Abstract
Objective:
The crisis of opioid use puts a strain on resources in the United States and worldwide. There are three U.S. Food and Drug Administration-approved medications for treatment of opioid use disorder: methadone, buprenorphine, and injectable naltrexone (XR-NTX). The comparative effectiveness and cost vary considerably among these three medications. Economic evaluations provide evidence that help stakeholders efficiently allocate scarce resources. Our objective was to summarize recent health economic evidence of pharmacologic treatment of opioid use disorder interventions.
Methods:
We searched PubMed for peer-reviewed studies in English from August 2015 through December 2019 as an update to a 2015 review. We used the Drummond checklist to evaluate and categorize economic evaluation study quality. We summarize results by economic evaluation methodology and pharmacologic treatment modality.
Results:
We identified 105 articles as potentially relevant and included 21 (4 cost-offset and 17 cost-effectiveness/cost-benefit). We found strengthened evidence on buprenorphine and methadone indicating these treatments are economically advantageous compared to no pharmacotherapy, but limited evidence on XR-NTX. Only half of cost-effectiveness studies used a generic preference-based measure of effectiveness, limiting broad comparison across diseases/disorders. The disease/disorder-specific cost-effectiveness measures vary widely, suggesting a lack of consensus on the value of substance use disorder treatment.
Conclusion:
We found studies that provide new evidence supporting the cost-effectiveness of buprenorphine compared to no pharmacotherapy. We found a lack of evidence supporting superior economic value for buprenorphine versus methadone suggesting both are attractive alternatives. Further economic research is needed on XR-NTX, other emerging pharmacotherapies, treatment modalities, and dosage forms.
Keywords: opioid use disorder, cost-effectiveness analysis, cost-benefit analysis, healthcare utilization, cost offset, systematic review
Précis
There is new evidence on buprenorphine and strengthened evidence on methadone indicating both are economically advantageous treatments for opioid use disorder.
INTRODUCTION
Approximately 27 million people worldwide have an opioid use disorder (OUD) including 2.1 million people in the United States.1,2 Opioids were involved in nearly 400,000 deaths worldwide and 48,000 deaths in the U.S. in 2017.3,4 The annual economic cost to the U.S. for OUD is estimated at $787 billion (2018USD) consisting of excess healthcare utilization, premature mortality, reduced workplace productivity, and criminal activity.5 Nonetheless, recent estimates indicate that only a third of people in the U.S. with OUD receive specialty treatment,1,2 and only one-fifth of those in treatment receive evidence-based pharmacotherapy.6
Experts agree OUD should be addressed as a chronic condition, without a recommended time limit on treatment, yet treatment typically lasts less than six months.7–9 As first-line OUD treatment, the American Society of Addiction Medicine recommends FDA-approved pharmacotherapy: methadone, buprenorphine, or extended-release naltrexone (XR-NTX)10; new delivery systems and pharmacologic treatments are under investigation.11 The opioid agonist, methadone, is restricted in the U.S. to certified opioid treatment programs.12 Buprenorphine, a partial agonist, is often combined with naloxone to prevent misuse, and can be prescribed in the U.S. in an office-based setting by providers who have received federal authorization (i.e., a DATA waiver) and can be administered daily in a sublingual form, or through an extended-release implant or injection.10,13 Naltrexone, an opioid antagonist, administered for OUD as a long-acting injection, approximately once every 28 days, requires patients to be abstinent from opioids prior to initiation. Oral naltrexone is characterized by low patient acceptance and high non-adherence, and not recommended for OUD treatment.10 In 2018, buprenorphine or methadone were available in 86 countries to treat people with OUD, with methadone being the most common.14 XR-NTX uptake is low compared to methadone and buprenorphine.15
Policymakers face limited and often shrinking budgets, and make decisions based on timeliness and clarity of evidence.16 A well-designed economic evaluation should inform decisions on how to allocate resources in a manner that will maximize desired outcomes (e.g., fewer opioid overdose events) subject to financial constraints. We sought to update the systematic literature review published by Murphy & Polsky in 2016,17 which found methadone economically advantageous but insufficient evidence for other medications, to improve our understanding of the quantity and quality of current evidence about the economic value of pharmacologic OUD interventions, and to identify policy-relevant gaps in the economic literature.
METHODS
Inclusion Criteria
We adopted the inclusion criteria used by Murphy & Polsky1 and excluded studies that were not economic evaluations of OUD interventions. For example, we excluded editorials; studies whose emphasis was treatment of a disorder other than opioids; and studies focused solely on identifying costs associated with OUD, as opposed to potential cost-offsets associated with treatment alternatives.
Search Strategy
We systematically searched PubMed, EMBASE, and Web of Science, according to recent recommendations,18 followed by a non-systematic, but meticulous review of Google Scholar to ensure we captured relevant articles. The search was conducted for the years 2015–2019 and included combinations of terms from the following categories: (1) OUD treatment and (2) health economic analysis; see Appendix Table B1 for search terms and a sample electronic search strategy.
Data Extraction
Author1 screened the search strategy results - study titles, abstracts, and additional text – to identify relevant studies and managed using EndNote™ X9 (Clarivate Analytics, Philadelphia, USA). Any uncertainty regarding study inclusion was resolved by consensus with Author2 and Author3. Author1 then reviewed the full text of all studies meeting the inclusion criteria, and extracted the following information: study details (author, publication year, population, and country); study design (e.g., cost-effectiveness, cost-benefit, observational, decision analytic model, etc.); intervention and comparator(s); stakeholder perspective; time horizon; location/setting; funding sources; reported outcomes; and findings. As in the Murphy & Polsky study,1 we used the Drummond checklist2 to assess quality and categorized the studies as: poor (1–3 points); average (4–7 points); or good (8–10 points) (Appendix B).
Data Synthesis
Given the diversity in patient populations and health economic methods, we conducted a structured narrative synthesis (rather than a meta-analysis) to summarize current evidence. We used the term “buprenorphine-naloxone” only when articles specified the use of the coformulated medication; otherwise, the medication was noted as “buprenorphine.” We reported author definition of OUD (e.g., DSM-V OUD) and used “OUD” when not specified. We classified detoxification, regardless of tapered medication use, to be non-pharmacologic treatment for OUD. We reported results in the currency and year in which they were published, if provided; otherwise, we inferred year from study references or denoted it as “unknown.” Strategies that were more-costly and less-effective (or less-costly and more-effective) than the comparator were labeled as “dominated” (or “dominant”), and, in accordance with best practices, we do not report a cost-effectiveness ratio. We summarized results by economic evaluation methodology (cost-offset/utilization vs. cost-benefit/cost-effectiveness studies), pharmacologic treatment (methadone, buprenorphine, or XR-NTX) or treatments (multiple medications), and treatment modality (e.g., patient-centered methadone versus methadone). If health economic results are not medication-specific (e.g. reported as opioid agonist treatment, OAT), we classified the study by majority medication.
Articles Excluded from Systematic Review after reading the abstract
We excluded 26 articles that were conference abstracts; 19 that focused solely on identifying the costs of opioid misuse, quality-of-life, or utilization outcomes; 17 that did not report health economic outcomes, or contain sufficient information on economic variables (e.g., no reported costs); 12 that were reviews of the literature; three that were trial protocols; four that did not include a pharmacologic treatment option; and three that were editorials.
RESULTS
We identified 3247 references for initial screening and ultimately removed 1051 duplications. We identified 105 abstracts as potentially relevant and included 21 in this review (Figure 1). We evaluated three articles as “average” quality per the Drummond checklist, and the remaining articles as “good” (Appendix B). We plotted the number of articles per year across both reviews and found approximately five articles per year from 2007–2019 (Figure 2). We identified five articles19–23 with at least one coauthor who reported industry sponsorships related to the published work (Appendix B); two sets of authors20,21 indicate employee sponsorship and all five studies indicate medication provision.
