Abstract
An 11-month-old, intact female Labrador retriever was presented with oligoanuric acute kidney injury and overhydration after grape ingestion. Percutaneous placement of a 12G × 30 cm Mila chest tube was done as an emergency temporary peritoneal dialysis catheter. Although no serious immediate complications were noted, an iatrogenic splenic injury had occurred. The catheter was used for peritoneal dialysis and urine output and hydration status improved over time. When the dialysis catheter was removed 3 d later, a synthetic hemostatic matrix, Surgiflo, was deposited through the catheter. No complications were noted. The dog recovered uneventfully and was doing well with normal kidney function.
Key clinical message:
To the authors’ knowledge, this report represents the first description of non-invasive management of iatrogenic splenic injury secondary to percutaneous peritoneal dialysis catheter placement in a dog.
Résumé
Prise en charge non invasive réussie de lésions spléniques iatrogènes associées à un cathéter de dialyse péritonéale chez un chien. Une femelle Labrador retriever intacte âgée de 11 mois a été présentée avec une lésion rénale aiguë oligoanurique et une surhydratation après ingestion de raisin. La mise en place percutanée d’un drain thoracique Mila de 12G × 30 cm a été réalisée en tant que cathéter de dialyse péritonéale temporaire d’urgence. Bien qu’aucune complication immédiate grave n’ait été notée, une lésion splénique iatrogène s’était produite. Le cathéter a été utilisé pour la dialyse péritonéale et le débit urinaire et l’état d’hydratation se sont améliorés au fil du temps. Lorsque le cathéter de dialyse a été retiré 3 jours plus tard, une matrice hémostatique synthétique Surgiflo a été déposée via le cathéter. Aucune complication n’a été notée. Le chien a récupéré sans incident et se portait bien avec une fonction rénale normale.
Message clinique clé:
À la connaissance des auteurs, ce rapport représente la première description de la gestion non invasive de lésions spléniques iatrogènes secondaires à la pose d’un cathéter de dialyse péritonéale percutanée chez un chien.
(Traduit par Dr Serge Messier)
Iatrogenic splenic injury is a relatively rare complication reported secondary to multiple procedures including chest tube insertions and nephrostomy catheters in humans (1–3). Injury to abdominal organs, including the spleen, is a recognized risk in veterinary medicine with placement of a peritoneal catheter; however, it has never been described.
Successful catheter placement and proper catheter selection are keys for successful peritoneal dialysis (PD) (4). Most of these catheters require surgical placement, but there are techniques to place them percutaneously in emergency situations. The benefit of percutaneous placement is that it can be done with local anesthetic and sedation without an abdominal incision. However, the disadvantage is that the catheters do not contain a cuff in the subcutaneous space, increasing the risk of dialysate leakage and peritonitis (5). There are several complications with percutaneous placement in humans including hollow organ perforation, but splenic laceration has not been described (6). Splenic laceration later in the course of PD has been described in human medicine due to complications of the catheter, as well as unknown causes.
Ingestion of grapes can lead to fatal kidney injury in dogs; tartaric acid and potassium bitartrate may be the toxic agents, but there is no antidote (7). Treatment usually consists of induction of emesis, activated charcoal for gastrointestinal decontamination, and intravenous fluid therapy. Peritoneal dialysis has been described for treatment of dogs with acute kidney injury requiring renal replacement therapy, especially when hemodialysis is not readily available (4,8).
In this report we describe a dog that incurred an iatrogenic splenic injury secondary to percutaneous PD catheter placement and was treated medically.
