Abstract
Background
Extubation failure is an important issue in ventilated patients and its risk factors remain a matter of research. We conducted a systematic review and meta-analysis to explore factors associated with extubation failure in ventilated patients who passed a spontaneous breathing trial and underwent planned extubation. This systematic review was registered in PROPERO with the Registration ID CRD42019137003.
Methods
We searched the PubMed, Web of Science and Cochrane Controlled Register of Trials for studies published from January 1998 to December 2018. We included observational studies involving risk factors associated with extubation failure in adult intensive care unit patients who underwent invasive mechanical ventilation. Two authors independently extracted data and assessed the validity of included studies.
Results
Sixty-seven studies (involving 26,847 participants) met the inclusion criteria and were included in our meta-analysis. We analyzed 49 variables and, among them, we identified 26 factors significantly associated with extubation failure. Risk factors were distributed into three domains (comorbidities, acute disease severity and characteristics at time of extubation) involving mainly three functions (circulatory, respiratory and neurological). Among these, the physiological respiratory characteristics at time of extubation were the most represented. The individual topic of secretion management was the one with the largest number of variables. By Bayesian multivariable meta-analysis, twelve factors were significantly associated with extubation failure: age, history of cardiac disease, history of respiratory disease, Simplified Acute Physiologic Score II score, pneumonia, duration of mechanical ventilation, heart rate, Rapid Shallow Breathing Index, negative inspiratory force, lower PaO2/FiO2 ratio, lower hemoglobin level and lower Glasgow Coma Scale before extubation, with the latest factor having the strongest association with extubation outcome.
Conclusions
Numerous factors are associated with extubation failure in critically ill patients who have passed a spontaneous breathing trial. Robust multiparametric clinical scores and/or artificial intelligence algorithms should be tested based on the selected independent variables in order to improve the prediction of extubation outcome in the clinical scenario.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03802-3.
Keywords: Airway extubation, Ventilator weaning, Risk factors
Background
As mechanical ventilation is associated with complications (e.g., ventilator-associated pneumonia) [1], the optimal time to wean patients from mechanical ventilation is a critical goal to achieve in intensive care unit (ICU) [2]. The decision to extubate is therefore usually taken as soon as a patient meet predefined weaning criteria and successfully pass a spontaneous breathing trial (SBT) [3]. Nevertheless, in 10–20% of patients who pass a spontaneous breathing trial and undergo planned extubation, extubation failure still occurs.
Extubation failure is usually defined as the need for reintubation within hours or days after a planned extubation. The time considered varies from 24 h [4, 5] until any time during the hospital stay [6, 7]. Extubation failure is associated with an overall increase in the duration of mechanical ventilation, a greater need for tracheostomy, higher medical costs and a 25–50% increased mortality rate [8–12]. There is some evidence that extubation failure is not just a marker of a more severe illness, but independently affects patients survival regardless of underlying illness severity [9, 13].
Unfortunately, the pathophysiology of extubation failure is not yet fully understood and a simple tool for predicting extubation failure is not available. Some studies suggested that the use of standardized weaning protocols reduced the total time of mechanical ventilation [14, 15] but the parameters that should be included in weaning protocols still remain controversial. Considering the complications associated with both a too early and delayed liberation from mechanical ventilation, the identification of robust risk factors for extubation failure would be extremely helpful in order to optimize the weaning process.
We therefore decided to conduct a systematic review of the literature and a meta-analysis to search risk factors associated with extubation failure, in adult critically ill patients who passed a SBT and underwent a planned extubation.
Methods
Search strategy and selection criteria
We performed this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16]. We searched PubMed, Web of Science and Cochrane controlled register of trials (CENTRAL) to identify articles on risk factors for extubation failure from January 1998 to December 2018. We used the following search algorithm: (extubation) AND (success OR failure OR factor OR predictor OR prediction OR risk OR score OR outcome OR mortality OR reintubation OR intensive care unit).
We included all studies that evaluated any risk factors for extubation failure in adult (at least 18 years old) ICU patients under invasive mechanical ventilation. We excluded studies in children and animals and studies not written in English. References of all selected articles were scanned for additional relevant manuscripts. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Registration Number CRD42019137003). Ethical approval was not required.
Data analysis
After the removal of the duplicates, two authors (FT, JM) independently screened titles and abstracts to obtain relevant articles for full text review. We obtained the full text of all potentially relevant studies and the authors independently decided for final inclusion in the review. We also reviewed the references of relevant articles to avoid missing any studies. Any disagreement was resolved by consensus or discussion with a third reviewer (AMD).
The review authors independently extracted data. The following data were recorded from each selected study: year of publication, study design, baseline characteristics of the population (age, comorbidities), severity scores on ICU admission and stay [Severity Acute Physiologic Score (SAPS), Acute Physiology and Chronic Health Evaluation (APACHE)], medications, characteristics of the SBT, definition of extubation failure, risk factors associated with extubation failure (respiratory, cardiovascular, neurologic, laboratory parameters) and primary outcome (extubation failure). We further excluded risk factors with excessive missing data (reported in less than 10% of studies; see Additional file 1: Table S1 in the additional material). Study quality was assessed in terms of risk of bias using the QUIPS tool for prognostic studies (Cochrane), rating the potential risk of bias as high, moderate or low for each of six domains, namely study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting. Two authors (FT, JM) independently assessed the risk of bias, implying a third author in case of disagreement (AMD).
Statistical analysis
We conducted a meta-analysis of observational prospective and retrospective studies. Data were summarized using medians and interquartile ranges (IQRs) or mean ± standard deviation (SD) were appropriate [17]. For binary variables, the odds ratio (OR) with 95% confidence interval (CI) was calculated for extubation failure. For continuous variables, we calculated the mean difference with 95% CI between extubation success and extubation failure groups. A natural log transformation of OR (lnOR) was derived from crude OR (for binary variables) [18, 19] and from standardized mean differences (for continuous variables) [19] in a symmetric scale, from minus infinity, to infinity, with zero defining no effect [18], to allow comparison between categorical and numeric variables.
We adopted the inverse variance method for developing weights for individual study effects. We quantified heterogeneity using I2 and Q statistics, with values greater than 50% regarded as being indicative of moderate-to-high heterogeneity [20]. We used a random effect model to assess the population average mean difference and 95% CI or OR and 95% CI for all the risk factors for extubation failure. In order to measure the dispersion of the pooled effect across study settings, we generated predictions intervals [21].
We performed prespecified subgroup analyses according to the type of ICU patients, e.g., medical, surgical, mixed, neurological or other type of ICU. A heatmap was created to present lnOR (scaled to adjust for extreme values) for each variable according to ICU type. We conducted a sensitivity analysis including only studies referring to the most used definition of extubation failure (death or reintubation within 48 h from extubation), to explore if it changed the significance of the results. Another sensitivity analysis focused on studies referring to death or reintubation (whatever the delay).
