Abstract
As an important olive component, hydroxytyrosol (HT) has good medicinal and health effects. However, its importance in alleviating immune suppression in broilers has not been established. Therefore, we aimed at evaluating the immunomodulatory and antioxidant effects of HT in immune suppressed broilers. Immune suppressed broiler models were established via intraperitoneal injection of 80 mg/kg cyclophosphamide (Cy). Thirty two Cobb 500 male broilers were randomly allocated into 4 groups of 8 each. Broilers in the model (Cy) and HT treatment (Cy+HT) groups were intraperitoneally administered with Cy (80 mg/kg BW) once a day for 3 d. From the 4th d, broilers in the Cy+HT and HT groups were treated with 0.5 mL of 200 mg/L HT solution, once a day, for 7 d. The Cy and Con groups were orally administered with normal saline. On the 14th and 28th d, serum and duodenal samples were obtained for testing. It was found that HT increased villi height (VH)/crypt depth (CD) ratio in the duodenum and suppressed serum tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels. Moreover, it elevated the expressions of CD4+ and CD8+ T lymphocytes. HT upregulated the mRNA expression levels of interleukin-2 (IL-2), interleukin-4 (IL-4), and interleukin-10 (IL-10), enhanced the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and downregulated malondialdehyde (MDA) levels in Cy-induced immune-suppressed broilers. In conclusion, HT can alleviate immune-suppression as well as enhance immunity and antioxidant activities in the local mucosa of small intestines in broilers. Therefore, it can be used as an immune stimulant. More studies should be performed to confirm our findings and to elucidate on the mechanisms of HT.
Key words: hydroxytyrosol, cyclophosphamide, broilers, intestinal immunity, antioxidant
INTRODUCTION
Olive contains a large number of polyphenolic compounds, such as oleuropein and hydroxytyrosol (HT) (Rigacci and Stefani, 2016), which have high nutritional and economic values. Olive leaves are important feed sources, and the nutritional values of olive by-products have been evaluated (Yáñez-Ruiz and Molina-Alcaide, 2007; Poudyal et al., 2010). In broilers, feed supplementation with 10% fermented olive leaves promotes protein utilization in feeds (Obikaonu et al., 2012). The chemical name of HT is 3, 4-dihydroxy phenylethanol and it exhibits a catechol structure. HT has strong antioxidant and immune-enhancing effects (Briante et al., 2001). In olive branches, leaves, fruits, oils, and related by-products, it exists in form of esters. Nousis et al. (2005) found that hydrogen peroxide caused oxidative damage to Jurkat T-lymphocytes. However, at 100 μM, HT significantly protected the Jurkat cells from oxidative damage. Moreover, increasing HT doses to millimole levels had no adverse effects on cells. The other beneficial effects of HT include promotion of immunity, antimicrobial, antiviral, antitumor, antiradiation, antiaging, and anti-inflammatory effects, lowering of blood lipid levels, and improving animal performance (Mulinacci et al., 2001; Allouche et al., 2004; Killeen et al., 2011). Therefore, we hypothesized that HT plays a key role in increasing ketone body muscle percentage.
Various factors, such as immune-suppression due to infection and stress, are associated with increased mortality rates, disease susceptibility and growth retardation. Cyclophosphamide (Cy), an alkylation agent, is a commonly used immunosuppression-inducing drug. Clinically, it is used in tumor and autoimmune disease treatment (Mirkes, 1985; Shirani et al., 2015; Kumar and Venkatesh, 2016). However, Cy is associated with serious adverse effects. It can reduce white blood cell counts by inhibiting hematopoiesis, which may have deleterious effects on animal life (Basu et al., 2017). In addition, the inhibitory effects of Cy on oxidative stress and immunity have been confirmed (Paul et al., 2011; Lapointe et al., 2016).
Intestinal mucosal immunity is an important component of the immune system. After hatching, chicks rely on maternal antibodies to provide immune protection, however, due to their susceptibility to pathogens, it is important to improve the immune capacities of chicks and enhance their intestinal mucosal immunity (Faulkner et al., 2013). Despite vaccinations, infectious diseases, especially acute infectious diseases with chronic disease courses, are still prevalent, which greatly affect poultry production. In addition, drug abuse, stress, and other diseases are important causes of immune-suppression in chicken. HT has a simple structure, a small molecular weight, is nontoxic, has no side effects, and has various pharmacological activities. Its antitumor activities and mechanisms have been evaluated (Granados-Principal et al., 2011 ). However, immune regulatory and antioxidant properties of HT in the duodenum of Cy-induced immune-suppressed chicken have not been evaluated. Therefore, we aimed at elucidating the effects of HT on immune functions and the mechanisms involved in intestinal mucosal immune regulation by assessing morphological changes in the duodenum, immune levels, cytokines, and oxidation indices of chicken after intragastric administration of HT. Our findings provide a theoretical basis for understanding the immune regulatory and antioxidant mechanisms of HT.