Case description
An 11-month-old, intact female Labrador retriever was presented to the Veterinary Medical Centre (VMC) Emergency Service, University of Saskatchewan, for vomiting, anorexia, and lethargy 2 d after ingesting an unknown quantity of grapes. At presentation, the dog had a body weight (BW) of 16.0 kg and was estimated to be 5% dehydrated with the remainder of the physical examination unremarkable. A complete blood (cell) count (Advia 2120i; Siemens, Oakville, Ontario) and serum biochemistry panel (Cobas c311; Roche Diagnostics, Laval, Quebec) were completed. There were significant elevations in urea 33.9 mmol/L [reference range (RR): 2.5 to 9.6 mmol/L], creatinine > 1202 μmol/L (RR: 44 to 159 μmol/L), total calcium > 4.0 mmol/L (RR: 1.98 to 3.0 mmol/L), and phosphorus 3.43 mmol/L (RR: 0.81 to 2.2 mmol/L). The dog was started on intravenous (IV) fluid therapy (Normosol-R; ICU Medical Canada, Saint-Laurent, Quebec), and 0.9% sodium chloride (Baxter Corporation, Mississauga, Ontario), and symptomatic supportive care including maropitant (Zoetis Canada, Kirkland, Quebec), 1 mg/kg BW, IV, q24h, and omeprazole (Sandoz Canada, Boucherville, Quebec), 1.25 mg/kg BW, PO, q12h. Over the next 12 h, the care of the dog was transferred to the family veterinarian. The dog stopped producing urine and developed severe hyperkalemia of 8.5 mmol/L (RR: 3.8 to 5.6 mmol/L), consistent with an abrupt decline in kidney function, and a life-threatening bradyarrhythmia suspected to be secondary to hyperkalemia. Furosemide (Intervet Canada, Kirkland, Quebec) was administered with no response in urine production. No urination was recorded for more than 24 h, and the dog had gained 2.0 kg BW (12.5% increase) in 19 h since initial presentation to the VMC. On the third day after grape ingestion (Day 0), persistent hyperkalemia of 7.93 mmol/L (RR: 3.8 to 5.6 mmol/L) was noted (Table 1) despite previous medical therapies including insulin (Eli Lilly Canada, Toronto, Ontario) and dextrose (Vétquinol N.-A., Lavaltrie, Quebec). An abdominal-focused sonographic assessment for triage scan revealed free fluid in the retroperitoneal space surrounding the kidneys bilaterally, consistent with fluid overload. Fluid therapy was decreased to 25 mL/h (1.35 mL/kg BW per hour). Given the lack of response to previous furosemide therapy, further administration of furosemide was withheld.
Table 1.
Summary of serial blood work, urine output, and dialysate volumes.
| Day/Time | Infused dialysate volume mL (mL/kg) | Recovered dialysate mL (%) | Urinary output (mL/kg/h) | Body weight (kg) | Potassium (mmol/L) | Creatinine (μmol/L) | Urea (mmol/L) | Packed cell volume (%) | Total protein (g/dL) | Venous pH | Phosphorus (mmol/L) | Albumin (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0/23:00 | — | — | 0 | 18.0 | 7.93 | — | — | — | — | 7.41 | — | — |
| Day 1/01:30 | — | — | 0 | — | 5.63 | — | — | — | — | 7.37 | — | — |
| Day 1/04:30 | — | — | 0 | — | 7.47 | 1364 | 41.4 | 41 | 5.1 | 7.39 | 3.37 | 2.8 |
| Day 1/09:30 | — | — | 0.21 | 18.4 | 7.79 | — | — | 41 | 7.5 | 7.35 | — | — |
| Day 1/14:30 | — | — | 1.53 | 18.2 | 7.96 | — | — | 37 | 5.7 | 7.34 | — | — |
| Day 1/16:00 | 270 (15) | 350 (130) | 1.55 | — | — | — | — | — | — | — | — | — |
| Day 1/19:00 | 270 (15) | 343 (127) | 2.7 | 18.0 | 7.32 | — | — | 40 | 5.3 | 7.37 | — | — |
| Day 1/22:30 | 270 (15) | 343 (127) | 3.8 | — | 6.11 | — | — | 39 | 5.6 | 7.32 | — | — |
| Day 2/01:30 | 270 (15.7) | 286 (106) | 3.7 | 17.2 | — | — | — | — | — | — | — | — |
| Day 2/08:00 | 270 (15.6) | 345 (128) | 6.0 | 17.3 | 6.73 | 1472 | 50.2 | 38 | 5.7 | 7.34 | 5.13 | 3.0 |
| Day 2/14:00 | 270 (16.6) | 289 (107) | 7.66 | 16.3 | — | — | — | — | — | — | — | — |
| Day 2/20:00 | 270 (16.5) | 200 (74) | 9.45 | 16.4 | 4.35 | — | — | 45 | 7.0 | 7.34 | — | — |
| Day 3/01:30 | 270 (16.5) | 286 (106) | 9.13 | 16.6 | 4.35 | — | — | 41 | 6.8 | 7.34 | — | — |
| Day 3/07:30 | 270 (16.5) | 135 (50) | 8.2 | 16.3 | — | — | — | — | — | — | — | — |
| Day 3/18:00 | — | — | — | 15.8 | 3.35 | 970 | 35.4 | 40 | 5.3 | — | 4.10 | 3.3 |
| Day 4/08:00 | — | — | — | 16.3 | 4.00 | 391 | 17.9 | 39 | 5.3 | — | 1.87 | 3.2 |
| Day 5/09:00 | — | — | — | 16.6 | 4.10 | 269 | 12.1 | 39 | 5.0 | — | 1.25 | 3.0 |
| Day 6/08:30 | — | — | — | 16.6 | 4.20 | 234 | 9.70 | 42 | 7.4 | — | 1.86 | 2.8 |
| Day 7/08:00 | — | — | — | 16.5 | 4.40 | 206 | 10.4 | 43 | 7.5 | — | 1.68 | 3.3 |
— = Not available.
