Abstract
A case of mycotic keratitis due to Curvularia senegalensis is reported. This case represents the third known reported infection caused by this rare species. Fungal hyphae were detected in corneal scrapings, and repeated cultures were positive for this fungi. The patient was presumed cured after a corneal transplant and treatment with itraconazole, but the infection recurred and the patient is waiting for a keratoplasty. The in vitro antifungal susceptibilities of the case strain and another 24 strains belonging to seven species of Curvularia were tested for six antifungal agents. With the exception of flucytosine, and occasionally fluconazole, the other drugs assayed (amphotericin B, miconazole, itraconazole, and ketoconazole) were highly effective in vitro.
Keratitis caused by filamentous fungi, such as Fusarium, Aspergillus, Acremonium, and Curvularia species, is relatively frequent (11, 21) and is usually induced by trauma. We report a case of human keratitis due to Curvularia senegalensis without known injury or trauma. C. senegalensis causing fungal keratitis has only been reported once before, approximately 20 years ago (10).
Case report.
A 38-year-old Brazilian housewife presented at the Institute Benjamin Constant, Rio de Janeiro, Brazil, on 14 July 1997. She complained of a painful, inflammation in her right eye, which was similar to conjunctivitis. The problem appeared spontaneously. One week earlier, she had noticed a white spot on the cornea and visited an ophthalmologist, who treated her with a combination of antibiotics and corticosteroid eye drops and sent her to the institute. She had previously suffered from pulmonary tuberculosis, and the treatment had ended 4 months before, but she had no history of preceding ocular trauma or previous eye disease. Examination revealed a visual acuity of 20/50 (acuity tested by patient counting fingers held up by examination 1 m away) and the presence of a corneal ulcer with diffuse infiltration through the corneal parenchyma. On 22 July 1997, she was examined in the Cornea Department and found to have a corneal abscess of approximately 8 mm in diameter, and treatment was initiated with 1% atropine eye drops twice daily. Deep corneal epithelium scrapings were obtained with a Kimura's spatula for Giemsa and Gram stains and culture.
Microscopic examination of corneal scrapings was positive for fungal elements (Fig. 1). Scrapings stained with Giemsa revealed abundant septate, darkly pigmented hyphae. On 12 August 1997, a new examination revealed a worsened eye condition, and pimaricin (5%) every 4 h was added to the treatment regiment. A corneal transplant was performed on 25 August 1997, and preoperatory complementary tests showed that the patient had diabetes mellitus (glycemia, 360 mg/dl). On 14 August 1997, inflammation recurred in the anterior chamber with pupillary block and hypopyon and hyperemic conjunctiva. Treatment with itraconazole (100 mg twice daily) and systemic corticoids was initiated. By 11 November 1997, the patient had improved substantially, and there was no more inflammation. She was discharged. On 9 December 1997, the patient returned, complaining of visual acuity reduction and, under examination, a uveal tract reaction, which was characterized by precipitates on the posterior surface of the cornea, was observed. Pred-fort and atropine drops were prescribed. On 28 January 1998, she had intense conjunctival hyperemia (injection), smarting, and cataract complication, still with uveal reaction. On 17 March 1998, she presented secondary glaucoma by pupil obstruction. She did not come back until 26 June 1998, when she showed a fetid yellowish secretion, a new corneal ulcer in the transplanted cornea, conjunctival injection (or hyperemia), and eyelid edema. A recurrence of the fungus was suspected, and ulcer scrapings were collected for new culture. The patient is still awaiting a new keratoplasty.
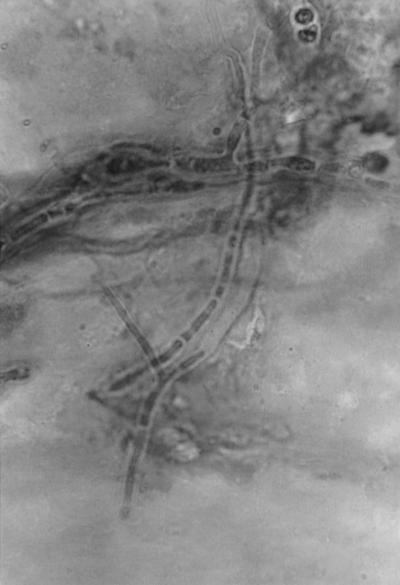
FIG. 1.
