Abstract
Introduction and importance
To describe an unusual case with a primary hepatic neuroendocrine tumour (PHNET) with multiple liver metastases.
Case presentation
We reported a 65-year-old woman with PHNET with multiple liver metastases. She was highly suspected of having primary liver cancer with multiple intrahepatic metastases before liver biopsy, but was diagnosed with PHNET with multiple liver metastases after histopathology and immunohistochemistry (IHC) examinations. The patient successfully underwent three times of transcatheter arterial chemoembolization (TACE), and is currently living in a good state without related complications.
Clinical discussion
Neuroendocrine tumors (NETs), also known as carcinoids or argyrophilic tumors, are very rare malignant tumors. The liver is the main metastasis site of NETs, but primary hepatic neuroendocrine tumors (PHNETs) are extremely rare. Histopathology and immunohistochemistry (IHC) examinations are still the main methods used for diagnosing NETs. There are no treatment guidelines for PHNETs, and surgical resection is generally the preferred treatment. For PHNET patients who are not suitable for surgery, TACE has been proven to be an effective alternative treatment that can effectively reduce the tumour burden and relieve symptoms, but the current evidence is still limited.
Conclusion
The clinical diagnosis of PHNET still faces great challenges, imaging examinations often lead to misdiagnosis, and its diagnosis mainly depends on histopathology and immunohistochemical examinations. For PHNET patients who are not suitable for surgery, TACE may be an effective alternative therapy.
Keywords: Primary hepatic neuroendocrine tumour, Neuroendocrine tumour, Liver metastasis, Immunohistochemistry, Transcatheter arterial chemoembolization, Case report
Highlights
-
•
We present a case of a primary hepatic neuroendocrine tumor (PHNETs) that is extremely rare.
-
•
Due to the lack of specificity in the clinical manifestations and imaging results of PHNET, the diagnosis is facing a huge challenge, and our case further confirms this.
-
•
We reported our experience with the diagnosis and treatment of a case of PHNET, adding additional information to the literature about the diagnosis and treatment of this rare disease.
-
•
Our case suggests that surgical treatment is at high risk in PHNET patients with well-differentiated multiple liver metastases and that TACE may be a good alternative to treatment.
1. Introduction
Neuroendocrine tumors (NETs) are very rare malignant tumors. They originate from peptidergic neurons and neuroendocrine cells. Common pathogenic organs include the stomach, duodenum, pancreas, gallbladder, etc., accounting for approximately 1% to 2% of all gastrointestinal cancer cases [1], [2], [3]. Liver metastases are common in NETs, but neuroendocrine tumors that originate in the liver are extremely rare. Since Edmonson first reported PHNETs in 1958, a total of 150 cases have been reported in the English literature, accounting for approximately 0.3% of all neuroendocrine tumour cases [4]. The clinical diagnosis of PHNET mainly relies on pathological results and immunohistochemical analysis, and long-term follow-up is required to exclude primary lesions outside the liver [5]. However, due to its rarity, the lack of specificity of clinical manifestations and imaging results, and no relevant diagnostic criteria and treatment guidelines, misdiagnosis of such patients is often inevitable. It is precisely due to the challenges in the diagnosis of PHNET that we reported our experience with the diagnosis and treatment of a case of PHNET, adding additional information to the literature about the diagnosis and treatment of this rare disease. This work has been reported in line with the SCARE criteria [6].