Figure 1:
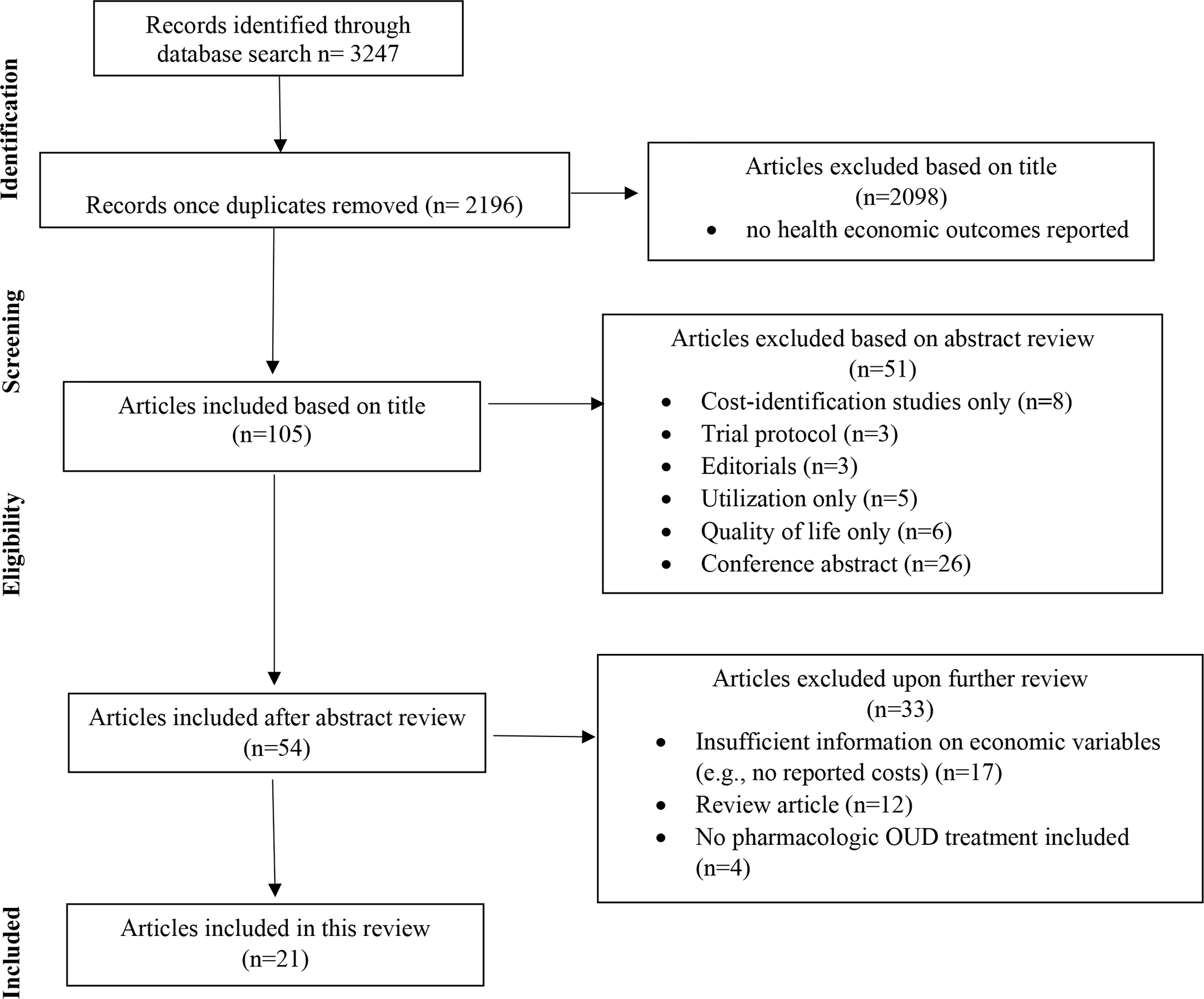
Article selection process. OUD = opioid use disorder.
Figure 2:
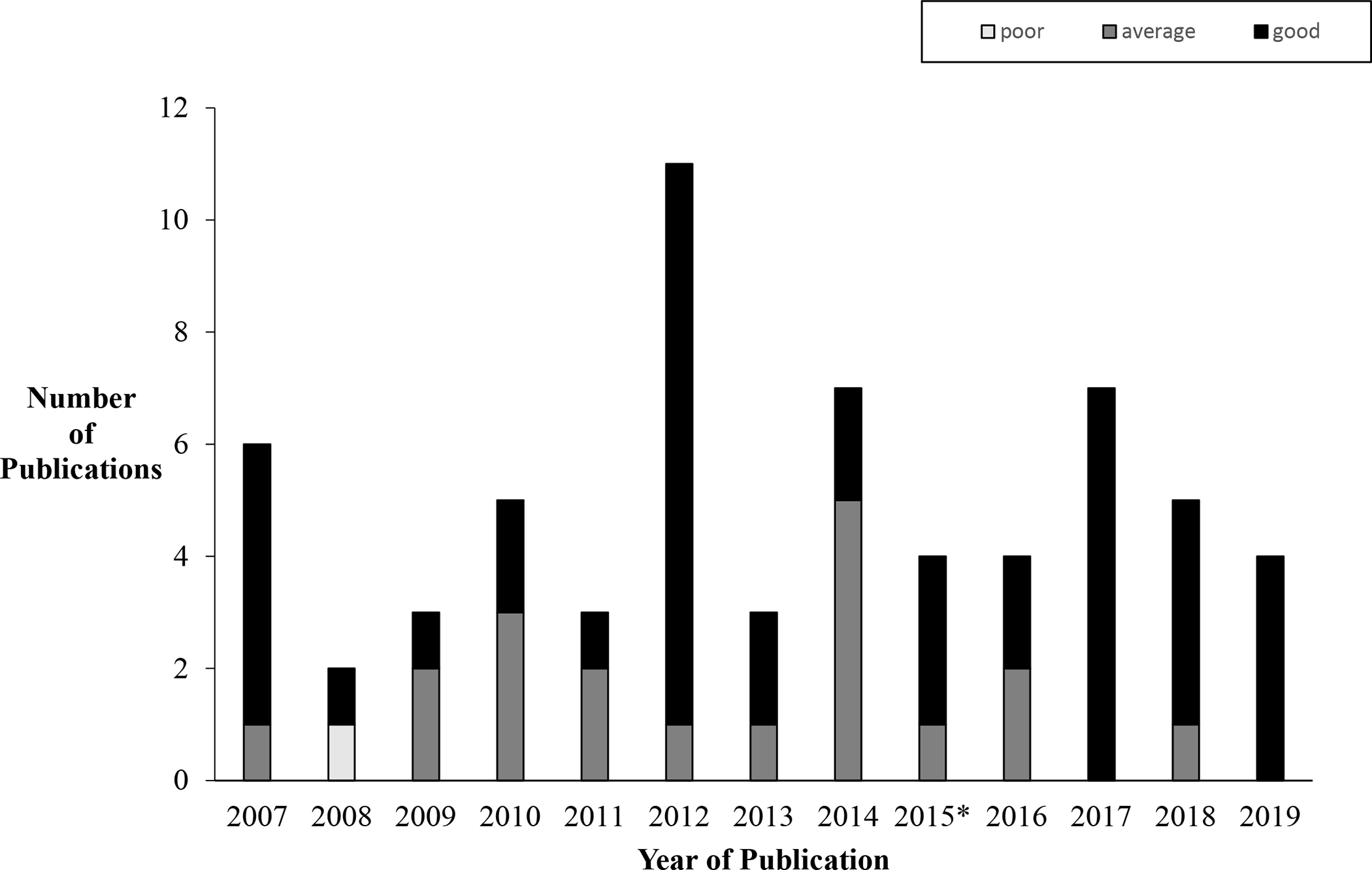
Number of published economic evaluations of medication to treat OUD (2007 – 2019). *3 of 4 publications in 2015 are summarized in Murphy & Polsky review;17 1 of 4 publications from 2015 are in included in this review.
Cost and Utilization
Four of the 21 articles focused primarily on identifying cost-offsets associated with OUD treatment by evaluating changes in healthcare resource utilization. The four studies were observational studies and took place in the U.S. (Table 1). We categorized three articles as “average” quality and one as “good” (Figure 3, Appendix B).
Table 1.
