Abstract
Superior vena cava syndrome is a life-threatening condition. Typically, the clinical presentations are gradual; hence, the diagnosis is often delayed until critical compression or obstruction has occurred. Pericardial hematoma is a rare condition that could occur after cardiac surgery. An asymptomatic, 25-year-old female, who underwent surgical atrial septal defect closure 5 days ago, was sent for routine echocardiography examination before discharge. An intrapericardiac hematoma was detected at the right atrium’s free wall without any intracardiac hemodynamic consequences. The patient was discharged and planned for monthly evaluation. During follow-up, the intrapericardiac hematoma was expanding. In the third month’s follow-up, the patient complained of shortness of breath, headaches, and coughs. Echocardiography evaluation revealed enlarged pericardial hematoma, which compressed the right atrium and superior vena cava orifice, without echo’ sign of cardiac tamponade. Computed tomography scan revealed superior vena cava compression by the pericardial hematoma and appearance of the collateral vessel. The patient was diagnosed with superior vena cava syndrome and sent for surgical evacuation. Pericardial hematoma after cardiac surgery should be evaluated meticulously. Chronic expanding hematoma could cause superior vena cava syndrome, which is fatal. Early diagnosis and appropriate treatment are essential in managing this condition.
Keywords: Expanding hematoma, pericardial thrombus, cardiac surgery, complication
Background
Pericardial hematoma is a relatively rare disorder that could be caused by several conditions, including after cardiac surgery. Pericardial hematoma could regress spontaneously or progress into a larger mass which could give rise to compression syndrome. 1 Only a few recorded cases of pericardial hematoma exist. To our knowledge, this is the rare case report describing superior vena cava syndrome (SVCS) caused by expanding pericardial hematoma after cardiac surgery.
Case presentation
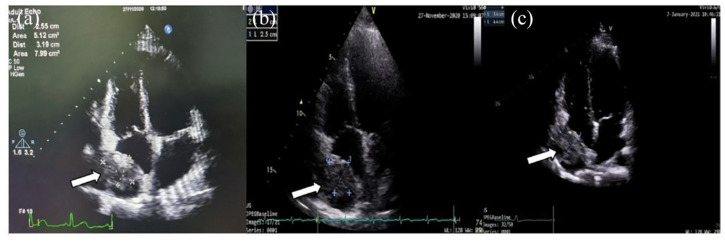
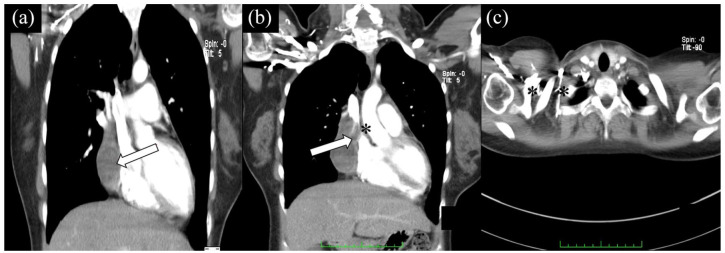
A 25-year-old asymptomatic woman was sent to echo lab for routine echocardiography evaluation before discharge. She underwent surgical atrial septal defect (ASD) closure with median sternotomy approach 5 days ago. Her vital signs were stable, with unremarkable physical findings. An echocardiogram showed normal chambers and functions; an ASD patch was visualized with no residual ASD shunt. An echo-lucent mass (2.9 × 1.9 cm2), suggestive hematoma, was visualized at the pericardial space near the right atrium (RA) (Figure 1(a)). It was mildly compressing the RA, but no abnormalities on intracardiac hemodynamic were observed. The patient was sent for cardiac computed tomography (CT) scan to confirm the diagnosis; CT scan showed distinct hematoma without any sign of compression of cardiac structures (Figure 2(a)). Considering that the patient was asymptomatic and hemodynamically stable, she was discharged from the hospital and planned for monthly follow-up.
Figure 1.