MATERIALS AND METHODS
Experimental Animals and Ethics Statement
Thirty-two 7 d Cobb 500 male broilers were purchased from the chicken farm of Yunnan Agriculture University. During acclimatization, broilers were kept in an air-conditioned room with a 12/12 h light/dark cycle at 37°C after which the temperature was gradually reduced to room temperature. The daylight test was performed in strict accordance with regulations of the ethics committee. Broilers were provided with free water and standard diet. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Guizhou University, Guizhou, China (EAE-GZU-2021-T096).
Immunosuppressive broiler models were established by intraperitoneal injection of 80 mg/kg Cy. Thirty two male broilers were randomized into the control (Con), cyclophosphamide model (Cy), Cy and hydroxytyrosol (HT) treatment (Cy+HT) as well as HT groups, 8 broilers per group. Broilers in the Cy and Cy+HT groups were intraperitoneally administered with Cy (80 mg/kg/d BW, Jiangsu Hengrui Medicine Co., Ltd., NO. 15073125) once a day for 3 d. From the 4th d, broilers in the Cy+HT and HT groups were treated with 0.5 mL HT solution (200 mg/L, HT net content 98%, Shanghai Yuanye Biotechnology Co. Ltd., China), once a day, for 7 d. The Cy and Con groups were orally administered with normal saline. On d 14 and 28, broilers were sacrificed by cervical dislocation for sample collection
Determination of Serum TNF-α and IL-6 Levels
Blood was collected from broiler wings for serum separation. Then, serum TNF-α and IL-6 levels were determined by ELISA kits (Shanghai Enzyme Link Biotechnology Co., Ltd., China), according to the manufacturer's instructions.
Morphological Evaluation of Duodenal Tissues
After broilers had been sacrificed, their duodenal tissues were obtained, sectioned, fixed in 4% paraformaldehyde, dehydrated using alcohol and embedded in paraffin. Duodenal tissues were sliced using a sledge microtome (Typ RM 2235, Leica, Germany) and stained by H&E. Histological observations were done using the Olympus CX43 microscope (Olympus Co., Ltd, Japan). The 3 longest villi heights (VH) and crypt depths (CD) for 3 sections in the obtained images from each group were measured using the Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD) to calculate the VH/CD ratios.
Immunohistochemistry for Expressions of CD4+ and CD8+ T Cells in the Duodenum
Paraffin blocks were sliced into 6 µm sections. Immunohistochemistry was performed using an Ultra-Sensitive SP kit-9701 and Diaminobenzidine (DAB) color liquid (Maixin Biotechnology products, Fuzhou, China), according to the manufacturer's instructions. Slices were incubated overnight at 4°C with Mouse Anti-Chicken CD4+ (NO. 8210-30, 1: 200) and Mouse Anti-Chicken CD8+ (NO. 8280-09, 1: 200) antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). Histological observations were done using the Olympus CX43 microscope (Olympus Co., Ltd, Japan) and the obtained images analyzed by Image Pro-Plus 6.0 software.
Flow Cytometry for CD8+ T Cells
On the 28th d after the last administration, blood was collected from under the broilers wings for lymphocyte isolation using the lymphocyte separation solution (Tianjin Hao Yang biological manufacture Co., Ltd, China). Cells were washed twice using pre-cooled PBS. Then, 400 μL 1 × binding buffer was added to make a single cell suspension after which cell concentrations were adjusted to about 1 × 106 /mL. Ten μL of mouse anti-chicken CD8α-PE antibody (NO. 8405-09, 1: 100, Southern Biotechnology Associates, Inc., Birmingham, AL) were added to the cell suspensions, gently mixed and incubated for 30 min at 4°C in the dark. Then, cells were washed using PBS and detected within 1 h by flow cytometry (Accuri C6, BD Company, Germany).
Quantitative Real Time-PCR
Table 1 shows the primer sequences (β-actin, IL-2, IL-4, and IL-10) for quantitative real time-PCR (qRT-PCR). Primers were synthesized by Sangon Biotech Corp (Shanghai, China). In this assay, mRNA were extracted from duodenal tissues to synthesize cDNA using the PrimeScript RT reagent kit (No. RR047A, TaKaRa, Dalian, China), according to the manufacturer's instructions. qRT-PCR reactions were performed using gene-specific primers, with SYBR Premix Ex Taq II (No. DRR820A, TaKaRa) according to the manufacturer's instructions. The qRT-PCR reaction conditions were: Pre-denaturation (94°C, 30 s), amplification ([95°C, 5 s; Tm, 20 s; 72°C, 30 s], 40 cycles). Relative mRNA expression level for each index gene was calculated using the 2−△△Ct method.
Table 1.