Potassium hydroxide wet preparation of the corneal scrapings. Magnification, ×960.
Portions of the scrapings obtained on both occasions were inoculated on plates of Sabouraud dextrose agar (with and without penicillin [20 U/ml], streptomycin [40 U/ml], and cycloheximide [0.5 mg/ml]), blood agar, and brain heart infusion agar. On all plates, an apparently identical grayish black mold developed with numerous thick-walled, septate conidia, borne on simple, club-shaped conidiophores. Both conidia and mycelium were dematiaceous. The fungal isolates were referred to the Microbiology Unit of the Rovira i Virgili University in Reus, Spain, for identification and an antifungal susceptibility study.
Morphological study.
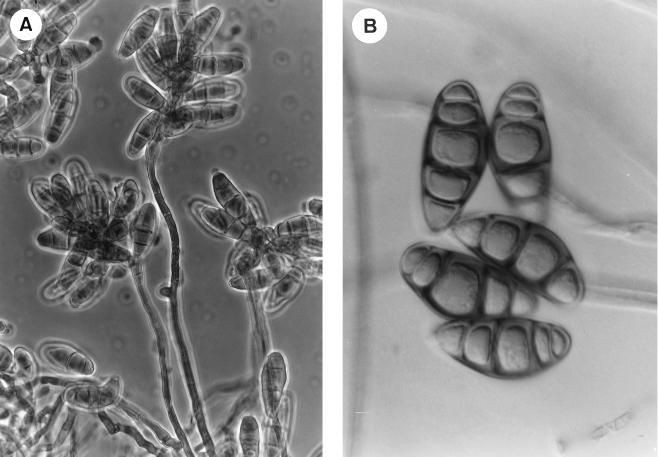
The isolates were subcultured on potato carrot agar (PCA) and oatmeal agar (OA), and incubated at ca. 25°C in the dark. After 7 days on PCA, the colonies were dark brown and velvety but loose cottony at the center and 60 to 62 mm in diameter. On OA, the macroscopic characteristics of the fungus were similar to those on PCA, but it grew more rapidly, reaching 65 to 68 mm in diameter after 7 days. Sporulation was abundant on both media. Conidiophores usually grew directly on the substrate; they were simple or branched, straight or flexuous, smooth walled, up to 130 μm long and 4 to 6 μm wide (Fig. 2A). Conidia had three to five septa (mostly four septa) and were dark brown, with subhyaline or pale brown cells at each end, ellipsoidal or broadly fusiform, slightly curved, 22 to 31 μm long by 11 to 14 μm wide at the broadest part (Fig. 2B). On the basis of these characteristics, the isolates were identified as C. senegalensis (Fig. 3). Curvularia geniculata, another species involved in some cases of keratomycosis (6), also has conidia, mostly with four septa, but they are markedly geniculate with the central cell usually very different from the rest (dark brown and swollen). The other reported pathogenic species of Curvularia, i.e., C. brachyspora, C. clavata, C. lunata, C. pallescens, and C. verruculosa, usually have conidia with three septa, which also differ from those of C. senegalensis mainly by their color, shape, size, or ornamentation. The two clinical isolates of C. senegalensis were kept in the Mycology Laboratory of the Faculty of Medicine in Reus, Spain, as FMR 6319 (first isolate) and FMR 6666 (second isolate). A living culture of the former has been deposited in the Centraalbureau voor Schimmelcultures, Baarn, The Netherlands, with the accession no. CBS 102171.
FIG. 2.
C. senegalensis FMR 6319. (A) Conidiophores bearing conidia photographed with phase-contrast optics. Magnification, ×384. (B) Conidia photographed with Nomarski optics. Magnification, ×1,200.