A multivariable meta-analysis of multiple factors was secondarily performed with variables significantly associated with extubation outcome, using effect sizes as lnOR [22] and the altmeta package for R [23, 24]. Among related significant univariate factors, only the most statistically robust (as per the lnOR), yet clinically relevant were entered into the models in order to minimize the effect of collinearity. Individual study effects and pooled effects were visualized through forest plots.
Publication biases were assessed graphically through funnel plot asymmetry [25]. Data were pooled and analyzed using Review Manager (Cochrane TC. Review Manager 5.3. Cph Nord Cochrane Cent, 2008) with a two-sided significance level of 5%, and R 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Studies
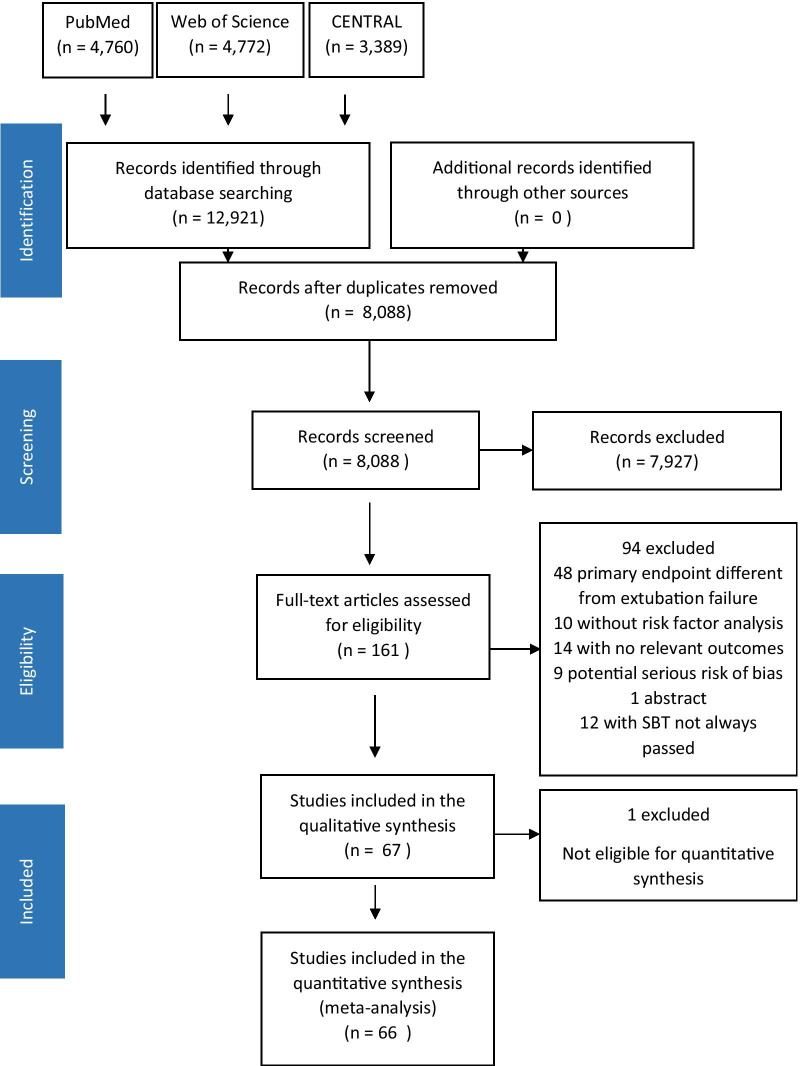
We identified a total of 12,921 references from our searches (Fig. 1). After removing 4833 duplicates, we screened the titles and abstracts of 8088 articles, of which 7927 were excluded. The full texts of 161 studies were reviewed and 94 were excluded. Thus, we included 67 studies in the narrative review, and 66 studies were included in the quantitative synthesis. Of the 67 studies included in the review, 50 were prospective observational studies [4–6, 9, 12, 13, 26–69] and 17 were retrospective studies [7, 70–85]. Fifty-seven studies were monocentric and ten were multicentric. Studies took place in medical (n = 19), surgical (n = 9), mixed (n = 28) and neurological (n = 11) ICUs.
Fig. 1.
Study flowchart
We included 67 studies involving 26,847 participants in the meta-analysis. The type of SBT and the definition of extubation failure varied among the included studies (Table 1). SBT types included: multiple choice for SBT (n = 15, with 4 studies allowing CPAP and 14 studies allowing T-tube), low pressure support with low positive end expiratory pressure (n = 30), flow-by (n = 1), standard pressure support ventilation (n = 1), proportional assist ventilation (n = 1) and automatic tube compensation with low PEEP (n = 1). Extubation failure was defined either as death or reintubation within hours to days after extubation (24 h, 48 h, 72 h or 7 days in 4, 30, 14 and 5 studies, respectively), or as the reinstitution of any mechanical ventilation after extubation, either invasive or noninvasive with a curative indication (10 studies).
Table 1.
Type of spontaneous breathing trial and definition of extubation failure
| Variable | Number of studies (%) |
|---|---|
| Spontaneous breathing trial | |
| T piece | 15 (22.4%) |
| Low pressure support with zero-end expiratory pressure | 6 (9.0%) |
| Continuous positive airway pressure | 3 (4.5%) |
| Other | 33 (49.3%) |
| Not available | 10 (14.8%) |
| Duration of SBT | |
| 30 min | 12 (17.9%) |
| 60 min | 8 (11.9%) |
| 120 min | 13 (19.4%) |
| Variable* | 21 (31.3%) |
| Not available | 12 (17.9%) |
| Definition of SBT failure | |
| Respiratory rate | 43 (64.2%) |
| Increased breathing work or distress signs | 31 (46.9%) |
| Arterial oxygen saturation | 39 (58.2%) |
| PaO2 | 8 (11.9%) |
| PaCO2 | 10 (14.9%) |
| Tidal volume or minute ventilation or RSBI | 11 (16.4%) |
| Heart rate | 36 (53.7%) |
| Arterial pressure or introduction of vasopressive drug | 36 (53.7%) |
| Neurological criteria | 38 (56.7%) |
| Not available | 20 (29.9%) |
| Definition of extubation failure | |
| Death or reintubation | |
| Within 24 h | 4 (6.0%) |
| Within 48 h | 30 (44.8%) |
| Within 72 h | 14 (20.9%) |
| Within 7 days | 5 (7.5%) |
| At any time until discharge or death | 4 (6.0%) |
| Reintubation or curative non-invasive mechanical ventilation | 10 (14.9%) |
SBT spontaneous breathing trial, PaO2 partial pressure of oxygen, PaCO2 partial pressure of carbon dioxide, RSBI Rapid Shallow Breathing Index, * from 30 min to 12 h
Risk factors for extubation failure
Among the 49 variables analyzed, we found 26 variables significantly associated with extubation outcome, distributed into three domains [comorbidities (n = 5), acute disease severity (n = 6) and characteristics at time of extubation (n = 15)] (Table 2, Fig. 2) involving mainly three functions [respiratory (n = 16), circulatory (n = 3) and neurological (n = 1)]; see Additional file 1 Fig. S1 in the additional material).
