Abstract
Chicken meat is an important source of high-quality animal protein. Its consumption continues to grow in both developed and developing countries. Muscle fiber characteristics are key determinants of meat quality and quantity. Skeletal muscle is a highly plastic tissue that is affected by breed differences and muscular tissues. However, studies regarding the effects of different breeds and muscular tissues on the fibers and meat quality traits in broilers are lacking. In this study, Ross 308 chickens (fast-growing [FG] broilers) and Xueshan chickens (slow-growing [SG] broilers) were selected, and their fiber and meat quality traits were characterized. The results showed that the breast muscle primarily comprised glycolytic fibers, whereas the leg muscle comprised glycolytic and a few oxidative fibers, regardless of the breed. The highest percentage of oxidative fibers (26.51%) appeared in the soleus muscle (SOL) of SG broilers. In addition, higher shear force, lower pressing loss, and thicker muscle fibers with less extracellular space were observed for SG meat than for FG meat. When comparing the different muscular tissues, a higher oxidative fiber percentage, ultimate pH, redness, and intramuscular fat (IMF) content were detected in the leg muscle than in the breast muscle in the 2 breeds. In summary, these data indicated that SG broilers had thicker muscle fibers than the FG broilers and that the leg muscle had more oxidative fibers than the breast muscle. Thicker fibers may contribute to increased firmness and more oxidative fibers lead to higher redness value and IMF content.
Key words: broiler, slow- and fast-growing, meat quality, muscle fiber
INTRODUCTION
Chicken meat is a healthy source of animal protein. It has lower raising costs and higher efficiency than other meat species (Dalle Zotte et al., 2020). Chicken meat is mainly from fast-growing (FG) broilers (i.e., Ross 308 broilers) and slow-growing (SG) broilers (i.e., Label Rouge). The slow-growing broilers are harvested at a more mature age (usually a minimum of 81 d) than conventional fast-growing lines (between 35 and 42 d) (Chabault et al., 2012). In addition, the Global broiler production generally defines slow growth as the “growth rate equal to or less than 50 g/D averaged over the growth cycle (Rayner et al., 2020). Recently, SG chickens have been attracting an increasing number of consumers, particularly in China and France. The SG broilers had expanded to 41.67% (5 billion) of broiler production in China and accounted for nearly 24% of French broiler production in 2019 (Singh et al., 2021). Moreover, SG broiler meat is considered to have a better flavor than FG broiler meat. Label Rouge chickens are widely used in France as chickens with higher sensory quality (Chabault et al., 2012). Indbro broiler is a strain developed to cater to the demand for SG broiler meat in India and contains significantly lower total fatty acid and saturated fatty acid contents than commercial white broiler chicken meat (Devatkal et al., 2019). Wen et al. (2017) found that the breast muscle of the Partridge Shank broilers (SG) had a lower cooking loss than that of the Arbor Acres broilers (FG). Therefore, there were great meat quality divergences among different broiler breeds in different regions. More efforts are needed to document quality variations between SG and FG broilers and characterize the underlying mechanisms.
The muscle fibers are directly related to poultry growth rate and meat quality traits (Joo et al., 2013). The number of muscle fibers formed before birth and the size of those fibers after birth determine the ultimate muscle mass, while the muscle fiber characteristics affect both appearance and eating quality traits in poultry (Ismail and Joo, 2017). The fiber characteristics include the fiber type composition and morphological traits of the fibers. In avian, skeletal muscles mainly comprise glycolytic (type II, fast-twitch) fibers and oxidative (type I, slow-twitch) fibers. However, compared with livestock, the skeletal muscles of poultry contain a relatively small number of oxidative fibers. Several studies have reported that breast muscle in broilers is entirely made up of 100% glycolytic fibers, regardless of breed (Roy et al., 2006; Verdiglione and Cassandro, 2013). Our previous studies also showed small number of oxidative fibers existed in geese and ducks at their marketable ages (Huo et al., 2021; Weng et al., 2021). However, Du et al. (2017) reported that the SOL muscle in the leg of broilers consisted of 76 to 79% oxidative fibers. Therefore, skeletal muscle is a plastic tissue that is affected by different breeds and muscular tissues. However, the fiber characteristics in SG and FG broilers are still not well understood, particularly in the leg muscles.
In this study, Xueshan (SG broilers, China) and Ross 308 (FG broilers, Britain) broilers were selected. Additionally, the composition of different fiber types and morphological traits of fibers in the breast and leg muscles were investigated. Furthermore, the physical properties and proximate composition of broiler meat were determined. These data will reveal the muscle fiber and meat quality divergences between SG and FG broilers.