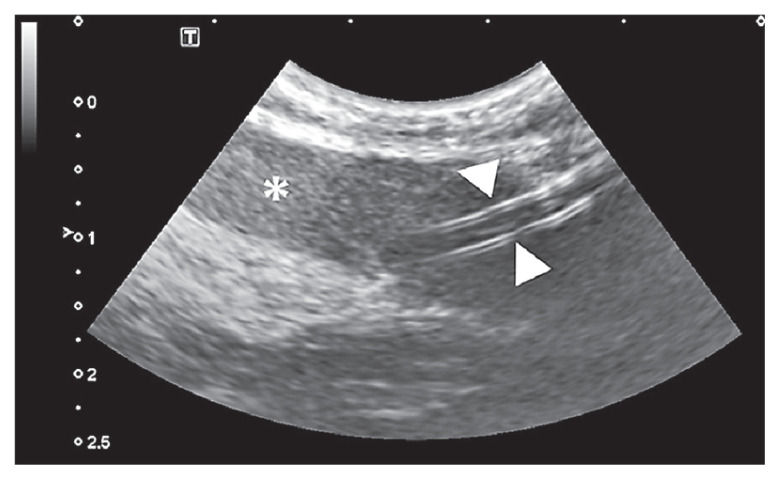
The following morning (Day 1), the dog still had not produced urine with worsening fluid overload (additional 0.4 kg BW, 2.2% BW) and ongoing uncontrolled hyperkalemia of 7.79 mmol/L (RR: 3.8 to 5.6 mmol/L) (Table 1). Therefore, the decision to proceed with PD was made. A central venous catheter to facilitate repeated blood sampling, a nasogastric tube for nutritional management and an indwelling urinary catheter to facilitate measuring urinary output and concentrating abilities, were placed. Under ultrasound guidance, a 12G × 30 cm Mila chest tube (Mila International, Florence, Kentucky, USA) was placed as an emergency temporary PD catheter. Upon placement of the catheter, the initial aspirated sample (~ 5 mL) was very hemorrhagic. The catheter flushed without resistance and the dog’s cardiovascular status as well as PCV/TP were stable (Table 1). Abdominal radiographs and an abdominal ultrasonographic examination were performed, and it was noted that the PD catheter went through the body of the spleen (Figure 1). It was elected to leave the catheter in place, due to concerns for hemorrhage if removed soon after placement and use it for PD. Cefazolin (Fresenius Kabi Canada, Toronto, Ontario) 22 mg/kg BW, IV, q8h was started at the time of catheter placement and continued while the dog had the PD catheter in place. During the next 12 h, PD was done every 3 to 4 h, as continual PD exchanges were not feasible with overnight staffing. The dialysate solution was Lactated Ringer’s Solution (ICU Medical Canada, Saint-Laurent, Quebec) with 2.5% dextrose (Vétquinol N.-A.). Initially, the PD system was flushed with 10 to 20 mL of dialysate solution, then 270 mL (15 mL/kg BW, as a standard starting volume) of dialysate solution was instilled into the patient’s abdominal cavity to allow increase in volume by ultrafiltration and to ensure the dog was comfortable. After a dwell time of 40 min the fluid was drained, and the amount of effluent was recorded. The dog’s fluid balance was monitored every 4 h according to the “fluidins” which included volume of IV fluids given during the period, volume of infused dialysate, and any fluid volume administered through nasogastric tube and “fluid-outs” which included urine output, volume of effluent, and any other sensible fluid losses if present. The hyperkalemia mildly improved over the course of the day (Table 1).