Specific primers for target genes.
| Gene | Primer (5’ -3’) | Fragment length |
|---|---|---|
| β-actin | F-TGATATTGCTGCGCTCGTTG | 143 |
| R-CTTTCTGGCCCATACCAACC | ||
| IL-2 | F-CAAGAGTCTTACGGGTCTAAATCAC | 100 |
| R-GTTGGTCAGTTCATGGAGAAAATC | ||
| IL-4 | F-GTGCCCACGCTGTGCTTAC | 82 |
| R-AGGAAACCTCTCCCTGGATGTC | ||
| IL-10 | F-TAAGGACTATTTTCAATCCAGGG | 142 |
| R-ACGGGGCAGGACCTCATC |
Analysis of SOD, GSH-Px, and MDA Activities
Duodenal tissues (0.6 g) were homogenized in 5.4 mL saline to make 10% tissue homogenates. The SOD, GSH-Px, and MDA activities in tissue homogenates were measured at 490 nm using an ELx800 Absorbance Microplate Reader (BioTek Instruments, Winooski, VT), according to the manufacturer's instructions (SOD, No. A001-3-1; GSH-Px No. A005-1-2; MDA No. A003-1-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical Analysis
Data are presented as mean ± SD for n = 3. Comparisons of means among groups were done by one-way ANOVA, followed by Duncan's post hoc test. SPSS 23.0 was used for statistical analyses (SPSS Science, Chicago, IL). Differences were regarded as significant at P ≤ 0.05 and highly significant at P ≤ 0.01.
RESULTS
Serum TNF-α and IL-6 Levels
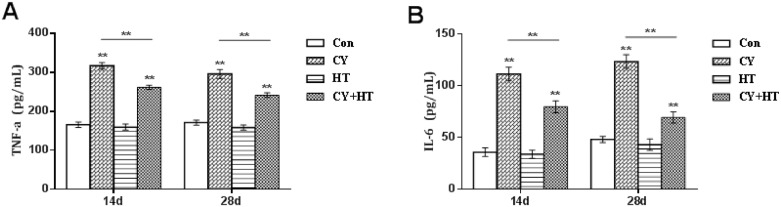
To evaluate the effects of HT on immune responses, serum levels of TNF-α and IL-6 were assayed by ELISA. Figure 1 shows that, compared to the Con group, serum TNF-α and IL-6 levels in the Cy group were significantly suppressed (P < 0.01). Compared to the Cy group, serum TNF-α, and IL-6 levels in the Cy+HT group were significantly suppressed (P < 0.01). Therefore, HT treatment significantly increased serum TNF-α and IL-6 levels in Cy-treated broilers.
Figure 1.
Effects of HT on serum TNF-α and IL-6 levels in chicken. (A) TNF-α; (B) IL-6. *Indicates minimum significant differences (P < 0.05), and ** indicates highly significant differences (P < 0.01). Abbreviations: HT, hydroxytyrosol; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
VH/CD Ratios
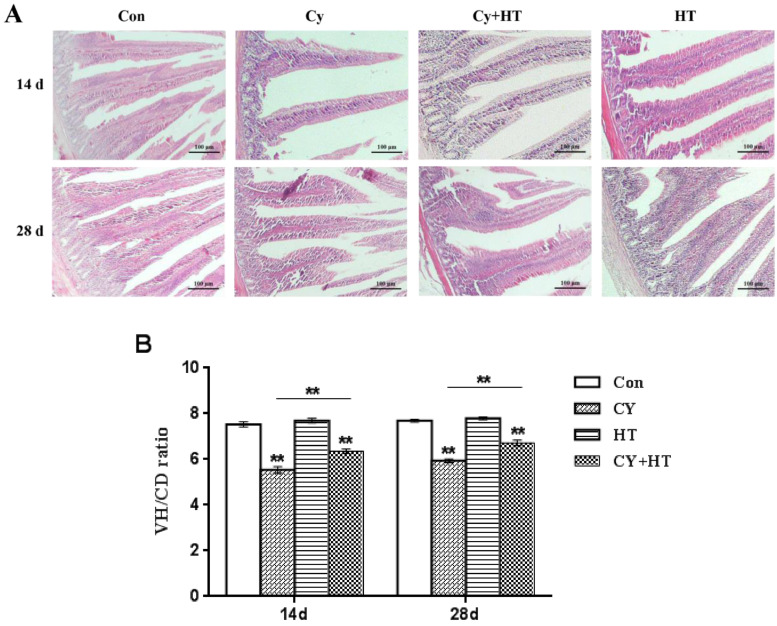
Duodenal structures after HT treatment are shown in Figure 2A. Villi from the Con and HY groups were neatly arranged while villi from the Cy group were short and unevenly arranged. Compared to the Cy group, duodenal villi were wider and longer in the Cy+HT group. Through H&E staining, chorionic gland ratios were determined to evaluate duodenal changes. Figure 2B shows that, on the 14th and 28th d after administration, VH/CD ratios in the Con group were significantly higher when compared to those of the Cy and Cy+HT groups (P < 0.01). However, VH/CD ratios of the Cy+HT group were significantly higher compared to those of the Cy group (P < 0.01). These findings imply that HT significantly increased duodenal VH/CD ratios of Cy-treated broilers. Indirectly, HT improved duodenal morphologies.