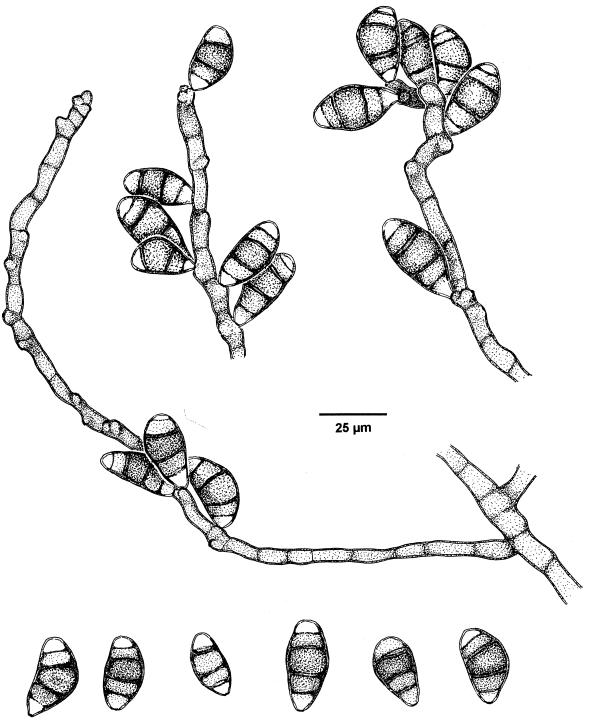
FIG. 3.
C. senegalensis conidiophores and conidia.
Antifungal susceptibility testing.
One of the case isolates (FMR 6319) and four additional isolates of C. brachyspora, three of C. clavata, four of C. geniculata, three of C. lunata, four of C. pallescens, two of C. senegalensis, and four of C. verruculosa from very diverse sources were tested to determine their susceptibility to antifungal drugs (Tables 1 and 2). Tests were accomplished by a previously described microdilution method (16) performed, where possible, according to the National Committee for Clinical Laboratory Standards' guidelines for filamentous fungi (14) by using RPMI 1640 medium buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid (MOPS), an inoculum of 4 × 102 to 4.5 × 104 CFU/ml, an incubation temperature of 30 or 35°C, reading the results after 2 or 3 days (48 or 72 h), and an additive drug dilution procedure.
TABLE 1.
In vitro susceptibilities of 25 isolates of Curvularia to six antifungal agents
| Antifungal | MIC (μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Amphotericin B | 0.06–32 | 0.250 | 0.5 |
| Fluconazole | 4–128 | 16 | 64 |
| Flucytosine | 128–256 | 256 | 256 |
| Itraconazole | 0.06–32 | 0.5 | 8 |
| Ketoconazole | 0.5–16 | 2 | 4 |
| Miconazole | 0.25–4 | 1 | 4 |
50% and 90%, MICs for which 50 and 90% of the isolates tested, respectively.
TABLE 2.
In vitro susceptibilities of Curvularia spp. to six antifungal agentsa
| Species | No. of isolates | AMB
|
MON
|
ITRA
|
KETO
|
FLU
|
5-FC
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | Range | MIC | Range | MIC | Range | MIC | Range | MIC | Range | MIC | Range | ||
| C. brachyspora | 4 | 0.29 | 0.06–2 | 0.84 | 0.250–4 | 0.70 | 0.125–32 | 0.84 | 0.5–4 | 22.62 | 4–128 | 180.9 | 128–256 |
| C. clavata | 3 | 0.31 | 0.250–0.5 | 1 | 1–2 | 0.19 | 0.125–0.5 | 0.62 | 0.5–1 | 16 | 16 | 256 | 256 |
| C. geniculata | 4 | 0.12 | 0.06–0.250 | 1.41 | 0.5–4 | 0.21 | 0.125–0.5 | 1 | 0.5–2 | 15.99 | 4–32 | 180.92 | 128–256 |
| C. lunata | 3 | 0.12 | 0.06–0.5 | 1 | 1–4 | 0.41 | 0.06–8 | 1 | 0.250–4 | 26.89 | 16–128 | 180.92 | 128–256 |
| C. pallescens | 4 | 0.41 | 0.06–32 | 1.99 | 0.55–4 | 2.82 | 0.5–16 | 4.70 | 0.06–32 | 76.07 | 16–128 | 215.15 | 128–256 |
| C. senegalensis | 3 | 0.50 | 0.250–2 | 1 | 1–2 | 0.50 | 0.25–1 | 1.41 | 1–2 | 50.77 | 16–128 | 203.07 | 128–256 |
| C. verruculosa | 4 | 0.25 | 0.125–0.5 | 1.41 | 1–2 | 0.70 | 0.5–2 | 1.18 | 0.5–2 | 13.45 | 4–64 | 180.92 | 128–256 |
Antifungal agents are abbreviated as follows: AMB, amphotericin B; MON, miconazole; ITRA, itraconazole; KETO, ketoconazole; FLU, fluconazole; 5-FC, flucytosine. Geometric mean MICs (expressed in micrograms per milliliter) are shown.
