Abstract
Introduction
Wildfire smoke (WFS) exposure is a growing threat to human health, and lower socioeconomic position (SEP) has been shown to increase pollution susceptibility. Studies of SEP-related susceptibility, however, are often compromised due to spatial confounding between lower-SEP and pollution. Here we examine outdoor-housed nonhuman primates, living in natural social hierarchy in a common location, born during years of high vs. low WFS, to examine the separate and combined effects of WFS and social rank, an analog to SEP, on lung and immune function.
Methods
Twenty-one females were born during extreme WFS events in summer 2008; 22 were born in summer 2009, during low WFS. Pulmonary function and circulating cytokines were measured three years later, in adolescence. We estimated fine particulate (PM2.5) and ozone exposures during each animal's first 90 days and three years of age using regulatory data. Early-life social status was estimated using maternal rank at birth, as rank in females is relatively stable throughout life, and closely approximates mother's rank. We tested associations among WFS exposure, rank, and endpoints using linear regression and ANOVA.
Results
Higher WFS exposure in infancy was, on average, associated with lower functional residual capacity (FRC), residual volume (RV), tissue compliance (Ct), and IL-8 secretion in adolescence. Higher social rank conferred significantly higher expiratory reserve volume (ERV) and functional residual capacity (FRC) solely among those born in the high-WFS year (2008). Differences in effects of rank between years were not significant after adjustment for multiple comparisons.
Conclusions
Exposure to WFS in infancy generally conferred lower adolescent respiratory volumes and inflammatory cytokines. Higher rank conferred higher respiratory volumes only among females born during WFS, suggesting the possibility that the health benefits of rank may be more apparent under environmental challenge.
Keywords: Wildfire smoke, Infancy exposure, Socioeconomic status, Lung volume, Cytokine response
Wildfire smoke; Infancy exposure; Socioeconomic status; Lung volume; Cytokine response.
1. Introduction
Wildfire smoke (WFS) has posed increasing risks to human health in recent years [1], with an estimated 212 million people exposed to WFS during a series of wildfire outbreaks in the western United States in 2011 alone [2]. WFS has been associated with impacts on respiratory disorders including asthma, bronchitis, dyspnea, and COPD, cardiovascular disease, and mortality [3, 4, 5, 6, 7, 8, 9, 10]. Limited evidence shows that the level of systemic inflammation markers, such as interleukin-8 (IL-8) and C-reactive protein (CRP), are also associated with WFS [11, 12]. Separately, there is growing evidence that social status may increase susceptibility to pollution [13, 14], possibly mediated via chronic stress and associated ‘allostatic load’ pathways [15, 16]. A persistent challenge in the study of social-environmental interactions, however, has been that of spatial confounding; lower-income communities are often located alongside highways and industrial corridors, with greater exposures to air pollution, including peak events (e.g., industrial accidents) [17, 18, 19]. As such, there have been few examples of ‘natural experiments,’ wherein both high- and low-status individuals are equally exposed to elevated pollution events, with high-quality objective health data.
WFS composition varies by location and weather conditions, but generally includes combustion products long associated with adverse impacts on health, including fine particulate matter (PM), carbon monoxide (CO), nitrogen oxides, volatile organic carbons (VOC), and secondary ozone formation [20]. Infants may be more vulnerable to air pollution exposures due to their immature immune system and smaller airways [21]. Studies have shown that early-life exposures can impact children's lung development, causing adverse effects in later life [22, 23, 24, 25, 26]. Our prior study reported that WFS exposure during infancy conferred impaired immune and pulmonary functions in adolescence among rhesus monkeys [27], though the short- and long-term health impacts of infancy WFS exposure remain under-studied.
Lower socioeconomic position (SEP) has been associated with a wide range of adverse health outcomes [28, 29, 30, 31], and early-life SEP impacts the trajectory of child development and severity of childhood illness, including greater cytokine (IL-5 and IL-13) and eosinophil counts in asthma [32]. Low childhood SEP is associated with childhood asthma [33], as well as adult illness including obesity, upper respiratory infections, chronic lung diseases, metabolic syndrome, and CVD [34, 35, 36, 37, 38, 39]; adults with lower early-life (first 2 years) SEP have shown higher circulating IL-6 levels [40].
Evidence suggests that lower SEP may increase susceptibility to environmental exposures [41, 42], and it is hypothesized that much of this susceptibility may be mediated through chronic life stress [15, 16]. People with lower SEP often experience higher levels of psychological stress, and consequent increases in urinary stress hormones (cortisol and epinephrine) [32, 43, 44, 45, 46, 47]. Chronic stress can alter secretion of pro-inflammatory cytokines, disturb lymphatic tissue function, and alter hormone levels via activating signaling pathways including the hypothalamic-pituitary-adrenocortical axis (HPA) and the sympathetic-adrenal-medullary (SAM) system [48, 49]. Studies have shown that children exposed to chronic stressors (neighborhood violence, parental stress) display greater susceptibility to the effects of traffic-related pollution in asthma etiology and exacerbation [50]. Likewise, controlled experiments in animals reveal stronger responses to environmental agents under chronic stress [51, 52, 53, 54, 55]. Finally, social status in some animal species (e.g., primates, rats) have proven effective models to study effects of social status and chronic stress in human populations [56, 57]; rhesus macaques, in particular, are strongly hierarchical in their social organization, with separate dominance hierarchies by sex [58]. While dominance hierarchy in rhesus monkeys does not map precisely onto human SEP [58], many studies have found important parallels, making this species a useful model for studying aspects of stress physiology, health, and immune function [59, 60, 61, 62, 63].