Table 2.
Variables analyzed in the meta-analysis
| Variables | n | N | Statistical method | Effect estimate [95%CI] |
|---|---|---|---|---|
| Age* | 59 | 23,426 | Mean difference | 3.43 [2.44, 4.41] |
| APACHE II score* | 33 | 15,696 | Mean difference | 1.63 [0.92, 2.35] |
| Body mass index* | 13 | 8483 | Mean difference | − 0.64 [− 1.21, − 0.08] |
| Male sex | 52 | 22,093 | Odds ratio | 0.90 [0.76, 1.07] |
| SAPS II* | 15 | 7159 | Mean difference | 4.20 [2.75, 5.65] |
| History of cardiac disease* | 16 | 7298 | Odds ratio | 1.35 [1.12, 1.64] |
| History of respiratory disease* | 11 | 6303 | Odds ratio | 1.49 [1.18, 1.87] |
| COPD* | 12 | 1984 | Odds ratio | 1.60 [1.16, 2.21] |
| Acute heart failure* | 11 | 3947 | Odds ratio | 1.40 [1.04, 1.89] |
| ARDS | 9 | 2842 | Odds ratio | 1.13 [0.75, 1.69] |
| COPD exacerbation* | 18 | 4183 | Odds ratio | 1.26 [1.01, 1.58] |
| Glasgow Coma Scale before ext.* | 13 | 5933 | Mean difference | − 0.75 [− 1.06, − 0.43] |
| Pneumonia* | 17 | 3692 | Odds ratio | 1.48 [1.21, 1.81] |
| Albumin | 9 | 5481 | Mean difference | − 0.21 [− 0.43, 0.02] |
| Hemoglobin* | 18 | 7277 | Mean difference | − 0.54 [− 0.72, − 0.35] |
| PaCO2 before ext | 34 | 12,328 | Mean difference | 0.81 [− 0.02, 1.64] |
| PaO2 before ext.* | 22 | 9677 | Mean difference | − 8.02 [− 12.39, − 3.66] |
| PaO2/FiO2 before ext.* | 30 | 11,960 | Mean difference | − 19.38 [− 26.92, − 11.84] |
| SaO2 before ext.* | 7 | 1893 | Mean difference | − 0.44 [− 0.87, − 0.01] |
| Duration of MV before ext.* | 46 | 19,775 | Mean difference | 1.03 [0.62, 1.43] |
| Respiratory rate before ext.* | 27 | 15,178 | Mean difference | 1.86 [1.19, 2.54] |
| RSBI* | 44 | 20,301 | Mean difference | 8.51 [6.20, 10.81] |
| RSBI after 1 min SBT* | 8 | 1606 | Mean difference | 10.26 [3.68, 16.84] |
| Tidal volume before ext.* | 25 | 12,070 | Mean difference | − 28.69 [− 44.61, − 12.78] |
| Heart rate before ext.* | 20 | 9848 | Mean difference | 2.99 [1.49, 4.49] |
| Maximal expiratory pressure* | 9 | 12,183 | Mean difference | − 10.22 [− 17.70, − 2.73] |
| Negative inspiratory force* | 14 | 13,448 | Mean difference | 5.30 [3.11, 7.48] |
| Cough* | 7 | 3337 | Odds ratio | 0.33 [0.16, 0.66] |
| Cough peak flow* | 8 | 1041 | Mean difference | − 27.50 [− 38.95, − 16.04] |
| Moderate/abundant secretions* | 7 | 2248 | Odds ratio | 1.98 [1.14, 3.43] |
| Acute respiratory failure | 7 | 1249 | Odds ratio | 1.43 [0.88, 2.32] |
| Coma | 8 | 2742 | Odds ratio | 0.77 [0.57, 1.03] |
| Creatinine | 9 | 5422 | Mean difference | 0.11 [− 0.06, 0.29] |
| Diastolic blood pressure before ext | 9 | 1651 | Mean difference | − 1.03 [− 2.57, 0.50] |
| Diabetes | 15 | 5976 | Odds ratio | 1.27 [0.96, 1.69] |
| FiO2 during SBT | 11 | 7818 | Mean difference | 0.00 [− 0.00, 0.01] |
| Glasgow Coma Scale upon admission | 14 | 9113 | Mean difference | − 0.28 [− 0.57, 0.00] |
| History of hypertension | 8 | 998 | Odds ratio | 1.09 [0.78, 1.52] |
| Mean arterial pressure before ext | 7 | 5161 | Mean difference | − 0.95 [− 2.36, 0.45] |
| Minute ventilation before ext | 17 | 14,383 | Mean difference | 0.00 [− 0.34, 0.34] |
| Neurologic diagnosis | 9 | 3357 | Odds ratio | 1.19 [0.76, 1.87] |
| PEEP during SBT | 11 | 7214 | Mean difference | 0.05 [− 0.00, 0.10] |
| pH before ext | 27 | 11,392 | Mean difference | − 0.00 [− 0.01, 0.00] |
| Postoperative respiratory failure | 7 | 2713 | Odds ratio | 1.01 [0.69, 1.48] |
| SBP before ext | 13 | 5240 | Mean difference | − 0.41 [− 1.95, 1.13] |
| Sepsis | 7 | 2903 | Odds ratio | 1.17 [0.92, 1.48] |
| Shock | 8 | 1722 | Odds ratio | 0.87 [0.50, 1.50] |
| Steroids | 7 | 3674 | Odds ratio | 0.84 [0.58, 1.24] |
| Trauma | 7 | 4916 | Odds ratio | 0.83 [0.63, 1.09] |
N number of participants, n number of studies, ext extubation, SBT spontaneous breathing trial, SAPS severity acute physiologic score, APACHE acute physiology and chronic health evaluation, COPD chronic obstructive pulmonary disease, ARDS acute respiratory distress syndrome, PaCO2 partial pressure of carbon dioxide in the arterial blood, PaO2 partial pressure of oxygen in the arterial blood, SaO2 oxygen saturation in the arterial blood, FiO2 fraction of inspired oxygen, RSBI Rapid Shallow Breathing Index, PEEP positive end expiratory pressure, *statistically significant by meta-analysis
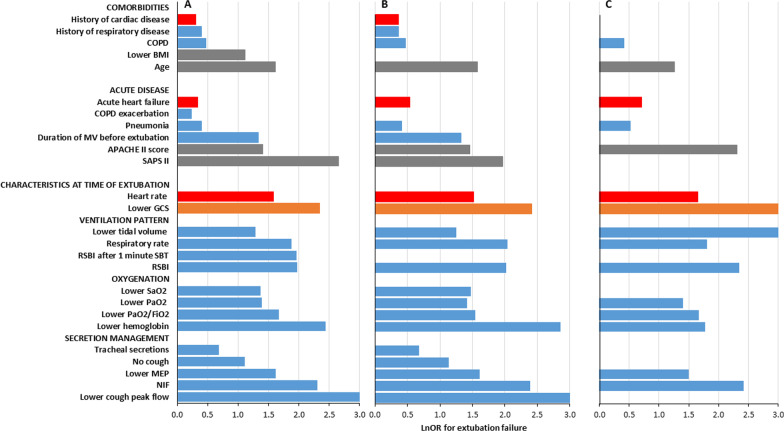
Fig. 2.