Study overview: cost offset and utilization studies
| Study | Country | Population | Intervention | Comparator | Study Design | Perspective and time horizon | Model and Outcomes | Findings | Ratings |
|---|---|---|---|---|---|---|---|---|---|
| Methadone compared to no pharmacologic treatment | |||||||||
| Krebs et al., 201724 | USA | criminal justice-involved adults admitted to publicly-funded OUD treatment in California from 2006 to 2010 | methadone | detoxification | observational (cohort analysis) | societal at 6 months | daily costs of crime, net criminal justice costs (2014 USD) | Daily costs of crime were $126 lower for methadone (95% CI: $116, $136) and $144 lower for detoxification (95% CI = $135, $154). Savings were estimated at $17,550 ($16,840, $18,383) at 6 months for adults enrolled in methadone compared to detoxification. | 10 Good |
| Buprenorphine treatment modalities | |||||||||
| Hsu et al. 201925 | USA | adults aged 18 to 64 enrolled in a Medicaid Managed Care plan in Maryland from 2008 to 2012 | integrated primary care and BUP onsite | BUP offsite | observational (cohort analysis) | third party payer at 6 and 12 months | BUP retention, hospital and ED utilization, healthcare costs (unknown USD) | Patients receiving integrated care compared to BUP offsite remained on treatment (79% vs 61%; p<0.001), experienced no significant difference in hospital and ED utilization, and had significantly lower health care costs ($4554; p<0.001). | 7 Average |
| Multiple Medications: combinations of two or more of the following buprenorphine, methadone, injectable hydromorphone, injectable diacetylmorphine, injectable naltrexone | |||||||||
| Shah et al. 201821 | USA | opioid dependent adults with 2 claims for XR-NTX, BUP, or methadone, or 3 claims for non-pharmacologic treatment between 2011 and 2014 | XR-NTX | methadone, BUP, or non-pharmacologic treatment | observational (cohort analysis) | third party payer baseline (1 year prior to index date) and follow-up (1-year post index date) |
healthcare costs including inpatient, outpatient, emergency department, and pharmacy cost (unknown USD) | XR-NTX patients experienced no change in healthcare costs. Healthcare costs increased for BUP by 43%, methadone by 48%, and non-pharmacologic cohort by 39%. | 7.5 Average |
| Mohlman et al. 201626 | USA | adults aged 18 to 64 enrolled in Vermont Medicaid receiving OUD treatment from 2008 to 2013 | methadone or BUP | non-pharmacologic treatment | observational (serial cross-sectional design) | Vermont Medicaid at 1 year | total healthcare expenditures including OUD treatment and other healthcare (unknown USD) | Beneficiaries receiving methadone or BUP had significantly lower non-OUD treatment expenditures than non-pharmacologic treatment (−2 409; p<0.01); when OUD treatment was included, total expenditures were not significantly different (−$412; p = 0.07) | 7 Average |
XR-NTX = injectable naltrexone; BUP = buprenorphine; OUD = opioid use disorder; USD = United States Dollars; CI = confidence interval; ED = emergency department
Score rubric: poor (1–3 points); average (4–7 points); good (8–10 points).
Figure 3.
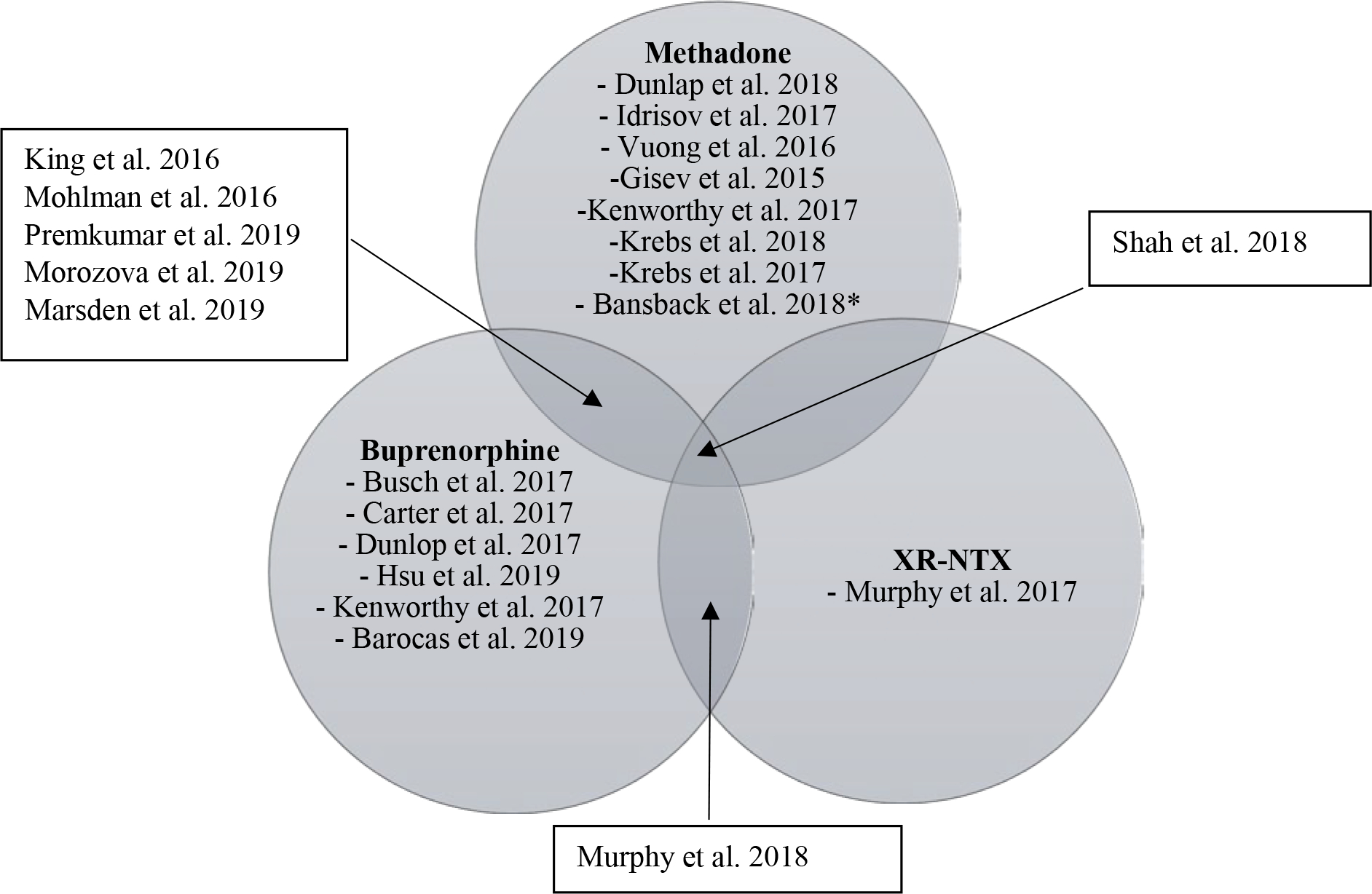
Results Venn diagram economic evaluation are OUD interventions. OUD = opioid use disorder; XR-NTX = injectable naltrexone
Methadone compared to no pharmacologic treatment
Krebs et al.24 conducted a cost-offset analysis from the societal perspective using retrospective cohort data from California-specific databases of criminal justice and death records of justice-involved adults admitted to publicly-funded OUD treatment from 2006 to 2010. Over a hypothetical 6-month period, the authors calculated the difference in criminal justice system costs for people recently de-incarcerated receiving OUD pharmacotherapy, compared to those who only received detoxification. The authors estimated the daily cost of crime to be $126 (2014USD) lower for people who received OUD pharmacotherapy (median duration 161 days) and $144 lower for people who received detoxification (median duration 19 days). Over 6 months, the state would save $17,550 in criminal justice and victimization costs for each justice-involved adult receiving OUD pharmacotherapy, compared to detoxification alone.
Buprenorphine treatment modalities
Hsu et al.25 conducted a retrospective cohort analysis of a comprehensive care practice integrating primary care and buprenorphine for OUD treatment, compared to buprenorphine offsite at outpatient practices participating in a large Medicaid managed care organization in Maryland. Patients receiving integrated primary care and buprenorphine had higher OUD treatment retention (79% vs 61%; p <0.001) and lower total healthcare costs (-$4554 unknown USD; p <0.001), with lower inpatient costs (-$2609, p = 0.001) offsetting the higher cost of buprenorphine ($987; p <0.001).
Multiple Medications: combinations of at least two of methadone, buprenorphine, XR-NTX
Shah et al.21 conducted a retrospective cohort analysis of adults meeting DSM-IV criteria for opioid dependence with at least two administrative claims (MarketScan Commercial®) for XR-NTX, buprenorphine, or methadone, or at least three claims for non-pharmacologic treatment within a period of 45 days of an initial OUD treatment visit from 2011–2014. Baseline costs were defined as twelve months before the initial treatment visit, and follow-up costs as the twelve months post initial OUD treatment visit. Total healthcare costs increased between baseline and follow-up for the buprenorphine, methadone, and no-medication cohorts (+43%, +48%, and +39%, p <0.05, respectively); the change in costs among the XR-NTX cohort was not statistically significant.
