁰C. Each well was inoculated with 20 µL of yeast cells into 180 µL of YPD broth. Growth curves were repeated in triplicate for each treatment to ensure reproducibility.
2.4. Model fitting and statistical analysis
To evaluate the effect of US treatment on the shape of the S. cerevisiae growth curve, the primary growth parameters were obtained by plotting and fitting OD660nm towards incubation time to three models: modified Gompertz, Scale-Free and Biphasic through the DMFit excel based tool [19]. The Gompertz equation (Eq. (1)) used in this study is common to all these models and can be described as follows [20].
| (1) |
Where Yt is the OD value in a specific incubation time (t, h), YEnd and Y0 are the maximum and initial OD values, Gmax represents the maximum relative growth (h−1) when time is equal to M, and M is the time in h when the absolute growth rate is maximum.
In addition, the parameters of maximum specific growth rate (μmax, h) and lag phase (λ, h) were calculated with Eq. (2) and Eq. (3) and determined in each of the former models and corresponding controls and US treatments.
| (2) |
| (3) |
Moreover, a Weibull model (Eq. (4)) was applied to fit the inactivation tendency observed in yeast counts after sonication with frequencies of 20 and 25 kHz. The excel tool of GInaFiT was employed for the purpose of data modelling and parameters like the time to reduce the yeast population in 1 log cfu/mL or Dt value ( min−1) and shape parameter (p) were calculated following the equation (4) [21].
| (4) |
Where N0 is the initial concentration of yeast cells and N is the maximum concentration of yeast cells in a specific time (t, min).
The validation of these models was expressed as the goodness of fit and parameters, such as R2, Regression coefficient (Eq. (5)); RMSE, root mean square error (Eq. (6)); AIC, Akaike’s Information Criterion (Eq. (7)); and BIC, Bayesian’s Information Criterion (Eq. (8)) were obtained [22].
| (5) |
| (6) |
| (7) |
| (8) |
Where Co and Cp are the observed and predictive results of yeast concentration, N is the observation number, SE represents the sum of the square error, and n is the number of parameters in the studied model.
Statistical analysis of the data was performed with Minitab® 17.1. The average and standard deviation of the yeast counts and ODs were calculated. Treated samples and controls were compared using a one-way ANOVA together with a post-hoc test of Tuckey at a significance level of p > 0.05.
2.5. Scanning electron microscope (SEM) analysis
S. cerevisiae suspensions at 7 log cfu/mL were sonicated at frequencies of 20 and 130 kHz for 30 min following same treatment procedure as described above. These samples together with a control were prepared following the protocol of Fratesy et al. [23] with minor adjustments for the analysis by image through scanning electron microscopy (FEI Quanta 3D FEG DaulBeam, FEI Ltd, Hillsboro, USA). Thus, cell suspensions were centrifuged at 4000 × g for 10 min at 4 ⁰C for fixation and pellets were covered with a solution of 2.5% glutaraldehyde (GA, Sigma Aldrich, Ireland) in 0.05 M sodium cacodylate buffer (SCB, pH 7.4, Sigma Aldrich, Ireland) for 24 h. The GA reagent was removed by repeated washes with SCB and subsequent centrifugations at 4000 × g for 10 min at 4 ⁰C. Afterwards, suspensions were transferred onto sterilized glass which were dehydrated with increasing concentrations of ethanol and Hexamethyldisilizane (HMDS, Sigma Aldrich, Ireland) until maximum concentrations of 99.5 and 100%, respectively. Samples just before the SEM analysis were immobilised on stubs through double-sided carbon tape and a sputter coater (Emitech K575X Coating Unit) was used to coat them with gold in order to avoid surface charging generated by the electron beam. Finally, representative images of the surface of yeast cells were chosen to compare between the selected treatments and control.