2. Presentation of case
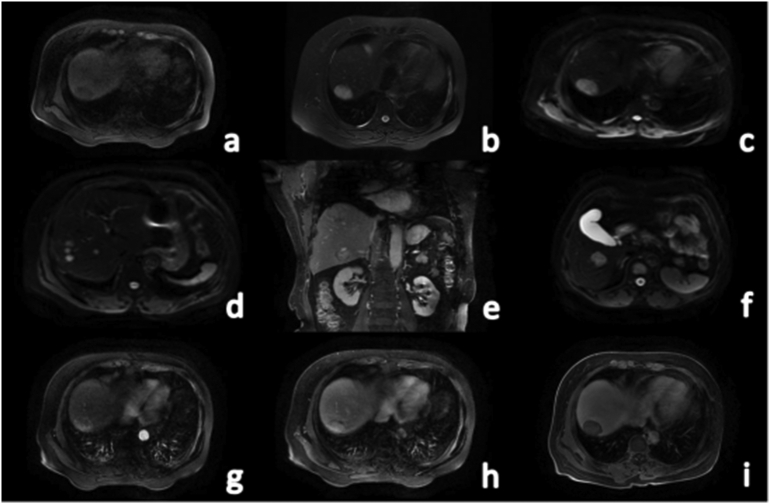
On April 7, 2017, A 65-year-old female patient came to the hospital 4 months after a physical examination found that her liver was enlarged. The patient had no obvious relevant medical history and has no relevant family history. On the first day after admission, laboratory parameters showed that her routine blood tests, urine tests, stool tests, blood coagulation function, liver and kidney function, and serum electrolytes were normal, and her tumour markers were also normal. Contrast-enhanced abdominal ultrasound showed that multiple hypoechoic nodules of varying sizes were detectable in the liver, with clear borders and halos around them; the arterial phase showed nodule-like overall high enhancement, and the portal phase and delayed phase microbubbles in the lesions quickly faded, showing a thick ring with low enhancement, suggesting the possibility of a rich blood supply for metastases. An enhanced computed tomography (CT) scan of the upper abdomen showed multiple abnormal densities in the liver, suggesting the possibility of liver cancer with multiple metastases in the liver, but atypical liver metastases could not be completely excluded. To rule out the possibility of metastasis from a primary lesion outside the liver, further gastric endoscopy and lung imaging were performed, and no extrahepatic lesions were found. Fluorodeoxyglucose positron emission tomography-CT (PET-CT) showed that multiple lesions in the liver had a high metabolic rate in their periphery and that their centres had low metabolism. No abnormally increased metabolism was found in any other organs. Magnetic resonance imaging (MRI) with a liver-specific contrast agent revealed multiple irregularities in the liver with irregular signal shadows, showing uneven long T1 and long T2 signals (Fig. 1a, b), and DWI showed a high-intensity shadow. The boundary was still clear. The largest lesion (36.4 × 23.7 mm) was found in segment VIII of the liver (Fig. 1c), and some lesions were lobulated (Fig. 1d). In the enhanced scan, the arterial phase showed uneven and obvious enhancement (Fig. 1g), the venous phase showed continuous uneven enhancement, and the enhancement was slightly reduced (Fig. 1h). The edge enhancement of some lesions in the delayed phase was significantly reduced, and the central area showed continuous uneven enhancement, a low signal in the hepatobiliary phase (Fig. 1i), and no obvious enhancement or filling defect in the portal vein.
Fig. 1.
MRI was performed on the first admission. A 65-year-old woman was diagnosed with PHNET, grade G1. a: Mixed low signal on T1WI; b: Axial T2WI lipid image showing mixed high signal; c: DWI image showing the largest lesion located in segment VIII of the liver; d: DWI image showing multiple lesions; e: Coronary T2WI weighted image; f: DWI image showing another large lesion; g: Arterial phase T1 weighted image; h: Portal phase T1 weighted image; i: Hepatobiliary phase T1 weighted image.
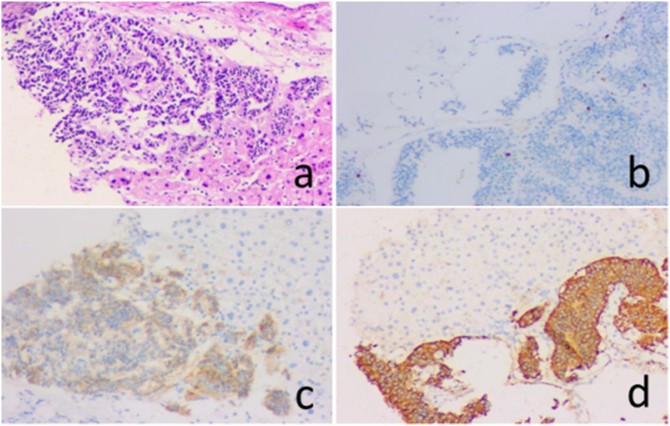
Since the source and nature of the liver lesions could not be determined, a CT-guided liver biopsy was performed. HE staining of the tumour tissue is shown in Fig. 2a. Immunohistochemical examinations showed that synaptophysin (Syn) was positive (Fig. 2b), chromogranin A (CgA) was positive (Fig. 2c), CKpan was positive, GS was positive, CD56 and CD19 were positive, and Ki-67 was 2% (Fig. 2d), while other markers, such as CEA, CD10, CD34, AFP, arginase and HepPar-1 immunohistochemical staining, were all negative. These findings suggest that they are from well-differentiated NETs (G1) Of malignant tumors.