MATERIALS AND METHODS
Animals and Sample Collection
The experiment was conducted at Yangzhou University, and all experimental animal procedures were approved by the Institutional Animal Care and Use Committee (Approval Number: 182-2019 YZUDWSY, Government of Jiangsu Province, China). A total of 60 chickens were sampled: Ross 308 broiler chickens (n = 30), white-feather broilers with a fast growth rate, and commercial Xueshan chickens (n = 30), yellow-feather broilers with a slow growth rate were provided by Jiangsu Lihua Animal Husbandry Co., Ltd., China. Xueshan chicken is a major slow-growing chicken breed which is popular mainly in some Asian countries and accounts a considerable market share in China (Wang et al., 2021). It was bred by cross-breeding 3 breeds, the White Rock chicken and 2 Chinese local breeds (Tibetan chicken and Chahua chicken). The average body weight of Xueshan broilers is approximately 1.5 kg at marketable age (a minimum of 90 d for males and 101 d for females). In the present study, female chickens were chosen and slaughtered at average live body weights of 2.02 ± 0.11 and 1.65 ± 0.13 kg at the age of 37 d for Ross 308 and 101 d for Xueshan, respectively. Samples for both groups were collected on the same day. All chickens were processed by an authorized commercial abattoir, which consisted of electrical stunning (120 V, 200 Hz) and exsanguination. Thereafter, the breast (pectoralis major) and leg samples were dissected from the carcasses. Samples were cooled on ice, packaged with plastic wraps and then transferred to the laboratory in chilled condition. At 24-h postmortem (4°C), the gastrocnemius (GAS), SOL, and extensor digitorum longus (EDL) muscles were separated from the leg muscle. Samples for hematoxylin and eosin staining and immunohistochemical staining were fixed in 4% paraformaldehyde. The remaining samples were kept at 4°C immediately for meat quality analysis at 24-h postmortem. The physical properties, proximate composition, and muscle fiber characteristics were measured on the right breast and leg samples.
Physical Property Analysis
Representative physical properties (pHu, meat color, tenderness, and pressing water loss) were measured at 24-h postmortem. pH analysis was performed with a penetration electrode using a portable meat pH meter (DELTA 320, Mettler Toledo, Columbus, OH). The pH meter was first calibrated at chilling temperature using pH 4.00 and pH 7.00 buffers. The pHu value analysis was then conducted at 3 random points in each breast or leg sample, at a depth of 10 mm into the meat. Each value was the average of three measurements. Color determinations were performed using a colorimeter (Minolta CR400, Konica Minolta, Tokyo, Japan). Readings were completed at 3 randomly selected points on the inner surface of the chicken muscle. Before testing, polyethylene bags were opened, and muscle samples were allowed to bloom for 30 min on ice (Wicklund et al., 2005). The samples were held on the ice during color evaluation. The results were collected using the CIE-LAB system: L* (lightness), a* (redness), and b* (yellowness). Tenderness was evaluated by shear force analysis conducted using a digital tenderness meter (C-LM3B, Tenovo, Beijing, China). Parallelepiped-shaped cuts of 2.0 × 1.0 × 1.0 cm) were made from the breast muscle and leg muscle at different positions, parallel to fiber orientation. The pressing loss analysis was performed using a dilatometer (C-LM3B, Tenovo, Beijing, China). Samples (approximately 1 g) were weighed 24-h postmortem (W1). Thereafter, 16 layers of filter paper were placed on the top and bottom of the sample. The sandwich was placed between the hard plastic plates on the platform of the dilatometer. The meat sample was pressurized (68.66 kPa) for 5 min and weighed again (W2). The pressing loss was calculated using the following equation: Pressing water loss (%) = [(W1-W2) / W1] × 100%. All measurements were taken on samples from 6 broilers in the same group, and each value was the average of three measurements.
Proximate Composition Analysis
The proximate composition was evaluated using a FoodScan Meat Analyzer (Foss, Hilleroed, Denmark). Samples were separated from tendons and muscle membranes, cut into pieces, ground into a paste with a high-speed universal crusher, and placed into sample cups. Moisture, intramuscular fat, protein, and collagen content were determined (Anderson, 2007). Measurements were taken on samples from 6 broilers in the same group and repeated 3 times.
Hematoxylin and Eosin Staining
Muscle fiber morphological traits were determined using hematoxylin and eosin (H&E) staining. Each muscle sample was fixed in 4% paraformaldehyde for 24 h and paraffin-embedded, and a microtome (Leica Biosystems, Wetzlar, Germany) was used to prepare 5-μm-thick sections. After drying overnight at 40°C, the sections were counterstained with H&E using a Leica Autostainer XL (Leica Biosystems). The samples were scanned using a NanoZoomer scanner (Hamamatsu, Sydney, Australia). The fiber diameter and cross-sectional area were calculated using an image analysis system (Image-Pro Plus, Media Cybernetics, Rockville, MD). For each sample, 3 different points on 3 images containing approximately 300 muscle fibers were estimated. Measurements were taken on samples from 6 broilers from the same group.