Figure 2.
Variations in histological structure of the duodenums and effects of HT on VH/CD ratios. (A) H&E staining; (B) VH/CD ratios. * Indicates minimum significant differences (P < 0.05), and ** indicates highly significant differences (P < 0.01). Abbreviations: HT, hydroxytyrosol; VH/CD, villi height/crypt depth.
Expressions of CD4+ and CD8+ T Cell Areas
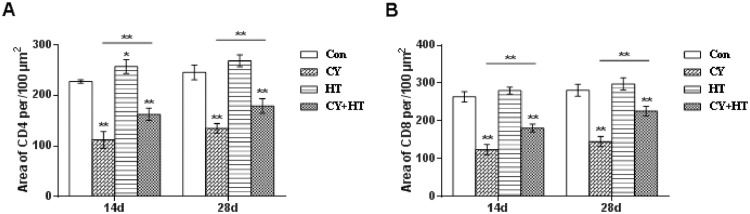
Figure 3 shows the expression areas of CD4+ and CD8+ T cells. After intragastric administration of HT, there were positive correlations between expression areas of CD4+ and CD8+ T cells on the 14th and 28th d. Compared to the Con group, expression areas of CD4+ and CD8+ T cells in the Cy group were significantly decreased (P < 0.01). Compared to the Cy group, expression areas of CD4+ and CD8+ T cells in the Cy+HT group were significantly increased (P < 0.01). On the 14th d after single intragastric administration of HT, CD4+ expression areas in the HT group were significantly different from those of the Con group (P < 0.05). There were no significant differences in CD8+ T expression areas between the Con and HT groups (P > 0.05). These findings show that HT significantly increased duodenal expressions of CD4+ and CD8+ T cells in Cy-treated broilers.
Figure 3.
CD4 and CD8 positive areas in the duodenum. (A) CD4; (B) CD8 *Indicates minimum significant differences (P < 0.05), and ** indicates highly significant differences (P < 0.01).
CD8+ T Cell Levels
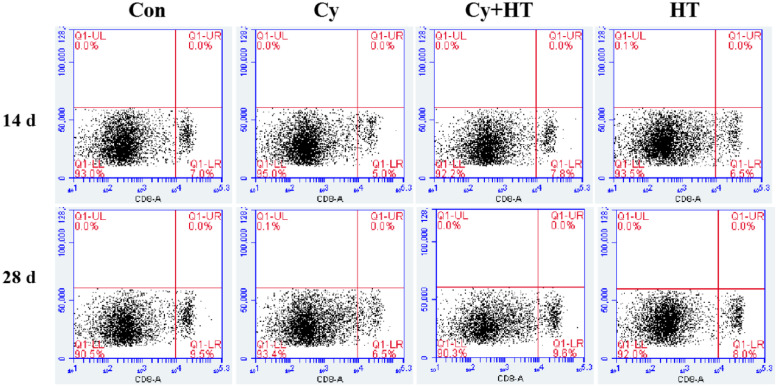
Figure 4 shows that the percentage of CD8+ T cells for chicken in the HT group was highest on the 28th d after HT administration. Compared to the control group, CD8+ T cell levels in the Cy group were significantly suppressed (P < 0.01). Compared to the Cy group, CD8+ T cell levels in the Cy+HT group were significantly elevated (P < 0.01). Therefore, HT increased the circulating numbers of CD8+ T cells in the blood of Cy-treated broilers.
Figure 4.
Abundance of CD8+ T cells detected by flow cytometry.