Figure 1.
An ultrasonographic view of PD catheter going through the body of spleen. The white arrowheads indicate the PD catheter and the asterisk (*) indicates the spleen.
On Day 2, PD was performed 4 times, approximately every 6 h. The IV fluid was delivered to match ongoing losses, and the hyperkalemia and overhydration improved over the next 12 h as urine output increased markedly (Table 1). The liquid feeding was increased to 50% resting energy requirements (RER). On Day 3, PD was performed twice, approximately 6 h apart and the amount of liquid feeding was increased to 75% RER. On Day 3, there was further improvement of the potassium to 3.35 mmol/L, which is considered a mild hypokalemia, as well as moderate to marked improvement in the degree of azotemia (Table 1). At this time, PD was discontinued, and potassium was supplemented in the IV fluids to ensure potassium concentrations remained within normal reference range. The dog remained markedly polyuric [urinary output (UOP) 8 to 13 mL/kg BW per hour] and started to voluntarily eat (Table 1).
On Day 4, given the stable patient condition as well as marked improvement in creatinine to 391 μmol/L (RR: 44 to 159 μmol/L) (Table 1), the temporary PD catheter was removed. The dog was sedated with butorphanol (Zoetis Canada), 0.2 mg/kg BW, IV, midazolam (Sandoz Canada), 0.25 mg/kg BW, IV, and alfaxalone (Central Sales, Brampton, Ontario), 0.5 mg/kg BW as an IV bolus, titrated to achieve adequate immobilization. The insertion site as well as the access port of the catheter were surgically prepared. A synthetic hemostatic matrix (Surgiflo; Ethicon, Bridgewater, New Jersey, USA) was reconstituted according to the manufacturer’s protocol. A syringe containing Surgiflo was aseptically attached to the catheter, which was slowly removed, and the matrix concurrently gently deposited. Throughout this procedure, location of the catheter in relation to the spleen was monitored by ultrasound. The dog required a dose of acepromazine [Boehringer Ingelheim (Canada), Burlington, Ontario], 0.01 mg/kg BW, IV for emergency delirium on recovery from the sedation. After removal of the PD catheter, the hemodynamic status and PCV/TP of the dog remained stable, and ultrasonographically there was no noticeable free fluid suggestive of hemorrhage, throughout the remaining 3 d of hospitalization (Table 1). The dog was discharged on Day 7. No immediate (e.g., anaphylaxis) or late complications (e.g., granuloma) associated with this hemostatic matrix were noted. At the time of writing (14 mo after discharge), the dog is doing well with normal kidney function.
Discussion
This case report describes non-invasive management of an iatrogenic splenic injury caused by an emergency temporary PD catheter (utilizing a chest tube) using a synthetic hemostatic matrix (Surgiflo).
Iatrogenic splenic injury accounts for 25% of indications for a splenectomy in humans; the 2 most common causes are reported to be left nephrectomy and lower gastrointestinal surgery ( colectomy) (9). Other sporadic causes of iatrogenic splenic injury such as a chest tube insertion, percutaneous nephrostomy catheter placement, or colonoscopy are also reported (1–3,10–12). This complication is managed by waiting an extended interval (~14 d) (1) or removal of the tube with deposition of hemostatic matrix (2,13). In veterinary patients, the former option may be problematic, as leaving in a catheter for an extended interval can contribute to bacterial colonization (14) or peritonitis (4) and add excessive costs for prolonged hospitalization. Leaving the PD catheter in place for 2 wk, therefore, was not deemed appropriate, so once the temporary PD catheter was no longer needed, the authors decided to remove it and concurrently deposit a hemostatic matrix. At catheter removal, the patient was polyuric, kidney function had substantially improved, and potassium had normalized. Although the authors used Surgiflo, which is composed of sterile porcine gelatin mixed with thrombin, other options include absorbable gelatin sponge or fibrin glue (13). There are potential risks, e.g., anaphylaxis, associated with gelatin materials (15). Surgiflo was as effective as absorbable gelatin sponge for hemostasis during lumbar spine surgery (16), and we considered the benefit of the liquified texture of this particular hemostatic material. Despite an inability to visualize infusion of the hemostatic materials with ultrasonography, the procedure was relatively easy and accomplished without complications. Furthermore, due to multiple fenestrations on the temporary PD catheter, it was impossible to control from which fenestration the material was deposited, or to determine whether the hemostatic material was deposited into the spleen. Perhaps the peritoneal catheter itself had contributed to formation of fibrin clots around the catheter, as this is a complication of peritoneal dialysis (4).