Natural log transformation of odd ratios summarizing variables significantly associated with extubation outcome. A Overall analysis; B Sensitivity analysis focusing on studies defining extubation failure as death or reintubation, whatever the delay; C Sensitivity analysis focusing on studies defining extubation failure at 48 h. Natural log transformation of odd ratios (lnOR) were derived from crude OR (for binary variables) and from standardized mean differences (for continuous variables) to summarize the effect of 26 variables significantly associated with extubation outcome, involving three main functions [respiratory (blue bars), circulatory (red bars), neurological (orange bars) and scores/physiological data (grey bars)]. COPD: chronic obstructive pulmonary disease; BMI: body mass index; GCS: Glasgow coma scale; NIF: negative inspiratory force; SAPSII: simplified acute physiology score; RSBI: rapid shallow breathing index; SBT: spontaneous breathing trial; MEP: maximal expiratory pressure; MV: mechanical ventilation
Comorbidities
We found a higher risk of extubation failure in older patients. Chronic obstructive respiratory disease, history of chronic cardiac or respiratory disease were associated with a higher risk of extubation failure as well. In contrast, a higher body mass index was associated with successful extubation.
Acute disease severity
Patients who failed extubation also differed from patients who succeeded in terms of acute disease severity, with higher values of SAPS II and APACHE II score in the former group. Acute heart failure, COPD exacerbation and pneumonia were the reasons for intubation significantly associated with a higher risk of extubation failure. Duration of mechanical ventilation before extubation was longer in patients with extubation failure.
Characteristics at the time of extubation
These variables involved the following physiological systems: (1) respiratory: related to secretion management (cough, cough peak flow, maximal expiratory pressure, presence of moderate to abundant secretions, negative inspiratory force), ventilation pattern [respiratory rate and tidal volume before extubation, rapid shallow breathing index (RSBI) after one minute from the SBT start, RSBI before extubation] and oxygenation (SaO2, PaO2 and PaO2/FiO2 before extubation, hemoglobin on the day of extubation); (2) cardiovascular (heart rate before extubation); and (3) neurological (Glasgow Coma Scale before extubation). The individual topic of secretion management was the one with the largest number variables (five).
Subgroup analysis
Subgroup analyses according to the type of ICU are presented in Additional file 1: Fig. S2 and Table S2 in the additional material. Among the 49 variables analyzed, 30 were significantly associated with extubation outcome in at least one ICU type. Eight factors were significant in the majority of ICU types (at least three among the five types), including age, SAPS II score, duration of mechanical ventilation before extubation, heart rate, respiratory rate, RSBI, PaO2 before extubation and cough peak flow. Duration of mechanical ventilation had the broadest association across ICU types (4/5 types), while cough peak flow had the strongest association across ICU types (Additional file 1: Fig. S2).
Sensitivity analysis
Due to the heterogeneity among studies in terms of definition of extubation failure, we performed an exploratory sensitivity analysis restricted to studies where extubation failure was defined as death or reintubation, whatever the delay (57 out of 67 included in the meta-analysis): the vast majority of variables identified by crude analysis (23) remained significant, while only three were not (see Fig. 2, and Additional file 1: Table S3 in the additional material). Another sensitivity analysis was restricted to studies where extubation failure was defined as death or reintubation within 48 h (30 articles out of 67 included in the meta-analysis): 15 variables remained significant while eleven were not, including factors related to cough and tracheal secretions (see Fig. 2, and Additional file 1: Table S4 in the additional material).
Multivariable analysis
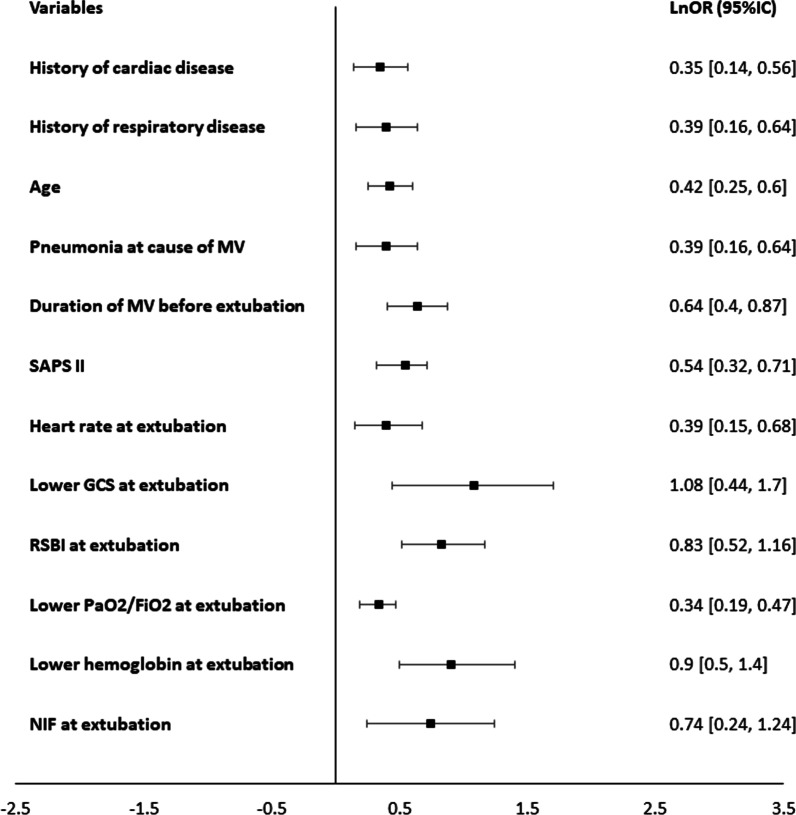
The 26 variables significantly associated with extubation outcome were assessed using a multivariable analysis for multiple factors (Additional file 1: Fig. S3 in the additional material). Twelve variables (age, history of cardiac disease, history of respiratory disease, SAPS II score, duration of mechanical ventilation, pneumonia, heart rate, RSBI, negative inspiratory force, lower PaO2/FiO2, lower Glasgow Coma Scale and lower hemoglobin level before extubation) were retained in the final model (Fig. 3). Glasgow Coma Scale (GCS) before extubation had the strongest independent association with extubation outcome.