Gene Expression Levels
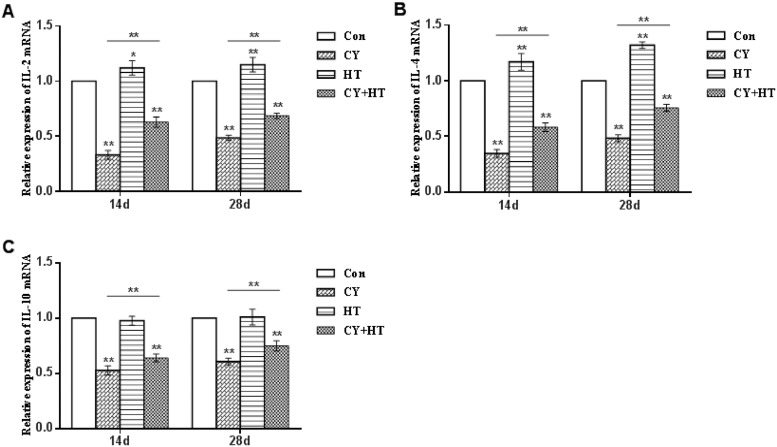
The relative duodenal mRNA expression levels of IL-2, IL-4, and IL-10 on the 14th and 28th d after intragastric administration of HT are shown in Figure 5. The mRNA expression levels of IL-2, IL-4, and IL-10 in the Cy group were significantly suppressed, when compared to the Con group (P < 0.01). However, after HT treatment, mRNA expression levels of IL-2, IL-4, and IL-10 in Cy+HT group were significantly elevated, when compared to the Cy group (P < 0.01). Moreover, after HT administration, mRNA expression levels of IL-2 and IL-4 were upregulated in the HT group, when compared to those of the Con group (P < 0.01 or P < 0.05). Therefore, HT upregulated duodenal mRNA expression levels of IL-2, IL-4, and IL-10 in Cy-treated broilers.
Figure 5.
mRNA expression levels of IL-2, IL-4, and IL-10 in the duodenum. (A) IL-2; (B) IL-4; (C) IL-10. *Indicates minimum significant differences (P < 0.05), and ** indicates highly significant differences (P < 0.01).
SOD, GSH-Px Activities, and MDA Levels
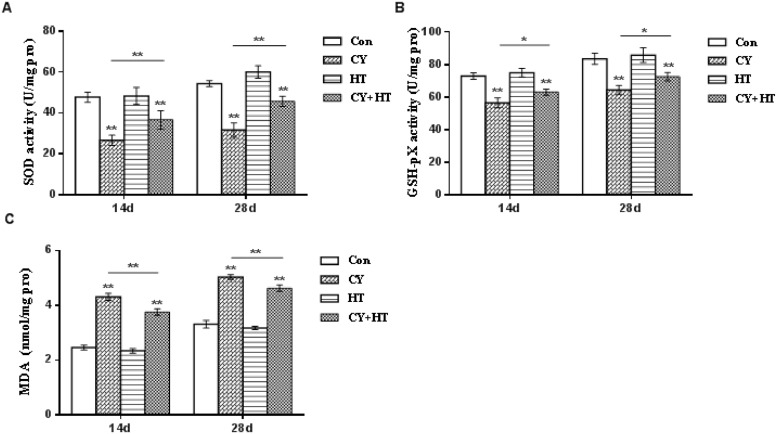
SOD, GSH-Px, and MDA levels are crucial oxidative stress indicators. Duodenal SOD and GSH-Px activities as well as MDA levels on the 14th and 28th d after HT treatment are shown in Figure 6. SOD and GSH-Px activities in the Cy group were significantly suppressed when compared to those of the Con group (P < 0.01), however, MDA levels were significantly elevated (P < 0.01). In the Cy+HT group, SOD and GSH-Px activities were significantly elevated, when compared to those of the Cy group (P < 0.01), while MDA levels were significantly suppressed (P < 0.01). In the HT group, SOD and GSH-Px activities as well as MDA levels were not significantly different from those of the Con group (P > 0.05). In conclusion, HT enhanced the activities of SOD and GSH-Px, suppressed MDA levels, enhanced antioxidant capacity in cells and effectively improved lipid peroxidation levels in Cy-induced immunosuppressed broilers.
Figure 6.
Effects of HT on SOD, GSH-Px activities, and MDA levels in the duodenum. (A) SOD; (B) GSH-Px; (C) MDA. *Indicates minimum significant differences (P < 0.05), and ** indicates highly significant differences (P < 0.01). Abbreviations: HT, hydroxytyrosol; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
DISCUSSION
In poultry, gastrointestinal microbiota play an important role in intestinal health and nutrition (Xiao et al., 2017). Bioactive phytochemicals can stimulate innate immunity, thereby inhibiting pathogenic invasion. Plant extracts have been shown to improve growth performance and feed efficiency of broilers by improving nutrient digestibility, and reducing intestinal pathogen-associated stress by enhancing digestive enzyme secretion, thereby affecting intestinal pathology, gastrointestinal motility, or bile acid secretion (Leskovec et al., 2018; Zhu et al., 2019). Olive leaf extracts exhibited intestinal anti-inflammatory activities in colitis model mice, and the extract was also shown regulate the activities of cells involved in inflammatory responses (Vezza et al., 2017). Supplementation of bioactive extracts from olive residues into sea bream feeds exerted positive effects on growth performance by improving lipid metabolism in the liver and by enhancing innate immune functions in the intestines (Gisbert et al., 2017). Olive is rich in polyphenols such as HT, whose content is about 0.058 to 0.768% (Ye et al., 2011). Metabolic benefits of polyphenols to the body depend on their bioavailability. The bioavailability of HT is low, and its plasma concentration ranges are in the nanomolar (nmol) levels (Peyrol et al., 2017). This could be because, HT plays a certain direct biological activity in the gastrointestinal tract before being absorbed. Intestinal flora regulation is the most likely local effect of HT, which can improve microbial community disturbance and protect intestinal wall integrity (Wang et al., 2018). After oral administration of HT in rats, it was shown to enter intestinal epithelial cells through passive diffusion (Manna et al., 2000), reached the highest plasma concentrations within 5 to 10 min, after which its concentration decreased rapidly.