The MICs of the six antifungal agents against the clinical isolate (FMR 6319) were as follows: 0.25 μg/ml for amphotericin B, 1 μg/ml for miconazole and ketoconazole, 0.25 μg/ml for itraconazole, 16 μg/ml for fluconazole, and 256 μg/ml for 5-fluorocytosine. This is the greatest number of species of Curvularia that has been tested so far in a single study for in vitro antifungal susceptibility. Tables 1 and 2 show the antifungal susceptibility results of the 25 isolates. In contrast to the results of You et al. (22), amphotericin B, itraconazole, miconazole, and ketoconazole were highly effective against almost all the species tested. Generally, C. pallescens displayed the highest MICs. These results are in agreement with those of Bent and Kuhn (2), who tested five strains of Curvularia spp. obtained from sinus aspirates from allergic fungal sinusitis patients. All five strains were sensitive to amphotericin B, ketoconazole, and nystatin; two strains were sensitive to itraconazole, and all were resistant to fluconazole. In addition, Sutton et al. (20) reported C. lunata as sensitive to practically all the antifungals available, with the exception of flucytosine. However, despite the fact that in vitro resistance to a particular agent does provide valuable information in selecting a proper antifungal agent for treatment, these results should be interpreted with caution because in vitro studies are only limited approximations of the in vivo situations. Studies of correlation of in vitro data with clinical outcome are needed for a more definitive evaluation of the predictive value of MICs for filamentous fungi.
The genus Curvularia comprises about 30 species. It was widely studied and monographed by Ellis (8, 9) and Sivanesan (19). Its telomorphs are included in the ascomycete genus Cochliobolus. Most of the species are pathogens of grains and plants common to tropical areas. They are also commonly found in agricultural soils (7). Curvularia spp. are darkly pigmented fungi with conidia efficiently adapted to aerial dispersal (7). They are, therefore, habitual components of air mycobiota and have a worldwide distribution.
Curvularia species had been previously considered nonpathogens or thought to affect humans only rarely, but these fungi are now increasingly being reported to cause human disease (3, 22). Seven species of Curvularia have been involved in human infections. They are morphologically very similar with the differences mainly in the conidial features (size, number of septa, shape, and ornamentation) (6). In human pathology, Curvularia spp. are frequently associated with allergic sinusitis (12), although several cases of ocular infections such as keratitis (1, 15) or endophthalmitis (17) have also been reported. Of the approximately 50 reported cases of Curvularia infection, approximately a third were keratitis. C. lunata is the species most commonly found in clinical specimens. Curvularia infections are usually acquired by either direct inoculation or inhalation (22). However, in the case reported here, there was no evidence of trauma to explain how or when the fungus entered the cornea. The only predisposing factor was that the patient was diabetic and had initially used eye drops containing steroids. Marcus et al. (13) also reported a case of Curvularia keratitis without trauma. Fungal keratitis without evidence of trauma has rarely been reported (5).
C. senegalensis was reported more than 20 years ago as the cause of two cases of keratitis (10). These patients were treated topically with 5% pimaricin suspensions, but little was reported about the evolution of the lesions. This species has also been involved in two cases of allergic bronchopulmonary disease (22). The management of Curvularia infections usually involves surgical treatment with or without the use of antifungal agents. Some patients have been successfully treated with amphotericin B (22). Despite their in vitro efficacy, therapy with azole agents, such as miconazole and ketoconazole, has been disappointing due to the frequent recurrence of infection (22). Itraconazole (18) and terbinafine (4) have also been used successfully. For localized infections such as sinusitis or keratitis, surgical treatment alone may be adequate (22). Our patient appeared to recover successfully after a cornea transplant and treatment with itraconazole for 3 months, which was also effective in vitro. However, a recurrence of the infection was probably due to remaining small hyphal elements which were not completely eliminated within 3 months of treatment with itraconazole at a dose of 200 mg/day.