Fig. 2.
The results of histopathology and immunohistochemical examinations. a: HE staining (10 × 10); b: IHC found the Ki-67 index was 2% (10 × 10); c: IHC showed that the tumour was positive for CgA (10 × 10); d: the tumour was also positive for Syn (10 × 10).
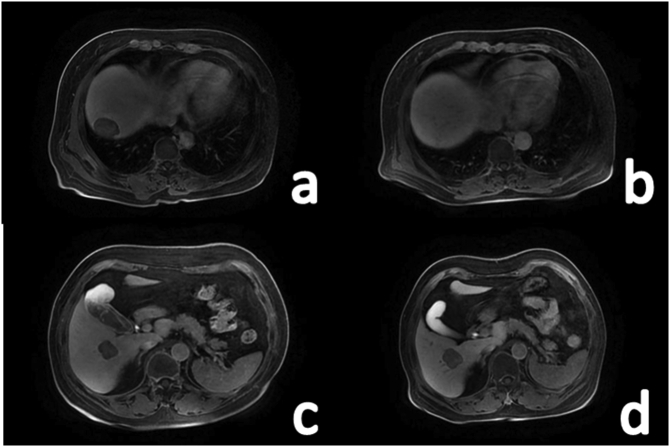
Because the patient had more than one lesion and multiple liver lobes were involved, there was a greater risk during surgical resection, so we decided to administer a TACE treatment, including injection of the chemotherapy drug pirarubicin 30 mg + hydroxycamptothecin 15 mg along the catheter and pirarubrum emulsion embolization treatment of 30 mg bisine +10 ml iodized poppy oil. The operation was performed by an experienced vascular surgeon. The patient received TACE treatment for the same tumour lesion 6 weeks after the first TACE. The first re-examination was performed 3 months after discharge and every six months thereafter. There was no abnormality in any laboratory tests, and the radiological examination showed no recurrence or metastasis of the lesion. Thirty months after the first TACE treatment, it was found that the largest lesion had shrunk and undergone necrosis (Fig. 3a, b), but some lesions were larger than before (Fig. 3c, d), and there was no abnormality in chest CT examination, so a third TACE treatment was performed. According to the imaging results during the follow-up, the possibility of metastatic cancer from the primary site outside the liver was ruled out. Based on the results of the imaging examination, the possibility of metastatic cancer from a primary site outside the liver was ruled out. Combined with the pathological and immunohistochemical examinations, the final diagnosis of this case was PHNET G1 (nonfunctional), TNM stage: T3NxMo·At present, the patient has no signs of tumour recurrence and has a good quality of life.
Fig. 3.
MRI hepatobiliary imaging before and after treatment. a: Before treatment of the largest lesion; b: Shrinkage and necrosis of the largest lesion occurred after 2 TACE procedures; c: Before treatment of another large lesion; d: Another lesion was larger than before at 30 months of follow-up.
3. Discussion
Neuroendocrine tumors (NETs), also known as carcinoids or argyrophilic tumors, are very rare malignant tumors. Their first report was in the 19th century by Oberndorfer, who described a gastrointestinal pancreatic neuroendocrine tumour (GEP- NET) [7]. The age-adjusted annual incidence of NETs was 1.09/100,000 in 1973, but it increased to 6.98/100,000 in 2012 [8]. The incidence of NETs is gradually increasing, which may be due to increasing awareness of NETs and advances in medical technology. Common pathogenic organs of NETs include the stomach, duodenum, pancreas, etc. The liver is the main metastasis site of NETs, but primary hepatic neuroendocrine tumors (PHNETs) are extremely rare.
At present, the pathogenesis of PHNET is still unclear, and three hypotheses about its origin have been proposed [9]: 1. from the multifunctional stem cells of the liver; 2. caused by the proliferation of neuroendocrine cells in the intrahepatic bile duct epithelium; and 3. from other ectopic tissues with endocrine functions. A recent study showed that mutations in the SET domain of the 1B gene might be related to the occurrence of PHNETs [10].