3. Results and discussion
3.1. Modelling the growth kinetics of S. cerevisiae cells
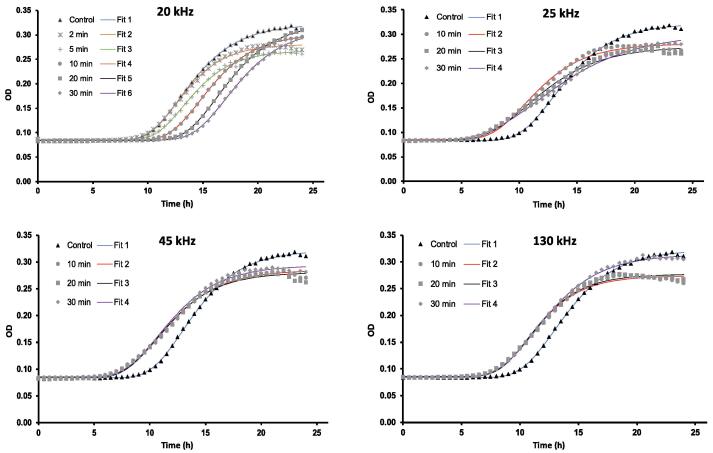
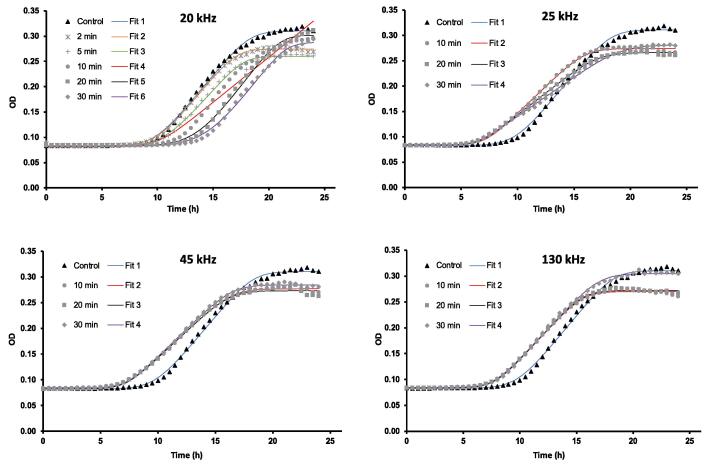
Growth curves of treated and control S. cerevisiae cells for each device, frequency, and treatment time were obtained from plotting OD660nm with incubation time (h) and fitted using the modified Gompertz model displayed in Fig. 1 and the Scale-Free model showed in Fig. A1 in the Appendix section. A Biphasic model was also considered; however, the validation of this model proved a poor goodness of the fit (Fig. A3). Models of modified Gompertz and Scale-Free are based on the Gompertz equation. This primary model has been identified in the literature as a powerful model for describing bacterial growth and incorporates two phases in its equation: a lag or latency phase and an inhibitory growth phase in the beginning and end of the incubation time, respectively [20], [24].
Fig. 1.
Growth curves of S. cerevisiae and corresponding Gompertz fits for each US treatment at the frequencies of 20, 25, 45 and 130 kHz, and control in which optical density (OD) is plotted against time (h).
Fig. A1.
Growth curves of S. cerevisiae and corresponding Scale-Free fits for each US treatment at frequencies of 20, 25, 45 and 130 kHz, and control in which optical density (OD) is plotted against time (h).
Fig. A3.
Growth curves of S. cerevisiae and corresponding Biphasic fits for each US treatment at frequencies of 20, 25, 45 and 130 kHz, and control in which optical density (OD) is plotted against time (h).
Table 1 shows parameters of Gompertz models obtained for every US treatment and control and Table A1 presents parameters of the Scale-Free model in the Appendix section. Within the fitted parameters, lag phase of samples treated with US at 20 kHz for 20 and 30 min observed a significant increase with respect to the control (p < 0.05). Furthermore, a reduction of 1 h in the lag time was detected in yeast suspensions treated with US at frequencies of 25, 45, and 130 kHz in the Gompertz model (Fig. 1) and Scale-Free model (Fig. A1 in Appendix), however, these differences were not statistically significant when compared with the control (p > 0.05) as presented in parameters of Gompertz (Table 1) and Scale-Free models (Table A1 in Appendix). The lag phase is the adaptation period of a microorganism to an environment or medium before the exponential growth occurs. In fermentation processes, efforts are intended to shorten this period of adjustment and thereby, improve process effectiveness [25]. Huezo et al. [9] studied the effect of direct and indirect ultrasound at 20 kHz on the fermentation rate of S. cerevisiae and observed that direct ultrasound increased the lag phase of yeast cells. In concordance with this, our study showed how treatment periods of US at 20 kHz of>20 min can induce stress conditions in the yeast population and delay for 3–4 h the beginning of the growth phase in parameters of Gompertz (Table 1) and Scale-free (Table A1 in Appendix). Nevertheless, lag phase of treated yeast suspensions was not significantly affected by sonication at frequencies of 25, 45, and 130 kHz (p > 0.05). The same applied to the maximum specific growth rate or μmax of yeast cells which was not interfered by all the studied US frequencies and treatment periods. Other studies also investigated the impact of this technology on the growth of S. cerevisiae and showed no signs of growth disruption when yeast suspensions were exposed to US frequencies above 25 kHz [7], [26], [27]. In contrast, Zhang et al. [8] reported that frequencies above 33 kHz induced damage of yeast cells compromising their growth rates. The authors suggested that these frequencies may stimulate the growth of cells which triggers the growth inhibition of some yeast cells [8]. US frequencies of 45 and 130 kHz investigated in our study did not negatively impacted the yeast growth. The different effect of US frequencies on the yeast growth can be associated with the cavitation phenomenon. Cavitation consists of the formation and collapse of gas bubbles with subsequent release of energy through US pressure waves which are generated in the vibrations of an ultrasonic field. These nanobubbles can be increased by raising the US frequency applied. Nevertheless, increasing the number of bubbles also reduces their diameter and the amount of energy released after implosion. This fact can explain that frequencies of 45 and 130 kHz did not affect the growth of S. cerevisiae. Furthermore, lower US frequencies than 40 kHz are capable of releasing large quantities of energy through the cavitation phenomenon which can lead to cell damage. The effectiveness of inactivation has been also shown to be improved with increased sonication time and the use of ultrasonic probe due to the reduced surface of its transducer [28]. Thus, frequencies of 20 and 40 kHz has been identified in the literature with the ability to disrupt living cells and therefore, this frequency range could be specifically considered for decontamination purposes [6].
