Abstract
Aims
There is conflicting evidence whether heart failure (HF) is a risk factor for incident cancer. Despite population-based cohorts demonstrating this association, an analysis of the Physician’s Health Study found no association in a cohort of mostly healthy males. We investigated the association of HF with incident cancer among a large cohort of post-menopausal women.
Methods and results
A prospective cohort study of 146 817 post-menopausal women age 50 to 79 years enrolled in the Women’s Health Initiative from 1993–1998, and followed through 2015. The primary exposure was adjudicated incident HF diagnosis, including preserved and reduced ejection fraction in a sub-cohort. The primary outcome was adjudicated incident total and site-specific cancers. Hazard ratios were calculated using multivariable-adjusted Cox proportional hazard regression models. Over a median follow-up of 8.4 years, 3272 and 17 474 women developed HF and cancer, respectively. HF developed in 235 women prior to cancer. HF was associated with subsequent incident cancer [hazard ratio (HR) 1.28, 95% confidence interval (CI) 1.11–1.48]. Associations were observed for obesity-related cancers (HR 1.24, 95% CI 1.02–1.51), as well as lung and colorectal cancers (HR 1.58, 95% CI 1.09–2.30 and HR 1.52, 95% CI 1.02–2.27, respectively). HF with preserved ejection fraction (HR 1.34, 95% CI 1.06–1.67), but not HF reduced ejection fraction (HR 0.99, 95% CI 0.74–1.34), was associated with total cancer.
Conclusion
Heart failure was associated with an increase in cancer diagnoses in post-menopausal women. This association was strongest for lung cancer. Further research is needed to appreciate the underlying mechanisms responsible for this association.
Keywords: Heart failure, Cancer, Risk, Prospective cohort, Cardio-oncology
Graphical Abstract
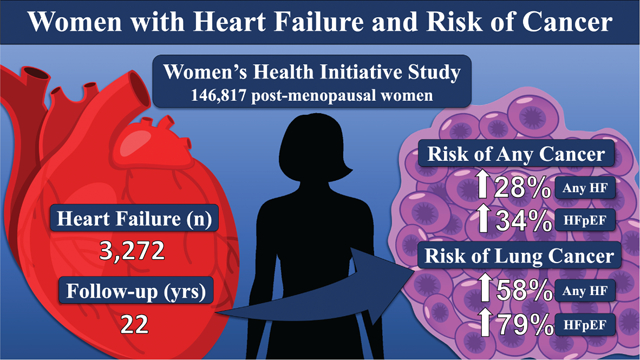
Heart failure (HF) is significantly associated with an increased risk of developing cancer among post-menopausal women. HFpEF, heart failure with preserved ejection fraction.
Introduction
Heart failure (HF) and cancer are clinical and public health issues associated with substantial morbidity, mortality, and healthcare expenditures.1,2 As our population ages in parallel to medical advancements, the prevalence of HF and cancer have both increased.2–4 Consequently, the coexistence of cancer among patients with HF has become more common and portends a worse prognosis than either HF or cancer alone.5–7 A cancer diagnosis after HF, specifically HF with preserved ejection fraction (HFpEF), is an independent predictor of mortality and the second leading cause of non-cardiovascular death.8,9
The literature to date has predominately focused on HF as a consequence of cancer therapy-related cardiotoxicity. However, several community-based and larger international studies have reported an association of HF with subsequent risk of incident cancer.6,7,10,11 Specifically, these studies suggest the association may be due to shared pathophysiological risk factors. A recent position statement by the European Society of Cardiology advocated for greater awareness of incident cancer among HF patients, further highlighting an emerging recognition of this association.12
Older women account for a disproportionate burden in HF, specifically HFpEF.13–15 Despite this, women have been historically underrepresented in clinical trials and studies within cardiology.16 In contrast to earlier studies, an analysis utilizing the all-male Physicians’ Health Study (PHS) I and II cohorts found no association between HF and incident cancer.17 Previous studies additionally did not differentiate HF by subtype: HFpEF vs HF with reduced ejection fraction (HFrEF). These contradictory findings and their clinical implications require clarification to determine whether these results were partially driven by gender differences or HF classification (HFpEF/HFrEF). In this study, we employ the strengths of the large, all-female, Women’s Health Initiative (WHI) cohort with adjudicated cancer and HF diagnoses capable of distinguishing HFpEF from HFrEF in order to examine the relative risk of incident cancer in patients with HF compared to similar women without HF.
Methods
Study population
The WHI is a national, prospective cohort study of post-menopausal women. A total of 161 808 women aged 50 to 79 were enrolled at 40 clinical centres nationwide between 1993 and 1998.18,19 Women were enrolled in either one or more of three clinical trials or an observational study with initial follow-up until 2005. At the end of the initial follow-up, WHI women were asked to participate in extended follow-up through 2015. Women who consented to the extension studies comprise two sub-groups that defined subsequent outcome ascertainment procedures: medical records cohort (MRC) and self-report cohort (SRC). The MRC consisted of all women who participated in the hormone therapy trials and all black and Hispanic participants in any other component of the clinical trial or observational study. A total of 41 503 women from the MRC were included where all major clinical events were documented and centrally adjudicated.
For the current study, women were excluded if they had no follow-up time (n = 691) in the main study, or if they had self-reported HF (n = 1518) or cancer (n = 12 782) at enrolment, resulting in a final sample size of 146 817 women for the main analysis (Figure 1).
Figure 1.