Immunohistochemical Staining
Muscle fiber types were determined by immunohistochemical staining. The slides were immersed 3 times in xylene for 15 min each, twice in anhydrous ethanol, twice in 95% ethanol, and twice in 80% ethanol for 5 min each, and then incubated in 3% methanol-H2O2 for 10 min. The slides were incubated with the primary antibody antifast myosin skeletal heavy chain (MYH1A, 1:1200, ab51263, Abcam, Cambridge, UK) or anti-Slow Myosin Skeletal Heavy chain (MYH7B, 1:4000, ab11083, Abcam,). After overnight incubation at 4°C, the slides were washed 3 times in PBS for 5 min each, incubated with the secondary antibody, rabbit anti-mouse IgG (ab6728, Abcam) for 30 min, washed 3 times again for 5 min each with PBS, and stained with 3,3′-diaminobenzidine (DAB) for 10 s. The fast- and slow-twitch fibers were separated and calculated using H&E staining. Measurements were taken on samples from 6 broilers from the same group.
Statistical Analysis
All data are presented as the mean ± standard error (SE). Comparisons of repeat measurements among different growth rate broilers and different muscular tissues were conducted using SPSS statistical software (version 18.0, SPSS, Chicago IL). Duncan's multiple range test was used to analyze the main effects among different growth rate broilers and different muscular tissues. Statistical significance was set at P ≤ 0.05.
RESULTS
Comparison of Muscle Fiber Morphological Traits Between SG and FG Broilers
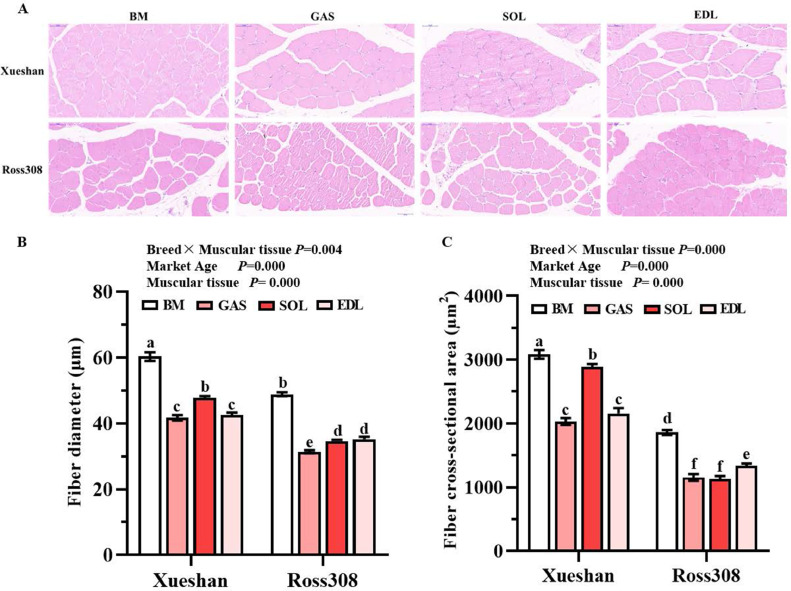
The fiber diameter and cross-sectional area (Figure 1) were measured to compare the morphology of muscle fibers in broilers. There was a significant interaction between factors (breeds and muscular tissues), and both indexes differed significantly among the groups. The largest muscle fiber cross-sectional area was observed in the breast muscle of SG broilers (3,085.26 μm2), whereas the smallest was found in GAS and SOL muscle in FG broilers (approximately 1,100 μm2). The fiber diameter of the breast muscle was significantly larger than that of the leg muscle in both SG and FG broilers (P < 0.05). When comparing the same muscular tissue in the 2 breeds, SG broilers had fibers with a larger cross-sectional area than FG broilers (P < 0.05). Additionally, the muscle fibers were more closely aligned with less extracellular space in SG broilers than in FG broilers (Figure 1A). The rule of fiber growth in the leg muscle was not the same in SG and FG broilers at their marketable ages. The cross-sectional area of SOL muscle was the largest among leg muscles in SG broilers; however, it was the smallest in FG broilers. Overall, the cross-sectional area was larger in SG broiler muscle than in FG broiler muscle. The thicker fibers were observed in breast muscle than that of leg muscle in both breeds at their marketable ages.
Figure 1.
Muscle fiber morphology traits of different muscular tissues from slow- and fast-growing broilers. (A) Hematoxylin and eosin staining. Magnification of 400 × was used (Bar = 50 μm). (B) Muscle fiber diameter. (C) Muscle fiber cross-sectional area. Vertical bars represent mean ± standard error (n = 6). Statistically significant differences are indicated by different letters (P < 0.05). BM, breast muscle. SOL, soleus muscle. Abbreviations: EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle.