Table 1.
S. cerevisiae growth curves parameters fitted with the Gompertz model and calculated for each US treatment and control, where μmax is the maximum specific growth rate in h, λ is the lag phase in h, and Y0 and Yend are the initial and maximum numbers of yeast cells. Results are expressed in average ± standard deviation. Significant differences (*, p < 0.05) of treatments compared to controls.
| Parameters | |||||
|---|---|---|---|---|---|
| Model | Treatment | µmax | λ | Y0 | YEnd |
| Gompertz | Control | 0.030 ± 0.002 | 10.060 ± 0.218 | 0.084 ± 0.003 | 0.322 ± 0.012 |
| 20 KHz 2 min | 0.030 ± 0.002 | 10.086 ± 0.114 | 0.085 ± 0.002 | 0.282 ± 0.028 | |
| 20 KHz 5 min | 0.027 ± 0.002 | 10.657 ± 0.051 | 0.085 ± 0.003 | 0.268 ± 0.007 | |
| 20 KHz 10 min | 0.029 ± 0.002 | 11.906 ± 0.149 | 0.084 ± 0.001 | 0.305 ± 0.026 | |
| 20 KHz 20 min | 0.031 ± 0.003 | 13.400 ± 0.381* | 0.084 ± 0.002 | 0.326 ± 0.009 | |
| 20 KHz 30 min | 0.029 ± 0.003 | 14.233 ± 0.461* | 0.085 ± 0.001 | 0.316 ± 0.011 | |
| 25 KHz 10 min | 0.033 ± 0.003 | 8.543 ± 1.873 | 0.085 ± 0.002 | 0.283 ± 0.022 | |
| 25 KHz 20 min | 0.032 ± 0.003 | 8.982 ± 2.722 | 0.084 ± 0.001 | 0.274 ± 0.017 | |
| 25 KHz 30 min | 0.032 ± 0.002 | 9.524 ± 3.304 | 0.085 ± 0.002 | 0.299 ± 0.034 | |
| 45 KHz 10 min | 0.030 ± 0.003 | 8.423 ± 1.718 | 0.086 ± 0.002 | 0.288 ± 0.028 | |
| 45 KHz 20 min | 0.031 ± 0.002 | 8.409 ± 1.751 | 0.084 ± 0.002 | 0.281 ± 0.018 | |
| 45 KHz 30 min | 0.032 ± 0.004 | 8.461 ± 1.831 | 0.085 ± 0.002 | 0.298 ± 0.023 | |
| 130 KHz 10 min | 0.031 ± 0.002 | 8.441 ± 1.089 | 0.085 ± 0.001 | 0.276 ± 0.023 | |
| 130 KHz 20 min | 0.031 ± 0.001 | 8.461 ± 1.181 | 0.085 ± 0.002 | 0.277 ± 0.022 | |
| 130 KHz 30 min | 0.030 ± 0.002 | 8.548 ± 1.216 | 0.085 ± 0.002 | 0.313 ± 0.010 | |
Additionally, the fitness grades of the modified Gompertz model displayed in Fig. 1 and the Scale-Free model presented in Fig. 1A (Appendix section) towards the experimental growth curves were measured and expressed as goodness of the fit. Thus, goodness of fit parameters like R2, RMSE, AIC, and BIC were calculated and are shown in Table 2 for the modified Gompertz model and Table A2 for the Scale-free model in the Appendix section. Models with higher fit performance are considered to present R2 values near to one and low values for the parameters RMSE, AIC and BIC [29]. In this study, both models exhibited R2 values higher than 0.991 and 0.982 for the modified Gompertz and Scale-Free models, respectively. Moreover, parameters of RMSE (0.001–0.013), AIC (-(659–417)) and BIC (-(664–422)) showed low values in both models and all the predicted growth curves as they are presented in Table 2 for the Gompertz model and Table A2 for the Scale-Free model. Studies evaluating the use of modelling tools for prediction of S. cerevisiae growth kinetics are scarce in the literature. Yang et al. [10] assessed the effect of low intensity ultrasound on the growth of S. cerevisiae using the predictive model of Luedeking-Piret with the Logistic regression tool and observed the capacity of this technology to promote the growth of yeast cells. Moreover, this model demonstrated a good correlation with the experimental growth curves and helped the authors to draw conclusions about the impact of this technology on the yeast cells [10]. Thus, the modelling technique may facilitate the understanding of potential growth promoter or disturbance effects of a technology on a population of microbial cells and simultaneously, validate and standardize the effectivity of a technology [16]. In fermentative processes, the application of the US technology has been proved beneficial in enhancing the microorganism performance [6]. A combination of this technology with predictive tools could facilitate its scaling up process and its subsequent implementation at industrial level.
Table 2.