Follow-up echocardiography. Echocardiography 1 week after surgery showed normal all chambers, normal left ventricles (LV) systolic function with normal kinetic at rest, normal diastolic function, mild mitral regurgitation (MR), low probability of pulmonary hypertension (PH), mild tricuspid regurgitation (TR), reduced right ventricle (RV) contractility, blood clot at pericardial cavity (2.9 × 1.9 cm2) detected at the right atrium’s free wall (a). The pericardial hematoma (arrows) was enlarged (2.5 × 3.0 cm2) (b), and echocardiography 3 months after surgery large pericardial hematoma (3.4 × 4.4 cm2) with dilated SVC orifice (c).
Figure 2.
CT scan shows distinct hematoma (arrows) without any sign of compression of cardiac structures (a). Three months after surgery, expanding pericardial hematoma compressing the SVC, extending anterosuperiorly to the level of the aortic arch (b). Collateral vessels were also visualized (c).
Note: Asterisk showing vein compression.
In the following few months, the patient came to the outpatient clinic monthly. She was asymptomatic and hemodynamically stable, with no remarkable physical findings. She was also sent to the echo lab for monthly evaluation. An echocardiogram revealed the hematoma was expanding and slightly compressing the RA (Figure 1(b) and (c)), with no intracardiac hemodynamic abnormalities. The patient was informed about her condition. Although stable, we suggested her to undergo hematoma evacuation due to fear that expanding hematoma will deteriorate her hemodynamic, but she refused and was discharged by her consent.
Three months later, the patient came to the outpatient clinic complaining of shortness of breath during daily activities, headaches, and coughs. On physical examination, the blood pressure was 110/70 mm Hg, heart rate 80×/min, respiratory rate 20×/min. Her physical examination showed increased jugular vein pressure with positive hepatojugular reflux. The cardiac examination showed cardiomegaly without muffled heart sound. The electrocardiography showed sinus rhythm, incomplete right bundle branch block, and the chest x-ray showed cardiomegaly without lung edema. The laboratory results were within normal limits. An echocardiogram revealed normal intracardiac hemodynamic without a sign of tamponade. Taking these findings into account, we suspected great vessel compression.
The patient was sent for a thorax CT scan, which revealed expanding hematoma compressed the SVC with dilation of the proximal vessel. Collateral vessels were also detected. The hematoma extended anterosuperiorly to the level of the aortic arch (Figure 2(b) and (c)). The patient was diagnosed with SVCS due to pericardial hematoma compression and sent for urgent surgery for evacuation. The patient then underwent a sternotomy, which found a large hematoma and hemostatic agents at the pericardium around the SVC, and the hematoma was successfully evacuated.
Discussion
Postoperative complications related to cardiac surgery are associated with increased morbidity and mortality. Postoperative complications could be classified as early complications, including lethality within 30 days and delayed complications. Several most common post–cardiac surgery complications involve cardiovascular, respiratory, kidneys, and central nervous systems (Table 1).2,3
Table 1.
| Cardiovascular system | Respiratory system | Kidney | Central nervous system |
|---|---|---|---|
| Cardiac tamponade Arterial graft occlusion Paravalvular regurgitation Myocardial infraction Arrhythmia Cardiac arrest |
Postoperative respiratory failure Acute respiratory distress syndrome Hypoxemia and atelectasis Pleural effusions Pneumothorax Bronchospasm |
Acute kidney injury | Stroke |
SVCS after cardiac surgery is a rare condition that has been described by several cases (Table 2). The most common etiology of SVCS after cardiac surgery is due to SVC blockage caused by intravascular thrombus. The management of those cases is thrombolysis, balloon dilation, and surgical evacuation, which have good outcomes.4–6 However, as in our case, the cause of SVCS was due to pericardial hematoma. Intrapericardial hematoma or thrombus with sufficient size may result in compression of the surrounding cardiac chambers or vessels. Cardiac chambers compressions could cause cardiac tamponade, which is fatal. 1
Table 2.