Here, we hypothesize that social rank modifies associations between early-life WFS exposure and measures of pulmonary and immune function in adolescence. We capitalized on the unique opportunity of a large population of rhesus monkeys, housed outdoors at the UC Davis California National Primate Research Center (CNPRC), during two summers with very different wildfire activity. We leverage an animal model of pulmonary function [64] that has been extremely useful in understanding the development and pathophysiology of lung function impairment in rhesus macaques. We hypothesize that the level and range of impaired lung volume and cytokine responses during adolescence could be the consequence of both WFS exposure and social rank in infancy. Ours is the first study, to our knowledge, to examine how social status influences susceptibility to WFS exposures.
2. Methods
2.1. Animals
We examine a cohort of female rhesus macaque monkeys that had been housed in outdoor facilities in CNPRC from birth through three years of life. Twenty-one females were born in the spring of 2008, and 22 in spring of 2009. We excluded 7 monkeys with missing rank information, for a final total of 43 females included in our study.
The study was approved by the University of California Institutional Animal Care and Use Committee. Animals were cared for, and housed under, the provisions and conforms of the Institute of Laboratory Animal Resources and the American Association for Accreditation of Laboratory Animal Care (AAALAC).
2.2. Air quality measurement
We examined daily average ambient fine particulate matter (PM2.5) and daily 8-hour peak ozone during summer months with and without wildfires. Air quality data from Jan 1st, 2008 to Dec 31st, 2012 was obtained from a California Air Resources Board air monitoring station (site number 57577) that is located 2.7 miles southeast of CNPRC. Because concentrations differed substantially between the year with (2008) and without wildfires (2009), we examined exposure as a dichotomous variable (wildfire/non-wildfire year).
2.3. Social status indicators
In rhesus monkey societies, female ranks are relatively stable, and generally comparable to those of the mother; in contrast, males typically emigrate to new groups at reproductive maturity, and physically compete with other males to establish rank, which can change substantially over the life course [65]. For these reasons, as our study extends from infancy through age 3 (adolescence), we opted to restrict the analysis to females only.
The original rank variable was constructed such that the highest-rank female in each cage would have a score of 1, and the lowest-rank female would have a score equivalent to the total number of females in the cage. To create a positive variable, wherein higher values indicate higher rank, across a consistent scale from 0-1, we calculated the ‘proportion outranked’ [= (total number of same-sex animals – animal's original rank)/total number of same-sex animals in the cage - 1]. The resultant variable is continuously coded from 0 (lowest rank) to 1 (highest rank). For example, if a female animal was originally ranked 40th among 50 females (a relatively poor rank), her new rank score (proportion outranked) is (50-40)/(50-1) = 0.20.
2.4. Pulmonary function and cytokine response measurements
All monkeys underwent pulmonary function tests (PFT) at approximately 3 years of age (2010 and 2011) and findings were previously reported [27]. Females were tested during summer months (June to early September) to avoid pregnancies. Seven pulmonary function measures were collected on all subjects, including six measures of lung capacity [inspiratory capacity (IC), vital capacity (VC), expiratory reserve volume (ERV), functional residual capacity (FRC), residual volume (RV), total lung capacity (TLC)], and tissue compliance (Ct), as described in our previous paper [27]. We calculated the measures of lung capacity as follows: (1) FRC = ERV + RV; (2). TLC = IC + FRC = IC + ERV + RV; (3). TLC = IC + ERV + RV. All measures were bodyweight adjusted. Lung capacity measures were reported in milliliters per kilogram body weight (mL/kg), and Ct was reported per kilogram body weight (/kg). Peripheral blood mononuclear cells (PBMCs) were challenged by TLR ligand in vitro. The TLR ligand was flagellin, at a concentration of 500 ng/ml, which is recognized by TLR5. Cytokines measured by ELISA. Methods for pulmonary function assessment and cytokine measurement are detailed in [7, 27].
2.5. Statistical analyses
We used the nonparametric Wilcoxon signed-rank test to test mean differences between groups, given relatively small sample sizes, and one-way ANOVA was used to compare differences across multiple groups.
Simple linear regression was used to test relationships between continuous rank and measures of pulmonary function or cytokine response. To examine associations between rank and each outcome (pulmonary function or cytokine response), we used Model 1, below, stratified by sex and birth-year [i.e., 2008 (high WFS) vs. 2009 (low WFS)]. Beta-coefficients were compared between each pair of models.
| Model 1: y = β0+ β1 ∗ Rank + ε |
The formal test of the interaction between social rank and WFS exposure in infancy (differ by birth year) was provided by Model 2, stratified by sex:
| Model 2: y = β0+ β1∗ Rank + β2∗ High Exposure (birth year) + β3∗ Rank ∗ High Exposure (birth year) + ε |
In Model 2, we retain a main effect by birth year, to account for secular trends and potential confounders differing by birth year. Likewise, we retain a main effect for continuous rank, to account for potential confounders varying by rank. Because animals are housed in supervised facilities where conditions which would vary by rank in the wild (e.g., food access) are more controlled, we are better able to observe the direct effects of social rank per se (e.g., stress, social stability) as directly relates to pollutant susceptibility, less confounded by other rank-related factors.