Fig. 3.
Forest plot for the twelve variables retained in the final model, significantly associated with extubation failure in multivariable meta-analysis. Effects are reported in natural log transformation of odd ratios (lnOR) derived from crude OR with 95% confidence interval margins (CI). NIF: negative inspiratory force; SAPSII: simplified acute physiology score; RSBI: rapid shallow breathing index; MV: mechanical ventilation
Quality
Included studies differed in their methodological quality (Fig. 4, and Additional file 1: Fig. S4 in the additional material). High risk of bias was related to the study participation in eight studies [47, 57, 78–82, 85], to study attrition in one study [33], to prognostic factor measurement in one study [7], to the outcome measurement in two studies [29, 77] and to study confounding in five studies [27, 65, 78, 81, 85]. The remaining studies had low or unclear risk of bias for each of the six domains.
Fig. 4.
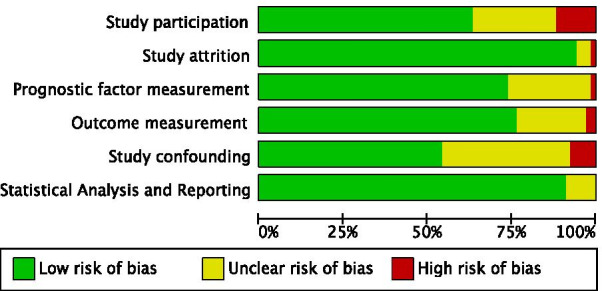
Summary of risk of bias in the included studies
Discussion
To the best of our knowledge, we herein report the first meta-analysis on factors associated with extubation failure. By multivariable analysis, twelve factors were significantly associated with extubation failure: age, history of cardiac disease, history of respiratory disease, SAPS II score, duration of mechanical ventilation, pneumonia, heart rate, RSBI, negative inspiratory force, lower PaO2/FiO2, lower hemoglobin level before extubation and lower Glasgow Coma Scale before extubation, with the latest factor having the strongest independent association with extubation outcome.
Definition of extubation failure
An important information that comes out from our systematic review is that there is lack of standardization about the definition of extubation failure. It was defined as death or reintubation within a time interval that varies from 24 h to 7 days and in some studies, it also comprised the need for curative noninvasive ventilation after extubation. This leads to a risk of bias in evaluating the prognostic factors for extubation failure. For this reason, we performed a sensitivity analysis considering only studies where extubation failure was defined as death or reintubation within 48 h, since this is the most used definition. In this sensitivity analysis, the majority (15 out of 26) of factors identified by the crude analysis remained significant. However, cough, cough peak flow and secretions were no longer associated with extubation outcome when considering a 48 h delay. Alteration of cough and/or excessive secretions may act as major cause of delayed reintubation. These findings, and the increasing use of prophylactic non-invasive ventilation and high flow oxygen after extubation, may suggest the use of death or reintubation within a seven-day delay to define extubation failure in future studies [86].
Risk factors for extubation failure
As highlighted in the present work, many factors may contribute to extubation failure in a given critically ill patient, suggesting an individual pathophysiological approach. The topic of secretion management was the one with the largest number of variables (five) significantly associated with extubation failure, pointing out that it should be carefully evaluated before extubation. The assessment of the “upper airway patency”, in terms of amount of secretions and the ability to clear them through an effective cough, has been increasingly used in the literature, even though these parameters are difficult to measure in an objective and standardized way. Cough peak flow is a parameter that has been proposed in the last few years to overcome this problem, but our multivariable analysis suggests negative inspiratory pressure as a relevant indicator.
Although the majority of statistically significant variables from our meta-analysis were related to the respiratory function (16 variables), the circulatory (three variables) and neurological (one variable) functions were also involved, with Glasgow Coma Scale having the strongest association with extubation outcome by multivariable analysis. These results are consistent with the plurality of often-intertwined mechanisms of extubation failure. Thus, restricting the clinical reasoning to the spectrum of a few variables related to the respiratory function may weaken the decisional process of liberation from mechanical ventilation. Robust multiparametric clinical scores and/or artificial intelligence algorithms should be tested based on the selected variables in order to improve the prediction of extubation outcome in the clinical scenario.
Strengths and limitations
Strengths of our study include the wide period of assessment and selection process. One limitation is the lack of standardization in the definition of extubation failure. However, we performed a sensitivity analysis using the most accepted definition. Another limitation is that, due to the lack of data, we could not analyze the postextubation stridor, which is considered a rare but important risk factor for extubation failure. Finally, we may have missed other potentially important factors since we chose to analyze only parameters evaluated in at least 10% of included studies.
Conclusion
We performed a systematic review and meta-analysis of a wide population of critically ill patients, finding 26 and 12 risk factors for extubation failure in patients who have successfully passed a spontaneous breathing trial by univariate and multivariable analysis, respectively. These factors were related to age, comorbidities, acute disease severity and physiological characteristics at the time of extubation. To further explore these factors and their combination, a unique definition of extubation failure is needed. An automated algorithm incorporating these factors would probably be very useful to inform the decisional process of extubation.
Supplementary Information
Additional file 1: Table S1. Variables excluded because of missing data. Figure S1. Forest plots for variables statistically significantly associated with extubation failure. Table S2. Subgroup analyses by intensive care unit type. Figure S2. Heatmap of natural log transformation of odd ratios (LnOR) for extubation failure, according to ICU type. Table S3. Sensitivity analysis focusing on studies defining extubation failure as death or reintubation, whatever the delay. Table S4. Sensitivity analysis focusing on studies defining extubation failure at 48 h. Figure S3. Individual risk of bias in the included studies. Figure S4. Forest plot for the 26 variables significantly associated with extubation failure, assessed by multivariate meta-analysis for multiple factors.
Acknowledgements
Not applicable.