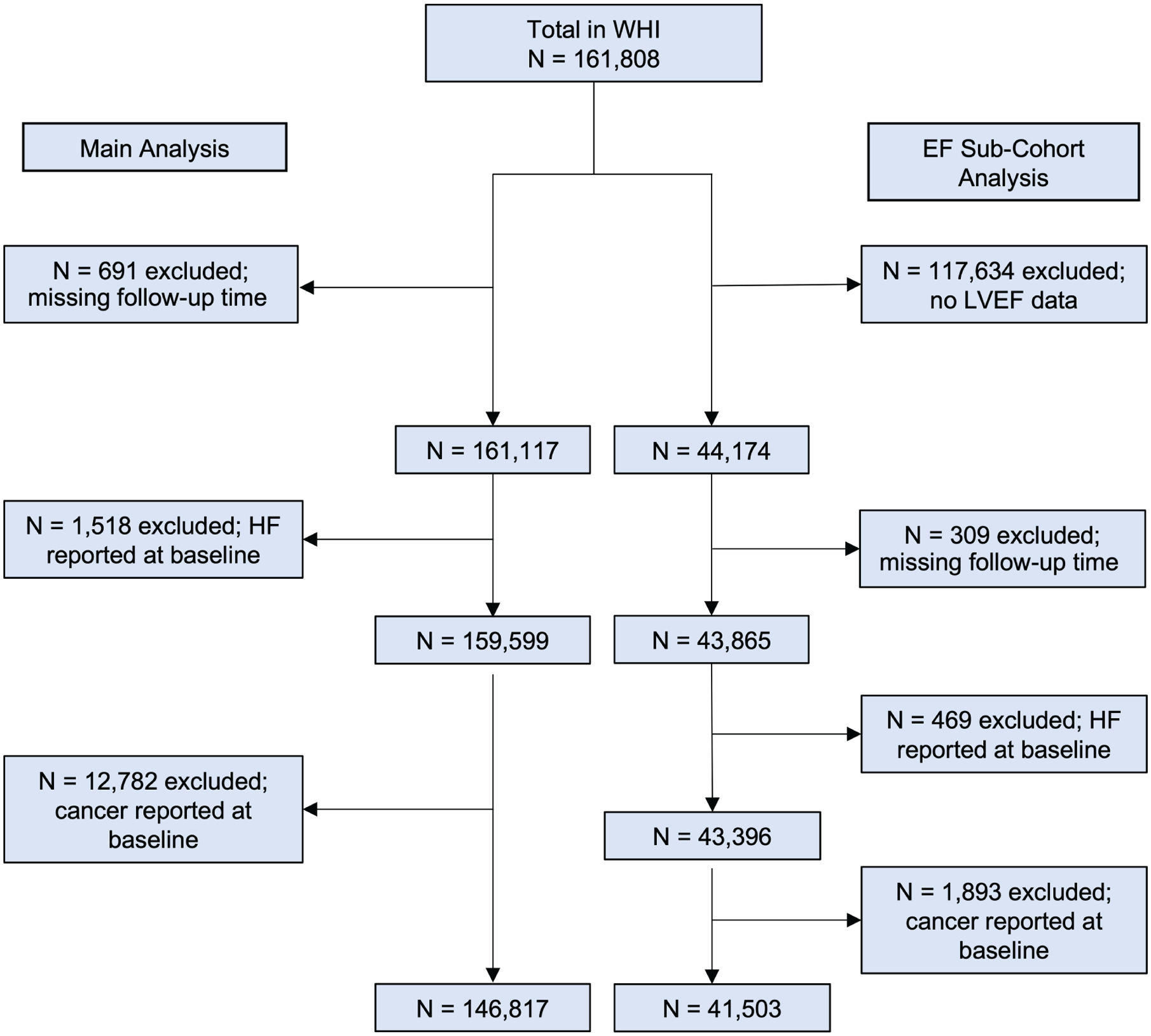
Flow diagram of the study population. EF, ejection fraction; HF, heart failure; LVEF, left ventricular ejection fraction; WHI, Women’s Health Initiative.
Exposure
The primary exposure of interest was incident, adjudicated HF diagnosis. HF that occurred during WHI follow-up was adjudicated by centrally trained physician adjudicators for all participants in the main study (through 2005) and from 2005–2015 for participants in the MRC. An adjudicated HF diagnosis, based on review of hospitalization records with a primary diagnosis of HF, was required to be included as an exposure.
In the MRC, hence referred to as ‘ejection fraction (EF) sub-cohort’, HF was further characterized as HFpEF or HFrEF among participants with available data on left ventricular ejection fraction (LVEF).18,20 HFrEF was defined as HF with a LVEF <50% and HFpEF as LVEF >50%. Sensitivity analysis was repeated with an LVEF threshold of 40%.
Outcome
The primary outcome was time to first incident cancer diagnosis (other than non-melanoma skin cancer). All self-reported cancers were documented with medical records, and centrally reviewed and coded according to SEER standards.21 Cancers first identified through regular linkage to the National Death Index (NDI) were coded based on death certificate information. For these cancers, diagnosis date was set to date of death. Site-specific cancers of breast, lung, and colorectal were analysed. To address our hypotheses regarding shared risk factors, we created mechanism-based categories of cancer diagnoses, specifically obesity-related and tobacco-related cancers. Obesity-related cancers were defined by International Agency for Research on Cancer (IARC) as having anyone of the following cancers: oesophageal, gastric, colorectal, liver, post-menopausal breast, pancreatic, ovarian, kidney, uterine, thyroid, or multiple myeloma.22 Tobacco-related cancers were defined by IARC as having anyone of the following cancers: lung, stomach, pancreas, liver, kidney, oral, oropharynx, nasopharynx, nasal, hypopharynx, larynx, oesophageal, bladder, ureter, or cervix.23
Statistical analysis
Continuous variables were assessed for normality. Baseline characteristics of the sample are reported using means and standard deviations or frequencies and proportions for continuous and categorical variables, respectively. Bivariate statistics employed t-tests and chi-square tests for continuous and categorical variables, respectively, to compare those with and without cancer.
Incidence rates (cases/1000 person-years) and 95% confidence intervals (CI) were calculated for any cancer, obesity-related cancer, tobacco-related cancer, and site-specific cancers of breast, lung, and colorectal comparing participants with HF vs. no HF, stratified by age strata at enrolment (all ages, ages 50–59, 60–69, and 70–79 years).