Analyses were performed using the Proc GLM procedure in SAS version 9.4 (Cary, NC, USA). A p-value < 0.05 was considered significant, after accounting for multiple comparisons using False Discovery Rate (FDR) adjustment.
3. Results
Early-life exposures to PM2.5 and ozone were significantly higher for animals born in 2008 (high WFS) than in 2009 (low WFS). During the first 90 days of life, mean daily PM2.5 exposures were 14.2 (SD = 1.28) μg/m3 for monkeys born in 2008, vs. 9.07 (SD = 0.44) μg/m3 for animals born in 2009 (p-value for difference <0.0001). Mean 8-hour peak ozone was 53.4 (SD = 0.35) ppb for monkeys born in 2008, vs. 48.3 (SD = 0.66) ppb for monkeys born in 2009 (p < 0.0001). Likewise, lifetime exposures (birth to PFT at age 3 years) were significantly higher for animals born in 2008 than in 2009 (Table 1).
Table 1.
Summary statistics: ambient air pollution concentrations by birth-year.
| Ambient air pollution exposure | Birth year (Infancy WFS exposure level) |
P-value± | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 (High WFS) |
2009 (Low WFS) |
||||||||||
| Mean | Median | SD | Min | Max | Mean | Median | SD | Min | Max | ||
| PM2.5: from birth to 90 days (μg/m3) | 14.2 | 14.1 | 1.28 | 11.6 | 15.7 | 9.07 | 9.12 | 0.44 | 8.51 | 9.70 | <.0001∗ |
| PM2.5: from 90 days to PFT (μg/m3) | 9.48 | 9.44 | 0.17 | 9.27 | 9.88 | 10.0 | 10.0 | 0.02 | 9.97 | 10.1 | <.0001∗ |
| 8-hour peak Ozone: from birth to 90 days (ppb) | 53.4 | 53.4 | 0.35 | 52.9 | 54.2 | 48.3 | 48.4 | 0.66 | 47.6 | 49.2 | <.0001∗ |
| 8-hour peak Ozone: from 90 days to PFT (ppb) | 40.3 | 40.2 | 0.29 | 39.8 | 40.9 | 40.5 | 40.5 | 0.17 | 40.1 | 40.7 | 0.01∗ |
SD: standard deviation; Min: minimum; Max: maximum.
Wilcoxon signed-rank test; differences between birth-year 2008 and 2009.
Indicates results robust to FDR adjustment for multiple comparisons, at p < .05.
Comparing mean pulmonary and cytokine measures between years (Table 2), females born in 2008 (high-WFS) had significantly lower FRC [μ = 26.7 (SD = 4.36) vs. 34.8 (SD = 6.92) mL/kg], RV [μ = 8.48 (SD = 3.75) mL/kg vs. 15.9 (SD = 6.56) mL/kg), Ct [μ = 8.2 × 10−4 (SD = 2.7 × 10−4)/kg vs. 1.0 × 10−3 (SD = 2.2 × 10−4)/kg), and IL-8 [μ = 1791.2 (SD = 1146.5) pg/mL vs. 3221.8 (SD = 1266.4) pg/mL) than females born in 2009 (low-WFS) (Table 2).
Table 2.
Summary statistics for lung function and cytokine response measures.
| Birth year (Infancy WFS exposure level) |
P-Value± | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 (High WFS) |
2009 (Low WFS) |
||||||||||||
| N | Mean | Median | SD | Min | Max | N | Mean | Median | SD | Min | Max | ||
| Lung Function | |||||||||||||
| Inspiratory Capacity (mL/kg) | 21 | 37.2 | 36.6 | 3.16 | 30.8 | 43.6 | 22 | 34.6 | 33.7 | 4.74 | 24.0 | 42.1 | 0.09 |
| Vital Capacity (mL/kg) | 21 | 55.7 | 55.5 | 5.44 | 47.7 | 67.9 | 22 | 53.2 | 53.4 | 4.96 | 45.2 | 64.9 | 0.22 |
| Expiratory Reserve Volume (mL/kg) | 21 | 18.6 | 17.7 | 4.89 | 8.81 | 30.0 | 22 | 18.6 | 17.7 | 4.77 | 12.3 | 29.7 | 0.91 |
| Functional Residual Capacity (mL/kg) | 21 | 26.7 | 27.1 | 4.36 | 17.2 | 32.4 | 22 | 34.8 | 35.5 | 6.92 | 23.1 | 54.5 | 0.0001∗ |
| Residual Volume (mL/kg) | 21 | 8.48 | 8.68 | 3.76 | 1.52 | 14.3 | 22 | 15.9 | 15.2 | 6.56 | 4.97 | 35.9 | <.0001∗ |
| Total Lung Capacity (mL/kg) | 21 | 64.2 | 65.3 | 5.66 | 49.2 | 73.1 | 22 | 69.1 | 68.3 | 8.94 | 56.5 | 96.3 | 0.051 |
| Tissue Compliance (/kg) | 21 | 8.2 × 10−4 | 7.8 × 10−4 | 2.7 × 10−4 | 3.8 × 10−4 | 1.7 × 10−3 | 22 | 1.0 × 10−3 | 9.7 × 10−4 | 2.2 × 10−4 | 6.5 × 10−4 | 1.5 × 10−3 | 0.001∗ |
| Cytokine Response | |||||||||||||
| IL8 Secretion Level (pg/mL) | 21 | 1791.2 | 1609.4 | 1146.5 | 460.7 | 4717.5 | 22 | 3221.8 | 3068.0 | 1266.4 | 1233.5 | 6090.4 | 0.001∗ |
| IL6 Secretion Level (pg/mL) | 21 | 81.5 | 71.2 | 57.9 | 15.4 | 248.3 | 22 | 62.3 | 59.1 | 44.7 | 8.83 | 152.3 | 0.26 |
SD: standard deviation; Min: minimum; Max: maximum.