Comparison of Myosin Heavy Chain-Based Fiber Characteristics Between SG and FG Broilers
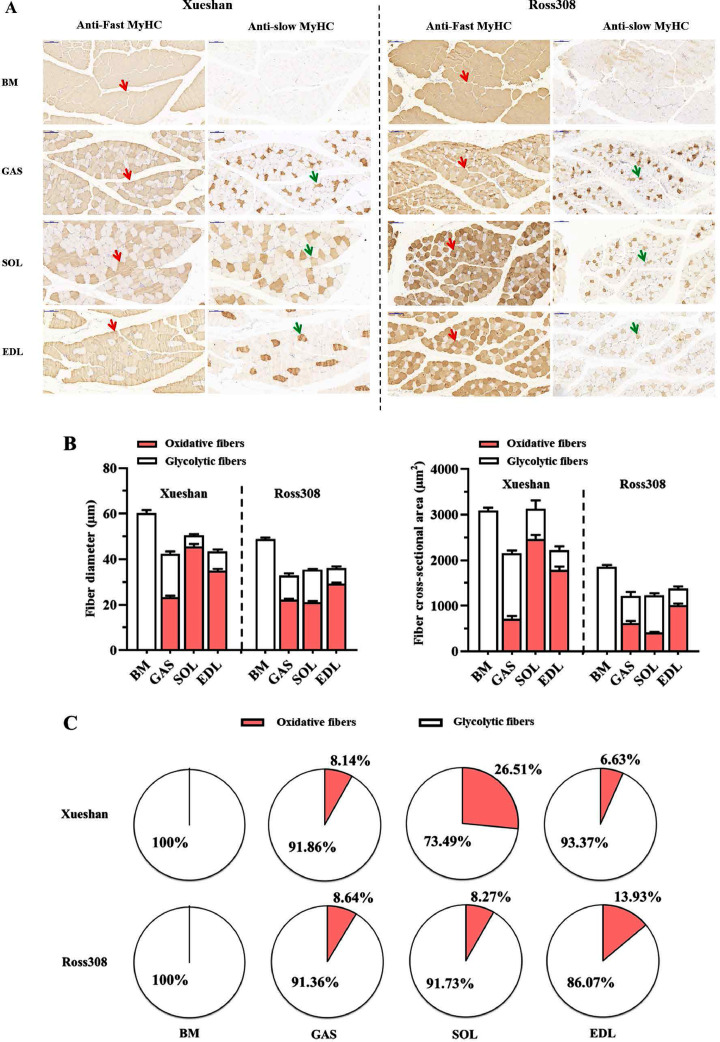
The relative area compositions of each fiber type were compared between the SG and FG broilers (Figures 2A and 2C). The variations in the fiber morphological traits between each fiber type were also investigated (Figure 2B). There was a significant interaction between factors (breeds and muscular tissues) for the fiber-type compositions. Breast muscle contained 100% glycolytic fibers and no oxidative fibers in either breed. However, all the muscle tissues in the leg muscle had oxidative fibers. The relative area composition of oxidative fibers in the SOL of SG broilers was higher (26.51%, P < 0.01) than that in other muscular tissues. FG broilers showed different trends in the leg muscle. There were more oxidative fibers in the EDL muscle (13.93%) than in the GAS and SOL muscles (8.64 and 8.27%, respectively). We also compared the fiber morphological traits between oxidative and glycolytic fibers. Higher fiber diameter was detected for glycolytic fibers than for oxidative fibers in all the muscular tissues. In summary, breast muscle contained 100% glycolytic fibers, whereas leg muscle contained both glycolytic and a few oxidative fibers in the 2 breeds. The relative area composition of oxidative fibers was the highest in the SOL muscle of SG broilers (26.51%). The sizes of the oxidative fibers were smaller than those of glycolytic fibers in all muscular tissues.
Figure 2.
The muscle fiber type composition of different muscular tissues from slow- and fast-growing broilers. (A) Immunohistochemical analyses for four muscular tissues using antifast and antislow myosin skeletal heavy chain. Red arrows point to examples of fibers with positive immunostaining for glycolytic fibers; Green arrows point to examples of fibers with positive immunostaining for oxidative fibers. Magnification of 200 × was used (Bar = 100 μm). (B) The muscle fiber diameter and cross-sectional area of glycolytic fibers and oxidative fibers in four muscular tissues of two breeds. (C) The muscle fiber type composition in four muscular tissues of two breeds. Abbreviations: BM, breast muscle; EDL, extensor digitorum longus muscle; GAS, gastrocnemius muscle; SOL, soleus muscle.
Comparison of Physical Properties of Meat From SG and FG Broilers
Representative physical properties were investigated to understand the variations in breast and leg meat from SG and FG broilers, including ultimate pH (pHu), meat color, shear force, and pressing loss (Table 1). Significant effects on shear force and pressing loss were observed for different breeds and muscular tissues. The breast meat was much tender than the leg meat in both SG and FG broilers (18.60 N vs. 24.01 N, P < 0.05). SG broiler meat possessed a higher shear force and a lower pressing loss than FG broiler meat (P < 0.05). In addition, there was a significant interaction between factors (breeds and muscular tissues) for pHu and meat color (L*, a*, and b*). The pHu value was significantly lower in the breast meat of SG broilers than in that of FG broilers. The highest lightness value was observed in the leg meat of FG broilers, but there were no significant differences among the other groups (P > 0.05). The highest redness and yellowness values were found in the leg and breast meat, respectively, of SG broilers. In summary, the breast and leg meat of SG broilers exhibited a higher shear force and lower pressing loss than those of FG broilers. The breast meat of SG broilers exhibited more yellowness and the leg meat of SG broilers exhibited more redness than those of other groups.