Time-dependent Kaplan–Meier curves were used to assess cancer-free survival probabilities stratified by those with HF and without HF. Follow-up time was defined as days from enrolment for all individuals to incident cancer (or censor). HF was modelled as a time-varying exposure. All participants began as unexposed (i.e. free of HF); then if HF developed, a participant became classified as exposed and follow-up time was henceforth counted in the exposed risk set until the end of follow-up. Censoring time was defined as time from enrolment to any other cancer diagnosis, non-cancer death, loss to follow-up, or 2005 for SRC participants (the end of adjudicated HF ascertainment) and 2015 for the EF sub-cohort (end of available follow-up). Hazard ratios (HRs) and 95% CI were calculated from multivariable-adjusted Cox proportional hazard regression models. Only participants with complete data were included in multivariable analysis (online supplementary Table S1). We fit separate models for total cancer, obesity-related cancers, tobacco-related cancers, and selected site-specific cancers.
Baseline covariates were decided a priori and were collected through questionnaires and clinic visits. Variables including WHI study component (observational study vs. clinical trial), age at enrolment, total physical activity (METs/week), alcohol use, cigarette smoking (pack-years), history of hypertension, cardiovascular disease (CVD) and hyperlipidaemia, family history of cancer, healthcare utilization (primary care visit within the last year), race/ethnicity, education, income, cardiac medications, and hormone therapy use were included in all multivariable-adjusted models. Additionally, the confounding effects of dietary intake were examined utilizing the healthy eating index into multivariable-adjusted models. In addition to baseline covariates, body mass index (BMI), cigarette smoking, and diabetes were modelled as time-varying covariates as these were hypothesized to be variables most strongly associated with HF and cancer.
The above analysis was repeated using HFpEF and HFrEF as exposures in the EF sub-cohort. For this, HF was classified as either HFpEF, HFrEF, or unknown EF and compared to no HF (reference group).
In secondary analyses, we explored baseline age, BMI, and smoking (pack-years) as potential effect modifiers by including an interaction term in the models. To account for surveillance bias, a longitudinal screening variable was created by calculating the average number of screening tests performed per year up until 1 year prior to cancer diagnosis (as not to include diagnostic tests). Screening tests were self-reported and included physical exam, blood pressure, cholesterol, electrocardiogram, breast exam, mammogram, Pap smear, rectal exam, hemoccult, and flexible sigmoidoscopy from the WHI annual questionnaire. For sensitivity analyses, we explored a LVEF cutoff of 40% for HFpEF and HFrEF. To test for residual confounding due to age, we explored time-dependent modelling with age in years as the time axis. Additionally, we repeated the main analysis including patients with self-reported HF at baseline that was later adjudicated. We also repeated the main analysis excluding participants with a cancer diagnosis identified through the NDI.
The proportional hazards assumption was confirmed using Schoenfeld residuals. A two-sided P-value of 0.05 was used to determine statistical significance. All analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study cohort
Baseline characteristics are presented in Table 1. Among 146 817 participants eligible for this analysis, median follow-up time was 8.4 years [interquartile range (IQR): 7.1–9.6 years]. The mean age was 63 ± 7 years and mean BMI 27 ± 6 kg/m2. Most women were Caucasian (82.6%) and never smokers (50.3%) with baseline comorbidities of diabetes (5.6%), hypertension (33.3%) and CVD (16.6%).
Table 1.
Baseline characteristics of participants in the main analysis and the ejection fraction sub-cohort stratified by adjudicated heart failure diagnosis during Women’s Health Initiative follow-up
| Total cohort (n = 146 817) | Total HF (n = 3272) | EF sub-cohort (n = 2528) | |
|---|---|---|---|
| Age (years), mean (SD) | 63.07 (7.20) | 68.10 (6.73) | 66.49 (6.84) |
| Ethnicity, n (%) | |||
| American Indian or Alaskan Native | 635 (0.4) | 17 (0.5) | 10 (0.4) |
| Asian or Pacific Islander | 3921 (2.7) | 33 (1.0) | 21 (0.8) |
| Black or African-American | 13 260 (9.1) | 375 (11.5) | 651 (25.8) |
| Hispanic/Latino | 5948 (4.1) | 78 (2.4) | 132 (5.2) |
| White | 120 984 (82.6) | 2721 (83.4) | 1693 (67.0) |
| Other | 1701 (1.2) | 37 (1.1) | 20 (0.8) |
| BMI categories, n (%) | |||
| Underweight (<18.5 kg/m2) | 1240 (0.9) | 28 (0.9) | 10 (0.4) |
| Normal (18.5–24.9 kg/m2) | 49 881 (34.3) | 746 (23.0) | 450 (17.8) |
| Overweight (25–29.9 kg/m2) | 50 741 (34.9) | 952 (29.4) | 764 (30.2) |
| Obesity (>30 kg/m2) | 43 691 (30.0) | 1514 (46.7) | 1284 (50.8) |
| Alcoholic servings/week, mean (SD) | 2.36 (4.86) | 1.77 (4.44) | 1.88 (4.91) |
| Physical activity (MET-h/week), mean (SD) | 12.46 (13.75) | 9.14 (11.32) | 9.14 (11.78) |
| Smoking (pack-years), mean (SD) | 9.83 (18.33) | 15.90 (25.03) | 14.20 (22.78) |
| Mammogram in last 2 years, n (%) | 118 591 (83.4) | 2470 (78.6) | 1803 (71.3) |
| PCP in last 1 year, n (%) | 115 644 (81.5) | 2784 (88.1) | 1989 (78.7) |
| Hormone use, n (%) | 95 795 (67.1) | 1840 (58.2) | 1153 (45.6) |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 8279 (5.6) | 716 (21.9) | 485 (19.2) |
| Hypertension | 48 425 (33.3) | 1926 (59.6) | 1371 (54.2) |
| Hyperlipidaemia | 18 992 (13.8) | 678 (22.5) | 422 (16.7) |
| Cardiovascular disease | 22 951 (16.6) | 1199 (22.5) | 633 (25.0) |
| Family history, n (%) | |||
| Any cancer | 29 003 (20.0) | 620 (19.3) | 481 (19.0) |
| Diabetes mellitus | 47 096 (32.2) | 1229 (37.7) | 1029 (40.7) |
BMI, body mass index; EF, ejection fraction; HF, heart failure; PCP, primary care physician; SD, standard deviation.