Abbreviations
- APACHE
Acute physiology and chronic health evaluation
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- CI
Confidence interval
- COPD
Chronic Obstructive Pulmonary Disease
- FiO2
Fraction of inspired oxygen
- GCS
Glasgow Coma Scale
- ICU
Intensive care unit
- IQRs
Interquartile ranges
- MEP
Maximal expiratory pressure
- MV
Mechanical ventilation
- NIF
Negative inspiratory force
- OR
Odds ratio
- PaCO2
Partial pressure of carbon dioxide in the arterial blood
- PaO2
Partial pressure of oxygen in the arterial blood
- PEEP
Positive end expiratory pressure
- RSBI
Rapid shallow breathing index
- SaO2
Oxygen saturation in the arterial blood
- SAPS
Severity acute physiologic score
- SBT
Spontaneous breathing trial
- SD
Standard deviation
Authors' contributions
AMD, FT and JM designed the meta-analysis. FT and JM searched for the articles, screened titles and abstracts and extracted data. AMD, FT and SG performed statistical analysis and interpretation of data. AWT, GC and MA contributed to the conception of the study. FT and AMD drafted the manuscript, and FT, SG, JM, GC, AWT, MA and AMD revised it for important intellectual content; all authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Flavia Torrini and Ségolène Gendreau have contributed equally to this work
References
- 1.Bigatello LM, Stelfox HT, Berra L, Schmidt U, Gettings EM. Outcome of patients undergoing prolonged mechanical ventilation after critical illness. Crit Care Med. 2007;35:2491–2497. doi: 10.1097/01.CCM.0000287589.16724.B2. [DOI] [PubMed] [Google Scholar]
- 2.Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–9. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 4.Segal LN, Oei E, Oppenheimer BW, Goldring RM, Bustami RT, Ruggiero S, et al. Evolution of pattern of breathing during a spontaneous breathing trial predicts successful extubation. Intensive Care Med. 2010;36:487–495. doi: 10.1007/s00134-009-1735-6. [DOI] [PubMed] [Google Scholar]
- 5.Beigmohammadi MT, Khan ZH, Samadi S, Mahmoodpoor A, Fotouhi A, Rahimiforoushani A, et al. Role of hematocrit concentration on successful extubation in critically ill patients in the intensive care units. Anesthesiol Pain Med. 2016 doi: 10.5812/aapm.32904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su WL, Chen YH, Chen CW, Yang SH, Su CL, Perng WC, et al. Involuntary cough strength and extubation outcomes for patients in an ICU. Chest. 2010;137:777–782. doi: 10.1378/chest.07-2808. [DOI] [PubMed] [Google Scholar]
- 7.Miu T, Joffe AM, Yanez ND, Khandelwal N, Dagal AH, Deem S, et al. Predictors of reintubation in critically ill patients. Respir Care. 2014;59:178–185. doi: 10.4187/respcare.02527. [DOI] [PubMed] [Google Scholar]
- 8.Thille AW, Richard JCM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187:1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 9.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39:2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 10.Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, et al. Effect of spontaneous breathing trial duration on outcome of attempts to 1999discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159:512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 11.Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998;158:1855–1862. doi: 10.1164/ajrccm.158.6.9712135. [DOI] [PubMed] [Google Scholar]
- 12.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664–1671. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 13.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;25:567–574. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH, Shapiro SD, Silver P, St. John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol-directed versus physician- directed weaning from mechanical ventilation. Crit Care Med. 1997;22:1052–1056. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Saura P, Blanch L, Mestre J, Vallés J, Artigas A, Fernández R. Clinical consequences of the implementation of a weaning protocol. Intensive Care Med. 1996;22:1052–1056. doi: 10.1007/BF01699227. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014 doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions. 2. Chichester: Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Deeks J. Chapter 6: Choosing effect measures and computing estimates of effect. Cochrane handbook systematic review interventions version 62 update Feb 2021. Chichester: Wiley; 2019. [Google Scholar]
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 22.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, Chu H. Bayesian multivariate meta-analysis of multiple factors. Res Synth Methods. 2018;9:261–272. doi: 10.1002/jrsm.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostatistics. 2008;9:172–186. doi: 10.1093/biostatistics/kxm023. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Smith GD, Schneider M, Minder C, Tabuso M, Dunlop A, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Wei LQ, Li GQ, Lv FY, Wang H, Zhang YH, et al. A decision-tree model for predicting extubation outcome in elderly patients after a successful spontaneous breathing trial. Anesth Analg. 2010;111:1211–1218. doi: 10.1213/ANE.0b013e3181f4e82e. [DOI] [PubMed] [Google Scholar]
- 27.White CE, Batchinsky AI, Necsoiu C, Nguyen R, Walker KP, Chung KK, et al. Lower interbreath interval complexity is associated with extubation failure in mechanically ventilated patients during spontaneous breathing trials. J Trauma Inj Infect Crit Care. 2010;68:1310–1316. doi: 10.1097/TA.0b013e3181da90db. [DOI] [PubMed] [Google Scholar]
- 28.Seely AJE, Bravi A, Herry C, Green G, Longtin A, Ramsay T, et al. Erratum to: Do heart and respiratory rate variability improve prediction of extubation outcomes in critically ill patients? Crit Care. 2014 doi: 10.1186/s13054-014-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CVR, Daigle JB, Foulkrod KH, Brouillette B, Clark A, Czysz C, et al. Risk factors associated with early reintubation in trauma patients: a prospective observational study. J Trauma Inj Infect Crit Care. 2011;71:37–42. doi: 10.1097/TA.0b013e31821e0c6e. [DOI] [PubMed] [Google Scholar]
- 30.Thille AW, Boissier F, Ghezala HB, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med. 2015;43:613–20. doi: 10.1097/CCM.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 31.Duan J, Zhou L, Xiao M, Liu J, Yang X. Semiquantitative cough strength score for predicting reintubation after planned extubation. Am J Crit Care. 2015;24:e86–e90. doi: 10.4037/ajcc2015172. [DOI] [PubMed] [Google Scholar]
- 32.Colonel P, Houzé MH, Vert H, Mateo J, Mégarbane B, Goldgran-Tolédano D, et al. Swallowing disorders as a predictor of unsuccessful extubation: a clinical evaluation. Am J Crit Care. 2008;17:504–510. [PubMed] [Google Scholar]
- 33.Boniatti VM, Boniatti MM, Andrade CF, Zigiotto CC, Kaminski P, Gomes SP, et al. The modified integrative weaning index as a predictor of extubation failure. Respir Care. 2014;59:1042–1047. doi: 10.4187/respcare.02652. [DOI] [PubMed] [Google Scholar]