Mohlman et al.26 conducted a serial cross-sectional analysis of Vermont Medicaid beneficiaries with ICD-9 codes indicative of OUD between 2008 and 2013. Beneficiaries who received methadone or buprenorphine were compared to beneficiaries who received non-pharmacologic treatment only. On average, beneficiaries receiving pharmacologic treatment accrued $412 (unknown USD; p=0.07) less, annually, in general healthcare expenditures. When OUD treatment costs were excluded, beneficiaries receiving pharmacologic treatment accrued $2,409 (p<0.01) less in annual healthcare costs than the non-pharmacologic treatment group due to significantly lower utilization rates of inpatient, outpatient, and ancillary healthcare services. Primary care physician and surgical specialist visits did not differ significantly between the two groups.
Cost-Effectiveness and Cost-Benefit
The remaining 17 articles were CEAs or cost-benefit analyses of OUD pharmacotherapies. We assigned a “good” quality score to all studies (Table 2, Figure 3, Appendix B). Cost-effectiveness thresholds varied amongst studies (Table 3).
Table 2.
Study overview: cost-benefit and cost-effectiveness studies
| Study | Country | Population | Intervention | Comparator | Study Design | Perspective and time horizon | Model and Outcomes | Findings | Rating |
|---|---|---|---|---|---|---|---|---|---|
| Methadone compared to no pharmacologic treatment | |||||||||
| Idrisov et al 201727 | Russia | adults with OUD | methadone at four different levels of treatment capacity: 3.1%, 12.5%, 25%, and 55% | no methadone | decision analytic model (decision tree) | healthcare system at 10 years | program implementation costs, DALYs averted, cost per DALY averted (2015 USD) | At increasing treatment capacities (3.1%, 12.5%, 25%, 55%), methadone resulted in 49 739, 201 234, 404 265, 898 958 DALYs averted at a cost of $17 068 524, $69 051 186, $138 707 623 and $308 382 234, respectively. methadone compared to no methadone resulted in an ICER of 343 per DALY averted. | 10 Good |
| Vuong et al. 201628 | Vietnam | heroin dependent adults in Hai Phong City, Vietnam† | community-based, voluntary methadone | center-based compulsory rehabilitation | observational (cohort analysis) | program and participant at 3 years | cost per self-reported drug-free days (2013 VND and 2013 USD) | Community-based methadone compared to center-based compulsory rehabilitation resulted in lower costs (-VND85.73 million or -$4108) and increase in drug-free days (344 drug-free days) (p<0.001). Findings were robust in sensitivity analyses. | 8.5 Good |
| Gisev et al. 201530 | Australia | 16,073 recently-released criminal justice involved individuals with a history of OUD | methadone | no medication | observational (cohort analysis) | treatment provider and criminal justice system at 6 months | cost per death avoided within 6 months of first prison release (2012 AUD) | Methadone dominated no medication. The probability of methadone to be cost-effective per life-year saved is 97% at a willingness to pay of $500 per life-saved | 10 Good |
| Krebs et al. 201831 | USA | individuals initially presenting for publicly funded treatment of opioid use disorder | immediate access to methadone | observed standard of care‡ | decision analytic model (semi-Markov model) | societal at lifetime horizon | QALYs and costs (2016 USD) | Immediate access to methadone dominates observed standard of care by costing less ($78 257) and being more effective (0.42) and in greater than 99% of probabilistic sensitivity analyses. Total lifetime savings estimated at $3.8 billion. | 10 Good |
| Methadone treatment modalities | |||||||||
| Dunlap et al. 201832 | USA | 300 adults initiating methadone in an outpatient treatment program in Baltimore, MD | patient-centered methadone* | methadone | randomized clinical trial | treatment program at 12 months | average treatment cost per patient, cost per self-reported day of heroin use abstinence in the past 30 days, cost per one percentage increase in patients with opioid-positive urine screen, cost per one percentage increase in participants not meeting DSM-IV opioid dependence criteria (2015 USD) | Patient-centered methadone and methadone had similar costs per patient ($2395 vs $2292; p =0.49). The ICER for patient-centered MET compared to methadone was $242 per self-reported day of heroin use abstinence in the past 30 day and $1160 per one percent point increase in participants not meeting DSM-IV opioid dependence criteria. Patient-centered methadone resulted in a decreased % of patients with positive opioid-positive urine screens at a higher cost when compared to methadone (i.e., is dominated by methadone). | 10 Good |
| Buprenorphine-Naloxone compared to no pharmacologic treatment | |||||||||
| Busch et al. 201734 | USA | 329 opioid-dependent adults presenting at an urban teaching hospital ED | brief intervention with buprenorphine-naloxone initiation in the ED and ongoing integrated primary care | brief intervention with referral to community-based treatment referral alone | randomized clinical trial | healthcare system at 30 days | cost per patient enrolled in formal addiction treatment at 30 days (2013 USD) cost per self-reported opioid-free day, in the past 7 days |
ED-initiated buprenorphine-naloxone dominated both comparators and has a greater than 99% of being cost-effective at a willingness-to-pay threshold of $30 per individual engaged in buprenorphine treatment at 30 days. ED-initiated buprenorphine has a 50% cost-effective at $100 per opioid-free day threshold and increases to 80% at $500 per opioid-free day. | 10 Good |
| Dunlop et al. 201735 | Australia | 50 patients with DSM-IV heroin dependence | outpatient buprenorphine-naloxone with weekly clinical visits | open-label waitlist§ | randomized clinical trial | healthcare at 12 weeks healthcare + criminal justice at 12 weeks |
Incremental cost per heroin-free day at 12 weeks (2009 AUS) | From the healthcare perspective, buprenorphine-naloxone compared to open-label waitlist resulted in an additional $18.24 per heroin-free day (95% CI: $4.50 to $28.49). Including cost of crime, buprenorphine -naloxone cost less and resulted in more heroin-free days (i.e., dominates). | 10 Good |
| Barocas et al. 201936 | USA | HIV/HCV co-infected patients who have OUD and are being considered for HCV treatment | BUP-NX in onsite care for HIV/HCV co-infected persons | referral to offsite OUD care | decision analytic model (Monte Carlo microsimulation) | healthcare at lifetime | Cost per QALY (2017 USD) | BUP-NX is cost-effective with an ICER of $57,100/QALY across a plausible range of parameter values assuming a WTP of $100,000/QALY | 9.5 Good |
| Buprenorphine-Naloxone treatment modalities | |||||||||
| Carter et al. 201719 | USA | clinically-stable adults with OUD | subdermal implantable buprenorphine | sublingual buprenorphine | decision analytic model (Markov model) | societal at 12 months | incremental cost-per QALY incremental net monetary benefit (2016 USD) |
Subdermal implantable BUP dominated sublingual BUP and is preferred in 89% of probabilistic sensitivity analyses. The incremental net monetary benefit of subdermal implantable BUP vs. sublingual BUP was $5,953 (p<.05), at a WTP of $50,000 per QALY. | 10 Good |
| Extended-Release Naltrexone compared to no pharmacologic treatment | |||||||||
| Murphy et al. 201723 | USA | community-dwelling adults aged 18 to 60 involved with the criminal justice system with prior DSM-IV OUD | XR-NTX | counseling and offsite referral | randomized clinical trial | taxpayer at 25 weeks and 78 weeks | cost per abstinent year cost per QALY (2014 USD) |
At threshold of $100,000/QALY, XR-NTX is unlikely to be cost-effective (10%) at 25 wks and likely (60%) cost-effective at 78 wks. XR-NTX is >50% cost-effective at 25 wks when WTP is >160,000/QALY. XR-NTX has >50% of being cost-effective at $50,000 /abstinent year at 25 weeks. At 78 wks, exceeds 95% at $10,000 per abstinent year. |
10 Good |
| Unspecified medications (methadone or buprenorphine) compared to no pharmacologic treatment | |||||||||
| Morozova et al. 201937 | Ukraine | people at risk and with OUD in 3 different Ukrainian cities | Plausible OAT (Methadone or Buprenorphine) scale-up strategies | Standard of care | compartmental modeling | Payer at 10 years | Incremental cost per QALY (2016 USD) | The optimal strategy and probability of cost-effectiveness varies according to WTP threshold, as well as other inputs such as baseline OAT demand, site of PWID population, and treatment retention. Cost-effectiveness was evaluated relative to a WTP range of $0/QALY to $6,555/QALY (3 x per GDP). | 9.5 Good |
| Multiple Medications:combinations of two or more of the following buprenorphine, methadone, injectable hydromorphone, injectable diacetylmorphine, injectable naltrexone | |||||||||