Table 1.
Comparison on physical properties of different muscular tissues from slow- and fast-growing broilers.
| Meat color |
Shear force (N) | Pressing loss (%) | |||||
|---|---|---|---|---|---|---|---|
| Item | pHu | L* | a* | b* | |||
| Xueshan | Breast | 6.07±0.04b | 46.35±1.59b | 2.38±0.51c | 13.84±1.44a | 21.16±2.40 | 17.94±1.99 |
| Leg | 6.34±0.07a | 44.73±1.89b | 6.59±1.51a | 10.73±1.41b | 25.17±2.17 | 16.14±3.42 | |
| Ross308 | Breast | 5.86±0.05c | 46.40±1.52b | 1.64±0.22c | 8.80±0.39c | 16.03±1.46 | 25.91±3.48 |
| Leg | 6.29±0.06a | 49.92±1.59a | 3.78±1.04b | 10.26±1.46bc | 22.86±1.66 | 22.61±2.66 | |
| Breeds | Xueshan | 6.21±0.15 | 45.54±1.86 | 4.48±2.46 | 12.28±2.12 | 23.17±3.02a | 17.04±2.80b |
| Ross308 | 6.07±0.23 | 48.16±2.37 | 2.71±1.33 | 9.53±1.27 | 19.44±3.89b | 24.26±3.40a | |
| Muscular tissues | Breast | 5.97±0.12 | 46.37±1.47 | 2.01±0.54 | 11.32±2.84 | 18.60±3.29b | 21.93±4.98 |
| Leg | 6.31±0.07 | 47.32±3.19 | 5.18±1.92 | 10.50±1.37 | 24.01±2.19a | 19.38±4.47 | |
| P-value (Two-way ANOVA) | |||||||
| Breeds | 0.000 | 0.003 | 0.001 | 0.000 | 0.001 | 0.000 | |
| Muscular tissues | 0.000 | 0.218 | 0.000 | 0.163 | 0.000 | 0.071 | |
| Breeds × Muscular tissues | 0.006 | 0.003 | 0.027 | 0.001 | 0.126 | 0.578 | |
Means ± SE with different superscript are significantly different in the same line (P < 0.05).
Comparison of Proximate Composition of Meat From SG and FG Broilers
The moisture, protein, IMF, and collagen contents were analyzed to compare the proximate composition of meat of SG and FG broilers (Table 2). Significant effects were observed for different breeds and muscular tissues on IMF content. The IMF content of breast and leg meat of SG broilers was lower than that of FG broilers (P < 0.05). Leg meat exhibited a higher IMF content than breast meat in the 2 breeds. In addition, there was a significant interaction between the factors (breeds and muscular tissues) for moisture content, protein content, and collagen content. The moisture content of breast meat of SG broilers was significantly lower than that of the other groups (P < 0.05). The highest protein content was found in the breast meat of SG broilers, and the lowest was detected in the leg meat of FG broilers. In contrast to the IMF content, leg meat exhibited lower protein content than breast meat in the 2 breeds. Furthermore, the lowest collagen content was observed in the breast meat of FG broilers, whereas there were no significant differences among the other groups (P > 0.05). Leg meat had a higher IMF content but lower protein content than breast meat in both breeds. The meat of SG broilers had higher protein content but a lower IMF content than that of FG broilers.
Table 2.
Comparison on proximate composition of different muscular tissues from slow- and fast-growing broilers.
| Item | Moisture content (%) | Protein content (%) | Intramuscular fat content (%) | Collagen content (%) | |
|---|---|---|---|---|---|
| Xueshan | Breast | 73.41±0.21b | 24.52±0.39a | 0.86±0.11 | 1.28±0.02a |
| Leg | 75.24±0.39a | 21.64±0.25c | 2.34±0.24 | 1.29±0.08a | |
| Ross308 | Breast | 74.94±0.37a | 22.55±0.23b | 1.62±0.41 | 1.15±0.05b |
| Leg | 75.12±0.67a | 20.96±0.50d | 3.18±0.52 | 1.32±0.05a | |
| Breeds | Xueshan | 74.33±1.01 | 23.08±1.55 | 1.60±0.80b | 1.28±0.05 |
| Ross308 | 75.03±0.51 | 21.76±0.91 | 2.40±0.93a | 1.24±0.10 | |
| Muscular tissues | Breast | 74.17±0.85 | 23.54±1.08 | 1.24±0.49b | 1.21±0.07 |
| Leg | 75.18±0.51 | 21.30±0.51 | 2.76±0.58a | 1.31±0.07 | |
| P-value (Two-way ANOVA) | |||||
| Breeds | 0.002 | 0.000 | 0.000 | 0.099 | |
| Muscular tissues | 0.000 | 0.000 | 0.000 | 0.001 | |
| Breeds × Muscular tissues | 0.001 | 0.001 | 0.791 | 0.004 | |
Means ± SE with different superscript are significantly different in the same line (P < 0.05).