Based on whether NETs have hormone secretion functions and whether there are clinical symptoms caused by the hormones, they can be divided into functional NETs and nonfunctional NETs. Unlike other NETs, PHNETs are usually nonfunctional, which may be related to the insufficient amount or quality of their secreted neuroendocrine hormones, so that the target organs of the hormones cannot be activated to produce biological effects [11]. According to previous reports on PHNETs, the patients are mostly over 50 years old, and there is no significant difference in their incidence between men and women. The most common clinical symptoms are nonspecific manifestations such as abdominal discomfort, abdominal pain, and abdominal masses, followed by no clinical symptoms, which are usually identified as liver space-occupying lesions found during a physical examination. In addition, there are also reports of Cushing syndrome, carcinoid syndrome or obstructive jaundice. Our case had no clinical symptoms and was a nonfunctional PHNET. Similar to other malignant tumors, PHNETs grow slowly and are not easy to detect early. At the time of diagnosis, it is often in the middle and late stages, and the tumour volume is very large [12], [13], [14], so the timing of treatment is often delayed.
PHNET tissue is usually greyish-yellow, the boundary between the tumour and the surrounding tissue is clear, and haemorrhage or cystic lesions can be seen in the centre of the lesion. Tumour cells stained by haematoxylin and eosin show nonspecific growth patterns, such as island, nest, trabecular or mixed cell growth. In some cases, a large number of interstitial blood vessels and fibrous capsules can be found. A small round nucleus can be seen in the centre of the tumour cells. Electron microscopy showed that there were some special neuroendocrine particles in the cytoplasm. These particles are round or elliptical, with different sizes, and are wrapped by a membrane [15]. The traditional biological markers of PHNETs include chromogranin A (CgA), pancreatin, neuron-specific enolase (NSE), synaptophysin (Syn), serotonin and its metabolite 5-hydroxyindole dole acetic acid (5-HIAA). Among them, chromogranin A and synaptophysin are considered to be specific immunohistochemical markers for the pathological diagnosis of NETs [13], while classic tumour markers such as AFP, CEA or CA19-9 are usually negative [5], [16].
The immunohistochemical examination of our case showed positive CgA and positive Syn, which is consistent with the above research conclusions. The WHO classification of neuroendocrine tumors of the digestive system (2019) divides NETs into three grades (G1, G2 and G3), namely, G1: mitosis <2/10 HPF and/or PI <3%; G2: mitosis 2– 20/10 HPF and/or PI3% ~ 20%; G3: mitosis>20/10 HPF and/or Ki-67 PI>20% [17]. Studies have shown that the mitotic index and Ki-67 index are of great value in evaluating the malignancy of PHNEN and its prognosis [18]. Wang et al. [19] analysed the survival of 40 PHNET patients and found that the tumour grade and Ki-67 index were significantly related to the overall survival rate. Our case showed a low cell proliferation index, such as 2% Ki-67 and less than 2 mitoses in every 10 high-power fields. According to the WHO standard, this case is classified as G1, which means that the prognosis is good. However, there are still controversies about the correlation between the number of tumors and the prognosis [5], [20].
Imaging examinations are of great value in the diagnosis of liver cancer. Long-term follow-up and regular imaging examinations can help detect potential extrahepatic primary lesions [16]. PHNETs usually have an abundant blood supply but lack specificity in their imaging manifestations, and preoperative imaging examination often leads to a misdiagnosis. To accurately identify whether the primary lesion is outside the liver, it is recommended to perform adequate imaging examinations, including CT, MRI and enhanced MRI, PET and ultrasound. PHNETs often show a single or multiple low-density shadows on CT, but enhanced CT shows uneven enhancement in the arterial phase or obvious circular enhancement and continuous enhancement in the venous phase, which is similar to the “fast-in and fast-out” enhancement of hepatocellular carcinoma or the “progressive” mild to moderate enhancement of cholangiocarcinoma [21]. MRI showed long T1 and long T2 signals, nodular or circular enhancement in the arterial phase, and continuous enhancement in the portal phase and delayed phase. Compared with the surrounding liver parenchyma, PHNETs show high signals on DWI sequences and decreased ADC values [22]. PHNETs in the G1 and G2 phases usually appear as a single lesion on the right lobe of the liver on CT or MRI, while in the G3 phase, there are often multiple lesions or a large tumour accompanied by several satellite lesions [23]. Studies have proven that the appropriate combination of imaging methods can optimize the evaluation of PHNET patients, but it is not yet possible to directly diagnose PHNETs through imaging examinations [24]. Histopathology and immunohistochemistry (IHC) examinations are still the main methods used for diagnosing NETs [16].