During follow-up, 17 474 (11.9%) participants (12.7 per 1000 person-years) developed any cancer. A total of 3272 (2.2%) participants developed HF during follow-up; of those, 235 (18.8 per 1000 person-years) developed HF prior to cancer. Age-adjusted Kaplan–Meier curves for survival free from cancer are shown in Figure 2. Median time from HF to cancer diagnosis was 2.0 years (IQR: 0.5–4.1 years). Incident rates for cancers by age strata for HF and non-HF participants are shown in online supplementary Table S2.
Figure 2.
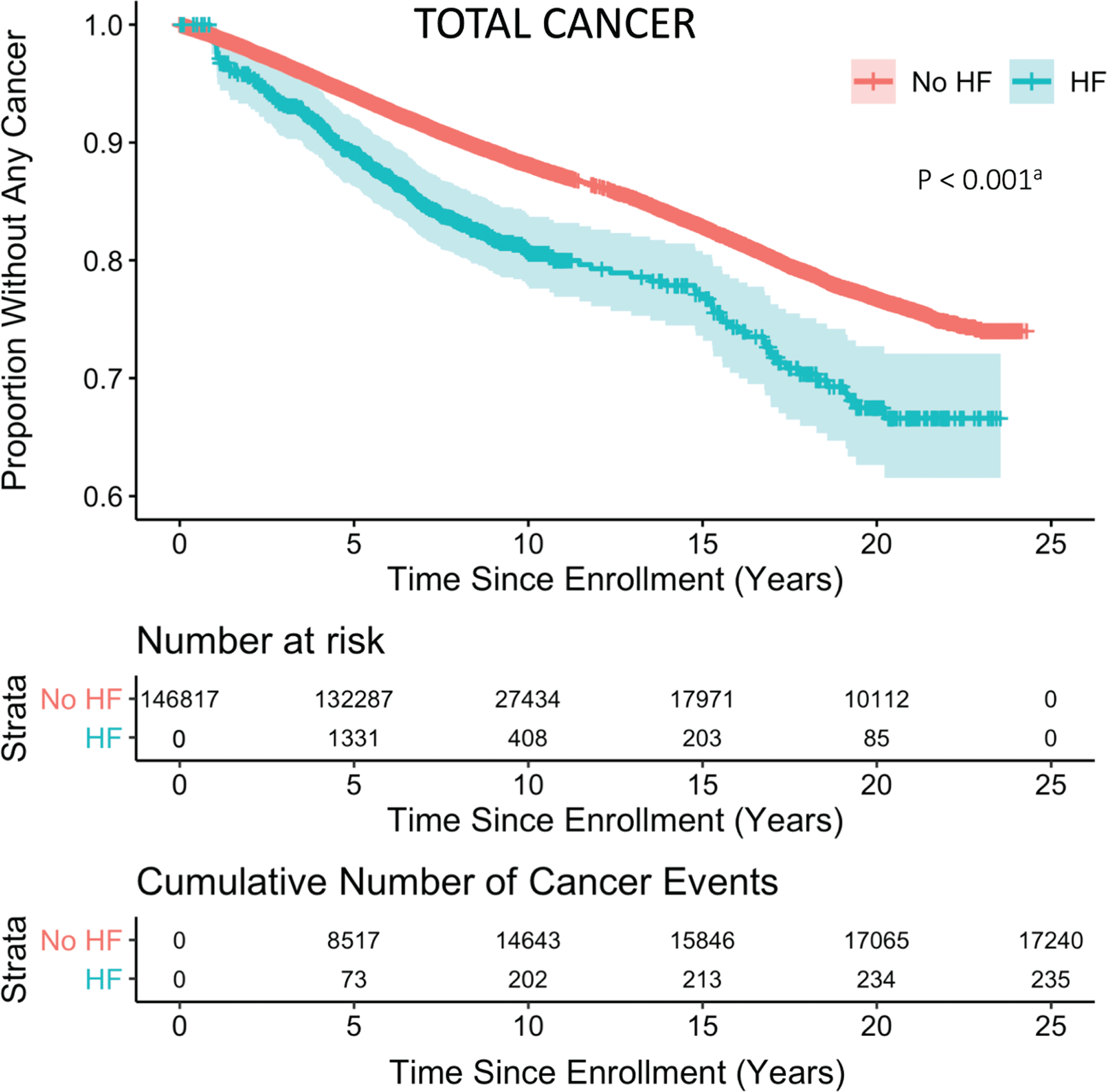
Kaplan–Meier analysis of cancer in heart failure (HF). Time-dependent Kaplan–Meier curve is shown for age-adjusted cancer-free survival probability of cohort participants stratified by HF and non-HF. Shaded regions represent 95% confidence intervals over time. aBased on unadjusted Cox proportional hazard model.
Ejection fraction sub-cohort
In the EF sub-cohort, where HFpEF and HFrEF could be distinguished, a total of 41 503 participants met the inclusion criteria. Median follow-up time was 13.4 years (IQR: 7.9–20.0 years). The mean age at time of enrolment was 63 ± 7 years. Baseline characteristics of the EF sub-cohort and total cohort are compared in online supplementary Table S3. Participants in the EF sub-cohort were more likely to identify as Black or African-American, have a greater BMI, be a current smoker, and have a history of diabetes, hypertension, and CVD.
Heart failure was diagnosed in 2528 (6.1%) participants during follow-up. Among those with available LVEF data, HFpEF and HFrEF accounted for 1193 (58.1%) and 859 (41.9%) of participants, respectively. Baseline characteristics of participants with HFpEF and HFrEF are presented in online supplementary Table S4.
A total of 7292 (17.6%) participants in the EF sub-cohort developed any cancer during follow-up at a mean age of 73 ± 8 years. Of those, 195 developed HF prior to cancer. Median time from HF to cancer was 2.2 years (IQR: 0.2–5.4 years).