Parameters of the goodness of the fit calculated for each growth curve of S. cerevisiae and fitted with the Gompertz model, where R2 is the regression coefficient, RMSE is the root mean square error, AIC is the Akaike’s Information Criterion, and BIC is the Bayesian’s Information Criterion.
| Model | Treatment | R2 | RMSE | AIC | BIC |
|---|---|---|---|---|---|
| Gompertz | Control | 0.999 | 0.002 | −611.053 | −615.517 |
| 20 KHz 2 min | 0.997 | 0.003 | −544.524 | −548.988 | |
| 20 KHz 5 min | 0.997 | 0.003 | −574.763 | −579.227 | |
| 20 KHz 10 min | 0.999 | 0.001 | −658.573 | −663.037 | |
| 20 KHz 20 min | 0.999 | 0.001 | −647.55 | −652.014 | |
| 20 KHz 30 min | 0.999 | 0.001 | −659.753 | −664.216 | |
| 25 KHz 10 min | 0.991 | 0.005 | −502.197 | −506.661 | |
| 25 KHz 20 min | 0.994 | 0.005 | −515.515 | −519.979 | |
| 25 KHz 30 min | 0.993 | 0.003 | −547.279 | −551.742 | |
| 45 KHz 10 min | 0.993 | 0.004 | −523.834 | −528.298 | |
| 45 KHz 20 min | 0.994 | 0.005 | −506.026 | −510.489 | |
| 45 KHz 30 min | 0.991 | 0.004 | −522.687 | −527.151 | |
| 130 KHz 10 min | 0.994 | 0.005 | −515.032 | −519.496 | |
| 130 KHz 20 min | 0.996 | 0.004 | −518.310 | −522.774 | |
| 130 KHz 30 min | 0.998 | 0.002 | −579.932 | −584.395 |
3.2. Modelling the inactivation effect of US and impact on external cell structures
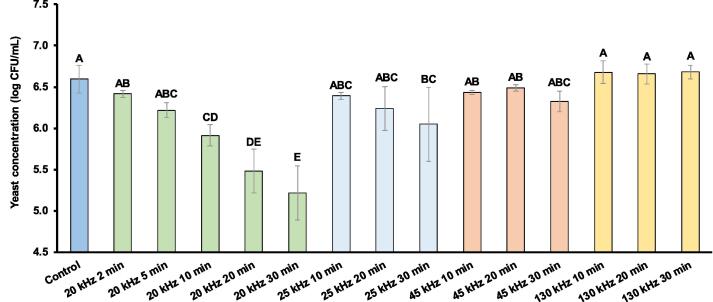
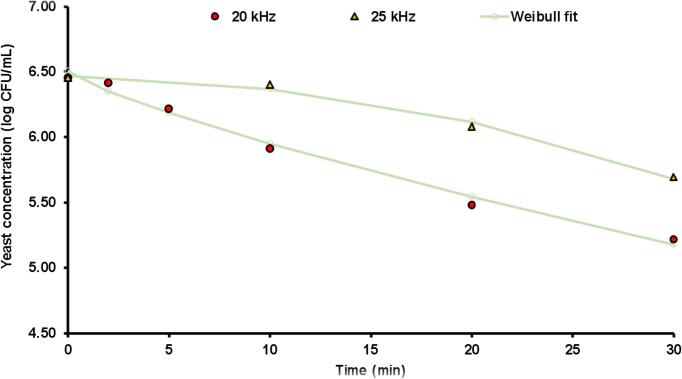
Fig. A2 shows the effect of US treatments on S. cerevisiae counts in control and after treatment. A significant decrease (p < 0.05) in total yeast counts was observed when yeast suspensions were exposed to the US frequency of 20 and 25 kHz. As shown in Fig. 2, inactivation curves of treated samples at frequencies of 20 and 25 kHz were obtained by plotting yeast concentration (log cfu/mL) versus US treatment time (min) and fitted using the Weibull model in all the selected US treatments. The Weibull model has been proved to predict effectively the inactivation kinetics of bacteria and yeast cells after sonication and therefore, this model was chosen to conduct the present study [11], [12], [29]. Parameters of the Weibull model and goodness of the fit shown in Table 3 were calculated for all the obtained inactivation curves. In general, the fitted Weibull models presented R2 between 0.950 and 0.975 and low values of RMSE (0.025–0.074), AIC (-(19–45)), and BIC (-(39–34)) which indicate the effective fitness of the Weibull model for predicting the kinetics of the inactivation curves. Thus, the Weibull model combines size and shape parameters ( and p) for describing more complex inactivation curves. Analysing the trend of these two parameters could give us some information about the effect of sonication on the growth inhibition [22]. In this study, the fitted Weibull model in the inactivation curve of US at 25 kHz showed parameters of shape (p) above 1 that produced more convex curves (Fig. 2 and Table 3). According to Scanlon et al. [30], convex curves could be a result of a slight resistance offered by the microbial population towards a disruptive effect. Furthermore, the inactivation curve obtained after US treatment at 20 kHz resulted in the lowest or Dt (22.91 min) value, corresponding to the treatment period of a decimal reduction (1 log cfu/mL), unlike treatment of 25 kHz which reduced yeast counts in 0.79 log cfu/mL. Overall, sonication at 20 kHz for 30 min was the most effective treatment with a maximum reduction of 1.3 log cfu/mL. In other studies, modelling tools were also used for evaluating the inactivation effect of thermosonication on S. cerevisiae cells [11], [12], [15]. The Weibull model was identified with the best fit for predicting inactivation curves of yeast cells exposed to thermosonication in all these studies. At the same time, the authors highlighted the combined effectiveness of temperature and sonication to inactivate S. cerevisiae in liquid foods.