| Bansback et al. 201838 | Canada | 202 persons who inject drugs with severe OUD in Vancouver | hydromorphone | diacetylmorphine or methadone | decision analytic cohort model (Markov model) | societal at 6 months and lifetime | Cost per QALY, cost per incremental costs (2015 USD) | Hydromorphone and diacetylmorphine had similar costs and benefits and dominate methadone when compared directly by providing more benefit at a lower cost. Hydromorphone and diacetylmorphine had a 67%, and 75%, respectively, of dominating methadone in probabilistic sensitivity analysis. Hydromorphone dominates diacetylmorphine and in 16% of probabilistic sensitive analyses. | 9.5 Good |
| Kenworthy et al. 201720 | United Kingdom | patients with OUD | buprenorphineor methadone | no medication | decision analytic model (decision tree) | societal UK National Health service & personal social service at 1 year |
cost per QALY (2016 UK) | Medication compared to no medication is cost-effective at £13,923/QALY for BUP and £14,206/QALY for methadone. Medication will have a net savings £14,032 for BUP or £17,174 for methadone /year. At WTP threshold of £30,000 /QALY BUP and methadone are cost-effective in >60% of simulations when compared individually to no medication. | 10 Good |
| King et al. 201639 | USA | hypothetical cohort of 1 000 opioid-dependent adults with no history of OUD treatment in past 30 days | office-based buprenorphine | clinic-based methadone | decision analytic model (Markov model) | third party payer at 1 year | cost per additional patient in treatment gained cost per additional opioid abuse-free week gained (2014 USD) |
The ICER for methadone vs buprenorphine was $10,437 per additional patient in treatment gained and $8,515 per additional opioid abuse-free week gained. Methadone is preferred in the base case at a threshold of $14,000 per patient retained in treatment at 1 year; results were sensitive to cost of methadone. | 9.5 Good |
| Premkumar et al. 201943 | USA | pregnant women with OUD | methadone or buprenorphine | detoxification w/ 14-day buprenorphine taper | decision analytical model (Markov model) | healthcare payer at 1 year | cost per QALY (2017 USD) | Buprenorphine dominated both methadone and detoxification at a WTP of $100,000 with a 70.5% of being cost-effective. Buprenorhpine no longer cost-effective if cost of MET was 8% less than base case or if overall costs for detox decreased by 79% or more. | 9.5 Good |
| Marsden et al. 201922 | London, UK | people who met DSM-IV criteria for opioid or cocaine dependence or both in the past 12 months | Methadone or buprenorphine with psychosocial intervention (PSI) | methadone or buprenorphine alone | randomized clinical trial | societal at 18 weeks | cost per QALY (2016 UK) Cost per 1% improvement in probability of treatment response |
The probabilities that the PSI were cost-effective relative to treatment as usual were 60% and 67%, respectively, at the NICE willingness-to-pay thresholds of £20 000 per QALY and £30 000 per QALY but decreases to 36% and 56%, respectively, from a limited healthcare perspective at £20 000 per QALY and £30 000 per QALY. The probability the cost per 1% improvement in treatment response is high as 87% at a WTP of £1000 and low as 50% at £30. | 10 Good |
| Murphy et al. 201945 | USA | adults with DSM-V OUD presenting at community based treatment programs offering detoxification services | XR-NTX | buprenorphine-naloxone | randomized clinical trial | healthcare and societal at 24 weeks and 36 weeks | cost per QALY cost per abstinent year (2016 USD) |
At a WTP of 100 000 per QALY, XR-NTX compared to buprenorphine-naloxone was unlikely to be cost-effective in the intention-to-treat (30%) and per-protocol samples (<50%) unless the time period was extended to 36 weeks; resulting in approximately 50% and 80%, respectively. | 10 Good |
defined as respectful of and responsive to individual patient preferences, needs, and values
defined as daily heroin use in the 3 months prior to treatment initiation
observed standard of care defined as 54.3% initiate opioid use disorder treatment with medically managed withdrawal
no clinical intervention
USD = United States Dollars; QALY = quality-adjusted life-year; DALY = disability-adjusted life-year; VND = Vietnamese Dongs; XR-NTX = injectable naltrexone; OUD = opioid use disorder
Score rubric: poor (1–3 points); average (4–7 points); good (8–10 points). 15 Modeling & 6 RCT
Table 3.
Summary of cost-effectiveness thresholds
| Cost-effectiveness Measure | Threshold Single Point Estimate | Threshold Range | Study or Studies | Supporting Evidence for Threshold |
|---|---|---|---|---|
| Generic preference-based outcome | ||||
| Cost per QALY | £20,000 to £30,000 per QALY | N/A | Marsden 201922 | UK NICE willingness-to-pay threshold44 |
| £30,000 per QALY | N/A | Kenworthy 201720 | UK NICE willingness-to-pay threshold44 | |
| $50,000 per QALY | N/A | Carter 201719 | no citation provided | |
| $100,000 per QALY | N/A | Premkumar 201943 Barocas 201936 |
Neumann 201451 Neumann 201652 |
|
| $100 000 to $200 000 per QALY | N/A | Murphy 201945 Murphy 201723 | Neumann 201451 | |
| averting one QALY for < 3x per-capita gross domestic product should be considered “cost-effective” | $0 to $6555 per QALY | Morozova 201937 | Neumann 201451 | |
| none listed | N/A | Bansback 201838 Krebs 201831 |
N/A | |
| Cost per DALY | averting one DALY for < 3x and <1x the per-capita gross domestic product should be considered “cost-effective” and “highly cost-effective”, respectively | N/A | Idrisov 201727 | World Health Organization’s Choosing Interventions that are Cost–Effective project (WHO-CHOICE)53 |
| Non-preference based outcome | ||||
| Treatment retention | ||||
| Cost per patient retained at treatment at 1 year | $14,000 per patient retained at treatment at 1 year | N/A | King 201639 | previous studies estimated $14,000 as the excess annual direct costs to third-party payers for patients diagnosed with OUD40–42 |
| Cost per enrollment in formal addiction treatment at 30 days (%) | N/A | $0 to $30 per enrollment in formal addiction treatment at 30 days | Busch 201734 | assumed by range provided in CEAC |
| Clinical outcome | ||||
| Cost per death avoided within 6 months of first prison release | $25,000 per death avoided within 6 months of first prison release | N/A | Gisev 201530 | half of the frequently used ceiling of $50 000 per additional life-year54 |
| Cost per change in days of self-reported illicit opioid use in the past 7 days (days) | N/A* | $0 to $500 per change in days of self-reported illicit opioid use in the past 7 days (days) | Busch 201734 | assumed by range provided in CEAC |
| Cost per abstinent year | N/A | $0 to $200,000 per abstinent year | Murphy 201723 | assumed by range provided in CEAC |
| Cost per one additional day abstinent in the past 30 days | N/A* | $0 to $600 per additional day abstinent in the past 30 days | Dunlap 201832 | assumed by range provided in CEAC |
| Cost per one percentage point increase in patients not opioid dependent | N/A* | $0 to $5000 per one percentage point increase in patients not opioid dependent | Dunlap 201832 | assumed by range provided in CEAC |
| Cost per one percentage point increase in patients with a negative urine test for opioids | N/A* | $0 to $5000 per one percentage point increase in patients with a negative urine test for opioids | Dunlap 201832 | assumed by range provided in CEAC |
| Cost per self-reported drug-free days | None listed | None listed | Vuong 201628 | N/A |
| Cost per heroin-free day at 12 weeks | None listed | None listed | Dunlop 201735 | N/A |
authors state threshold should be set by decision maker N/A = not applicable; QALY = quality-adjusted life-year; CEAC = cost-effectiveness acceptability curve; DALY = disability-adjusted life-year; OUD = opioid use disorder
Methadone compared to no pharmacologic treatment
Idrisov et al.27 conducted a modeling study from the healthcare-system perspective in Russia to predict the implementation costs and disability-adjusted life years (DALYs) associated with methadone, compared to no methadone. The authors modeled hypothetical cohorts of people with OUD across scenarios where 3.1% to 55.0% of the population has access to methadone, resulting in a projected 49,739 to 898,958 DALYs averted and $17 million to $308 million (2015USD) saved over a 10-year period, resulting in a cost-per-DALY averted of $343 across all scenarios.
Vuong et al.28 conducted a cohort analysis of adults in Vietnam with OUD in a community-based voluntary methadone program, versus a compulsory rehabilitation center, for up to 2 years.29 The authors conducted analyses from the OUD treatment-sector perspective and included participant costs in sensitivity analyses. Over a 3-year time-period, methadone cost $4,108 (2013USD) less than the compulsory rehabilitation center, and participants taking methadone had 344 (p<0.001) additional drug-free days compared to compulsory rehabilitation center participants, suggesting that voluntary methadone dominates compulsory rehabilitation.