- 34.Fujii E, Fujino K, Tanaka-Mizuno S, Eguchi Y. Variation of risk factors for cause-specific reintubation: a preliminary study. Can Respir J. 2018;2018:3654251. [DOI] [PMC free article] [PubMed]
- 35.Dos Reis HFC, Almeida MLO, Da Silva MF, Moreira JO, De Seixas RM. Associação entre o índice de respiração rápida e superficial e o sucesso da extubação em pacientes com traumatismo cranioencefálico. Rev Bras Ter Intensiva. 2013;25:212–217. [Google Scholar]
- 36.Teixeira C, Da Silva NB, Savi A, Vieira SRR, Nasi LA, Friedman G, et al. Central venous saturation is a predictor of reintubation in difficult-to-wean patients. Crit Care Med. 2010;38:491–496. doi: 10.1097/CCM.0b013e3181bc81ec. [DOI] [PubMed] [Google Scholar]
- 37.Chien JY, Lin MS, Huang YCT, Chien YF, Yu CJ, Yang PC. Changes in B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Crit Care Med. 2008;36:1421–1426. doi: 10.1097/CCM.0b013e31816f49ac. [DOI] [PubMed] [Google Scholar]
- 38.Smailes ST, McVicar AJ, Martin R. Cough strength, secretions and extubation outcome in burn patients who have passed a spontaneous breathing trial. Burns. 2013;39:236–242. doi: 10.1016/j.burns.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 39.dos Reis HFC, Gomes-Neto M, Almeida MLO, da Silva MF, Guedes LBA, Martinez BP, et al. Development of a risk score to predict extubation failure in patients with traumatic brain injury. J Crit Care. 2017;42:218–222. doi: 10.1016/j.jcrc.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Thille AW, Boissier F, Ben-Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C, et al. Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: a prospective before-after study. Crit Care. 2016 doi: 10.1186/s13054-016-1228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Solh AA, Bhat A, Gunen H, Berbary E. Extubation failure in the elderly. Respir Med. 2004;98:661–668. doi: 10.1016/j.rmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez R, Raurich JM, Mut T, Blanco J, Santos A, Villagra A. Extubation failure: diagnostic value of occlusion pressure (P0.1) and P0.1-derived parameters. Intensive Care Med. 2004;30:234–240. doi: 10.1007/s00134-003-2070-y. [DOI] [PubMed] [Google Scholar]
- 43.Jaber S, Quintard H, Cinotti R, Asehnoune K, Arnal J-M, Guitton C, et al. Risk factors and outcomes for airway failure versus non-airway failure in the intensive care unit: a multicenter observational study of 1514 extubation procedures. Critical Care. 2018 doi: 10.1186/s13054-018-2150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asehnoune K, Seguin P, Lasocki S, Roquilly A, Delater A, Gros A, et al. Extubation success prediction in a multicentric cohort of patients with severe brain injury. Anesthesiology. 2017;127:338–346. doi: 10.1097/ALN.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi LY, Gazzotti MR, Vidotto MC, Jardim JR. Incidence, indication and complications of postoperative reintubation after elective intracranial surgery. Sao Paulo Med J. 2017;131:158–165. doi: 10.1590/1516-3180.2013.1313440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norisue Y, Kataoka J, Homma Y, Naito T, Tsukuda J, Okamoto K, et al. Increase in intra-abdominal pressure during airway suctioning-induced cough after a successful spontaneous breathing trial is associated with extubation outcome. Ann Intensive Care. 2018 doi: 10.1186/s13613-018-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seymour CW, Halpern S, Christie JD, Gallop R, Fuchs BD. Minute ventilation recovery time measured using a new, simplified methodology predicts extubation outcome. J Intensive Care Med. 2008;23:52–60. doi: 10.1177/0885066607310302. [DOI] [PubMed] [Google Scholar]
- 48.Martinez A, Seymour C, Nam M. Minute ventilation recovery time: a predictor of extubation outcome. Chest. 2003;123:1214–1221. doi: 10.1378/chest.123.4.1214. [DOI] [PubMed] [Google Scholar]
- 49.Takaki S, Kadiman SB, Tahir SS, Ariff MH, Kurahashi K, Goto T. Modified rapid shallow breathing index adjusted with anthropometric parameters increases predictive power for extubation failure compared with the unmodified index in postcardiac surgery patients. J Cardiothorac Vasc Anesth. 2015;29:64–68. doi: 10.1053/j.jvca.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30:1334–1339. doi: 10.1007/s00134-004-2231-7. [DOI] [PubMed] [Google Scholar]
- 51.Banner MJ, Euliano NR, Martin AD, Al-Rawas N, Layon AJ, Gabrielli A. Noninvasive work of breathing improves prediction of post-extubation outcome. Intensive Care Med. 2012;38:248–255. doi: 10.1007/s00134-011-2402-2. [DOI] [PubMed] [Google Scholar]
- 52.Tadié JM, Behm E, Lecuyer L, Benhmamed R, Hans S, Brasnu D, et al. Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med. 2010;36:991–998. doi: 10.1007/s00134-010-1847-z. [DOI] [PubMed] [Google Scholar]
- 53.Mokhlesi B, Tulaimat A, Gluckman TJ, Wang Y, Evans AT, Corbridge TC. Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care. 2007;52:1710–1717. [PubMed] [Google Scholar]
- 54.Gobert F, Yonis H, Tapponnier R, Fernandez R, Labaune M-A, Burle J-F, et al. Predicting extubation outcome by cough peak flow measured using a built-in ventilator flow meter. Respir Care. 2017;62:1505–1519. doi: 10.4187/respcare.05460. [DOI] [PubMed] [Google Scholar]
- 55.Piriyapatsom A, Williams EC, Waak K, Ladha KS, Eikermann M, Schmidt UH. Prospective observational study of predictors of re-intubation following extubation in the surgical ICU. Respir Care. 2016;61:306–315. doi: 10.4187/respcare.04269. [DOI] [PubMed] [Google Scholar]
- 56.Kutchak FM, Debesaitys AM, Rieder MM, Meneguzzi C, Skueresky AS, Forgiarini LA, et al. Reflex cough PEF as a predictor of successful extubation in neurological patients. J Bras Pneumol. 2015;41:358–364. doi: 10.1590/S1806-37132015000004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim AS, Aly MG, Abdel-Rahman KA, Mohamed MA, Mehany MM, Aziz EM. Semi-quantitative cough strength score as a predictor for extubation outcome in traumatic brain injury: a prospective observational study. Neurocrit Care. 2018;29:273–279. doi: 10.1007/s12028-018-0539-3. [DOI] [PubMed] [Google Scholar]
- 58.Kutchak FM, Rieder MM, Victorino JA, Meneguzzi C, Poersch K, Forgiarini LA, et al. Simple motor tasks independently predict extubation failure in critically ill neurological patients. J Bras Pneumol. 2017;43:183–189. doi: 10.1590/S1806-37562016000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez Martinez G, Fernandez R, Luzon E, Cuena R, Montejo JC. The early phase of the minute ventilation recovery curve predicts extubation failure better than the minute ventilation recovery time. Chest. 2007;131:1315–1322. doi: 10.1378/chest.06-2137. [DOI] [PubMed] [Google Scholar]
- 60.Steidl C, Boesel J, Suntrup-Krueger S, Schoenenberger S, Al-Suwaidan F, Warnecke T, et al. Tracheostomy, extubation, reintubation: airway management decisions in intubated stroke patients. Cerebrovasc Dis. 2017;44:1–9. doi: 10.1159/000471892. [DOI] [PubMed] [Google Scholar]
- 61.Bai L, Duan J. Use of cough peak flow measured by a ventilator to predict re-intubation when a spirometer is unavailable. Respir Care. 2017;62:566–571. doi: 10.4187/respcare.05260. [DOI] [PubMed] [Google Scholar]