CD4+ and CD8+ T lymphocytes are important in immune regulation (Parel and Chizzolini, 2004), therefore, changes in the number of CD4+ and CD8+ T lymphocytes can directly reflect immune states of a person (Yin et al., 2015). As an important T lymphocyte subgroup, CD4 + T lymphocytes can induce and enhance cellular as well as humoral immune responses. By secreting various cytokines, such as IL-2, IL-4, IL-6, and IL-10 among others, CD4+ T lymphocytes promote the activation and proliferation of Thl or Th2 T lymphocytes as well as B lymphocytes, which produce the corresponding antibodies. CD8+ T lymphocytes can kill and inhibit target cells, inhibit the differentiation and proliferation of B lymphocytes as well as regulate cellular and humoral immunity (Nhiem et al., 2010). Th1 cells secrete IL-2, IL-12, and TNF-α, which are involved in cell-mediated immune responses. Th2 cells secrete IL-4, IL-5, and IL-10 among others, which promote humoral or allergic reactions (Sakthivel and Guruvayoorappan, 2015). In this study, immunosuppressive chicken models were established by Cy injection at a dose of 80 mg/kg/d, which resulted in slow development of duodenal tissues, lower VH/CD ratios and decreased expressions of CD8+ T lymphocytes. However, after HT treatment, VH/CD ratios and CD8+T lymphocyte counts were increased. In addition, HT alleviated Cy-induced immune-suppression in broilers, elevated duodenal CD4+ and CD8+ T lymphocyte counts, upregulated the mRNA expression levels of IL-2, IL-4, and IL-10, and suppressed IL-6 and TNF-α levels (Vilaplana-Pérez et al., 2014; Echeverría et al., 2017). These findings are consistent with ours, which proves that HT can enhance immune functions in chicken. High Cy doses affect T cell expression areas in lymphatic tissues (McCormick et al., 1987).
Oxidative stress, which is attributed to excess free radicals, is a pathological feature of aging and various diseases. Due to its free radical scavenging activities, HT is an effective antioxidant that can inhibit intracellular oxidative stress injuries (Manna et al., 1999). For example, HT significantly elevated glutathione (GSH) levels while suppressing the levels of ROS and those of other free radicals in cells treated with Sudan red, thereby regulating oxidative stress and preventing oxidative DNA damage. In a previous study, there was a dose-dependent relationship between the degree of damage and concentrations of light tyrosol, that is, the higher the concentration of light tyrosol, the lower the degree of cell breakage (Qi and Jin, 2006). Prevention and treatment of cardiovascular and cerebrovascular diseases by HT is based on its strong antioxidant activities, which regulate bile flow, increasing cholesterol and bile acid levels in bile (Jemai et al., 2008). In addition, HT corrects redox imbalances by scavenging for peroxides and by enhancing antioxidant activities, thereby improving obesity and insulin resistance (IR), which are attributed to toxic substances or high fat diet (HFD) exposure (Liu et al., 2007; Cao et al., 2014).
Cyclophosphamide can damage the DNA of normal cells, cause immunosuppression and oxidative stress in various tissues and organs, and sometimes death (Chen et al., 2012). We found that Cy inhibited duodenal SOD and GSH-Px activities while elevating MDA levels. However, HT treatment significantly increased SOD and GSH-Px activities, and decreased MDA levels. There were significant differences in oxidation parameters between the Cy+HT and Cy groups. These findings imply that HT can effectively scavenge for various free oxygen radicals and their products, enhance antioxidant capacities, and effectively improve lipid peroxidation in immune-suppressed broilers. Therefore, HT has a protective effect on Cy-induced oxidative stress in vivo. The exact repair mechanisms of HT and its interactions with other drugs should be further evaluated.
CONCLUSIONS
In conclusion, HT stimulates suppressed immune systems, promotes free radical scavenging and enhances local immunity as well as antioxidant activities in local intestinal mucosa of broilers. The antioxidant functions of HT may be correlated with their immune-enhancing effects. However, other possible molecular mechanisms, such as its anti-inflammatory mechanisms, should be further evaluated.
Acknowledgments
ACKNOWLEDGMENTS
We are grateful to the staff of Yunnan academy of forestry and grassland for their assistance during the experimental work.
Data availability: All materials, data, and associated protocols promptly available to readers without undue qualifications in material transfer agreements.