Fig. A2.
Concentrations of S. cerevisiae in controls and after US treatment at different frequencies (20, 25, 45 and 130 kHz) and times (min). Significant differences of p < 0.05 in samples that do not share a letter.
Fig. 2.
Inactivation curves of S. cerevisiae after US treatment at the frequencies of 20 and 25 kHz, where the yeast concentration (log cfu/mL) is plotted against US treatment time (min) and Weibull model is fitted for each US treatment.
Table 3.
Parameters of the Weibull model and goodness of the fit calculated for the inactivation curves of S. cerevisiae at frequencies of 20 and 25 kHz, where is Dt value in min−1, p is a shape parameter, R2 is the regression coefficient, RMSE is the root mean square error, AIC is the Akaike’s Information Criterion, and BIC is the Bayesian’s Information Criterion.
| Parameters | Goodness of fit | |||||
|---|---|---|---|---|---|---|
| US treatment | p | R2 | RMSE | AIC | BIC | |
| 20 kHz | 22.913 ± 7.731 | 0.796 ± 0.013 | 0.975 | 0.074 | −19.004 | −34.924 |
| 25 kHz | 32.714 ± 4.762 | 2.388 ± 1.226 | 0.965 | 0.057 | −44.978 | −39.906 |
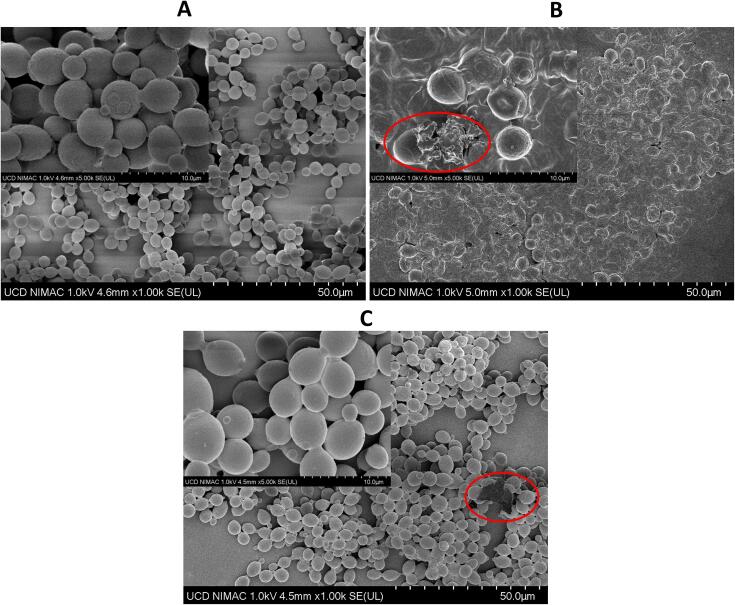
The impact of US treatments on the external structures of yeast cells was also studied. Representative images of yeast cells subjected to US frequencies of 20 and 130 kHz for 30 min together with non-treated cells were obtained by SEM as represented in Fig. 3. US treatments were selected according to the yeast growth kinetics obtained. Structures of yeast cells were not affected by exposure to a frequency of 130 kHz, although some wall breakage occurred in isolated yeast cells (Fig. 3C). On the contrary, significant changes in the morphology of yeast cells were noted after treatment with 20 kHz for 30 min (Fig. 3B). General modifications consisted of wall breakage, cell deformation, and aggregate formation. These changes on the bacterial structures can be associated with the acoustic cavitation generated in US frequencies ranging from 20 to 40 kHz. Ultrasonic waves can induce the permeabilization of the cell membrane creating pores (sonoporation) that could cause the disruption of microbial cells. In US frequencies higher than 40 kHz, the cavitation effect is lower and therefore, cells were minimally impacted as it was shown in yeast cells treated with US at 130 kHz (Fig. 3C) [6]. Other authors, such as Ferrario and Guerrero [13] and Wordon et al. [31] also investigated the impact of US on structures of yeast cells and similar structural changes were observed in yeast cells subjected to sonication at the frequency of 20 kHz. Thus, the inactivation effect of US at 20 kHz on yeast cells like S. cerevisiae has been well documented [11], [13], [14], [16], [31], [32], [33]. For instance, Jambrak et al. [3] achieved yeast reductions between 3 and 5 log cfu/mL in blueberry, cranberry, and apple juice subjected to a temperature of 60 ⁰C, although no significant reductions were observed in US treatment conducted at 20 ⁰C. Contamination of juices and other beverages with yeasts can cause undesirable fermentations, quality alteration and even posed a potential food safety risk [3]. The US technology at low frequencies has been identified as a disinfection strategy causing cell membranes damage due to the cavitation phenomenon [34]. In the present study, US frequency of 20 kHz was the most powerful treatment towards S. cerevisiae showing the highest reductions and thereby, could be considered for its decontamination in liquid media.