Primary analysis
Depicted in Table 2, diagnosis of HF was associated with incident total cancer in the multivariate-adjusted model (HR 1.28, 95% CI 1.11–1.48). Significant associations were observed for obesity-related (HR 1.24, 95% CI 1.02–1.51), as well as site-specific lung (HR 1.58, 95% CI 1.09–2.30) and colorectal cancers (HR 1.52, 95% CI 1.02–2.27). No significant association was found between HF and breast or tobacco-related cancers (HR 1.17, 95% CI 0.87–1.56 and HR 1.24, 95% CI 0.94–1.63, respectively).
Table 2.
Association of overall heart failure with incident total and site-specific cancers
| Outcome | Age-adjusteda | Multivariable-adjusteda,b | ||||
|---|---|---|---|---|---|---|
| No. of events | HR (95% CI) | P-value | No. of events | HR (95% CI) | P-value | |
| Total | 17 475 | 1.39 (1.22–1.58) | <0.001 | 13 966 | 1.28 (1.11–1.48) | <0.001 |
| Obesity-related | 10 209 | 1.27 (1.07–1.52) | 0.008 | 8215 | 1.24 (1.02–1.51) | 0.03 |
| Tobacco-related | 3834 | 1.85 (1.47–2.32) | <0.001 | 3017 | 1.24 (0.94–1.63) | 0.13 |
| Breast | 5757 | 1.06 (0.81–1.39) | 0.67 | 4604 | 1.17 (0.87–1.56) | 0.29 |
| Lung | 1596 | 2.51 (1.85–3.40) | <0.001 | 1243 | 1.58 (1.09–2.30) | 0.02 |
| Colorectal | 1657 | 1.64 (1.13–2.38) | 0.009 | 1337 | 1.52 (1.02–2.27) | 0.04 |
CI, confidence interval; HR, hazard ratio.
All models stratified by Women’s Health Initiative study (clinical trial vs. observational study).
Variables: body mass index (kg/m2), diabetes (yes/no), and smoking (never, former, current) as time-varying covariates; smoking (pack-years), age at enrolment, baseline primary care physician visit within 1 year, physical activity (MET-h/week), alcohol (non-drinker, past drinker, <1 drink/month, <1 drink/week, 1–< 7 drinks/week, ≥7 drinks/week), ethnicity (American Indian or Alaskan Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, White, other), education (high school or less, high school or GED, >high school–Bachelor’s degree, >Bachelor’s degree), income (less than $34 999, $35 000–$74 999, $75 000–$99 999, >$100 000), hormone use ever (yes/no), hypertension (yes/no), cardiac medication use [beta-blockers (yes/no), calcium channel blockers (yes/no), antiarrhythmic (yes/no), and antihypertensives (yes/no)], family history of cancer, history of cardiovascular disease (yes/no), high cholesterol (yes/no).
The association of HFpEF and HFrEF with incident total and site-specific cancers among women in the EF sub-cohort is presented in Table 3, using parallel models to those in the main analysis. HFpEF was significantly associated with incident total cancer (HR 1.28, 95% CI 1.09–1.50) and site-specific cancers including lung (HR 1.79, 95% CI 1.09–2.92) and colorectal (HR 1.83, 95% CI 0.99–3.35) cancers. There was no statistically significant association with HFrEF and total cancer (HR 0.99, 95% CI 0.74–1.34). However, HFrEF was significantly associated with decreased incidence of obesity-related cancer (HR 0.59, 95% CI 0.35–0.99) and increased incidence of lung cancer (HR 1.76, 95% CI, 1.03–3.01).
Table 3.
Association of heart failure with preserved or reduced ejection fraction with incident total and site-specific cancers in the ejection fraction sub-cohort
| Age-adjusteda | Multivariable-adjusteda,b | |||||
|---|---|---|---|---|---|---|
| No. of eventsc | HR (95% CI) | P-value | No. of events | HR (95% CI) | P-value | |
| Totald | 7292 | 5701 | ||||
| No HF | 6753 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 539 (195) | 1.37 (1.19–1.58) | <0.001 | 1.28 (1.09–1.50) | 0.002 | |
| HFpEF | 253 (91) | 1.45 (1.18–1.79) | <0.001 | 1.34 (1.06–1.67) | 0.01 | |
| HFrEF | 169 (56) | 1.08 (0.83–1.41) | 0.56 | 0.99 (0.74–1.34) | 0.96 | |
| Obesity-related | 4122 | 3260 | ||||
| No HF | 3841 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 281 (80) | 1.03 (0.83–1.29) | 0.77 | 1.08 (0.85–1.37) | 0.52 | |
| HFpEF | 129 (35) | 1.03 (0.74–1.44) | 0.83 | 1.11 (0.79–1.57) | 0.55 | |
| HFrEF | 78 (17) | 0.60 (0.37–0.98) | 0.04 | 0.59 (0.35–0.99) | 0.05 | |
| Tobacco-related | 1974 | 1529 | ||||
| No HF | 1832 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 142 (67) | 1.56 (1.22–1.99) | <0.001 | 1.21 (0.92–1.61) | 0.18 | |
| HFpEF | 68 (31) | 1.63 (1.14–2.33) | 0.007 | 1.31 (0.88–1.96) | 0.18 | |
| HFrEF | 50 (25) | 1.62 (1.09–2.40) | 0.02 | 1.18 (0.74–1.89) | 0.49 | |
| Breast | 2176 | 1710 | ||||
| No HF | 2019 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 157 (35) | 0.87 (0.61–1.23) | 0.43 | 0.96 (0.67–1.37) | 0.82 | |
| HFpEF | 77 (16) | 0.91 (0.55–1.52) | 0.72 | 0.99 (0.58–1.67) | 0.96 | |
| HFrEF | 48 (9) | 0.57 (0.29–1.15) | 0.12 | 0.71 (0.35–1.42) | 0.33 | |
| Lung | 1006 | 771 | ||||
| No HF | 923 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 83 (51) | 2.18 (1.61–2.94) | <0.001 | 1.62 (1.14–2.30) | 0.008 | |
| HFpEF | 41 (27) | 2.48 (1.63–3.77) | <0.001 | 1.79 (1.09–2.92) | 0.021 | |
| HFrEF | 30 (19) | 2.36 (1.48–3.78) | <0.001 | 1.76 (1.03–3.01) | 0.038 | |
| Colorectal | 854 | 681 | ||||
| No HF | 787 | 1.0 (reference) | 1.0 (reference) | |||
| Any HF | 67 (3) | 1.66 (1.12–2.46) | 0.01 | 1.76 (1.16–2.67) | 0.008 | |
| HFpEF | 29 (13) | 1.63 (0.89–2.96) | 0.11 | 1.83 (0.99–3.35) | 0.05 | |
| HFrEF | 14 (3) | 0.53 (0.17–1.65) | 0.27 | 0.40 (0.10–1.60) | 0.19 | |
CI, confidence interval; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
All models stratified by Women’s Health Initiative study (clinical trial vs. observational study).