Gisev et al.30 conducted a cost-effectiveness analysis (CEA) of methadone compared to no pharmacotherapy in an observational cohort of recently-released, justice-involved persons in Australia with OUD. The authors included treatment provider, criminal justice system, and crime costs to calculate the cost per life-year-saved within 6 months of first prison release. The point estimates indicate that methadone dominated no-pharmacotherapy, and was cost-effective with 97% certainty at a willingness to pay of $500 per life-year-saved (2012AUD).
Krebs et al.31 conducted a decision analysis using a semi-Markov cohort model to compare immediate access to methadone, to short-term, medically-managed withdrawal in California among adults initially presenting for publicly-funded treatment for OUD. The authors used a societal perspective, including healthcare and criminal justice costs, over a lifetime horizon. In the base-case, immediate access to medication dominated detoxification, providing an additional 0.42 QALYs at a lower average cost of $78,257 (2016USD) per-person. The estimated lifetime savings were $3.8 billion for the nearly 50,000 people in California with OUD.
Methadone treatment modalities
Dunlap et al.32 conducted a CEA alongside a U.S. randomized controlled trial (RCT) of adults newly admitted to patient-centered methadone, a strategy based on patient preferences, values, and needs,33 compared to methadone alone. The authors determined that patient-centered methadone would be cost-effective with at least 50% certainty at a willingness-to-pay threshold of $220 per abstinent-day (2015USD), and with ~50% certainty at a willingness-to-pay threshold of $1,300 per percentage-point increase in patients no longer meeting DSM-IV criteria for opioid dependence at 12 months; however, methadone alone would be cost-effective with ~75% certainty at willingness-to-pay threshold of $5,000 per percentage-point increase in patients with a negative opioid urine screen.
Buprenorphine-naloxone compared to no pharmacologic treatment
Busch et al.34 conducted a CEA alongside an RCT of patients with a DSM-IV OUD diagnosis presenting at an urban ED in the U.S. The study arms were: 1) brief intervention with buprenorphine-naloxone initiated in the ED, 2) brief intervention with facilitated referral to community-based treatment, and 3) referral alone. From a healthcare-system perspective, ED-initiated buprenorphine-naloxone was cost-effective compared to brief intervention or referral alone with 99% certainty, assuming a willingness-to-pay of $30 (2013USD) per one percentage-point increase in the probability a patient is engaged in treatment at 30 days, and 50% certainty assuming a willingness-to-pay of $100 per opioid-free day.
Dunlop et al.35 estimated the cost-effectiveness of buprenorphine-naloxone to an open-label waitlist (i.e., no clinical intervention) alongside an RCT including 1) healthcare perspective only and 2) healthcare + criminal justice (i.e. cost-of-crime) perspectives in 50 patients with DSM-IV heroin dependence in Australia. From the healthcare perspective, buprenorphine-naloxone compared to waitlist cost an additional $18.42 (95% CI: 4.50 to 28.49, 2009AUD) per heroin-free day. When crime costs were included, the authors found an estimated net savings of $8,273 over the 12-week intervention period.
Barocas et al.36 constructed a decision analytic model to evaluate the cost-effectiveness of OUD treatment for persons co-infected with HIV and HCV. The treatment arms were 1) standard HIV/HCV care integrated with onsite buprenorphine-naloxone, and 2) standard HIV/HCV care with referral to offsite OUD care (treatment as usual, TAU). The authors conducted analyses from the U.S. healthcare perspective with a lifetime horizon. In the base case, buprenorphine-naloxone was cost-effective at $57,100/QALY (2017USD) compared to a willingness-to-pay threshold of $100,000/QALY. The cost-effectiveness results were robust in sensitivity analyses unless buprenorphine-naloxone was 75% less effective than the base case and the cost was equal to or greater than the base-case estimate.
Buprenorphine-naloxone treatment modalities
Carter et al.19 developed a Markov model to analyze the cost-effectiveness and cost-benefit of subdermal implantable buprenorphine, compared to sublingual buprenorphine-naloxone, from a U.S. societal perspective. Subdermal implantable buprenorphine dominated sublingual buprenorphine in the base-case and 89% of probabilistic sensitivity analyses, assuming a willingness-to-pay threshold of $50,000/QALY (2016USD). The authors also calculated incremental net monetary benefit. Valuing QALYs at $50,000, the authors found favorable results for subdermal implantable buprenorphine compared to sublingual buprenorphine ($20,812 vs $15,099; p <0.05).
XR-NTX versus no pharmacologic treatment
Murphy et al.23 conducted a CEA alongside an RCT comparing XR-NTX to counseling with offsite referral among community-dwelling, justice-involved persons. The authors conducted analyses from the U.S. taxpayer perspective over the 25-week intervention period, and the entire 78-week observation period. The probability that XR-NTX was cost-effective at 25 weeks ranged from 10% with a willingness-to-pay threshold of $100,000/QALY, to 62% with a threshold of $200,000/QALY; at 78 weeks the respective probabilities were 59% and 76%.
Unspecified medications (methadone or buprenorphine) compared to no pharmacologic treatment
Morozova et al.37 developed a compartmental model of people at-risk for, or living with OUD in 3 Ukrainian cities to assess the cost-effectiveness of plausible, scale-up strategies of opioid agonist treatment (methadone or buprenorphine) versus standard of care (no pharmacological treatment), from the payer perspective at 10 years. The authors used a willingness-to-pay threshold of 3 times the Ukrainian GDP ($6,555/QALY, 2016USD) and found increases in capacity (Kyiv: 12.2-fold increase, Mykolaiv: 2.4-fold increase, Lviv: 13.4-fold increase) to be cost-effective with modest amounts of people in treatment (20%, 11%, 17%, respectively), as reaching maximum capacity was not efficient due to limited demand.
Multiple Medications: combinations of two or more of: buprenorphine, methadone, injectable hydromorphone, injectable diacetylmorphine, injectable naltrexone
Bansback et al.38 developed a lifetime decision analytic cohort model informed by a 6-month randomized clinical trial of people who inject opioids long-term in Canada, with at least two attempts at treatment (including one with methadone). The authors included three strategies: injectable hydromorphone, injectable diacetylmorphine, and methadone. Injectable hydromorphone and injectable diacetylmorphine had similar costs and benefits. Hydromorphone and diacetylmorphine had a 67% and 75% probability of dominating methadone, respectively.
Kenworthy et al.20 modelled the cost-effectiveness of buprenorphine and methadone compared to no pharmacologic treatment for persons with OUD. From the U.K. healthcare-system perspective, the cost-effectiveness ratios were £13,923/QALY (2016GBP) for buprenorphine and £14,206/QALY for methadone, compared to no medication; buprenorphine and methadone were not compared directly. At a willingness-to-pay threshold of £30,000/QALY, buprenorphine and methadone were cost-effective in >60% of simulations. From the societal perspective, buprenorphine and methadone dominated no medication, resulting in a savings of £14,032 for buprenorphine or £17,174 for methadone, however, no level of certainty was indicated.
King et al.39 developed a Markov model to evaluate the cost-effectiveness of office-based buprenorphine compared to methadone dispensed at a specialized clinic, among a U.S. cohort of adults with OUD. The evaluation was conducted from a third-party-payer perspective, with effectiveness measures of patients retained in treatment and number of opioid-free weeks. The incremental cost-effectiveness ratio for methadone compared to buprenorphine was $10,437 (2014USD) per-patient-retained-in-treatment at 1 year; however, the results were sensitive to the cost of methadone: a 10% reduction of the cost led methadone to dominate buprenorphine, while a 10% increase led methadone to exceed the a priori willingness-to-pay threshold of $14,000 per-patient-retained-in-treatment at 1 year. The $14,000 threshold was based on the estimated excess annual cost to third party payers for adults with OUD.40–42 Methadone had an incremental cost-effectiveness ratio of $8,515 per-additional-opioid-free week gained compared to buprenorphine, but no value threshold was defined.
Premkumar et al.43 developed a Markov model to assess the cost-effectiveness of 1) methadone; 2) buprenorphine; or 3) detoxification, which included a 14-day taper of buprenorphine, for the management of OUD during pregnancy in the U.S. The authors conducted the analysis from a healthcare-payer perspective at 1 year. At a willingness-to-pay threshold of $100,000/QALY (2017USD), buprenorphine (71%) was preferred versus methadone (4%) or detoxification (26%). In deterministic sensitivity analyses, buprenorphine remained cost-effective except in cases of modest reductions in cost of methadone (> 8%) or substantial reduction in detoxification costs (>79%).