DISCLOSURES
All authors declare no conflicts of interest.
REFERENCES
- Allouche N., Fki I., Sayadi S., Fki Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004;52:267–273. doi: 10.1021/jf034944u. [DOI] [PubMed] [Google Scholar]
- Basu A., Bhattacharjee A., Baral R., Biswas J., Samanta A., Bhattacharya S. Vanadium(III)-l-cysteine enhances the sensitivity of murine breast adenocarcinoma cells to cyclophosphamide by promoting apoptosis and blocking angiogenesis. Tumour Biol. 2017;39 doi: 10.1177/1010428317705759. [DOI] [PubMed] [Google Scholar]
- Briante R., La Cara F., Tonziello MP., Febbraio F., Nucci R. Antioxidant activity of the main bioactive derivatives from oleuropein hydrolysis by hyperthermophilic beta-glycosidase. J. Agric. Food Chem. 2001;49:3198–3203. doi: 10.1021/jf001342r. [DOI] [PubMed] [Google Scholar]
- Cao K., Xu J., Zou X., Li Y., Chen C., Zheng A., Li H., Li H., Szeto IM., Shi Y., Long J., Liu J., Feng Z. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014;67:396–407. doi: 10.1016/j.freeradbiomed.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Chen X., Nie W., Fan S., Zhang J., Wang Y., Lu J., Jin L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012;90:1114–1119. doi: 10.1016/j.carbpol.2012.06.052. [DOI] [PubMed] [Google Scholar]
- Echeverría F., Ortiz M., Valenzuela R., Videla LA. Hydroxytyrosol and cytoprotection: a projection for clinical interventions. Int. J. Mol. Sci. 2017;18:930. doi: 10.3390/ijms18050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner OB., Estevez C., Yu Q., Suarez DL. Passive antibody transfer in chickens to model maternal antibody after avian influenza vaccination. Vet. Immunol. Immunopathol. 2013;152:341–347. doi: 10.1016/j.vetimm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Gisbert E., Andree KB., Quintela JC., Calduch-Giner JA., Ipharraguerre IR., Pérez-Sánchez J. Olive oil bioactive compounds increase body weight, and improve gut health and integrity in gilthead sea bream (Sparus aurata) Br. J. Nutr. 2017;117:351–363. doi: 10.1017/S0007114517000228. [DOI] [PubMed] [Google Scholar]
- Granados-Principal S., Quiles JL., Ramirez-Tortosa C., Camacho-Corencia P., Sanchez-Rovira P., Vera-Ramirez L., Ramirez-Tortosa MC. Hydroxytyrosol inhibits growth and cell proliferation and promotes high expression of sfrp4 in rat mammary tumours. Mol. Nutr. Food Res. 2011;55(Suppl. 1):S117–S126. doi: 10.1002/mnfr.201000220. [DOI] [PubMed] [Google Scholar]
- Jemai H., Bouaziz M., Fki I., El Feki A., Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Killeen MJ., Pontoniere P., Crea R. Hydroxytyrosol: an examination of its potential role in cardiovascular disease, inflammation, and longevity. Agro. Food Ind. Hi Tech. 2011;22:16–19. [Google Scholar]
- Kumar VP., Venkatesh YP. Alleviation of cyclophosphamide-induced immunosuppression in Wistar rats by onion lectin (Allium cepa agglutinin) J. Ethnopharmacol. 2016;186:280–288. doi: 10.1016/j.jep.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Lapointe JM., Valdez RA., Ryan AM., Haley PJ. Evaluation of the utility of popliteal lymph node examination in a cyclophosphamide model of immunotoxicity in the rat. J. Immunotoxicol. 2016;13:449–452. doi: 10.3109/1547691X.2015.1122117. [DOI] [PubMed] [Google Scholar]
- Leskovec J., Levart A., Žgur S., Jordan D., Pirman T., Salobir J., Rezar V. Effects of olive leaf and marigold extracts on the utilization of nutrients and on bone mineralization using two different oil sources in broilers. J. Poult. Sci. 2018;55:17–27. doi: 10.2141/jpsa.0170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun L., Zhu L., Jia X., Li X., Jia H., Wang Y., Weber P., Long J., Liu J. Hydroxytyrosol protects retinal pigment epithelial cells from acrolein-induced oxidative stress and mitochondrial dysfunction. J. Neurochem. 2007;103:2690–2700. doi: 10.1111/j.1471-4159.2007.04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna C., Galletti P., Cucciolla V., Montedoro G., Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J. Nutr. Biochem. 1999;10:159–165. doi: 10.1016/s0955-2863(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Manna C., Galletti P., Maisto G., Cucciolla V., D'Angelo S., Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000;470:341–344. doi: 10.1016/s0014-5793(00)01350-8. [DOI] [PubMed] [Google Scholar]