Fig. 3.
Microscopic images of external structures of S. cerevisiae obtained through the SEM technique in: A) control, B) sample treated with US at 20 kHz for 30 min, and C) sample treated with US at 130 kHz for 30 min. Red circles indicate cellular breakage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
The influence of the US technology on the growth of S. cerevisiae was investigated in this study for different frequencies, treatment times and devices types using modelling tools and SEM analysis. Application of US treatments at 45 and 130 kHz on yeast suspensions did not disrupt the lag phase and maximum specific growth rate of the yeast total population. At the same time, total yeast counts were not affected after the application of US treatments at the same frequencies. Additionally, reductions of 1.3 and 0.79 log cfu/mL were achieved in yeast suspensions exposed to US frequencies of 20 and 25 kHz, respectively. Representative SEM images of yeast populations also demonstrated the disruptive effect of low US frequencies on the external structures of S. cerevisiae. Furthermore, the importance of predictive models to evaluate the US technology was expressed. The selected models presented high level of fitness towards the inactivation and growth kinetics of S. cerevisiae. In this regard, this study demonstrated the significant effect of applying different US frequencies on the yeast growth for potential application in the food industry.
CRediT authorship contribution statement
Arturo B. Soro: Data curation, Formal analysis, Investigation, Writing – original draft. Márcia Oliveira: Conceptualization, Data curation, Investigation, Writing – review & editing. Colm P. O'Donnell: Conceptualization, Writing – review & editing. Brijesh K. Tiwari: Conceptualization, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the Teagasc Walsh Fellowship program, Department of Agriculture, Food, and Marine (DAFM) under the Food Institutional Research Measure (FIRM) program [Grant number: DAFM/17/F/275].
Contributor Information
Arturo B. Soro, Email: arturo.blazquezsoro@ucdconnect.ie.
Márcia Oliveira, Email: marciapsoliveira82@gmail.com.
Colm P. O'Donnell, Email: colm.odonnell@ucd.ie.
Brijesh K. Tiwari, Email: Brijesh.tiwari@teagasc.ie.
Appendix.
Table A1.
S. cerevisiae growth curves parameters fitted with the Scale-Free model and calculated for each US treatment and control, where μmax is the maximum specific growth rate in h, λ is the lag phase in h, and Y0 and Yend are the initial and maximum numbers of yeast cells. Results are expressed in average ± standard deviation. Significant differences (*, p < 0.05) of treatments compared to controls.
| Parameters | |||||
|---|---|---|---|---|---|
| Model | Treatment | µmax | λ | Y0 | YEnd |
| Scale-Free | Control | 0.027 ± 0.002 | 9.700 ± 0.250 | 0.082 ± 0.003 | 0.311 ± 0.011 |
| 20 KHz 2 min | 0.026 ± 0.002 | 9.841 ± 0.168 | 0.083 ± 0.002 | 0.275 ± 0.024 | |
| 20 KHz 5 min | 0.024 ± 0.001 | 10.515 ± 0.096 | 0.084 ± 0.003 | 0.260 ± 0.007 | |
| 20 KHz 10 min | 0.020 ± 0.003 | 10.610 ± 0.780 | 0.081 ± 0.001 | 0.260 ± 0.003 | |
| 20 KHz 20 min | 0.023 ± 0.003 | 12.507 ± 0.777* | 0.082 ± 0.002 | 0.299 ± 0.012 | |
| 20 KHz 30 min | 0.027 ± 0.004 | 14.189 ± 1.274* | 0.082 ± 0.001 | 0.286 ± 0.008 | |
| 25 KHz 10 min | 0.027 ± 0.001 | 8.151 ± 2.049 | 0.083 ± 0.002 | 0.278 ± 0.019 | |
| 25 KHz 20 min | 0.026 ± 0.004 | 8.428 ± 2.733 | 0.082 ± 0.002 | 0.269 ± 0.017 | |
| 25 KHz 30 min | 0.024 ± 0.003 | 8.606 ± 3.126 | 0.083 ± 0.002 | 0.275 ± 0.022 | |
| 45 KHz 10 min | 0.025 ± 0.001 | 7.954 ± 1.820 | 0.084 ± 0.002 | 0.282 ± 0.024 | |
| 45 KHz 20 min | 0.026 ± 0.001 | 7.982 ± 1.860 | 0.082 ± 0.002 | 0.276 ± 0.019 | |
| 45 KHz 30 min | 0.026 ± 0.002 | 7.982 ± 1.943 | 0.083 ± 0.002 | 0.291 ± 0.018 | |
| 130 KHz 10 min | 0.026 ± 0.001 | 8.023 ± 1.194 | 0.084 ± 0.001 | 0.271 ± 0.021 | |
| 130 KHz 20 min | 0.026 ± 0.001 | 8.081 ± 1.329 | 0.084 ± 0.002 | 0.272 ± 0.021 | |
| 130 KHz 30 min | 0.030 ± 0.001 | 8.096 ± 1.321 | 0.084 ± 0.002 | 0.305 ± 0.010 | |
Table A2.
