Abstract
Background
Prostate cancer is the most common malignancy in men globally. This study aims at investigating the incidence rates and trends of prostate cancer in Lebanon, and to compare them to those of countries from different regions in the world.
Methods
Data on prostate cancer were obtained from the Lebanese national cancer registry for the years 2005 to 2016. The calculated age-standardized incidence and age-specific rates were expressed as per 100 000 population.
Results
In Lebanon, prostate cancer is ranked as the most common cancer in men. The age-standardized incidence rate of prostate cancer has increased from 29.1 per 100 000 in 2005 to 37.3 per 100 000 in 2016; the highest rate was in 2012, surpassing the global average incidence rate for that year. The age-specific incidence rate of prostate cancer has increased exponentially starting at the age of 50 years to reach its peak in men aged 75 years or more. Two trends were identified in the age-standardized incidence rate of prostate cancer; an average significant increase of 7.28% per year for the period 2005–2009 (P-value < .05), followed by a non-significant decrease of around .99% for the period between 2009 and 2016 (P-value > .05). The age-standardized incidence rate in Lebanon was higher than most countries in the Middle East and North Africa region and Asia, but lower than the rates reported in Australia, America, and different European countries.
Conclusion
Prostate cancer is the leading cancer among men in Lebanon. Screening practices, changes in population age structure, and prevalence of genetic and risky lifestyle factors may explain the increased incidence rates of prostate cancer. Given the controversy of screening recommendations and the slow growing nature of prostate cancer, increasing public awareness on ways of prevention, and implementing the latest screening recommendation of the United States Preventive Services Task Force are the suggested way forward.
Keywords: prostate cancer, epidemiology, incidence, prevention, cancer screening, cancer detection, risk factors
Background
Prostate cancer (PCa) is the fourth most common cancer globally, and the second most common malignancy among men. 1 PCa has had an increasing incidence over the years, with a staggering 1,414,259 new cases in 2020, compared to 1,276,000 reported in 2018; 1,112,000 in 2012; and 913,000 cases in 2008.2-4 It is the fifth most common cause of cancer-related mortality in men worldwide, leading to around 375,304 deaths in 2020 (6.8% of all cancer-related deaths in men), 358,989 deaths in 2018, 307,000 deaths in 2012, and 261,000 deaths in 2008.1-4 Its projected age-standardized incidence rate adjusted to the world population (ASRw) per 100,000 person-years was estimated to be 30.7 in 2020, compared to 29.3 in 2018, 31.1 in 2012, and 28.5 in 2008.1-4 Globally, the incidence of PCa shows a substantial geographic disparity that can exceed 25-fold between one region and another.2,3
Countries with high or very high standard human development index (HDI, a measure of the average achievement of a country in 3 key dimensions of human development: long and healthy life, being knowledgeable, and having a decent standard of living), 5 were reported to have the highest estimated incidence of PCa in 2018. These countries are located mainly in Oceania, Northern and Western Europe, and Northern America regions. 2 The estimated incidence of PCa is lower in less developed regions such as the Caribbean, Southern Africa, and South America, and much lower in Asia and Africa (particularly Northern and Eastern Africa).2,3 This geographic variation in PCa incidence may be explained by a higher frequency of screening for PCa in some developed countries, as well as differences in the prevalence of risk factors or protective factors for PCa among different populations.2,3,6-9
The introduction of prostate-specific antigen (PSA) in 1986 as a screening test for the early detection of PCa was associated with a significant upsurge in the incidence of PCa globally for many years to come. 2 Another major shift occurred in the last decade, particularly in the United States of America (USA) and most Nordic countries subsequent to the recommendation of the United States Preventive Services Task Force (USPSTF) against screening for PCa in 2012, following which there was a decline in the global incidence of PCa.2,10 However, USPSTF changed their recommendation again in 2018 and proposed a shared decision for PCa screening 11 ; the impact of this change on the incidence of PCa remains to be seen.