REFERENCES
- 1.Abdul-Samad S, Salleh M M, Gurunathan A, Salaton M. Laboratory diagnosis of keratomycosis. Trop Biomed. 1996;13:25–27. [Google Scholar]
- 2.Bent J P, Kuhn F A. Antifungal activity against allergic fungal sinusitis organisms. Laryngoscope. 1996;106:1331–1334. doi: 10.1097/00005537-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Berg D, García J A, Schell W A, Perfect J R, Murray J R. Cutaneous infection caused by Curvularia pallescens: a case report and review of the spectrum of disease. J Am Acad Dermatol. 1995;32:375–378. doi: 10.1016/0190-9622(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 4.Bryan C S, Smith C W, Berg D E, Karp R B. Curvularia lunata endocarditis treated with terbinafine: case report. Clin Infect Dis. 1993;16:30–32. doi: 10.1093/clinids/16.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Cepero de García M C, Arboleda M L, Barraquer F, Grose E. Fungal keratitis caused by Metarhizium anisopliae var. anisopliae. J Med Vet Mycol. 1997;35:361–363. [PubMed] [Google Scholar]
- 6.de Hoog G S, Guarro J, editors. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 7.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. I. London, United Kingdom: Academic Press; 1980. [Google Scholar]
- 8.Ellis M B. Dematiaceous hyphomycetes. VII. Curvularia brachyspora. Mycol Pap. 1966;106:1–57. [Google Scholar]
- 9.Ellis M B. Dematiaceous hyphomycetes. Kew, United Kingdom: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 10.Forster R K, Rebell G, Wilson L A. Dematiaceous fungal keratitis. Clinical isolates and management. Br J Ophthalmol. 1975;59:372–376. doi: 10.1136/bjo.59.7.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarro J, Gams W, Pujol I, Gené J. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin Infect Dis. 1997;25:1222–1229. doi: 10.1086/516098. [DOI] [PubMed] [Google Scholar]
- 12.Kinsella J B, Bradfield J J, Gourley W K, Calhoun K H, Rassekh C H. Allergic fungal sinusitis. Clin Otolaryngol. 1996;21:389–392. doi: 10.1046/j.1365-2273.1996.00807.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcus L, Vismer H F, van der Hoven H J, Gove E, Meewes P. Mycotic keratitis caused by Curvularia brachyspora (Boedjin). A report of the first case. Mycopathologia. 1992;119:29–33. doi: 10.1007/BF00492227. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 15.Panda A, Sharma N, Das G, Kumar N, Satpathy G. Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea. 1997;16:295–299. [PubMed] [Google Scholar]
- 16.Pujol I, Guarro J, Llop C, Soler L, Fernández-Ballart J. Comparison of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2103–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satpathy G, Vishalakshi P. Microbiology of infectious endophthalmitis: a 3-year study. Ann Ophthalmol. 1997;29:50–53. [Google Scholar]
- 18.Sharkey P K, Graybill J R, Rinaldi M G, Stevens D A, Tucker R M, Peterie J D, Hoeprich P D, Greer D L, Frenkel L, Counts G W, Goodrich J, Zellner S, Bradsher R W, van der Horst C M, Israel K, Pankey G A, Barranco C P. Itraconazole treatment of phaeohyphomycosis. J Am Acad Dermatol. 1990;23:577–586. doi: 10.1016/0190-9622(90)70259-k. [DOI] [PubMed] [Google Scholar]
- 19.Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap. 1987;158:1–261. [Google Scholar]
- 20.Sutton D A, Fothergill A W, Rinaldi M G. Guide to clinically significant fungi. Baltimore, Md: Williams & Wilkins; 1998. [Google Scholar]
- 21.Thomas P A. Mycotic keratitis—an underestimated mycosis. J Med Ved Mycol. 1994;32:235–256. doi: 10.1080/02681219480000321. [DOI] [PubMed] [Google Scholar]
- 22.You Y C W, de Nanassy J, Summerbell R C, Matlow A G, Richardson S E. Fungal sternal wound infection due to Curvularia lunata in a neonate with congenital heart disease: case report and review. Clin Infect Dis. 1994;19:735–740. doi: 10.1093/clinids/19.4.735. [DOI] [PubMed] [Google Scholar]