Bolded values were intended to highlight statistical significance.
Wilcoxon signed-rank test; differences between birth-year 2008 and 2009.
Indicates results robust to FDR adjustment for multiple comparisons, at p < .05.
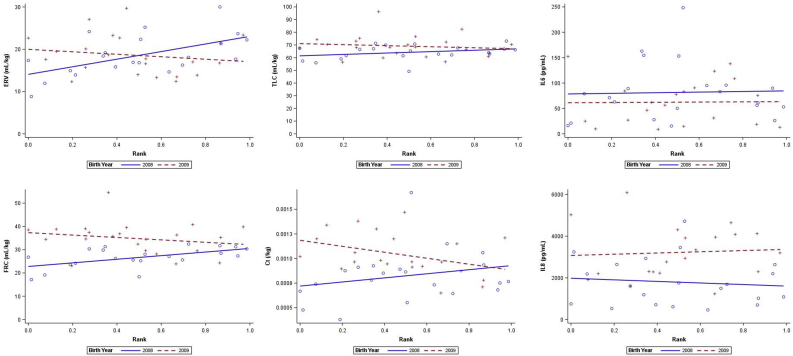
In fully-stratified linear regression models, we found that higher social rank was significantly associated with higher ERV and FRC in 2008 (high-WFS); a 1-unit increase in rank (from lowest to highest rank in cage) conferred a 8.98 (95% CI = 2.84–15.1) mL/kg increase in ERV, and a 7.74 (95% CI = 2.19–13.3) mL/kg increase in FRC, robust to adjustment for multiple comparisons Figure 1. In 2009 (low-WFS), however, rank was not significantly associated with any outcome. Significant differences in the effect of rank between years were not robust to FDR adjustment (Table 3).
Figure 1.
Associations between rank and pulmonary function and cytokine measures, stratified by WFS exposure (birth year). Top row (left – right): ERV, TLC, IL-6. Bottom row (left - right): FRC, Ct, IL-8.
Table 3.
Simple linear regression models for association between social rank and each outcome, stratified by birth year (High/Low WFS).
| Birth year (Infancy WFS) |
Difference in β (year 2008–2009) | P-Value for difference∗∗ | ||||
|---|---|---|---|---|---|---|
| 2008 (High WFS) |
2009 (Low WFS) |
|||||
| β (95% CI) | P-value∗ | β (95% CI) | P-value∗ | |||
| Lung Function | ||||||
| Inspiratory Capacity (mL/kg) | -4.01 (-8.46, 0.43) | 0.07 | 0.61 (-7.54, 8.76) | 0.88 | -4.62 | 0.30 |
| Vital Capacity (mL/kg) | 4.97 (-3.03, 13.0) | 0.21 | -2.32 (-10.8, 6.15) | 0.57 | 7.29 | 0.20 |
| Expiratory Reserve Volume (mL/kg) | 8.98 (2.84, 15.1) | 0.006 | -2.93 (-11.0, 5.18) | 0.46 | 11.91 | 0.018 |
| Functional Residual Capacity (mL/kg) | 7.74 (2.19, 13.3) | 0.009 | -5.17 (-16.8, 6.51) | 0.37 | 12.91 | 0.039 |
| Residual Volume (mL/kg) | 0.54 (-5.22, 6.30) | 0.85 | -1.78 (-13.1, 9.50) | 0.75 | 2.31 | 0.70 |
| Total Lung Capacity (mL/kg) | 5.51 (-2.75, 13.8) | 0.18 | -4.1 (-19.4, 11.2) | 0.58 | 9.61 | 0.25 |
| Tissue Compliance (/kg) | 2.1 × 10−4 (-1.9 × 10−4, 6.0 × 10−4) | 0.28 | -3.0 × 10−4 (-6.5 × 10−4, 4.3 × 10−5) | 0.08 | 5.1 × 10−4 | 0.051 |
| Cytokine Response | ||||||
| IL8 Secretion Level (pg/mL) | -375.4 (-2123.8, 1372.9) | 0.66 | 293.4 (-2011.7, 2598.5) | 0.79 | -668.8 | 0.63 |
| IL6 Secretion Level (pg/mL) | 6.07 (-82.6, 94.8) | 0.89 | 2.28 (-79.3, 83.9) | 0.95 | 3.79 | 0.95 |
SE: standard error; 95% CI: 95% confidence interval.∗ rank: ranges from 0 (lowest) to 1 (highest rank). ∗∗ difference between coefficients, calculated by SAS proc glm procedure.
Bolded values were intended to highlight statistical significance.