Risk factors for PCa can be classified as non-modifiable or modifiable. Non-modifiable factors include increasing age, male sex, taller stature, African ancestry among American men, a positive family history, and presence of predisposing genetic mutations; these variables have been well established as risk factors for PCa. 8 Modifiable risk factors include obesity, tobacco use, hormonal imbalances, dietary intake of certain foods (food rich in calcium and high consumption of dairy products), exposure to certain environmental agents, occupational exposure, and various lifestyle factors; the mechanisms by which some of these factors impact the development of PCa remain unclear, although there is strong medical evidence to link excess body fat and cigarette smoking to the risk of advanced or fatal PCa (Table 1).6,8,12-17
Table 1.
Risk factors of PCa.
| Risk factors of PCa | Strength of evidenceReferences | |
|---|---|---|
| Risk factors of total PCa | Increased risk | |
| Older age | Strong-convincing 8 | |
| African and Caribbean ethnic backgrounds | Strong-convincing 8 | |
| Family history | Strong-convincing 8 | |
| Genetic factors | Strong-convincing 8 | |
| Development factor (taller height) | Strong-probable
8
No relation 12 |
|
| High serum insulin-like growth factor (IGF)-1 | Probable 8 | |
| Body fatness | Limited-inconclusive
8
Possible 12 |
|
| Dairy products-high consumption | Limited-suggestive 8 | |
| Diets high in calcium | Limited-suggestive 8 | |
| Low plasma alpha-tocopherol concentrations | Limited-suggestive 8 | |
| Low plasma selenium concentrations | Limited-suggestive 8 | |
| Cereals (grains) and their products; dietary fiber; potatoes; non-starchy vegetables; fruits; pulses (legumes); processed meat; red meat; poultry; fish; eggs; total fat; saturated fatty acids; monounsaturated fatty acids; polyunsaturated fatty acids; plant oils; sugar (sucrose); sugary foods and drinks; Coffee; tea; alcoholic drinks; Carbohydrate; protein; vitamin A; retinol; alpha Carotene; lycopene; folate; thiamin; riboflavin; niacin; vitamin C; vitamin D; vitamin E supplements; gamma-tocopherol; multivitamins; selenium supplements; iron; phosphorus; calcium supplements; zinc; physical activity; energy expenditure; vegetarian diets; seventh-day adventist diets; individual dietary patterns; birth weight; and energy intake | Limited-inconclusive 8 | |
| Consumption of beta-carotene (in the diet or as supplements) | Strong; unlikely 8 | |
| Occupational exposure to some pesticides | Possible 13 | |
| Decreased risk | ||
| Lycopene and tomato-based products (canned and cooked) | Possible 14 | |
| Some risk factors of advanced or lethal PCa | Increased risk | |
| Development factor (taller height) | Strong 7 | |
| Lipid levels | Possible 7 | |
| Greater body fatness (marked by BMI; waist circumference; and waist-hip ratio) | Strong-probable7,8 | |
| Smoking | Strong7,15 | |
| High consumption of dairy products or calcium | Possible 7 | |
| High levels of Omega-3 fatty acids and fish oil | Possible (limited evidence) 8 | |
| Occupational exposure to some pesticides | Possible 16 | |
| Decreased risk | ||
| Physical activity | Strong7,17 | |
| Lycopene and tomato-based products | Probable 7 | |
| Fish | Possible (limited evidence) 7 | |
| Vitamin D | Possible (limited evidence) 7 | |
| Statins | Possible (limited evidence) 7 | |
Lebanon is a small developing country in the Middle East (population estimated at 6 million in 2016) with health indices close to those of the western world, 18 and it has a high HDI based on the standard HDI formula (.757 in 2017). 5 PCa is one of the most common cancers in men in the country, and it accounted for 16.5% of cancer-related deaths amongst men in 2014. 19 The Lebanese national cancer registry (NCR) was officially restarted in 2002 after years of inactivity due to multiple wars; this registry provides an almost absolute count of all incident cancer cases nationwide. NCR data are being continuously collected from the capture system (data collected passively from physicians’ reports) and recapture system (data collected actively from histopathological and hematological laboratories) 20 ; the recapture system confirms and complements the capture approach. 21 The NCR covers most cancer cases (more than 90%) in Lebanon, 20 but it excludes in situ lesions. The NCR periodically publishes its cancer incidence data electronically on the Ministry of Public Health (MoPH) website; the currently available data cover the period extending from 2005 to 2016. 21
This study aims at analyzing the 12-year incidence rates and temporal trends for PCa in Lebanon. It also compares the incidence rates in Lebanon, the Middle East and North Africa (MENA) region, and other countries and regions worldwide. Recommendations for effective PCa screening and prevention are highlighted, taking into consideration the different risk factors prevalent in the Lebanese context.