Grapes are known to cause acute kidney injury in dogs which can lead to death. The current mechanism for the development of kidney injury is not fully known. However, proximal renal tubular degeneration is common (17) and tartaric acid and its metabolites may be the causative agent, although additional investigation is required to confirm this (7). The dose response is also unknown, as some dogs can ingest many grapes with no consequence, whereas others can ingest only few and develop severe acute kidney injury (17). In severe acute kidney injury cases, renal replacement therapy is recommended (18–20).
Peritoneal dialysis is a viable option for severe acute kidney injury cases requiring renal replacement therapy, especially when the animals have a relative contraindication for hemodialysis (e.g., small body size; < 2.5 kg BW, anemia without available donor blood, or are hemodynamically unstable) or there is no availability of hemodialysis machines (4). In the present case, we used a multi-fenestrated chest tube as a temporary PD catheter, placed percutaneously using a modified Seldinger technique. There are other types of PD catheters, including soft silicone-based catheter with 1 to 2 Dacron cuffs that are typically placed surgically. A small incision can enable use of appropriately located Dacron cuffs which promote fibroblast ingrowth and better sealing; furthermore, if the placement is done by laparotomy, concurrent omentectomy can minimize catheter obstruction (19). However, these other dialysis catheters are not regularly stocked at the authors’ clinic, and the expectation of relatively short dialysis-dependent period for this patient prompted placing a chest tube as a temporary PD catheter. The authors recognize inherent risks of using a chest tube for PD, including catheter malfunction as a common complication (4). In the present case, ultimately the use of a PD catheter with Dacron cuff was not necessary, but such a catheter would have been a superior option if the dog remained dialysis-dependent for longer than 3 d (21). Waiting 2 wk to remove a cuffed chronic catheter is recommended to prevent dialysate leakage in humans (21), but is not practical in most veterinary PD patients. To prevent future occurrence of the iatrogenic splenic injury due to percutaneous PD catheter placement, distending the peritoneal cavity by pre-infusion of warmed saline into the cavity prior to catheter insertion (19), and ultrasound guidance at catheter insertion should be considered.
Administration of Cefazolin was continued while the dog had the PD catheter in place, pending a negative urine culture. Although administration during placement is recommended, prolonged unnecessary use of antimicrobials can increase risk of bacterial resistance (19,22). The use of intraperitoneal or prophylactic administration of antimicrobials was historically advised, but not currently recommended (4,19). Microbiological specimen of the PD catheter was not obtained and therefore, it is impossible to retrospectively evaluate bacterial film or colonization on the catheter.
This is the first report in veterinary medicine describing iatrogenic splenic injury secondary to an emergency peritoneal dialysis catheter placement. Similar to human patients, in the absence of an immediate, ongoing urgent hemorrhagic complication, iatrogenic splenic injury itself should not dissuade clinicians to stop patient care. The use of hemostatic matrix at catheter removal was effective in the present case.