Similarly, in linear regression models for the effect of rank on each outcome, with an interaction between rank and WFS (year), we observe significantly lower FRC, RV, and Ct, on average, in the high-WFS year (2008). There were, however, positive benefits of higher rank in the high-WFS year, for ERV, FRC, and Ct. These significant differences in rank effects by year were not robust to adjustment for multiple comparisons (Table 4).
Table 4.
Linear regression models for modification of rank effects by WFS exposure (birth year).
| Intercept |
Rank∗ |
High WFS |
Rank × High WFS |
|||||
|---|---|---|---|---|---|---|---|---|
| β0 (95% CI) | p-value | β1 (95% CI) | p-value | β2 (95% CI) | p-value | β3 (95% CI) | p-value± | |
| Lung Function | ||||||||
| Inspiratory Capacity (mL/kg) | 34.3 (30.8, 37.9) | <.0001 | 0.61 (-5.99, 7.21) | 0.8528 | 4.83 (-0.13, 9.79) | 0.06 | -4.62 (-13.4, 4.19) | 0.30 |
| Vital Capacity (mL/kg) | 54.3 (49.7, 58.9) | <.0001 | -2.32 (-10.8, 6.15) | 0.5826 | -1.09 (-7.46, 5.28) | 0.73 | 7.29 (-4.02, 18.6) | 0.20 |
| Expiratory Reserve Volume (mL/kg) | 20.0 (16.0, 24.0) | <.0001 | -2.93 (-10.3, 4.39) | 0.4230 | -5.92 (-11.4, -0.42) | 0.036 | 11.9 (2.13, 21.7) | 0.018 |
| Functional Residual Capacity (mL/kg) | 37.3 (32.3, 42.3) | <.0001 | -5.17 (-14.3, 3.98) | 0.2600 | -14.5 (-21.4, -7.64) | 0.0001∗ | 12.9 (0.70, 25.1) | 0.039 |
| Residual Volume (mL/kg) | 16.8 (11.9, 21.7) | <.0001 | -1.78 (-10.8, 7.20) | 0.6913 | -8.55 (-15.3, -1.80) | 0.014∗ | 2.31 (-9.68, 14.3) | 0.70 |
| Total Lung Capacity (mL/kg) | 71.1 (64.4, 77.8) | <.0001 | -4.10 (-16.4, 8.24) | 0.5055 | -9.64 (-18.9, -0.36) | 0.042 | 9.61 (-6.87, 26.1) | 0.25 |
| Tissue Compliance (/kg) | 1.2 × 10−3 (9.8 × 10−4, 1.4 × 10−3) | <.0001 | -3.0 × 10−4 (-6.9 × 10−4, 8.2 × 10−5) | 0.1192 | -4.7 × 10−4 (-7.5 × 10−4, 1.8 × 10−4) | 0.0024∗ | 5.1 × 10−4 (3.0 × 10−6, 1.0 × 10−3) | 0.051 |
| Cytokine Response | ||||||||
| IL8 Secretion Level (pg/mL) | 3074.8 (1878.2, 4271.5) | <.0001 | 293.4 (-1819.6, 2406.4) | 0.7800 | -1095.8 (-2685.7, 494.1) | 0.17 | -668.8 (-3435.1, 2097.5) | 0.63 |
| IL6 Secretion Level (pg/mL) | 61.2 (9.51, 112.8) | 0.0216 | 2.28 (-88.9, 93.5) | 0.9598 | 17.4 (-51.2, 86.0) | 0.61 | 3.79 (-115.6, 123.2) | 0.95 |
‘High WFS’ = 1 for birth year 2008; ‘High WFS’ = 0 for birth year 2009.
Bolded values were intended to highlight statistical significance.
Wilcoxon signed-rank test; differences between birth years 2008 and 2009.
Indicates results robust to FDR adjustment for multiple comparisons, at p < .05.
4. Discussion
We found that WFS exposure in infancy, on average, conferred lower adolescent respiratory volumes and inflammatory response, but social rank influenced these measures as well. Among females, higher rank conferred larger lung volumes solely among those born during high WFS, suggesting that, under adverse environmental conditions, benefits of higher rank may be more apparent.
We found significantly lower average FRC, RV and Ct among 3-year-old female monkeys exposed to high levels of WFS during infancy, compared to low-exposed females. As previously reported, high WFS exposure in infancy was associated with decreased lung volume and possibly altered structure during adolescence. We also explored if social rank was associated with WFS health effects. We found that among female monkeys with high infancy WFS exposure, higher social rank was associated with better ERV and FRC, but observed the reverse among females with low infancy WFS exposure.
Our data suggests that health effects of social rank may be particularly apparent under environmental pressures, and that social rank effects vary by health outcome. Another possible explanation is that the offspring of higher-ranked females may be healthier after birth, grow faster, and have higher chances of survival to age three [66, 67]. Finally, effects of WFS in infancy may well differ by individual's baseline health conditions shortly after birth, plausibly altered by maternal rank.
Our results are not likely generalizable to males, as social status among male primates changes substantially over the lifecourse. Other studies have reported sex-specific pollution effects on lung function and cytokines in primates and humans [68, 69, 70, 71], and rank operates very differently in male primates (i.e., males physically fight to establish and re-establish rank, and mid-ranked males, often in conflict with animals both above and below them, display particularly high social vigilance [72] and plasma vasopressin [73]. Likewise, lung development, function, and volume vary by sex in human [74, 75, 76] and animal populations; one human study found that maternal and early-life stress reduced postnatal lung function among boys but not girls [71]. In mice, males have been shown more sensitive to in-utero second-hand smoke exposure than females during lung development [77].