Materials and Methods
Twelve-Year Trend Analysis From 2005 Until 2016
In this study, PCa data include diagnosis with the code C61 of the International Classification of Diseases, 10th revision (ICD-10). The age-standardized incidence rate adjusted to the world population (ASRw) and the age-specific incidence rates, expressed per 100,000 men, were retrieved from the Lebanese NCR data published on the MoPH website for twelve consecutive years from 2005 to 2016. 21 The ASRw is a weighted average of the age-specific incidence rates per 100,000 persons, with weights being the proportions of persons in the corresponding age groups of a standard population. Standardization is important when comparing different populations with different age structures. The most used standard population is the world standard population, which is drawn from a pooled population of several countries. In this study, the ASRw was calculated using the modified world standard population by Doll as the reference population. 22 The age-specific incidence rate is the number of new cancer cases occurring during a specific period, in a population of a specific age and sex group, divided by the number of mid-year population of that age and sex group. 23
The annual percent change (APC) and the average annual percent change (AAPC) of PCa incidence rates over the years were calculated using the Joinpoint Regression Program (JRP) 4.7.0.0 with a statistical significance level defined at a P-value < .05. The APC is based on the hypothesis that cancer rates change at a constant percentage of the previous year’s rate. 24
Projections of the ASRw of PCa in Lebanon for the Year 2025
Projections were estimated using the currently available PCa data over the 12-year period (2005–2016). Log-linear regression model was found to be the best fit model in this study (R-squared or R2: .607); therefore, future ASRw for PCa was projected by fitting a log-linear regression model to observed ASRw against observed years (y = 3.375ln(x) + 31.562). Linear and log-linear regression models, which assume a Poisson distribution for the observed number of incident cases, have been reported to be the most practical methods that can be used to estimate future patterns of cancer incidence for periods up to 10 years, while assuming that there is no change in underlying trends. 25
The new PCa incidence coefficients were generated. R-squared (R2 or the coefficient of determination), adjusted R-squared, and the standard error of estimate were used to assess the goodness-of-fit of the model. R2 permits to measure how well the model explains the total variations, that is, the proportion of variance in the dependent variable (ASRw) that can be explained by the independent variable (year). In other words, R2 shows how well the data fit the regression model (the goodness of fit). The P-value for the model was also computed to determine the significance of the model compared with a null model, that is, there is a non-zero relationship between the independent and dependent variables described by the model. A P-value less than .05 is considered statistically significant. The sample linear regression model used in estimating the ASRw of PCa is included below. It is worth noting that the independent variable is time.
| R | R-square | Adjusted R-square | Standard error of the estimate | |
|---|---|---|---|---|
| Log-linear model | .779 | .607 | .567 | 2.154 |
| Linear model | .578 | .334 | .268 | 2.802 |
Comparison of Prostate Cancer Incidence Rates in Lebanon to Regional and Worldwide Countries
Prostate cancer ASRw and age-specific rates in Lebanon were subsequently compared with age-standardized and age-specific incidence rates from selected regional and worldwide countries that have cancer data available for a comparable period. These data were extracted from the Cancer Incidence in 5 Continents Time Trends (CI5plus), 26 Cancer Incidence in 5 Continents Volume XI (CI5 XI) 27 , or from the Regional or Country National Cancer Registry.28-32 The rates mentioned in CI5plus and CI5 XI are built on high-quality population-based cancer registries. For comparison, we included countries that surround Lebanon geographically, as well as randomly selected countries from the MENA region and other regions of the world.