DISCUSSION
At present, meat quality in broilers has raised great concern among consumers. Meat quality traits have also been considered for breeding and production. However, these traits are complex and are influenced by numerous factors. Meanwhile, there existed a few unfavorable genetic correlations between growth rate and meat quality traits. Therefore, it is necessary to evaluate chicken meat quality divergences and understand the mechanisms behind it.
Muscle fiber characteristics are closely related to fresh meat quality in poultry (Ismail and Joo, 2017). Therefore, we compared the fiber characteristics of SG and FG broilers. In both breeds, the breast muscle contained 100% glycolytic fibers. To the best of our knowledge, there have been no reports of oxidative fibers in the breast muscle of broilers. Petracci and Cavani (2012) stated that the glycolytic muscles of chickens and turkeys are capable of short bursts of activity for the “fight or flight” response. Further studies are needed to investigate why the breast muscle of broilers has no oxidative fibers. However, we detected significant differences in morphological traits. The cross-sectional area was larger in the breast muscle of SG broilers than in that of FG broilers. In addition, we found that the breast muscle grew faster than the leg muscle in both breeds. In the leg muscle, we detected the highest relative composition of oxidative fibers (26.51%) in SOL and lowest in EDL (6.63%) of SG broilers. The SOL muscle is often considered a pure oxidative fiber, whereas the EDL muscle is considered a pure glycolytic muscle in mice (Barclay et al., 1993). However, in FG broilers, the SOL muscle contained less oxidative fibers (8.64%) than that of EDL muscle (13.93%). We attribute the different trend of fiber type composition in FG broilers to the high-intensity selection of growth rate. Furthermore, we compared the fiber diameter and cross-sectional area between the oxidative fibers and glycolytic fibers. The sizes of the oxidative fibers were smaller than those of glycolytic fibers in all muscular tissues. Our results were consistent with the findings of Hwang et al. (2010), who showed that the diameters of type I (oxidative) fibers were significantly lower than those of type II (glycolytic) fibers in Korean native cattle.
A large proportion of oxidative fibers can positively affect muscle to meat conversion since oxidative fibers have slow, aerobic metabolism, which delays the postmortem metabolic rate (Lefaucheur, 2010). Kim et al. (2018) found that higher oxidative fibers and lower glycolytic fibers in biopsied muscle will improve pork quality. Wright et al. (2018) reported that a greater cross-sectional area of glycolytic fibers is associated with tougher steaks. Moreover, fibers of the same type but different sizes would also lead to different meat quality traits. Kim et al. (2013) reported that pig muscles with a higher percentage of large glycolytic fibers exhibit tougher, lighter, and more exudative meat than pig muscles with a higher proportion of small- or normal-sized glycolytic fibers. This study detected significant variations in fiber types or fiber morphological traits in the leg and breast muscles of SG and FG broilers. Therefore, we compared the physical properties of broiler meat. More yellowness, higher shear force, and lower pressing loss were observed in the breast muscle of SG broilers than in that of FG broilers. Since there was no difference in fiber types in breast muscle, the higher shear force and lower pressing loss in SG meat than in FG meat may be attributed to the larger muscle fibers with less extracellular space in the former. Similar results were obtained for pork; the muscle fibers of meat with a high drip loss were much smaller and less closely aligned than meat with a low drip loss (Hou et al., 2020). People in China tend to judge poultry products with high pigments as prolonged raising cycle meat with high nutrient levels. The breast muscle showed lower pHu and redness than the leg muscle in both breeds. We speculated that these phenomena were mainly caused by variations in the fiber type compositions in the breast and leg muscles. Oxidative fibers in the leg muscle use oxidative phosphorylation as a source of energy and have more mitochondria than glycolytic fibers. Oxidative fibers are also more vascularized and store more myoglobin in the sarcoplasm. This gives a reddish color to the leg meat. Glycolytic fibers contain more glycogen than oxidative fibers (Henckel et al., 2002). The higher percentage of glycolytic fibers in the breast muscle than in the leg muscle was related to an increase in the postmortem metabolic rate and a reduction in the pHu (Ryu and Kim, 2005). However, we did not detect PSE-like (pale, soft, and exudative, pHu <5.7 and lightness >53) meat in either breed (Alnahhas et al., 2014).