- McCormick S., Dowler K., Armstrong JA., Hsiung GD. Cyclophosphamide immunosuppression during lymphotropic herpesvirus infection in the guinea pig model. A histopathologic and virologic study. Am. J. Pathol. 1987;127:538–548. [PMC free article] [PubMed] [Google Scholar]
- Mirkes PE. Cyclophosphamide teratogenesis: a review. Teratog. Carcinog. Mutagen. 1985;5:75–88. doi: 10.1002/tcm.1770050202. [DOI] [PubMed] [Google Scholar]
- Mulinacci N., Romani A., Galli G., Galli C. Hydroxytyrosol, as a component of olive mill waste water, is dose-dependently absorbed and increases the antioxidant capacity of rat plasma. Free Radic. Res. 2001;34:301–305. doi: 10.1080/10715760100300271. [DOI] [PubMed] [Google Scholar]
- Nhiem NX., Kiem PV., Minh CV., Tai BH., Tung NH., Ha do T., Soung KS., Kim JH., Ahn JY., Lee YM., Kim YH. Structure-activity relationship of lupane-triterpene glycosides from Acanthopanax koreanum on spleen lymphocyte IL-2 and IFN-gamma. Bioorg. Med. Chem. Lett. 2010;20:4927–4931. doi: 10.1016/j.bmcl.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Nousis L., Doulias P.T., Aligiannis N., Bazios D., Agalias A., Galaris D., Mitakou S. DNA protecting and genotoxic effects of olive oil related components in cells exposed to hydrogen peroxide. Free Radic. Res. 2005;39:787–795. doi: 10.1080/10715760500045806. [DOI] [PubMed] [Google Scholar]
- Obikaonu H.O., Okoli I.C., Opara M.N., Okoro V.M.O., Ogbuewu I.P., Etuk E.B., Udedibie A.B.I. Heamatological and serum biochemical indices of starter broilers fed leaf meal of neem (Azadirachta indica) J. Agr. Tech. 2012;8:71–79. [Google Scholar]
- Parel Y., Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun. Rev. 2004;3:215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Paul R., Kulkarni P., Ganesh N. Avocado fruit (Persea americana Mill) exhibits chemo-protective potentiality against cyclophosphamide induced genotoxicity in human lymphocyte culture. J. Exp. Ther. Oncol. 2011;9:221–230. [PubMed] [Google Scholar]
- Peyrol J., Riva C., Amiot MJ. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients. 2017;9:306. doi: 10.3390/nu9030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudyal H., Campbell F., Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010;140:946–953. doi: 10.3945/jn.109.117812. [DOI] [PubMed] [Google Scholar]
- Qi D., Jin ZX. Application of plant polyphenol in healthfood. HeiLongJiang Med. J. 2006;19:120–122. (in Chinese) [Google Scholar]
- Rigacci S., Stefani M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci. 2016;17:843. doi: 10.3390/ijms17060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthivel KM., Guruvayoorappan C. Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice. J. Immunotoxicol. 2015;12:154–163. doi: 10.3109/1547691X.2014.914988. [DOI] [PubMed] [Google Scholar]
- Shirani K., Hassani FV., Razavi-Azarkhiavi K., Heidari S., Zanjani BR., Karimi G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ. Toxicol. Pharmacol. 2015;39:1262–1275. doi: 10.1016/j.etap.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Vezza T., Algieri F., Rodríguez-Nogales A., Garrido-Mesa J., Utrilla MP., Talhaoui N., Gómez-Caravaca AM., Segura-Carretero A., Rodríguez-Cabezas ME., Monteleone G., Gálvez J. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201601066. 10.1002. [DOI] [PubMed] [Google Scholar]
- Vilaplana-Pérez C., Auñón D., García-Flores LA., Gil-Izquierdo A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014;1:18. doi: 10.3389/fnut.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhou J., Chen M., Huang X., Xie X., Li W., Cao Q., Kan H., Xu Y., Ying Z. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model. Part. Fibre Toxicol. 2018;15:17. doi: 10.1186/s12989-018-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yáñez-Ruiz DR., Molina-Alcaide E. A comparative study of the effect of two-stage olive cake added to alfalfa on digestion and nitrogen losses in sheep and goats. Animal. 2007;1:227–232. doi: 10.1017/S1751731107340032. [DOI] [PubMed] [Google Scholar]
- Ye JZ., Wang CZ., Chen HX., Zhou H. Variation rule of hydroxytyrosol contention olive leaves. Chem. Indus. Forest Prod. 2011;31:69–74. (in Chinese) [Google Scholar]
- Yin Y., Qin J., Dai Y., Zeng F., Pei H., Wang J. The CD4+/CD8+ ratio in pulmonary tuberculosis: systematic and meta-analysis article. Iran J. Public Health. 2015;44:185–193. [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]