Parameters of the goodness of the fit calculated for each growth curve of S. cerevisiae and fitted with the Scale-Free model, where R2 is the regression coefficient, RMSE is the root mean square error, AIC is the Akaike’s Information Criterion, and BIC is the Bayesian’s Information Criterion.
| Model | Treatment | R2 | RMSE | AIC | BIC |
|---|---|---|---|---|---|
| Scale-Free | Control | 0.997 | 0.004 | −528.016 | −532.480 |
| 20 KHz 2 min | 0.998 | 0.003 | −574.697 | −579.161 | |
| 20 KHz 5 min | 0.996 | 0.003 | −547.012 | −551.476 | |
| 20 KHz 10 min | 0.982 | 0.013 | −417.669 | −422.134 | |
| 20 KHz 20 min | 0.987 | 0.004 | −521.758 | −526.222 | |
| 20 KHz 30 min | 0.992 | 0.004 | −529.168 | −533.632 | |
| 25 KHz 10 min | 0.993 | 0.003 | −568.122 | −572.586 | |
| 25 KHz 20 min | 0.991 | 0.002 | −577.245 | −581.709 | |
| 25 KHz 30 min | 0.986 | 0.002 | −590.407 | −594.871 | |
| 45 KHz 10 min | 0.994 | 0.002 | −577.532 | −581.995 | |
| 45 KHz 20 min | 0.996 | 0.004 | −541.143 | −545.607 | |
| 45 KHz 30 min | 0.992 | 0.002 | −589.361 | −593.825 | |
| 130 KHz 10 min | 0.996 | 0.003 | −575.560 | −580.023 | |
| 130 KHz 20 min | 0.997 | 0.002 | −592.068 | −596.532 | |
| 130 KHz 30 min | 0.997 | 0.003 | −559.657 | −564.121 |
References
- 1.Walker G., Stewart G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages. 2016;2(4):30. doi: 10.3390/beverages2040030. [DOI] [Google Scholar]
- 2.Albergaria H., Arneborg N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: role of physiological fitness and microbial interactions. Appl Microbiol Biotechnol. 2016;100(5):2035–2046. doi: 10.1007/s00253-015-7255-0. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez A., Perez-Nevado F., Ruiz-Moyano S., Serradilla M.J., Villalobos M.C., Martin A., Cordoba M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int J Food Microbiol. 2018;286:98–110. doi: 10.1016/j.ijfoodmicro.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Gallo M., Ferrara L., Naviglio D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods. 2018;7 doi: 10.3390/foods7100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojha K.S., Mason T.J., O'Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Dai C., Xiong F., He R., Zhang W., Ma H. Effects of low-intensity ultrasound on the growth, cell membrane permeability and ethanol tolerance of Saccharomyces cerevisiae. Ultrason Sonochem. 2017;36:191–197. doi: 10.1016/j.ultsonch.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z., Xiong F., Wang Y., Dai C., Xing Z., Dabbour M., Mintah B., He R., Ma H. Fermentation of Saccharomyces cerevisiae in a one liter flask coupled with an external circulation ultrasonic irradiation slot: Influence of ultrasonic mode and frequency on the bacterial growth and metabolism yield. Ultrason Sonochem. 2019;54:39–47. doi: 10.1016/j.ultsonch.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Huezo L., Shah A., Michel F. Effects of Ultrasound on Fermentation of Glucose to Ethanol by Saccharomyces cerevisiae. Fermentation. 2019;5(1):16. doi: 10.3390/fermentation5010016. [DOI] [Google Scholar]
- 10.Yang Y., Xiang J., Zhang Z., Umego E.C., Huang G., He R., Ma H. Stimulation of in situ low intensity ultrasound on batch fermentation of Saccharomyces cerevisiae to enhance the GSH yield. J Food Process Eng. 2020;43 [Google Scholar]
- 11.Milani E.A., Silva F.V.M. Ultrasound assisted thermal pasteurization of beers with different alcohol levels: Inactivation of Saccharomyces cerevisiae ascospores. J Food Eng. 2017;198:45–53. [Google Scholar]
- 12.Sasikumar R., Pradhan D., Deka S.C. Effects of thermosonication process on inactivation of Escherichia coli and Saccharomyces cerevisiae and its survival kinetics modeling in khoonphal (Haematocarpus validus) juice to extend its shelf life. J Food Process Preserv. 2019;43(11) doi: 10.1111/jfpp.v43.1110.1111/jfpp.14220. [DOI] [Google Scholar]
- 13.Ferrario M., Guerrero S. Impact of a combined processing technology involving ultrasound and pulsed light on structural and physiological changes of Saccharomyces cerevisiae KE 162 in apple juice. Food Microbiol. 2017;65:83–94. doi: 10.1016/j.fm.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Samani B.H., Khoshtaghaza M.H., Lorigooini Z., Minaei S., Zareiforoush H. Analysis of the combinative effect of ultrasound and microwave power on Saccharomyces cerevisiae in orange juice processing. Innov Food Sci Emerg Technol. 2015;32:110–115. [Google Scholar]