Finally, we analyzed the proximate composition of broiler meat. The breast meat of SG broilers contained higher protein and collagen contents, although lower IMF and moisture content. Meats rich in proteins are considered to exhibit good nutritional quality with a proportion of essential amino acids (Listrat et al., 2016). Collagen is associated with the texture of meat, which determines the toughness (El-Senousey et al., 2013). The chewier meat of SG broilers may be related to the higher collagen content. However, IMF content was significantly lower in SG broilers than in FG broilers. IMF content is often recognized as a key factor for meat flavor in cattle and swine (Aass et al., 2009; Schwab et al., 2009), and it directly influences consumers’ preferences. However, IMF is not visible in poultry products and is much less common than in livestock. Therefore, it is necessary to incorporate IMF into breeding programs to improve chicken meat quality. In addition, we found that the leg muscle contained more IMF content than the breast muscle in the 2 breeds. Hwang et al. (2010) found that oxidative muscles contain higher IMF and phospholipid contents than glycolytic muscles, which may explain our results. Moreover, skeletal muscle is an extremely plastic tissue (Honda et al., 2019). Zhang et al. (2017) showed that the overexpression of PGC-1α induces the conversion of fast glycolytic fibers to oxidative fibers, which could regulate the content of IMF in the PGC-1α transgenic pig. Still in pigs, conjugated linoleic acid can improve the meat quality by altering the expression of fiber type-related genes (Huang et al., 2014). In addition, endurance exercise training increases oxidative metabolism which can remodel fiber types (Lessard et al., 2018). In summary, genetic engineering or increasing the exercise amount of broilers may be potential strategies for fiber type regulation, further improving meat quality.
Based on these results, we conclude that breast muscle contains 100% glycolytic fibers, whereas leg muscle contains both glycolytic and oxidative fibers in the 2 breeds. Thicker muscle fibers exist in SG broilers than in FG broiler and more oxidative fibers are found in the leg muscle than in the breast muscle, thus contributing to increased firmness, redness, and IMF content. This study might provide perspectives for the genetic improvement of poultry meat quality traits by controlling skeletal muscle fiber characteristics.
ACKNOWLEDGMENTS
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province [grant number KYCX21_3262] and the earmarked fund for Jiangsu Agricultural Industry Technology System [grant number JATS(2020)6]
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Aass L., Fristedt C.G., Gresham J.D. Ultrasound prediction of intramuscular fat content in lean cattle. Livest. Sci. 2009;125:177–186. [Google Scholar]
- Alnahhas N., Berri C., Boulay M., Baéza E., Jégo Y., Baumard Y., Le Bihan-Duval E. Selecting broiler chickens for ultimate pH of breast muscle: analysis of divergent selection experiment and phenotypic consequences on meat quality, growth, and body composition traits. J Anim. Sci. 2014;92:3816–3824. doi: 10.2527/jas.2014-7597. [DOI] [PubMed] [Google Scholar]
- Anderson S. Determination of fat, moisture, and protein in meat and meat products by using the FOSS FoodScan Near-Infrared Spectrophotometer with FOSS Artificial Neural Network Calibration Model and Associated Database: collaborative study. J. AOAC Int. 2007;90:1073–1083. [PubMed] [Google Scholar]
- Barclay C.J., Constable J.K., Gibbs C.L. Energetics of fast- and slow-twitch muscles of the mouse. J. Physiol. 1993;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabault M., Baéza E., Gigaud V., Chartrin P., Chapuis H., Boulay M., Le Bihan-Duval E. Analysis of a slow-growing line reveals wide genetic variability of carcass and meat quality-related traits. BMC Genet. 2012;13:90. doi: 10.1186/1471-2156-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Zotte A., Gleeson E., Franco Ruiz D., Cullere M., Lorenzo J.M. Proximate composition, amino acid profile, and oxidative stability of slow-growing indigenous chickens compared with commercial broiler chickens. Foods. 2020;9:546. doi: 10.3390/foods9050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devatkal S.K., Naveena B.M., Kotaiah T. Quality, composition, and consumer evaluation of meat from slow-growing broilers relative to commercial broilers. Poult. Sci. 2019;98:6177–6186. doi: 10.3382/ps/pez344. [DOI] [PubMed] [Google Scholar]
- Du Y.F., Ding Q.L., Li Y.M., Fang W.R. Identification of differentially expressed genes and pathways for myofiber characteristics in soleus muscles between chicken breeds differing in meat quality. Anim. Biotechnol. 2017;28:83–93. doi: 10.1080/10495398.2016.1206555. [DOI] [PubMed] [Google Scholar]
- El-Senousey H.K., Fouad A.M., Yao J.H., Zhang Z.G., Shen Q.W. Dietary alpha lipoic Acid improves body composition, meat quality and decreases collagen content in muscle of broiler chickens. Asian Austral. J. Anim. 2013;26:394–400. doi: 10.5713/ajas.2012.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckel P., Karlsson A., Jensen M.T., Oksbjerg N., Petersen J.S. Metabolic conditions in Porcine longissimus muscle immediately pre-slaughter and its influence on peri- and post mortem energy metabolism. Meat Sci. 2002;62:145–155. doi: 10.1016/s0309-1740(01)00239-x. [DOI] [PubMed] [Google Scholar]