Literature review describing SVC syndrome after cardiac surgery.
| Author | Sex/age | Operation/incident | Diagnostic modality | Symptoms | Disease progression | Outcome |
|---|---|---|---|---|---|---|
| Maggiano et al. 4 | Man/70 years | Coronary artery bypass surgery (CABG) and aortic valve replacement | Chest radiograph | Dyspnea | Apical pericardial thrombus and a large extrapericardial blood clot causing obstruction of the SVC | Surgical evacuation, patient feeling well |
| Daniel et al. 5 | Woman/5 years | Heart transplantation | Transesophageal echocardiogram | Facial swelling and occasional oxygen desaturations | Thrombus in the SVC | Balloon dilatation of the SVC and thrombolysis, patient feeling well |
| Woman/47 years | Heart transplantation | Venography | Right heart failure, hypoxemia | Complete occlusion of the SVC, with nonocclusive thrombus extending into the right brachiocephalic vein to the level of the internal jugular/subclavian confluence | Thrombolysis, patient feeling well | |
| Delgado et al. 6 | Woman/56 years | Mitral valve replacement | Cavography | Progressive edema of the head, neck, and upper limbs. | Fixed obstruction at the SVC | Balloon dilatation of the SVC, patient feeling well |
| Man/63 years | Mitral valve replacement | Cavography | Hemodynamic deterioration, edema, and cyanosis of the head, chest, and upper limbs. | Extensive venous thrombosis involving the SVC, right jugular, and both subclavian veins | Balloon dilatation of the SVC, patient feeling well |
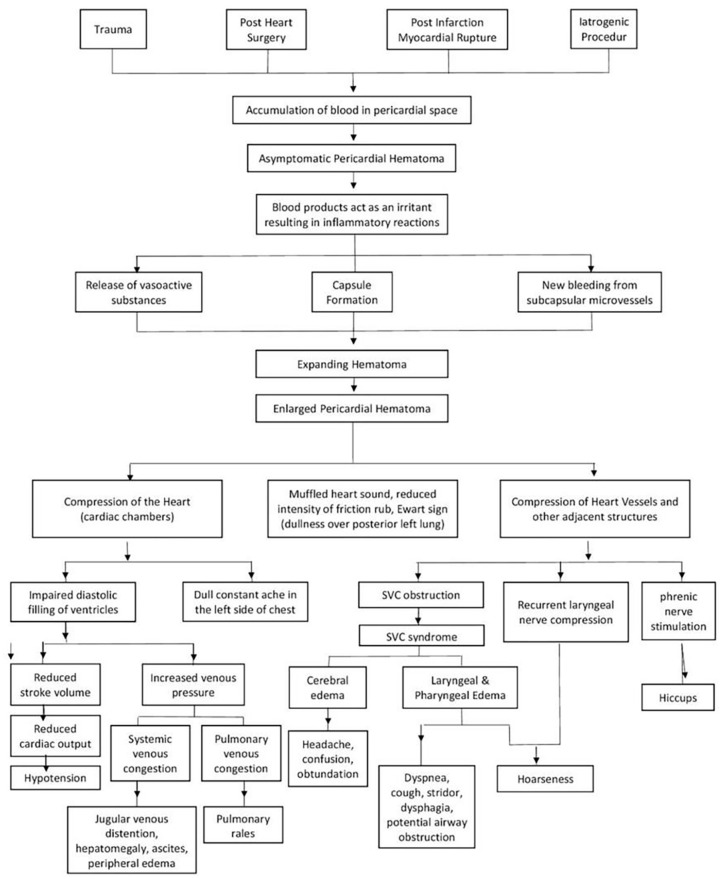
The mechanism of hematoma expansion is still not clearly understood. Labadie et al. described those inflammatory reactions could cause the expanded nature of hematoma due to the effect of irritants on blood. Irritation releases vasoactive substances and induces capsule formation, while repeated inflammation can result in effusion and new bleeding from damaged microvessels (Figure 3). 7
Figure 3.
Pathomechanism of expanding cardiac hematoma and consequences of large pericardial hematoma.
Clinical diagnosis of pericardial hematoma is impossible. Hence, imaging modalities are vital in diagnosis, such as echocardiography, CT scan, and cardiac magnetic resonance imaging (MRI). Echocardiography is the first modality that could detect pericardial hematoma, which also can evaluate intracardiac hemodynamic. CT scan and cardiac MRI are if further evaluation is needed. 8 As in our cases, the pericardial hematoma was detected as an incidental finding by routine echocardiography after surgery. A CT scan was performed to evaluate the surrounding great vessel, revealing compression of the superior vena cava (SVC).