Variables: body mass index, diabetes, and smoking as time-varying covariates; smoking (pack-years), primary care physician visit within 1 year, physical activity (MET-h/week), alcohol (non-drinker, past drinker, <1 drink/month, <1 drink/week, 1–< 7 drinks/week, ≥7 drinks/week), ethnicity (American Indian or Alaskan Native, Asian or Pacific Islander, Black or African-American, Hispanic/Latino, White, other), education (high school or less, high school or GED, >high school–Bachelor’s degree, >Bachelor’s degree), income (less than $34 999, $35 000–$74 999, $75 000–$99 999, >$100 000), hormone use ever (yes/no), hypertension (yes/no), cardiac medication use [beta-blockers (yes/no), calcium channel blockers (yes/no), antiarrhythmic (yes/no), and antihypertensives (yes/no)], family history of cancer, cardiovascular diseaase (yes/no), high cholesterol (yes/no).
Unknown ejection fraction: total (n = 117), obesity-related (n = 74), tobacco-related (n = 24), breast (n = 32), lung (n = 30), colorectal (n = 24).
Number of participants with HF before a cancer diagnosis represented in parentheses.
When testing for differences between HFpEF and HFrEF, there was no significant difference between HFpEF and HFrEF in total or site-specific cancer (online supplementary Table S5). However, there were significant differences in obesity-related and colorectal cancers (HR 1.89, 95% CI 1.01–3.53 and HF 4.61, 95% CI 1.02–20.83, respectively).
Exploratory analysis
There was no evidence of effect modification for age or smoking on any cancer outcome. There was an interaction with BMI for breast cancer in which the 4th quartile (highest BMI) had the largest association between HF and breast cancer but contained a wide CI (HR 1.66, 95% CI 0.31–8.92) (online supplementary Table S6). Additionally, results were consistent with the main analysis when exploring a LVEF cutoff of 40% for HFpEF and HFrEF (online supplementary Table S7). When adjusting for a longitudinal screening variable up to 1 year before a cancer diagnosis, results for the association of any incident HF with total cancer remained significant (online supplementary Table S8). Similarly, the association between HFpEF and total cancer persisted in the EF sub-cohort (online supplementary Table S9). In the time-dependent analysis using age as the time scale to account for potential confounding by age, results were again consistent with our main analyses (online supplementary Table S10). After excluding participants with a cancer diagnosis identified through NDI, any HF remained significantly associated with any cancer; however, colorectal cancer was no longer significant in either the age- or multivariable-adjusted models (online supplementary Table S11). The association between HF and any cancer remained significant after including patients with adjudicated HF at baseline (online supplementary Table S12). The addition of healthy eating index did not change the results (online supplementary Table S13).
Discussion
In a large cohort of 146 817 post-menopausal women followed over 22 years, incident HF was significantly associated with subsequent risk of cancer. Increased risk was observed for obesity-related, lung, and colorectal cancers, but not for breast or tobacco-related cancer. After stratifying by LVEF, HFpEF appeared to be more strongly associated with total cancer as well as site-specific lung and colorectal cancers than HFrEF. Findings did not vary by baseline age, BMI, or smoking status.
Our finding of an increased risk of cancer among HF patients is consistent with some, but not all, prior studies (Figure 3). In two studies by Hasin et al.,7,10 a 2013 case-control study comparing cancer among community subjects newly diagnosed with HF with matched controls and a 2016 prospective cohort study of patients with myocardial infarction who later developed HF, there was a statistically significant increased risk of cancer among HF patients. These results were further reproduced in two large cohort studies of Danish and Japanese HF patients.6,11
Figure 3.