4.1. Strengths
This study is the first, to our knowledge, to leverage, together, the ‘natural experiments’ of primate social rank and real wildfire smoke. We prospectively assess WFS exposure in infancy and pulmonary and immune functions in adolescence, as modified by social rank. As animals were housed outdoors and exposed to naturally-occurring wildfire events, we capture effects of real wildfire exposures on animals of very different social ranks, experiencing the same environmental conditions.
Importantly, WFS exposures were similar for animals across all ranks, eliminating the confounding between social status and pollution exposures that commonly occurs in human societies. Future studies would benefit from a larger sample, longer observational period, and blood cytokine measures.
4.2. Limitations
The sample size is relatively small, limiting our statistical power in tests for multiple comparisons. PBMCs were used for cytokine secretion analysis, though future studies would benefit from direct blood cytokine measures. Only maternal rank was used to represent each animal's own rank, which is appropriate during infancy, and captures the period of wildfire exposure, to which rank is hypothesized to impact susceptibility. Because female rhesus monkeys rank is generally stable – females ranking similarly to, but generally just below, their mothers [78] - the difference in rank between infancy and adolescence is likely minimal. Substantial changes in rank over time (rare among females, but common among males), have been shown to alter immune response [70], and may consequently alter health and susceptibility, but are not seen in our cohort.
5. Conclusion
In summary, we found that exposure to WFS in infancy is associated with lower lung volume changes in adolescence among female rhesus monkeys. However, we also observed that, only among females born during WFS, higher status conferred higher respiratory volumes, indicating the resilience benefits of higher early-life social status. Future studies with longer observational periods could determine whether these effects persist into adulthood. Social hierarchy in macaques is a simple model, compared to the complexities of social status and SEP in humans – but, in this controlled setting, avoids inherent confounding by environmental co-exposures, food access, housing quality, and innumerable other physical and material factors which vary with SEP in humans [79]. Finally, there are substantial differences in the determination of social status among male and female primates - and social status appears to differently affect WFS susceptibility by sex - pointing to the potential utility of animal models to help elucidate some direct status-related aspects of pollution susceptibility.
Declarations
Author contribution statement
Heng Bai & Jane E. Clougherty: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
John P. Capitanio & Lisa A. Miller: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by California Air Resources Board (10-303 & 15-303) and National Institutes of Health (PS1OD011107 & R21HL089148).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Dennison P.E., et al. Large wildfire trends in the western United States, 1984–2011. Geophys. Res. Lett. 2014;41(8):2928–2933. [Google Scholar]
- 2.Knowlton K. NRDC Issue Brief; 2013. Where There's Fire, There's Smoke: Wildfire Smoke Affects Communities Distant from Deadly Flames. [Google Scholar]
- 3.Khilnani G.C., Tiwari P. Air pollution in India and related adverse respiratory health effects: past, present, and future directions. Curr. Opin. Pulm. Med. 2018;24(2):108–116. doi: 10.1097/MCP.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 4.Sierra-Vargas M.P., Teran L.M. Air pollution: impact and prevention. Respirology. 2012;17(7):1031–1038. doi: 10.1111/j.1440-1843.2012.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugha R., Grigg J. Urban air pollution and respiratory infections. Paediatr. Respir. Rev. 2014;15(2):194–199. doi: 10.1016/j.prrv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C., et al. Association between air pollution and cardiovascular mortality in Hefei, China: a time-series analysis. Environ. Pollut. 2017;229:790–797. doi: 10.1016/j.envpol.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Black C., et al. Wildfire smoke exposure and human health: significant gaps in research for a growing public health issue. Environ. Toxicol. Pharmacol. 2017;55:186–195. doi: 10.1016/j.etap.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J.C., et al. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ. Res. 2015;136:120–132. doi: 10.1016/j.envres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid C.E., et al. Associations between respiratory health and ozone and fine particulate matter during a wildfire event. Environ. Int. 2019;129:291–298. doi: 10.1016/j.envint.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 10.Lipsett M., et al. The respiratory health impact of a large urban fire. Am. J. Publ. Health. 1994;84(3):434–438. doi: 10.2105/ajph.84.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adetona A.M., et al. Impact of work task-related acute occupational smoke exposures on select proinflammatory immune parameters in wildland firefighters. J. Occup. Environ. Med. 2017;59(7):679–690. doi: 10.1097/JOM.0000000000001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C.M., et al. Measuring acute pulmonary responses to occupational wildland fire smoke exposure using exhaled breath condensate. Arch. Environ. Occup. Health. 2020;75(2):65–69. doi: 10.1080/19338244.2018.1562413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheffield P.E., et al. Violent crime and socioeconomic deprivation in shaping asthma-related pollution susceptibility: a case-crossover design. J. Epidemiol. Community Health. 2019;73(9):846–853. doi: 10.1136/jech-2018-211816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shmool J.L., et al. Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environ. Res. 2015;142:624–632. doi: 10.1016/j.envres.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clougherty J.E., Shmool J.L., Kubzansky L.D. The role of non-chemical stressors in mediating socioeconomic susceptibility to environmental chemicals. Curr. Environ. Health Rep. 2014;1(4):302–313. [Google Scholar]