- 62.Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27:221.e1–221.e8. doi: 10.1016/j.jcrc.2011.07.079. [DOI] [PubMed] [Google Scholar]
- 63.McCredie VA, Ferguson ND, Pinto RL, Adhikari NKJ, Fowler RA, Chapman MG, et al. Airway management strategies for brain-injured patients meeting standard criteria to consider extubation: a prospective cohort study. Ann Am Thorac Soc. 2017;14:85–93. doi: 10.1513/AnnalsATS.201608-620OC. [DOI] [PubMed] [Google Scholar]
- 64.Godet T, Chabanne R, Marin J, Kauffmann S, Futier E, Pereira B, et al. Extubation failure in brain-injured patients. Anesthesiology. 2017;126:104–114. doi: 10.1097/ALN.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 65.Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care. 2017;30:37–43. doi: 10.1016/j.aucc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Mu Y, Li GQ, Yu X, Li PJ, Shen ZQ, et al. Extubation outcome after a successful spontaneous breathing trial: a multicenter validation of a 3-factor prediction model. Exp Ther Med. 2015;10:1591–1601. doi: 10.3892/etm.2015.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo H-J, Chiu H-W, Lee C-N, Chen T-T, Chang C-C, Bien M-Y. Improvement in the prediction of ventilator weaning outcomes by an artificial neural network in a medical ICU. Respir Care. 2015;60:1560–1569. doi: 10.4187/respcare.03648. [DOI] [PubMed] [Google Scholar]
- 68.Teixeira C, Zimermann Teixeira PJ, Hohër JA, de Leon PP, Brodt SFM, da Siva MJ. Serial measurements of f/VT can predict extubation failure in patients with f/VT ≤ 105? J Crit Care. 2008;23:572–576. doi: 10.1016/j.jcrc.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Xiao M, Duan J. Weaning attempts, cough strength and albumin are independent risk factors of reintubation in medical patients. Clin Respir J. 2018;12:1240–1246. doi: 10.1111/crj.12657. [DOI] [PubMed] [Google Scholar]
- 70.Menon N, Joffe AM, Deem S, Yanez ND, Grabinsky A, Dagal AH, et al. Occurrence and complications of tracheal reintubation in critically ill adults. Respir Care. 2012;57:1555–1563. doi: 10.4187/respcare.01617. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh M-H, Hsieh M-J, Chen C-M, Hsieh C-C, Chao C-M, Lai C-C. An artificial neural network model for predicting successful extubation in intensive care units. J Clin Med. 2018;7:240. doi: 10.3390/jcm7090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoo JW, Lee SJ, Lee JD, Kim HC. Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success. Korean J Intern Med. 2018;33:331–339. doi: 10.3904/kjim.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chao CM, Lai CC, Cheng AC, Chiang SR, Liu WL, Ho CH, et al. Establishing failure predictors for the planned extubation of overweight and obese patients. PLoS ONE. 2017;12:e0183360. doi: 10.1371/journal.pone.0183360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai CC, Chen CM, Chiang SR, Liu WL, Weng SF, Sung MI, et al. Establishing predictors for successfully planned endotracheal extubation. Med U S. 2016;95:e4852. doi: 10.1097/MD.0000000000004852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nantsupawat N, Nantsupawat T, Limsuwat C, Sutamtewagul G, Nugent K. Factors associated with reintubation in patients with chronic obstructive pulmonary disease. Qual Manag Health Care. 2015;24:200–206. doi: 10.1097/QMH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 76.Silva C, Timenetsky K, Taniguchi C, Calegaro S, Azevedo C, Stus R, et al. Low mechanical ventilation times and reintubation rates associated with a specific weaning protocol in an intensive care unit setting: a retrospective study. Clinics. 2012;67:995–1000. doi: 10.6061/clinics/2012(09)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saugel B, Rakette P, Hapfelmeier A, Schultheiss C, Phillip V, Thies P, et al. Prediction of extubation failure in medical intensive care unit patients. J Crit Care. 2012;27:571–577. doi: 10.1016/j.jcrc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Guru PK, Singh TD, Pedavally S, Rabinstein AA, Hocker S. Predictors of extubation success in patients with posterior fossa strokes. Neurocrit Care. 2016;25:117–127. doi: 10.1007/s12028-016-0249-7. [DOI] [PubMed] [Google Scholar]
- 79.Mahmood S, Alani M, Al-Thani H, Mahmood I, El-Menyar A, Latifi R. Predictors of re-intubation in trauma intensive care unit: Qatar experience. Oman Med J. 2014;29:289–293. doi: 10.5001/omj.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dupont H, Le Port Y, Paugam-Burtz C, Mantz J, Desmonts J. Reintubation after planned extubation in surgical ICU patients: a case-control study. Intensive Care Med. 2001;27:1875–1880. doi: 10.1007/s001340101101. [DOI] [PubMed] [Google Scholar]
- 81.Wojak JF, Ditz C, Abusamha A, Smith E, Gliemroth J, Tronnier V, et al. The impact of extubation failure in patients with good-grade subarachnoid hemorrhage. World Neurosurg. 2018;117:e335–e340. doi: 10.1016/j.wneu.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 82.El Khoury MY, Panos RJ, Ying J, Almoosa KF. Value of the PaO2:FiO2 ratio and Rapid Shallow Breathing Index in predicting successful extubation in hypoxemic respiratory failure. Heart Lung J Acute Crit Care. 2010;39:529–536. doi: 10.1016/j.hrtlng.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Okabe Y, Asaga T, Bekku S, Suzuki H, Kanda K, Yoda T, et al. Lung-thorax compliance measured during a spontaneous breathing trial is a good index of extubation failure in the surgical intensive care unit: a retrospective cohort study. J Intensive Care. 2018 doi: 10.1186/s40560-018-0313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robriquet L, Georges H, Leroy O, Devos P, D’escrivan T, Guery B. Predictors of extubation failure in patients with chronic obstructive pulmonary disease. J Crit Care. 2006;21:185–190. doi: 10.1016/j.jcrc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 85.Segura A, Carvajal N, Chavarro PA, Wilches EC, Carvajal A. Sensitivity and specificity of the Yang Tobin Index to predict extubation success in critical patients. Colomb Med. 2011;42:458–467. [Google Scholar]
- 86.Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med. 2017;195:772–783. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Variables excluded because of missing data. Figure S1. Forest plots for variables statistically significantly associated with extubation failure. Table S2. Subgroup analyses by intensive care unit type. Figure S2. Heatmap of natural log transformation of odd ratios (LnOR) for extubation failure, according to ICU type. Table S3. Sensitivity analysis focusing on studies defining extubation failure as death or reintubation, whatever the delay. Table S4. Sensitivity analysis focusing on studies defining extubation failure at 48 h. Figure S3. Individual risk of bias in the included studies. Figure S4. Forest plot for the 26 variables significantly associated with extubation failure, assessed by multivariate meta-analysis for multiple factors.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.