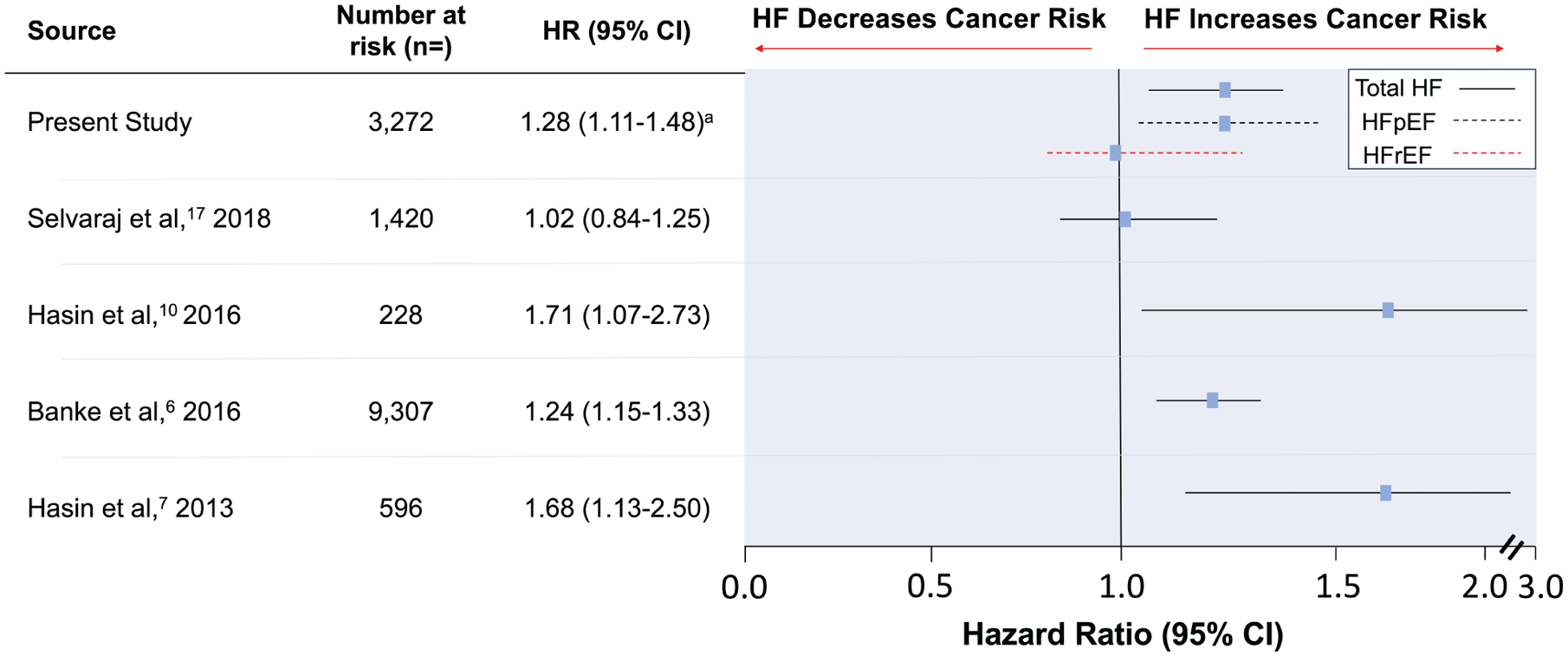
Studies investigating the association of heart failure (HF) with incident cancer. Hazard ratios (HR) and 95% confidence intervals (CI) are shown from studies evaluating the risk of incident cancer among patients with HF. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. aHR for HFpEF and HFrEF 1.28 (1.06–1.67) and 0.99 (0.78–1.34), respectively. bNumber of participants in each study with HF at risk of developing cancer.
In contrast, a more recent study by Selvaraj et al.17 reported a lack of association between HF and cancer. The study used the two large prevention trials of the all-male PHS.24 Although the study was supported by a large sample size and median follow-up period of 19.9 years, there were several key differences from prior studies. First, the PHS was restricted to relatively healthy, all-male participants. This is salient as in Hasin et al.’s 2013 study, there was a higher risk of any cancer among women compared to men and similar findings in the Japanese cohort. Women were underrepresented in these prior studies and thus gender differences may account for our findings. Second, the study did not specifically address the heterogeneous conditions of HFpEF vs. HFrEF as HF was determined by self-reporting rather than echocardiography or adjudication. Related to our findings of an association with HFpEF, the Japanese study found increased cancer risk with decreasing left ventricular diastolic dimensions – a hallmark of HFpEF – without an observed correlation in LVEF. HFpEF may have been underrepresented in the all-male PHS, as HFpEF is more common among women than men.25
When investigating specific cancers, HF was not associated with increased risk of breast or tobacco-related cancers in our adjusted models. The lack of association with breast cancer, in contrast to prior Danish and Japanese studies, may reflect differences in our post-menopausal cohort. For instance, obesity may be protective in pre-menopausal women in contrast to a linearly increased risk in post-menopausal women.26–28 It is thus plausible that shared risk factors for HF and breast cancer differ between pre- and post-menopausal women.
The elevation in tobacco-related cancers with HF was no longer significant after controlling for current vs. former and pack-years of smoking, whereas lung cancer remained significantly increased. This suggests our modelling of cigarette smoking was adequate to detect a confounding impact, but smoking does not completely explain the elevated lung cancer risk.
Although the basis for increased cancer incidence in HF cannot be inferred from our present study, there is a growing body of research into potential mechanisms. Among several hypotheses, interest surrounding shared risk factors between HF and cancer has emerged as one possible explanation. Traditional cardiovascular risk factors have been associated with increased risk of cancer; however, our findings remained significant after adjusting for these factors.29,30 Our findings may be due to unmeasured risk factors shared in the development of HF and cancer, as described below, rather than a cause-and-effect relationship.
Shared pathophysiological pathways by ways of oxidative stress, neuro-hormonal activation, and chronic inflammation may explain the association. In our study we observed increased risk of lung cancer among HF participants, regardless of LVEF, despite controlling for smoking at baseline and during follow-up. Trials such as the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) lend insight into these findings as well as the inflammatory intersect between HF and cancer.31 In the CANTOS trial, specific targeting of interleukin-1β conferred both a cardiovascular benefit – including a reduction in HF-related hospitalization – and a dramatic decrease in lung cancer incidence.32,33 Similar findings of an increased risk of lung cancer death in patients with HF and elevated C-reactive protein has been reported.34
Mutual pathogenic gene variants may also predispose individuals to both cancer and HF. Clonal hematopoiesis of indeterminate potential (CHIP) is an emerging entity in which somatic mutations in the blood are present in individuals without established haematologic abnormalities. Studies have demonstrated the presence of CHIP to be associated with increased risk of haematologic cancer, CVD, and both disease progression and mortality in HF.35–37 Additionally, a recent study examining the incidence of cancer in peripartum cardiomyopathy (PPCM), with mostly LVEF >50%, found women with PPCM had a nine-fold higher risk of cancer following diagnosis compared to matched controls in German and Swedish cohorts.38 This was correlated with exome sequencing showing PPCM participants had a higher prevalence of genetic variants in cancer promoting syndrome and DNA damage repair genes.