There are no treatment guidelines for PHNETs, and surgical resection is generally the preferred treatment. According to reports by Knox CD [25], the success rate of PHNET liver resection is approximately 70%, and the 5-year survival rate after surgery is as high as 78%. Shi et al. [5] conducted long-term follow-up on 22 PHNET patients undergoing surgical treatment and found that the 1-year, 3-year and 5-year overall survival rates could reach 95.5%, 81.8% and 64.7%, respectively. For PHNET patients who are not suitable for surgery, TACE has been proven to be an effective alternative treatment that can effectively reduce the tumour burden and relieve symptoms, but the current evidence is still limited [26], [27]. Li et al. [21] reported 6 cases of PHNET patients treated with TACE, and they obtained satisfactory results during a 4-year follow-up.
In our case, due to the involvement of multiple liver lobes, surgery had a greater risk, so TACE treatment was chosen. After 30 months of follow-up, most of the lesions shrank and underwent necrosis. The patient is currently in stable condition and has no related complications. This indicates that TACE containing anthracycline is an effective treatment for PHNET patients. Liver transplantation (LT) can also be selected for patients who cannot be surgically resected if interventional therapy has failed. A retrospective study evaluated the outcomes of 213 NET patients who underwent LT and found that LT is an effective treatment for patients with unresectable metastatic hepatic neuroendocrine tumors (MHNETs) [28].
The somatostatin receptor is a possible target for other systemic treatments. Low- and medium-grade NETs tend to express somatostatin receptors at high levels. The use of somatostatin analogues (SSAs), such as long-acting octreotide, lanreotide, pasireotide, etc. can effectively control the carcinoid synthesis of such NETs, prolonging the patient's progression-free survival time. Shen et al. [29] reported the outcomes of 233 patients with advanced GEP-NETs who received octreotide injection, with a median survival time of 35.22 months. Long-acting somatostatin lanreotide has also been shown to have antihormonal and antiproliferative effects on NETs, but its role in the treatment of PHNETs remains to be determined [30], [31].
Peptide-receptor radionuclide therapy (PRRT) is an emerging treatment option that has promising effects [32]. PRRT refers to the specific binding of somatostatin analogues (SSTAs) to somatostatin receptors (SSTRs) to introduce labeled radionuclides into tumors with high expression of SSTRs so that SSTAs and radionuclides can be co-localized in the primary site of the tumour to play a dual therapeutic role of chemotherapy and internal radiation. Kwekkeboom et al. [33] reported that MHNET patients treated with a radiolabelled somatostatin analogue, [(177)Lu-DOTA(0), Tyr(3)] octreotide, had a survival benefit of 40–72 months compared with historical controls.
Systemic chemotherapy is mainly used for the treatment of metastatic NETs. For high-grade neuroendocrine tumors, especially those with Ki-67 > 50%, systemic chemotherapy with drugs such as cisplatin and etoposide has been shown to have a better effect [34]. Targeted therapy is mainly for low- and medium-grade advanced chronic NETs (G1 or G2). Commonly used molecular targets include SSTRs, mammalian rapamycin receptor (mTOR) and angiogenic factors. A study reported that the mTOR inhibitor everolimus and the antiangiogenic multitarget tyrosine kinase inhibitor (TKI) sunitinib could be applied to pancreatic NETs [35]. Targeted therapy can also be combined with SSA to treat low- and medium-grade NETs [36], [37], [38]. Some NET cells highly express PD-1, and immunotherapy can prolong the survival time of these patients. Y Fan et al. [39] studied the expression of PD-L1 and PD-1 in 80 patients diagnosed with NETs, suggesting that PD-L1 and PD-1 may become a new choice for poorly differentiated G3 NET patients. In addition, radiofrequency ablation and cryoablation have a positive effect in the treatment of MHNETs [40], [41]. Whether the above treatment methods can be routinely applied to the treatment of PHNETs needs further study.