Acknowledgment
We thank Dr. Samantha Bray for her contributions in management and data collection for this manuscript. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was not supported by a grant. Neither of the authors of this article has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- 1.Ohtaka K, Hase R, Chiba R, et al. Noninvasive management for iatrogenic splenic injury caused by chest tube insertion: A case report. Clin Case Rep. 2016;4:1157–1160. doi: 10.1002/ccr3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas AA, Pierce G, Walsh RM, Sands M, Noble M. Splenic injury during percutaneous nephrolithotomy. J Soc Lap Robot Surg. 2009;13:23323–23326. [PMC free article] [PubMed] [Google Scholar]
- 3.Feola A, Niola M, Conti A, et al. Iatrogenic splenic injury: Review of the literature and medico-legal issues. Open Med (Wars) 2016;11:307–315. doi: 10.1515/med-2016-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross LA, Labato MA. Current techniques in peritoneal dialysis. J Vet Emerg Crit Care (San Antonio) 2013;23:230–240. doi: 10.1111/vec.12035. [DOI] [PubMed] [Google Scholar]
- 5.Peppelenbosch A, van Kuijk WH, Bouvy ND, van der Sande FM, Tordoir JH. Peritoneal dialysis catheter placement technique and complications. NDT Plus. 2008;1:iv23–iv8. doi: 10.1093/ndtplus/sfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abreo K, Sequeira A. Bowel perforation during peritoneal dialysis catheter placement. Am J Kidney Dis. 2016;68:312–315. doi: 10.1053/j.ajkd.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Wegenast C, Meadows I, Anderson R, Southard T. Letter to the editor: Unique sensitivity of dogs to tartaric acid and implications for toxicity of grapes. J Amer Vet Med Assoc. 2021;258:1181–1183. [Google Scholar]
- 8.Mazzaferro EM, Eubig PA, Hackett TB, et al. Acute renal failure associated with raisin or grape ingestion in 4 dogs. J Vet Emerg Critic Care. 2004;14:203–212. [Google Scholar]
- 9.Tan K, Lewis GR, Chahal R, et al. Iatrogenic splenectomy during left nephrectomy: A single-institution experience of eight years. Urol Int. 2011;87:59–63. doi: 10.1159/000326761. [DOI] [PubMed] [Google Scholar]
- 10.Cassar K, Munro A. Iatrogenic splenic injury. J Roy Coll Surg Edinb. 2002;47:731–741. [PubMed] [Google Scholar]
- 11.Carey RI, Siddiq FM, Guerra J, Bird VG. Conservative management of a splenic injury related to percutaneous nephrostolithotomy. J Soc Lap Robot Surg. 2006;10:504–506. [PMC free article] [PubMed] [Google Scholar]
- 12.Singla S, Keller D, Thirunavukarasu P, et al. Splenic injury during colonoscopy — A complication that warrants urgent attention. J Gastrointest Surg. 2012;16:1225–1234. doi: 10.1007/s11605-012-1871-0. [DOI] [PubMed] [Google Scholar]
- 13.Desai AC, Jain S, Benway BM, Grubb RL, Picus D, Figenshau RS. Splenic injury during percutaneous nephrolithotomy: A case report with novel management technique. J Endourol. 2010;24:541–545. doi: 10.1089/end.2009.0290. [DOI] [PubMed] [Google Scholar]
- 14.Perondi F, Petrescu VF, Fratini F, et al. Bacterial colonization of non-permanent central venous catheters in hemodialysis dogs. Heliyon. 2020;6:e03224. doi: 10.1016/j.heliyon.2020.e03224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lied GA, Lund KB, Storaas T. Intraoperative anaphylaxis to gelatinbased hemostatic agents: A case report. J Asthma Allergy. 2019;12:163–167. doi: 10.2147/JAA.S202784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Dai L, Yang Y, Liu H. Comparison the efficacy of hemorrhage control of Surgiflo Haemostatic Matrix and absorbable gelatin sponge in posterior lumbar surgery: A randomized controlled study. Medicine (Baltimore) 2018;97:e13511. doi: 10.1097/MD.0000000000013511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostrom MS. Grapes and raisins. In: Peterson ME, Talcott PA, editors. Small Animal Toxicology. St. Louis, Missouri: Elsevier; 2006. pp. 727–731. [Google Scholar]
- 18.Acierno MJ, Labato MA. Continuous renal replacement therapy/ hemodialysis. In: Ettinger SJ, Feldman EC, Côté E, editors. Textbook of Veterinary Internal Medicine. 8th ed. Vol. 1. St. Louis, Missouri: Elsevier; 2017. pp. 425–430. [Google Scholar]
- 19.Bersenas AME. Peritoneal dialysis. In: Ettinger SJ, Feldman EC, Côté, editors. Textbook of Veterinary Internal Medicine. 8th ed. Vol. 1. St. Louis, Missouri: Elsevier; 2017. pp. 423–425. [Google Scholar]
- 20.Langston CE. Acute kidney injury. In: Ettinger SJ, Feldman EC, Côté E, editors. Textbook of Veterinary Internal Medicine. 8th ed. Vol. 2. St. Louis, Missouri: Elsevier; 2017. pp. 1919–1934. [Google Scholar]
- 21.Crabtree JH. Peritoneal access devices, placement techniques, and maintenance. In: Nissenson AR, Fine RN, editors. Handbook of Dialysis Therapy. 5th ed. Philadelphia, Pennsylvania: Elsevier; 2017. pp. 97–120. [Google Scholar]
- 22.Weese JS, Giguère S, Guardabassi L, et al. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med. 2015;29:487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]