- 16.Clougherty J.E., Kubzansky L.D. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ. Health Perspect. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark L.P., Millet D.B., Marshall J.D. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One. 2014;9(4):e94431. doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratt G.C., et al. Traffic, air pollution, minority and socio-economic status: addressing inequities in exposure and risk. Int. J. Environ. Res. Publ. Health. 2015;12(5):5355–5372. doi: 10.3390/ijerph120505355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell M.L., Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbanski S. Combustion efficiency and emission factors for wildfire-season fires in mixed conifer forests of the northern Rocky Mountains, US. Atmos. Chem. Phys. 2013;13:7241–7262. [Google Scholar]
- 21.Malilay J. World Health Organization; Lima, Peru, 1988: 1999. A Review of Factors Affecting the Human Health Impacts of Air Pollutants from forest Fires. [Google Scholar]
- 22.Jung C.R., et al. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J. Allergy Clin. Immunol. 2019;143(6):2254–2262. doi: 10.1016/j.jaci.2019.03.024. e5. [DOI] [PubMed] [Google Scholar]
- 23.Pennington A.F., et al. Exposure to mobile source air pollution in early-life and childhood asthma incidence: the Kaiser air pollution and pediatric asthma study. Epidemiology. 2018;29(1):22–30. doi: 10.1097/EDE.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He B., et al. The association of early-life exposure to air pollution with lung function at ∼17.5 years in the "Children of 1997" Hong Kong Chinese Birth Cohort. Environ. Int. 2019;123:444–450. doi: 10.1016/j.envint.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Patelarou E., Tzanakis N., Kelly F.J. Exposure to indoor pollutants and Wheeze and asthma development during early childhood. Int. J. Environ. Res. Publ. Health. 2015;12(4):3993–4017. doi: 10.3390/ijerph120403993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz E.S., et al. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am. J. Respir. Crit. Care Med. 2016;193(2):171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 27.Black C., et al. Early life wildfire smoke exposure is associated with immune dysregulation and lung function decrements in adolescence. Am. J. Respir. Cell Mol. Biol. 2017;56(5):657–666. doi: 10.1165/rcmb.2016-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M.W., et al. Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harv. Rev. Psychiatr. 2018;26(2):57–69. doi: 10.1097/HRP.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siart B., Pflüger L.S., Wallner B. Pulling rank: military rank affects hormone levels and fairness in an allocation experiment. Front. Psychol. 2016;7:1750. doi: 10.3389/fpsyg.2016.01750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golbidi S., Frisbee J.C., Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015;308(12):H1476–H1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 31.Baritaki S., et al. Chronic stress, inflammation, and colon cancer: a CRH system-driven molecular crosstalk. J. Clin. Med. 2019;8(10) doi: 10.3390/jcm8101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen E., et al. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J. Allergy Clin. Immunol. 2006;117(5):1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Deng Q., et al. Parental stress and air pollution increase childhood asthma in China. Environ. Res. 2018;165:23–31. doi: 10.1016/j.envres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Li M., Mustillo S., Anderson J. Childhood poverty dynamics and adulthood overweight/obesity: unpacking the black box of childhood. Soc. Sci. Res. 2018;76:92–104. doi: 10.1016/j.ssresearch.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S., et al. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom. Med. 2004;66(4):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z., et al. Association between exposure to the Chinese famine during infancy and the risk of self-reported chronic lung diseases in adulthood: a cross-sectional study. BMJ Open. 2017;7(5):e015476. doi: 10.1136/bmjopen-2016-015476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., et al. Chinese famine exposure in infancy and metabolic syndrome in adulthood: results from the China health and retirement longitudinal study. Eur. J. Clin. Nutr. 2019;73(5):724–732. doi: 10.1038/s41430-018-0211-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee M., Khan M.M., Wright B. Is childhood socioeconomic status related to coronary heart disease? Evidence from the health and retirement study (1992-2012) Gerontol. Geriatr. Med. 2017;3 doi: 10.1177/2333721417696673. 2333721417696673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laitinen T.T., et al. Association of socioeconomic status in childhood with left ventricular structure and diastolic function in adulthood: the cardiovascular risk in young Finns study. JAMA Pediatr. 2017;171(8):781–787. doi: 10.1001/jamapediatrics.2017.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll J.E., Cohen S., Marsland A.L. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav. Immun. 2011;25(7):1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerrett M., et al. Do socioeconomic characteristics modify the short term association between air pollution and mortality? Evidence from a zonal time series in Hamilton, Canada. J. Epidemiol. Community Health. 2004;58(1):31–40. doi: 10.1136/jech.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krewski D., et al. Overview of the reanalysis of the harvard six cities study and American cancer society study of particulate air pollution and mortality. J. Toxicol. Environ. Health. 2003;66(16-19):1507–1551. doi: 10.1080/15287390306424. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S., Doyle W.J., Baum A. Socioeconomic status is associated with stress hormones. Psychosom. Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 44.Garratt E.A., et al. Income and social rank influence UK children's behavioral problems: a longitudinal analysis. Child Dev. 2017;88(4):1302–1320. doi: 10.1111/cdev.12649. [DOI] [PubMed] [Google Scholar]