4. Conclusion
PHNET is an extremely rare malignant tumour. For those who have no clinical symptoms, normal tumour markers, and suspected malignant tumour signs but no typical manifestations on imaging examination, the diagnosis of a primary hepatic neuroendocrine tumour should be considered after excluding the presence of a primary extrahepatic lesion. The lack of specificity of PHNET radiological characteristics often leads to its misdiagnosis as some other type of liver tumour. The diagnosis depends on histopathology and immunohistochemical examinations. Surgery is the first choice for treatment, but the treatment plan should be individualized, and if necessary, a combination of multiple treatments can be used to improve the prognosis. Our case confirmed the effectiveness of TACE as an alternative therapy for patients with well-differentiated PHNETs.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Source of funding
The research did not receive and specific grant form funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This paper as a case report, therefore does not require ethics approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
N/a.
Guarantor
Hong-Fang Tuo.
CRediT authorship contribution statement
Wei-Ming Yu: study concept design, literature review, writing the paper.
Ri Li, Bing-Lun Sun, Ji-Kang Du:data collection, data analysis or interpretation.
Hong-Fang Tuo: main surgical provider for the patient, operator and the lead of this project. Concept and design of the manuscript, revisions of the manuscript, and final approval of the version to be published.
Declaration of competing interest
Wei-Ming Yu declares no conflict of interest.
Ri Li declares no conflict of interest.
Bing-Lun Sun declares no conflict of interest.
Ji-Kang Du declares no conflict of interest.
Hong-Fang Tuo declares no conflict of interest.
References
- 1.Burad D.K., Kodiatte T.A., Rajeeb S.M., Goel A., Eapen C.E., Ramakrishna B. Neuroendocrine neoplasms of liver - A 5-year retrospective clinico-pathological study applying World Health Organization 2010 classification. World J Gastroenterol. 2016;22:8956–8966. doi: 10.3748/wjg.v22.i40.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster D.S., Jensen R., Norton J.A. Management of liver neuroendocrine tumors in 2018. JAMA Oncol. 2018;4:1605–1606. doi: 10.1001/jamaoncol.2018.3035. [DOI] [PubMed] [Google Scholar]
- 3.Song J.E., Kim B.S., Lee C.H. Primary hepatic neuroendocrine tumor: a case report and literature review. World J. Clin. Cases. 2016;4:243–247. doi: 10.12998/wjcc.v4.i8.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quartey B. Primary hepatic neuroendocrine tumors: what do we know now? World J Oncol. 2011;2:209–216. doi: 10.4021/wjon341w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C.Y., Zhao Q., Dai B.H., Xie F., Yang J.M. Primary hepatic neuroendocrine neoplasm: long-time surgical outcome and prognosis. Medicine (Baltimore) 2018;97:e11764. doi: 10.1097/MD.0000000000011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Oberg K. The genesis of the neuroendocrine tumors concept: from Oberndorfer to 2018. Endocrinol Metab Clin North Am. 2018;47:711–731. doi: 10.1016/j.ecl.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Dasari A., Shen C., Halperin D., Zhao B., Zhou S., Xu Y., Shih T., Yao J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z., Xiao H.E., Ramchandra P., Huang H.J. Imaging and pathological features of primary hepatic neuroendocrine carcinoma: An analysis of nine cases and review of the literature. Oncol Lett. 2014;7:956–962. doi: 10.3892/ol.2014.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang P.H., Huang X.L., Lai C.C., Li L., Li T.L., Huang P.D., Ouyang S.Y., Yan J., Cheng S.J., Lei G.L., Wang J.G., Yu L.X., Hong Z.X., Li R.S., Dong H., Wang C., Yu Y.H., Wang X., Li X.H., Wang L.M., Lv F.D., Yin Y., Yang H.M., Song J.H., Gao Q., Wang X.L., Zhang S.G. SET domain containing 1B gene is mutated in primary hepatic neuroendocrine tumors. Int J Cancer. 2019;145:2986–2995. doi: 10.1002/ijc.32334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akahori, Sho M., Tanaka T., Nishiofuku H., Kinoshita S., Nagai M., Kichikawa K., Nakajima Y. Significant efficacy of new transcatheter arterial chemoembolization technique for hepatic metastases of pancreatic neuroendocrine tumors. Anticancer Res. 2013;33:3355–3358. doi: 10.3109/0284186X.2013.806820. [DOI] [PubMed] [Google Scholar]