Lastly, another hypothesis suggests HF may promote oncogenesis through release of circulating factors. Meijers et al.39 presented evidence suggesting HF may promote intestinal precancerous polyp growth in mouse models. Evaluation of serum samples suggested a role for myocardium-secreted factors in HF and further provided translational evidence by showing these secreted factors were predictive of incident cancer in patients enrolled in the PREVEND study (Prevention of Renal and Vascular End-Stage Disease), independent of cancer risk factors.40
Strengths and limitations
The current study was conducted among a large cohort of women examining the association of incident HF with incident cancer where all endpoints were adjudicated rigorously in this study. Additionally, we were able to stratify HF by LVEF, which was not possible in prior studies.
Due to the observational nature of the study, causality cannot be determined. Although we were able to control for many potential confounding variables related to both HF and cancer not available in previous studies, the potential role for residual confounding remains. Repeated measures of longitudinal data for some variables, i.e. physical activity and dietary intake, were not represented in this analysis. Most variables were measured at enrolment and several years prior to HF diagnosis. Secondly, the study population was an all-female, post-menopausal cohort and is not generalizable to men or a younger population. Nonetheless, women are historically underrepresented in cardiology trials. The WHI was a predominantly White/Caucasian cohort and cancer risk may not be generalizable to a more diverse population. However, the EF sub-cohort (MRC) was racially diverse and yielded similar findings to the main WHI cohort analysis.
Thirdly, with a median time from HF to cancer diagnosis of around 2 years, our findings could reflect detection bias related to intensified medical evaluation following the diagnosis of HF. However, results remained largely unchanged after controlling for health care utilization and cancer screening. With 82% of WHI participants visiting a primary care physician in the year prior to enrolment, results may not be applicable in underserved populations with more limited access to medical care. EF data were not available for all participants due to the data ascertainment structure of the WHI; however, this is the largest cohort to date with EF data available addressing this question. Additionally, as HF adjudication required a hospitalization for HF as the primary diagnosis, our cohort may represent moderate-severe HF and excluded those with milder forms of HF. Lastly, these findings are generalizable only to a population who live long enough to develop HF with subsequent cancer diagnosis.
Conclusion
Among a large cohort of post-menopausal women, HF was associated with increased incident cancer. This association was strongest for lung cancer but was also seen for colorectal cancer and obesity-related cancers. There was no association with breast or tobacco-related cancer. The association was more pronounced in HFpEF than in HFrEF. Further research is needed to identify potential mechanisms driving this association.
Supplementary Material
Acknowledgement
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the programme possible.
Funding
This work was supported by the Women’s Health Initiative (WHI) programme which is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and the U.S. Department of Health and Human Services [HHSN268 201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C]. Additional funding for statistical support of this study was provided by the John L Locke Jr Charitable Trust Fund [RC].
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of interest: none declared.
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 4.MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, McMurray JJ. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102:1126–1131. [DOI] [PubMed] [Google Scholar]
- 5.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banke A, Schou M, Videbæk L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail 2016;18:260–266. [DOI] [PubMed] [Google Scholar]
- 7.Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail 2008;1:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J 2007;29:339–347. [DOI] [PubMed] [Google Scholar]
- 10.Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto M, Hasegawa T, Asakura M, Kanzaki H, Takahama H, Amaki M, Mochizuki N, Anzai T, Hamasaki T, Kitakaze M. Does the pathophysiology of heart failure prime the incidence of cancer? Hypertens Res 2017;40:831–836. [DOI] [PubMed] [Google Scholar]
- 12.Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler-Klein J, Cohen-Solal A, Farmakis D, López-Fernández T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, Von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG; Heart Failure Association Cardio-Oncology Study Group of the European Society of Cardiology. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018;20:879–887. [DOI] [PubMed] [Google Scholar]
- 13.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004;43:1432–1438. [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 2009;119: 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct pheno-types within the heart failure spectrum. Circulation 2011;123:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O’Connor CM, Butler J. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol 2018;3:1011–1019. [DOI] [PubMed] [Google Scholar]
- 17.Selvaraj S, Bhatt DL, Claggett B, Djoussé L, Shah SJ, Chen J, Imran TF, Qazi S, Sesso HD, Gaziano JM, Schrag D. Lack of association between heart failure and incident cancer. J Am Coll Cardiol 2018;71:1501–1510. [DOI] [PubMed] [Google Scholar]
- 18.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 2003;13(9 Suppl):S18–S77. [DOI] [PubMed] [Google Scholar]
- 20.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K, Klein L. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail 2016;9: e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curb JD, Mctiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S; WHI Morbidity and Mortality Committee. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol 2003;13 (9 Suppl):S122–S128. [DOI] [PubMed] [Google Scholar]
- 22.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer – viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 24.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 25.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209–2216. [DOI] [PubMed] [Google Scholar]
- 26.Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, Weiss LK, Liff JM, McDonald JA, Strom BL, Simon MS, Deapen D, Press MF, Burkman RT, Spirtas R, Ursin G. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev 2010;19:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015;107:djv088. [DOI] [PubMed] [Google Scholar]
- 28.Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, Hainaut P. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev 2013;14:665–678. [DOI] [PubMed] [Google Scholar]
- 29.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 33.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 34.Oikawa T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, Sato M, Aoyanagi H, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H; CHART-2 Investigators. Increased risk of cancer death in patients with chronic heart failure with a special reference to inflammation – a report from the CHART-2 Study. Int J Cardiol 2019;290:106–112. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 2018;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeffer TJ, Schlothauer S, Pietzsch S, Schaufelberger M, Auber B, Ricke-Hoch M, List M, Berliner D, Moulig VA, König T, Arany Z, Sliwa K, Bauersachs J, Hilfiker-Kleiner D. Increased cancer prevalence in peripartum cardiomyopathy. JACC CardioOncol 2019;1:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meijers WC, Maglione M, Bakker SJ, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Silljé HHW, de Boer RA. Heart failure stimulates tumor growth by circulating factors. Circulation 2018;138:678–691. [DOI] [PubMed] [Google Scholar]
- 40.Hillege HL, Janssen W, Bak A, Diercks G, Grobbee D, Crijns H, Van Gilst W, De Zeeuw D, De Jong P; PREVEND Study Group. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001;249:519–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