- 45.Cucciare M.A., et al. Associations between deployment, military rank, and binge drinking in active duty and Reserve/National Guard US servicewomen. Drug Alcohol Depend. 2015;153:37–42. doi: 10.1016/j.drugalcdep.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Nader M.A., et al. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berlin) 2012;224(1):57–67. doi: 10.1007/s00213-012-2843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisman O., et al. Social rank and affiliation in social anxiety disorder. Behav. Res. Ther. 2011;49(6-7):399–405. doi: 10.1016/j.brat.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Cohen S., et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 50.Shankardass K., et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc. Natl. Acad. Sci. U. S. A. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clougherty J.E., et al. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010;118(6):769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider J.S., et al. Sex-dependent effects of lead and prenatal stress on post-translational histone modifications in frontal cortex and hippocampus in the early postnatal brain. Neurotoxicology. 2016;54:65–71. doi: 10.1016/j.neuro.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cory-Slechta D.A., et al. Brain hemispheric differences in the neurochemical effects of lead, prenatal stress, and the combination and their amelioration by behavioral experience. Toxicol. Sci. 2013;132(2):419–430. doi: 10.1093/toxsci/kft015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cory-Slechta D.A., et al. Enhanced learning deficits in female rats following lifetime pb exposure combined with prenatal stress. Toxicol. Sci. 2010;117(2):427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virgolini M.B., et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol. Sci. 2005;87(2):469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 56.Uno H., et al. Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 1989;9(5):1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sapolsky R.M., Mott G.E. Social subordinance in wild baboons is associated with suppressed high density lipoprotein-cholesterol concentrations: the possible role of chronic social stress. Endocrinology. 1987;121(5):1605–1610. doi: 10.1210/endo-121-5-1605. [DOI] [PubMed] [Google Scholar]
- 58.Melnick D.J. The genetic consequences of primate social organization: a review of macaques, baboons and vervet monkeys. Genetica. 1987;73(1-2):117–135. doi: 10.1007/BF00057443. [DOI] [PubMed] [Google Scholar]
- 59.Shively C.A., Day S.M. Social inequalities in health in nonhuman primates. Neurobiol. Stress. 2015;1:156–163. doi: 10.1016/j.ynstr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capitanio J.P. The Oxford Handbook of Health Psychology. 2011. Health and social relationships in nonhuman primates: toward a comparative health psychology. [Google Scholar]
- 61.Kaplan J.R., Manuck S.B. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann. N. Y. Acad. Sci. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 62.Sapolsky R.M. The endocrine stress-response and social status in the wild baboon. Horm. Behav. 1982;16(3):279–292. doi: 10.1016/0018-506x(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 63.Sapolsky R.M., Alberts S.C., Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch. Gen. Psychiatr. 1997;54(12):1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- 64.Phillips K.A., et al. Why primate models matter. Am. J. Primatol. 2014;76(9):801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clutton-Brock T.H., Huchard E. Social competition and selection in males and females. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368(1631):20130074. doi: 10.1098/rstb.2013.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pusey A.E., Schroepfer-Walker K. Female competition in chimpanzees. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368(1631):20130077. doi: 10.1098/rstb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson S.E. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am. J. Phys. Anthropol. 2003;120(1):83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- 68.Klein S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fink A.L., Klein S.L. Sex and gender impact immune responses to vaccines among the elderly. Physiology. 2015;30(6):408–416. doi: 10.1152/physiol.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snyder-Mackler N., et al. Social status alters immune regulation and response to infection in macaques. Science. 2016;354(6315):1041–1045. doi: 10.1126/science.aah3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee A.G., et al. Association of prenatal and early childhood stress with reduced lung function in 7-year-olds. Ann. Allergy Asthma Immunol. 2017;119(2):153–159. doi: 10.1016/j.anai.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Capitanio J.P., Boccia M.L., Colaiannia D.J. The influence of rank on affect perception by pigtailed macaques (Macaca nemestrina) Am. J. Primatol. 1985;8(1):53–59. doi: 10.1002/ajp.1350080106. [DOI] [PubMed] [Google Scholar]
- 73.Weinstein T.A., et al. Early involvement in friendships predicts later plasma concentrations of oxytocin and vasopressin in juvenile rhesus macaques (Macaca mulatta) Front. Behav. Neurosci. 2014;8:295. doi: 10.3389/fnbeh.2014.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laube M., et al. Epidermal growth factor strongly affects epithelial Na(+) transport and barrier function in fetal alveolar cells, with minor sex-specific effects. Sci. Rep. 2021;11(1):15951. doi: 10.1038/s41598-021-95410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauerslev M., et al. Pediatr Pulmonol; 2021. Long-term Predictors of Loss of Asthma Control in School-Aged Well-Controlled Children with Mild to Moderate Asthma: a 5-year Follow-Up. [DOI] [PubMed] [Google Scholar]
- 76.Heraganahally S.S., Howarth T.P., Sorger L. Chest computed tomography findings among adult Indigenous Australians in the Northern Territory of Australia. J. Med. Imag. Radiat. Oncol. 2021 doi: 10.1111/1754-9485.13295. [DOI] [PubMed] [Google Scholar]
- 77.Noël A., et al. Sex-specific lung functional changes in adult mice exposed only to second-hand smoke in utero. Respir. Res. 2017;18(1):104. doi: 10.1186/s12931-017-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walters J.R., Seyfarth R.S. In: Primate Societies. Smuts B.B., et al., editors. University of Chicago Press; Chicago: 1987. Conflict and cooperation; pp. 306–317. [Google Scholar]
- 79.Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.