Marsden et al.22 conducted a CEA alongside an RCT of patients in the U.K. engaged in OUD pharmacotherapy for at least 6 weeks, who used cocaine or opioids in the past 28 days. Participants were randomized to pharmacotherapy or pharmacotherapy plus a psychosocial intervention (PSI). From a societal perspective at 18 weeks, the probability that the PSI was cost-effective relative to TAU was 60% and 67% at the NICE44 willingness-to-pay thresholds of £20,000/QALY and £30,000/QALY (2016GBP), respectively, and decreased to 36% and 56%, respectively, from a limited healthcare perspective. PSI was cost-effective in at least 50% of simulations at a willingness-to-pay threshold of £30 per 1% improvement in treatment response probability and 87% at £1,000 per 1% improvement in treatment response probability.
Murphy et al.45 conducted a CEA alongside an RCT, comparing XR-NTX to buprenorphine-naloxone among 570 adults with OUD seeking treatment in a U.S. inpatient or residential treatment program. The authors conducted analyses from the healthcare-sector and societal perspectives over the 24-week intervention and 36-week observation periods. From the healthcare-sector perspective, XR-NTX had less than a 5% chance of being cost-effective, compared to buprenorphine-naloxone, using the recommended range of $100,000 – $200,000 per QALY at 24 weeks, and a 20% chance of being cost-effective at 36 weeks. The respective probabilities of XR-NTX being cost-effective increased to 30% and 50% from a societal perspective. The likelihood of XR-NTX being cost-effective increased for each scenario when analyzing the per-protocol sample (i.e., participants who successfully initiated their assigned medication).
DISCUSSION
We identified 21 articles, 10 modeling (2 decision trees, 5 Markov models, 1 Monte Carlo microsimulation, 1 serial cross-sectional design, 1 compartmental model), 5 cohort analyses, and 6 randomized clinical trials, published from August 2015 through December 2019 that provide new health economic evidence supporting the use of OUD pharmacotherapy. Similar to Murphy and Polsky,17 we continue to find evidence supporting the economic value of methadone compared to no pharmacotherapy. Much of the evidence from this review supports buprenorphine as a cost-effective treatment compared to no pharmacotherapy, whereas prior findings on buprenorphine were quite limited.17 We found two RCTs on the economic value of XR-NTX. Although this is an improvement over the prior review, which only included one XR-NTX study, the evidence on the economic value of XR-NTX remains limited. We found an additional health economic study suggesting diacetylmorphine is preferred to methadone, adding to the mixed results on diacetylmorphine from the previous review. There was no previous evidence on hydromorphone.
We found four studies focused on potential reductions in healthcare costs associated with treatment for OUDs.21,24–26 Results from these studies suggest OUD pharmacotherapy leads to lower healthcare resource utilization and expenditures than non-pharmacological therapies. Krebs et al.24 also found significantly lower criminal justice-related costs among participants who received methadone, compared to those who received detoxification only.
Of the 17 cost-effectiveness articles, only 11 reported cost/QALY or cost/DALY, which limits broad comparison of economic value across diseases/disorders, as QALYs and DALYs are the only effectiveness measures with commonly-accepted value thresholds. Of note, thresholds in the U.S., the U.K., Canada, and developing countries vary (Table 3, Appendix A). Five studies reported OUD-specific outcomes (e.g., cost/opioid-free-day), and one reported cost-benefit outcomes (e.g., net societal costs).
Three studies34–36 evaluated the cost-effectiveness of buprenorphine compared to no pharmacotherapy; however, only Barocas et al.36 reported QALYs gained as the effectiveness measure. Busch and colleagues34 used two OUD-specific outcomes, cost/enrollment in formal addiction treatment at 30 days and cost/change in days of self-reported illicit opioid use in the past 7 days, while Dunlop et al.35 used heroin-free days as the effectiveness measure. The results in each case appeared favorable for buprenorphine, depending on the stakeholder’s willingness-to-pay. Four studies compared methadone to a non-pharmacological alternative,27,28,30,31 and two assessed methadone or buprenorphine relative to a non-pharmacological therapy.20,37 Altogether, these studies indicate buprenorphine and methadone are a good value, compared to non-pharmacological alternatives.
Two studies39,43 assessed the cost-effectiveness of methadone relative to buprenorphine. King et al.39 compared methadone to buprenorphine and the findings indicated methadone is preferred over buprenorphine, but results were sensitive to the cost of methadone. Premkumar et al.43 found buprenorphine preferred to methadone and no pharmacological treatment.
There were a limited number of studies evaluating alternative pharmacotherapies, modalities, and dosage forms. Dunlap et al.32 and Marsden et al.22 compared medication alone to medication plus a psychosocial intervention, and both found the latter option was preferred. Carter et al.19 compared subdermal implantable buprenorphine to sublingual buprenorphine, and found the former dominated the latter. Additionally, buprenorphine was compared to XR-NTX by Murphy et al.45 who found that buprenorphine was favored in most scenarios from a healthcare-sector perspective. In a separate study, Murphy et al.23 compared XR-NTX to no-pharmacologic treatment and found the probability of XR-NTX being cost-effective varied from 10% to 76%.
Finally, Bansback et al.38 estimated the cost-effectiveness of injectable diacetlymorphine and injectable hydromorphone compared to methadone. Both diacetlymorphine and injectable hydromorphone had a high likelihood of dominating methadone, suggesting these treatment alternatives would be viable options for individuals with long-term injection opioid use who have not benefited from other pharmacotherapies.
Limitations
First, our systematic review of the economics of OUD treatment did not include economic evaluations of harm reduction strategies for people with OUD, such as syringe exchange and naloxone distribution programs to prevent overdose. We identified a wide range of study designs, which limits cross-study comparability. Additionally, there was wide variation in study time horizons ranging from 30 days to lifetime. The studies also took place in seven countries, which may diminish comparability, as healthcare systems and OUD care delivery differ, although the majority of studies were conducted in the U.S. We used the Drummond checklist, as opposed to other potential rubrics,46,47 to ensure consistency with previously conducted reviews on this topic.17,48 We identified few articles on XR-NTX and scant studies on special populations, such as people with mental health comorbidities, who may require specific services during treatment.49,50
CONCLUSION
We found additional evidence that buprenorphine and methadone are economically advantageous OUD treatments; however, there remains no clear evidence supporting superior economic value for either one. We identified few research studies on XR-NTX, other emerging pharmacotherapies, treatment modalities, and dosage forms, indicating further economic research is needed. Similarly, there continues to be wide variation in research designs, perspectives, and outcomes, including disorder-specific measures, all of which limit comparisons to economic evaluations in general, as well as within the substance use disorder literature.
Supplementary Material
Key Points for Decision Makers.
There is new evidence on buprenorphine and strengthened evidence on methadone indicating both are economically advantageous treatments for opioid use disorder compared to no pharmacotherapy.
Approximately half of recent cost-effectiveness studies used a generic preference-based measure of effectiveness (i.e., quality-adjusted life years (QALYs) or disability-adjusted life years (DALYs)) limiting broad comparison across diseases/disorders as QALYs/DALYs are the only health economic effectiveness measures with commonly accepted value thresholds. There is wide variation in disease/disorder-specific measures thereby limiting comparisons within the substance use disorder literature.
More economic evidence is needed on injectable naltrexone and novel treatment delivery modalities.
ACKNOWLEDGEMENTS
Sources of Financial Support
All authors report funding from NIDA (P30DA040500); JAL, BRS, KEM, DP and SMM report additional funding from NIDA (R01DA035808); and JAL, DP, and SMM report additional funding from NIDA (R01DA046721).
Funding:
This research was supported by the National Institute on Drug Abuse (P30DA040500, R01DA035808, and R01DA046721). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies or the U.S. government.
Footnotes
Conflicts of Interest
Dr. Murphy reports having consulted for Sandoz Inc. outside the submitted work.
Compliance with Ethical Standards
Not applicable.
REFERENCES
- 1.World Health Organization. Information sheet on opioid overdose. https://www.who.int/substance_abuse/information-sheet/en/. Accessed 4 September 2019
- 2.Center for Behavioral Health Statistics and Quality. Reports from the 2018 National Survey on Drug Use and Health (NSDUH): detailed tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetailedTabs2018.pdf. Accessed 20 February 2020.
- 3.United Nations. World Drug Report https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_1_EXECUTIVE_SUMMARY.pdf. Accessed 12 September 2019
- 4.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MWWR Morb Mortal Wkly Rep. 2019;67(5152):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy SM. The Cost of Opioid Use Disorder and the Value of Aversion. Drug Alcohol Depend. 2020:108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Behavioral Health Statistics and Quality. Treatment epiosde data set (TEDS): 2017. Admissions to and discharges from publicly-funded substance use treatment. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/TEDS-2017.pdf. Accessed 18 July 2019.