- 15.Hashemi S.M.B., Roohi R., Mahmoudi M.R., Granato D. Modeling inactivation of Listeria monocytogenes, Shigella sonnei, Byssochlamys fulva and Saccharomyces cerevisiae and ascorbic acid and β-carotene degradation kinetics in tangerine juice by pulsed-thermosonication. Lwt. 2019;111:612–621. [Google Scholar]
- 16.Paniagua-Martínez I., Ramírez-Martínez A., Serment-Moreno V., Rodrigues S., Ozuna C. Non-thermal Technologies as Alternative Methods for Saccharomyces cerevisiae Inactivation in Liquid Media: a Review. Food Bioprocess Tech. 2018;11(3):487–510. [Google Scholar]
- 17.Garre A., Zwietering M.H., den Besten H.M.W. Multilevel modelling as a tool to include variability and uncertainty in quantitative microbiology and risk assessment. Thermal inactivation of Listeria monocytogenes as proof of concept, Food Res Int. 2020;137 doi: 10.1016/j.foodres.2020.109374. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari B.K., Muthukumarappan K., O'Donnell C.P., Cullen P.J., Tiwari B. Effects of sonication on the kinetics of orange juice quality parameters. J Agric Food Chem. 2008;56:2423–2428. doi: 10.1021/jf073503y. [DOI] [PubMed] [Google Scholar]
- 19.Baranyi J., Roberts T.A. A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 20.Ojha K.S., Kerry J.P., Alvarez C., Walsh D., Tiwari B.K. Effect of high intensity ultrasound on the fermentation profile of Lactobacillus sakei in a meat model system. Ultrason Sonochem. 2016;31:539–545. doi: 10.1016/j.ultsonch.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Geeraerd A.H., Valdramidis V.P., Van Impe J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int J Food Microbiol. 2005;102:95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Soro A.B., Whyte P., Bolton D.J., Tiwari B.K. Modelling the effect of UV light at different wavelengths and treatment combinations on the inactivation of Campylobacter jejuni. Innov Food Sci Emerg Technol. 2021;69:102626. doi: 10.1016/j.ifset.2021.102626. [DOI] [Google Scholar]
- 23.Fratesi S.E., Lynch F.L., Kirkland B.L., Brown L.R. Effects of SEM Preparation Techniques on the Appearance of Bacteria and Biofilms in the Carter Sandstone. J Sediment Res. 2004;74(6):858–867. [Google Scholar]
- 24.Tjorve K.M.C., Tjorve E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D. Ferreira V. Galeote I. Sanchez J.-L. Legras A. Ortiz-Julien S. Dequin Yeast multistress resistance and lag-phase characterisation during wine fermentation 17 6 2017 2017 10.1093/femsyr/fox051. [DOI] [PubMed]
- 26.Nikolić S., Mojović L., Rakin M., Pejin D., Pejin J. Ultrasound-assisted production of bioethanol by simultaneous saccharification and fermentation of corn meal. Food Chem. 2010;122:216–222. [Google Scholar]
- 27.Stobienia M., Kalschne D.L., Peron-Schlosser B., Colla L.M., Baraldi I.J., Colla E. Evaluation of Ultrasound Waves on S. cerevisiae Stimulation in the Bioethanol Production from Rice Bran. BioEnergy Res. 2020;13:314–324. [Google Scholar]
- 28.Bu X., Alheshibri M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason Sonochem. 2021;76:105629. doi: 10.1016/j.ultsonch.2021.105629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inguglia E.S., Tiwari B.K., Kerry J.P., Burgess C.M. Effects of high intensity ultrasound on the inactivation profiles of Escherichia coli K12 and Listeria innocua with salt and salt replacers. Ultrason Sonochem. 2018;48:492–498. doi: 10.1016/j.ultsonch.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Scanlon K.A., Tiwari U., Cagney C., Walsh D., McDowell D.A., Duffy G. Modelling the thermal inactivation of five Campylobacteraceae species. Food Control. 2015;47:135–140. [Google Scholar]
- 31.Wordon B.A., Mortimer B., McMaster L.D. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20kHz) and heat for the application of hurdle technology. Food Res Int. 2012;47:134–139. [Google Scholar]
- 32.Liu J., Li L., Zhou L., Li B., Xu Z. Effect of ultrasound treatment conditions on Saccharomyces cerevisiae by response surface methodology. Microb Pathog. 2017;111:497–502. doi: 10.1016/j.micpath.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Rezek Jambrak A., Simunek M., Evacic S., Markov K., Smoljanic G., Frece J. Influence of high power ultrasound on selected moulds, yeasts and Alicyclobacillus acidoterrestris in apple, cranberry and blueberry juice and nectar. Ultrasonics. 2018;83:3–17. doi: 10.1016/j.ultras.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Pagnossa J.P., Rocchetti G., Ribeiro A.C., Piccoli R.H., Lucini L. Ultrasound: beneficial biotechnological aspects on microorganisms-mediated processes. Curr Opin Food Sci. 2020;31:24–30. [Google Scholar]