- 12.Li W., Zhuang B.W., Wang Z., Liao B., Hong L.Y., Xu M., Lin X.N., Xie X.Y., Lu M.D., Chen L.D. Case report of contrast-enhanced ultrasound features of primary hepatic neuroendocrine tumor: a care-compliant article. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003450]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia C., Zhang Y., Xu J., Ke S. Experience in primary hepatic neuroendocrine tumor. Turk J Gastroenterol. 2012;23:546–551. doi: 10.4318/tjg.2012.0370. [DOI] [PubMed] [Google Scholar]
- 14.Lin C.W., Lai C.H., Hsu C.C., Hsu C.T., Hsieh P.M., Hung K.C., Chen Y.S. Primary hepatic carcinoid tumor: a case report and review of the literature. Cases J. 2009;2:90. doi: 10.1186/1757-1626-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty P.K., Baliga S.V., Balaiah K., Gnana P.S. Primary hepatic neuroendocrine tumor: an unusual cystic presentation. Indian J Pathol Microbiol. 2010;53:760–762. doi: 10.4103/0377-4929.72078. [DOI] [PubMed] [Google Scholar]
- 16.Song J.E., Kim B.S., Lee C.H. Primary hepatic neuroendocrine tumor: a case report and literature review. World J Clin Cases. 2016;4:243–247. doi: 10.12998/wjcc.v4.i8.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagtegaal I.D., Odze R.D., Klimstra D., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A., Paradis V. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Z., Shi C., Wen H., Li F.L., Wang B.L., Wang J. The potential of carcinoembryonic antigen, p53, Ki-67 and glutathion Stransferase-pi as clinico-histopathological markers for colorectal cancer. J Biomed Res. 2010;24:51–57. doi: 10.1016/S1674-8301(10)60008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H.H., Liu Z.C., Zhang G., Li L.H., Li L., Meng Q.B., Wang P.J., Shen D.Q., Dang X.W. Clinical characteristics and outcome of primary hepatic neuroendocrine tumors after comprehensive therapy. World J. Gastrointest. Oncol. 2020;12:1031–1043. doi: 10.4251/wjgo.v12.i9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A., Xiang J., Zhang M., Zheng S. Primary hepatic carcinoid tumors: clinical features with an emphasis on carcinoid syndrome and recurrence. J. Int. Med. Res. 2008;36:848–859. doi: 10.1177/147323000803600428. [DOI] [PubMed] [Google Scholar]
- 21.Li X.S., Zhang M.C., Qu Y.C., Zhang X.Q., Pan F., Liu Y.X. Diagnostic imaging of primary hepatic neuroendocrine tumors and treatment with transarterial chemoembolization: analysis of 6 cases. Chin. J. Hepatol. 2018;26:294–297. doi: 10.3760/cma.j.issn.1007-3418.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Sheng R.F., Xie Y.H., Zeng M.S., Ji Y., Rao S.X., Chen C.Z. MR imaging of primary hepatic neuroendocrine neoplasm and metastatic hepatic neuroendocrine neoplasm:a comparative study. Radiol. Med. 2015;120:1012–1020. doi: 10.1007/s11547-015-0544-y. [DOI] [PubMed] [Google Scholar]
- 23.Wang L.X., Liu K., Ling W., Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13. doi: 10.1186/s40644-015-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K., Cheng Y.S., Yang J.J., Jiang X., Guo J.X. Primary hepatic neuroendocrine tumors: multi-modal imaging features with pathological correlations. Cancer Imaging. 2017;17:20. doi: 10.1186/s40644-017-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knox C.D., Anderson C.D., Lamps L.W., Adkins R.B., Pinson C.W. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171–1175. doi: 10.1245/aso.2003.04.533. [DOI] [PubMed] [Google Scholar]