- 7.The National Academies of Sciences, Engineering, and Medicine. Medications for Opioid Use Disorder Saves Lives. https://www.nap.edu/resource/25310/032019_OUDhighlights.pdf. Accessed 30 April 2019
- 8.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND, Wargo EM. Overdose Prevention Through Medical Treatment of Opioid Use Disorders. Ann Intern Med. 2018;169(3):190–192. [DOI] [PubMed] [Google Scholar]
- 10.Kampman K, Jarvis M. American society of addiction medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. The Lancet. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Whelan PJ, Remski K. Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parida S, Carroll KM, Petrakis IL, Sofuoglu M. Buprenorphine treatment for opioid use disorder: recent progress. Expert Review of Clinical Pharmacology. 2019;12(8):791–803. [DOI] [PubMed] [Google Scholar]
- 14.Harm Reduction International. The Global State of Harm Reduction 2018. https://www.hri.global/files/2019/02/05/global-state-harm-reduction-2018.pdf. Accessed 6 September 2019.
- 15.Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid Detoxification and Naltrexone Induction Strategies: Recommendations for Clinical Practice. Am J Drug Alcohol Abuse. 2012;38(3):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisel ZF, Mitchell J, Polsky D, Boualam N, et al. Strengthening partnerships between substance use researchers and policy makers to take advantage of a window of opportunity. Subst Abuse Treat Pr. 2019;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions. Pharmacoeconomics. 2016;34(9):863–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Systematic reviews. 2017;6(1):245–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter JA, Dammerman R, Frost M. Cost-effectiveness of subdermal implantable buprenorphine versus sublingual buprenorphine to treat opioid use disorder. J Med Econ. 2017;20(8):893–901. [DOI] [PubMed] [Google Scholar]
- 20.Kenworthy J, Yi Y, Wright A, Brown J, Maria Madrigal A, Dunlop WC. Use of opioid substitution therapies in the treatment of opioid use disorder: results of a UK cost-effectiveness modelling study. J Med Econ. 2017;20(7):740–748. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Duncan M, Atreja N, Tai KS, Gore M. Healthcare utilization and costs associated with treatment for opioid dependence. J Med Econ. 2018;21(4):406–415. [DOI] [PubMed] [Google Scholar]
- 22.Marsden J, Stillwell G, James K, Shearer J, et al. Efficacy and cost-effectiveness of an adjunctive personalised psychosocial intervention in treatment-resistant maintenance opioid agonist therapy: a pragmatic, open-label, randomised controlled trial. The lancet Psychiatry. 2019;6(5):391–402. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SM, Polsky D, Lee JD, Friedmann PD, et al. Cost‐effectiveness of extended release naltrexone to prevent relapse among criminal justice‐involved individuals with a history of opioid use disorder. Addiction. 2017;112(8):1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs E, Urada D, Evans E, Huang D, Hser YI, Nosyk B. The costs of crime during and after publicly funded treatment for opioid use disorders: a population‐level study for the state of California. Addiction. 2017;112(5):838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu Y-J, Marsteller JA, Kachur SG, Fingerhood MI. Integration of buprenorphine treatment with primary care: comparative effectiveness on retention, utilization, and cost. Popul Health Manag. 2018;22(4):292–299. [DOI] [PubMed] [Google Scholar]
- 26.Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat. 2016;67:9–14. [DOI] [PubMed] [Google Scholar]
- 27.Idrisov B, Murphy SM, Morrill T, Saadoun M, Lunze K, Shepard D. Implementation of methadone therapy for opioid use disorder in Russia–a modeled cost-effectiveness analysis. Subst Abuse Treat Pr. 2017;12(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vuong T, Shanahan M, Nguyen N, Le G, et al. Cost-effectiveness of center-based compulsory rehabilitation compared to community-based voluntary methadone maintenance treatment in Hai Phong City, Vietnam. Drug Alcohol Depend. 2016;168:147–155. [DOI] [PubMed] [Google Scholar]
- 29.Hall W, Babor T, Edwards G, Laranjeira R, et al. Compulsory detention, forced detoxification and enforced labour are not ethically acceptable or effective ways to treat addiction. Addiction. 2012;107(11):1891–1893. [DOI] [PubMed] [Google Scholar]
- 30.Gisev N, Shanahan M, Weatherburn DJ, Mattick RP, et al. A cost‐effectiveness analysis of opioid substitution therapy upon prison release in reducing mortality among people with a history of opioid dependence. Addiction. 2015;110(12):1975–1984. [DOI] [PubMed] [Google Scholar]
- 31.Krebs E, Enns B, Evans E, Urada D, et al. Cost-effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med. 2018;168(1):10–19. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap LJ, Zarkin GA, Orme S, Meinhofer A, et al. Re-engineering methadone—Cost-effectiveness analysis of a patient-centered approach to methadone treatment. J Subst Abuse Treat. 2018;94:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barry MJ, Edgman-Levitan S. Shared decision making—the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. [DOI] [PubMed] [Google Scholar]
- 34.Busch SH, Fiellin DA, Chawarski MC, Owens PH, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addiction. 2017;112(11):2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunlop AJ, Brown AL, Oldmeadow C, Harris A, et al. Effectiveness and cost-effectiveness of unsupervised buprenorphine-naloxone for the treatment of heroin dependence in a randomized waitlist controlled trial. Drug Alcohol Depend. 2017;174:181–191. [DOI] [PubMed] [Google Scholar]
- 36.Barocas JA, Morgan JR, Fiellin DA, Schackman BR, et al. Cost-effectiveness of integrating buprenorphine-naloxone treatment for opioid use disorder into clinical care for persons with HIV/hepatitis C co-infection who inject opioids. The International journal on drug policy. 2019;72:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morozova O, Crawford FW, Cohen T, Paltiel AD, Altice FL. Cost-effectiveness of expanding the capacity of opioid agonist treatment in Ukraine: dynamic modeling analysis. Addiction (Abingdon, England). 2020;115(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bansback N, Guh D, Oviedo-Joekes E, Brissette S, et al. Cost-effectiveness of hydromorphone for severe opioid use disorder: findings from the SALOME randomized clinical trial. Addiction. 2018;113(7):1264–1273. [DOI] [PubMed] [Google Scholar]
- 39.King JB, Sainski-Nguyen AM, Bellows BK. Office-based buprenorphine versus clinic-based methadone: a cost-effectiveness analysis. J Pain Palliat Care Pharmacother. 2016;30(1):55–65. [DOI] [PubMed] [Google Scholar]
- 40.Rice JB, Kirson NY, Shei A, Cummings AKG, et al. Estimating the costs of opioid abuse and dependence from an employer perspective: a retrospective analysis using administrative claims data. Appl Health Econ Hea. 2014;12(4):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice JB, Kirson NY, Shei A, Enloe CJ, et al. The economic burden of diagnosed opioid abuse among commercially insured individuals. Postgrad Med. 2014;126(4):53–58. [DOI] [PubMed] [Google Scholar]
- 42.White AG, Birnbaum HG, Mareva MN, Daher M, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11(6):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Premkumar A, Grobman WA, Terplan M, Miller ES. Methadone, Buprenorphine, or Detoxification for Management of Perinatal Opioid Use Disorder: A Cost-Effectiveness Analysis. Obstetrics and gynecology. 2019;134(5):921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institute for Health and Clinical Excellence. The guidelines manual. http://www.nice.org.uk/. Accessed 08 Oct 2019.
- 45.Murphy SM, McCollister KE, Leff JA, Yang X, et al. Cost-Effectiveness of Buprenorphine–Naloxone Versus Extended-Release Naltrexone to Prevent Opioid Relapse. Ann Intern Med. 2019;170(2):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husereau D, Drummond M, Petrou S, Carswell C, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Resour Alloc. 2013;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 48.Doran C Economic evaluation of interventions to treat opiate dependence. Pharmacoeconomics. 2008;26(5):371–393. [DOI] [PubMed] [Google Scholar]
- 49.Ecker J, Abuhamad A, Hill W, Bailit J, et al. Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of Addiction Medicine. Am J Obstet Gynecol. 2019;221(1):B5–B28. [DOI] [PubMed] [Google Scholar]
- 50.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. The Lancet. 2019;393(10182):1760–1772. [DOI] [PubMed] [Google Scholar]
- 51.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 52.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. Oxford University Press; 2016. [Google Scholar]
- 53.Tan-Torres Edejer T, Acharya A, Ta Adam, Baltussen R, et al. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 54.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