SVCS is the clinical manifestation of SVC obstruction. It could be caused by external compression, thrombosis, or invasion of the vein, which more than 90% is secondary to malignancy. 9 Critical signs and symptoms in life-threatening SVC syndrome include headaches caused by cerebral edema, cough, and stridor caused by laryngeal edema and potential airway obstruction, or hemodynamic disturbances leading to syncope and hypotension. 10 Our patient has a headache, dyspnea, and cough, which also supported imaging findings of SVCS. Hence, we suggested to undergo surgical evacuation. Surgery enables inspection, identification, and correction of a responsible lesion or site to prevent a recurrence. 1
Conclusion
Physicians should evaluate pericardial hematoma after cardiac surgery with meticulous attention. SVCS should be considered in the patient with deteriorating conditions. Early diagnosis and appropriate treatment are essential in managing this condition.
Footnotes
Author note: Andra Naufal Pramanda is now affiliated to Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin General Hospital, Bandung, Indonesia.
Melawati Hasan is now affiliated to Cardiovascular Imaging Division, Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin General Hospital, Bandung, Indonesia.
Euis Maryani is now affiliated to Cardiothoracic Surgery Division, Department of Surgery, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin General Hospital, Bandung, Indonesia.
Undang Ruhimat is now affiliated to Department of Radiology, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin General Hospital, Bandung, Indonesia.
Charlotte Johanna Cool is now affiliated to Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin General Hospital, Bandung, Indonesia.
Author contributions: Aninka Saboe contributed to the conceptualization, data curation, writing-original draft, review & editing, and supervision. Andra Naufal Pramanda contributed to the conceptualization, writing-original draft, and data curation. Melawati Hasan contributed to writing—review & editing—and data curation. Euis Maryani contributed to the writing—review & editing. Undang Ruhimat contributed to writing—review & editing—and data curation. Nuraini Yasmin Kusumawardhani contributed to writing—review & editing—and data curation. Charlotte Johanna Cool contributed to writing—review & editing.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Our institution does not require ethical approval for reporting individual cases or case series. This study was conducted following the fundamental principles of the Declaration of Helsinki.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Aninka Saboe  https://orcid.org/0000-0002-2653-8621
https://orcid.org/0000-0002-2653-8621
References
- 1. Hutchinson S. Pericardial diseases: clinical diagnostic imaging atlas. Amsterdam: Elsevier, 2009. [Google Scholar]
- 2. Ball L, Costantino F, Pelosi P. Postoperative complications of patients undergoing cardiac surgery. Curr Opin Crit Care 2916; 22(4): 386–392. [DOI] [PubMed] [Google Scholar]
- 3. Udzik J, Sienkiewicz S, Biskupski A, et al. Cardiac complications following cardiac surgery procedures. J Clin Med 2020; 9(10): 3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maggiano HJ, Higgins TL, Lobo W, et al. Superior vena cava syndrome after open heart surgery. Cleve Clin J Med 1992; 59(1): 93–95. [DOI] [PubMed] [Google Scholar]
- 5. Sze DY, Robbins RC, Semba CP, et al. Superior vena cava syndrome after heart transplantation: percutaneous treatment of a complication of bicaval anastomoses. J Thorac Cardiovasc Surg 1998; 116(2): 253–261. [DOI] [PubMed] [Google Scholar]
- 6. García-Delgado M, Navarrete-Sánchez I, Colmenero M, et al. Superior vena cava syndrome after cardiac surgery: early treatment by percutaneous stenting. J Cardiothorac Vasc Anesth 2007; 21(3): 417–419. [DOI] [PubMed] [Google Scholar]
- 7. Labadie E, Glover D. Physiopathogenesis of subdural hematomas. Part 1: histological and biochemical comparisons of subcutaneous hematoma in rats with subdural hematoma in man. J Neurosurg 1976; 45(4): 382–392. [DOI] [PubMed] [Google Scholar]
- 8. Saboe A, Sanjaya F, Soeriadi REA, et al. Multiple pericardial hematomas: a case report and mini-review in multimodality imaging. BMC Med Imaging 2021; 21(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen R, Mena D, Carbajal-Mendoza R, et al. Superior vena cava syndrome: a medical emergency? Int J Angiol 2008; 17(1): 43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu J, Wilson L, Detterbeck F. Superior vena cava syndrome—a proposed classification system and algorithm for management. J Thorac Oncol 2008; 3(8): 811–814. [DOI] [PubMed] [Google Scholar]