- 26.Morishita A., Yoneyama H., Nomura T., Sakamoto T., Masaki T. Primary hepatic neuroendocrine tumor: a case report. Mol Clin Oncol. 2016;4:954–956. doi: 10.3892/mco.2016.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y.J., Zhang L., Wu H.J., Qiao L., Xia L. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with literature review. J Int Med Res. 2020;48 doi: 10.1177/0300060520932114. 300060520932114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.YPL Treut, Gregoire E., Klempnauer J., Belghiti J., Adam R. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg. 2013;257:807–815. doi: 10.1097/SLA.0b013e31828ee17c. [DOI] [PubMed] [Google Scholar]
- 29.Shen C., YCT Shih, Xu Y., Yao J.C. Octreotide LAR among elderly patients with neuroendocrine tumors: a survival analysis of SEER-medicare data. Cancer Epidemiol Biomarkers Prev. 2015;24:1656–1665. doi: 10.1158/1055-9965.EPI-15-0336. [DOI] [PubMed] [Google Scholar]
- 30.Wängberg B., Nilsson O., Johanson V.V., Kölby L., Forssell-Aronsson E., Andersson P., Fjälling M., Tisell L., Ahlman H. Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumor. Oncologist. 1997;2:50–58. doi: 10.1634/theoncologist.2-1-50. [DOI] [PubMed] [Google Scholar]
- 31.Hwang S., Lee Y.J., Lee S.G., Kim C.W., Kim K.H., Ahn C.S., Moon K.M., Ko K.H., Kim K.W., Choi N.K., Ha T.Y. Surgical treatment of primary neuroendocrine tumors of the liver. J Gastrointest Surg. 2008;12(4):725–730. doi: 10.1007/s11605-007-0418-2. [DOI] [PubMed] [Google Scholar]
- 32.Wong P.C., She W.H., Khoo U.S., Cheung T.T. A case of primary hepatic neuroendocrine tumor and literature review. Case Rep Oncol. 2021;14:90–97. doi: 10.1159/000510935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwekkeboom D.J., de Herder W.W., Kam B.L., van Eijck C.H., van Essen M., Kooij P.P., Feelders R.A., van Aken M.O., Krenning E.P. Treatment with the radio-labeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 34.Raj N., Valentino E., Capanu M., Tang L.H., Basturk O., Untch B.R., Allen P.J., Klimstra D.S., Reidy-Lagunes D. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology:Well differentiated versus poorly differentiated. Pancreas. 2017;46:296–301. doi: 10.1097/MPA.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E., Hobday T.J., Okusaka T., Capdevila J., de Vries E.G., Tomassetti P., Pavel M.E., Hoosen S., Haas T., Lincy J., Lebwohl D., Öberg K., <collab>RAD001 in Advanced Neuroendocrine Tumors Third Trial R.A.D.I.A.N.T.-3Study Groupcollab. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberg K., Knigge U., Kwekkeboom D., Perren A., ESMO Guidelines Working Group Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 37.Fazio N., Granberg D., Grossman A., Saletan S., Klimovsky J., Panneerselvam A., Wolin E.M. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest. 2013;143:955–962. doi: 10.1378/chest.12-1108. [DOI] [PubMed] [Google Scholar]
- 38.Pavel M.E., Becerra C., Grosch K., Cheung W., Hasskarl J., Yao J.C. Effect of everolimus on the pharmacokinetics of octreotide long-acting repeatable in patients with advanced neuroendocrine tumors: An analysis of the randomized phase III RADIANT-2 trial. Clin Pharmacol T-her. 2017;101:462–468. doi: 10.1002/cpt.559. [DOI] [PubMed] [Google Scholar]
- 39.Fan Y., Ma K., Wang C., Hu Y., Dong D., Dong X., Geng Q., Li E., Wu Y., Ning J. Prognostic value of PD-L1 and PD-1 expression in pulmonary neuroendocrine tumors. Onco Targets Ther. 2016;9:6075–6082. doi: 10.2147/OTT.S115054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atwell T.D., Charboneau J.W., Que F.G., Rubin J., Lewis B.D., Nagorney D.M., Callstrom M.R., Farrell M.A., Pitot H.C., Hobday T.J. Treatment of neuroendocrine cancer metastatic to the liver: the role of ablative techniques. Cardiovasc Intervent Radiol. 2005;28:409–421. doi: 10.1007/s00270-004-4082-6. [DOI] [PubMed] [Google Scholar]
- 41.Cozzi P.J., Englund R., Mprris D.L. Cryotherapy treatment of patients with hepatic metastases from neuroendocrine tumors. Cancer. 1995;76:501–509. doi: 10.1002/1097-0142(19950801)76:3<501::aid-cncr2820760322>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]