- Honda M., Tsuchimochi H., Hitachi K., Ohno S. Transcriptional cofactor Vgll2 is required for functional adaptations of skeletal muscle induced by chronic overload. J. Cell Physiol. 2019;234:15809–15824. doi: 10.1002/jcp.28239. [DOI] [PubMed] [Google Scholar]
- Hou X., Liu Q., Meng Q., Wang L., Yan H., Zhang L., Wang L. TMT-based quantitative proteomic analysis of porcine muscle associated with postmortem meat quality. Food Chem. 2020;328 doi: 10.1016/j.foodchem.2020.127133. [DOI] [PubMed] [Google Scholar]
- Huang J.X., Qi R.L., Chen X.L., You X.Y., Liu X.Q., Yang F.Y., Liu Z.H. Improvement in the carcass traits and meat quality of growing-finishing Rongchang pigs by conjugated linoleic acid through altered gene expression of muscle fiber types. Genet. Mol. Res. 2014;13:7061–7069. doi: 10.4238/2014.March.24.25. [DOI] [PubMed] [Google Scholar]
- Huo W., Weng K., Gu T., Zhang Y., Zhang Y., Chen G., Xu Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y.-H., Kim G.-D., Jeong J.-Y., Hur S.-J., Joo S.-T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010;86:456–461. doi: 10.1016/j.meatsci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Ismail I., Joo S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017;37:873–883. doi: 10.5851/kosfa.2017.37.6.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kim G.-D., Jeong J.-Y., Jung E.-Y., Yang H.-S., Lim H.-T., Joo S.-T. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013;94:267–273. doi: 10.1016/j.meatsci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Kim J.-M., Lim K.-S., Ko K.-B., Ryu Y.-C. Estimation of pork quality in live pigs using biopsied muscle fibre number composition. Meat Sci. 2018;137:130–133. doi: 10.1016/j.meatsci.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Lefaucheur L. A second look into fibre typing - Relation to meat quality. Meat Sci. 2010;84:257–270. doi: 10.1016/j.meatsci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lessard S.J., MacDonald T.L., Pathak P., Han M.S., Coffey V.G., Edge J., Goodyear L.J. JNK regulates muscle remodeling via myostatin/SMAD inhibition. Nat. Commun. 2018;9:3030. doi: 10.1038/s41467-018-05439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Bugeon J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016;2016 doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner A.C., Newberry R.C., Vas J., Mullan S. Slow-growing broilers are healthier and express more behavioural indicators of positive welfare. Sci. Rep. 2020;10:15151. doi: 10.1038/s41598-020-72198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B.C., Oshima I., Miyachi H., Shiba N., Nishimura S., Tabata S., Iwamoto H. Effects of nutritional level on muscle development, histochemical properties of myofibre and collagen architecture in the pectoralis muscle of male broilers. Br. Poult. Sci. 2006;47:433–442. doi: 10.1080/00071660600828334. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Schwab C.R., Baas T.J., Stalder K.J., Nettleton D. Results from six generations of selection for intramuscular fat in Duroc swine using real-time ultrasound. I. Direct and correlated phenotypic responses to selection. J. Anim. Sci. 2009;87:2774–2780. doi: 10.2527/jas.2008-1335. [DOI] [PubMed] [Google Scholar]
- Singh M., Lim A.J., Muir W.I., Groves P.J. Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdiglione R., Cassandro M. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult. Sci. 2013;92:2433–2437. doi: 10.3382/ps.2013-03013. [DOI] [PubMed] [Google Scholar]
- Wang L.-D., Zhang Y., Kong L.-I., Wang Z.-I., Bai H., Jiang Y., Chen G.-H. Effects of rearing system (floor vs. cage) and sex on performance, meat quality and enteric microorganism of yellow feather broilers. J. Integr. Agric. 2021;20:1907–1920. [Google Scholar]
- Wen C., Jiang X.Y., Ding L.R., Wang T., Zhou Y.M. Effects of dietary methionine on growth performance, meat quality and oxidative status of breast muscle in fast- and slow-growing broilers. Poult. Sci. 2017;96:1707–1714. doi: 10.3382/ps/pew432. [DOI] [PubMed] [Google Scholar]
- Weng K., Huo W., Gu T., Bao Q., Hou L.-E., Zhang Y., Chen G. Effects of marketable ages on meat quality through fiber characteristics in the goose. Poult. Sci. 2021;100:728–737. doi: 10.1016/j.psj.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund S.E., Homco-Ryan C., Ryan K.J., McKeith F.K., McFarlane B.J., Brewer M.S. Aging and enhancement effects on quality characteristics of beef strip steaks. J. Food Sci. 2005;70:S242–S248. [Google Scholar]
- Wright S.A., Ramos P., Johnson D.D., Scheffler J.M., Elzo M.A., Mateescu R.G., Scheffler T.L. Brahman genetics influence muscle fiber properties, protein degradation, and tenderness in an Angus-Brahman multibreed herd. Meat Sci. 2018;135:84–93. doi: 10.1016/j.meatsci.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhou Y., Wu W., Hou L., Chen H., Zuo B., Yang J. Skeletal muscle-specific overexpression of PGC-1α induces fiber-type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int. J. Biol. Sci. 2017;13:1152–1162. doi: 10.7150/ijbs.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]