Results
Incidence Trends (2005–2016) and Average Annual Percent Change (AAPC)
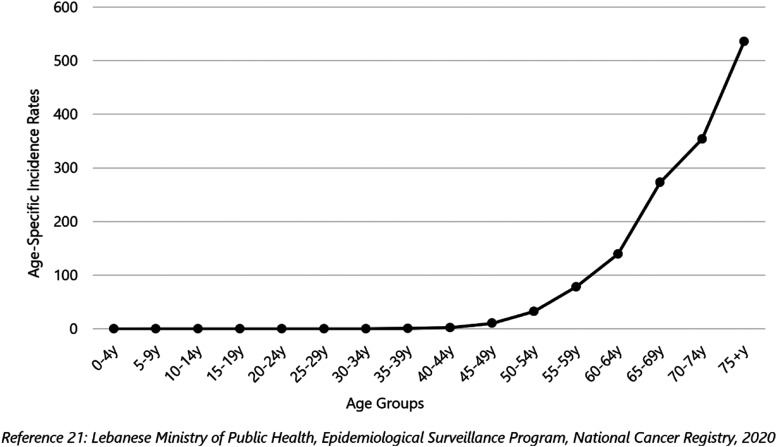
In Lebanon, PCa is ranked as the most common cancer in men (followed by lung, trachea, and bladder cancer) over the 12-year study period (2005–2016). A total of 9790 incident cases of PCa were reported during this period, with 92.4% of cases affecting men aged 50 years or more, and 70.3% affecting men aged 65 years or more. The average number of new cases of PCa per year was 816. The age-specific incidence rate increased exponentially starting at age of 50 years and reached its peak in men aged 75 years or more (Figure 1).
Figure 1.
Age-specific incidence rates (per 100,000 population) for prostate cancer, Lebanon 2005–2016.
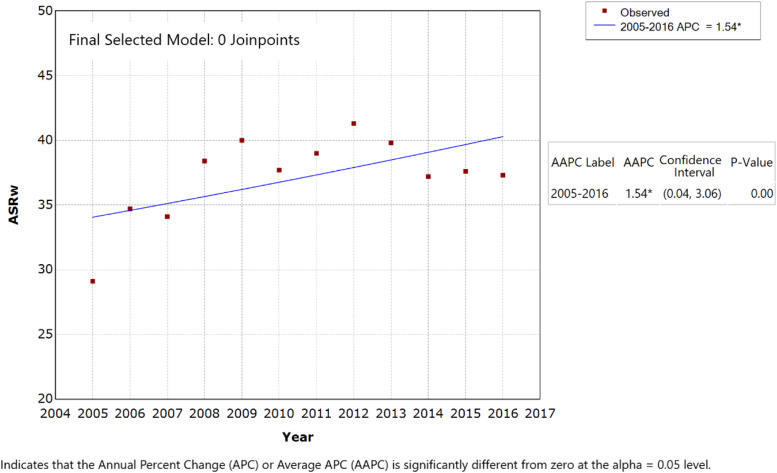
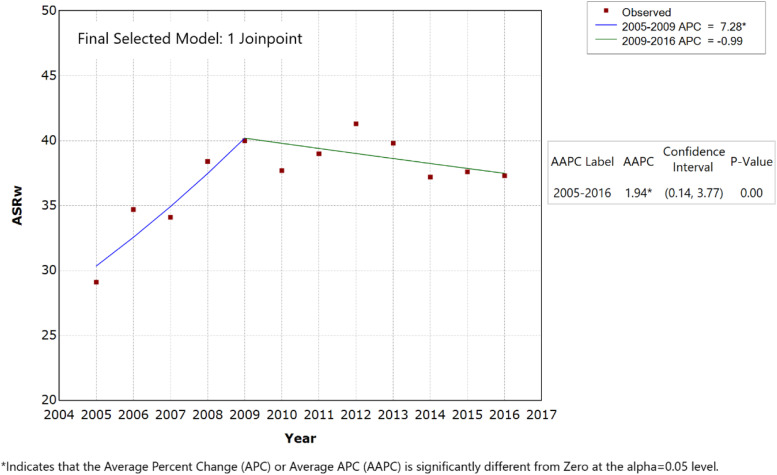
The APC and AAPC were computed for all study participants to reflect the trends in ASRw of PCa over the 12-year study period; they were also calculated for various age groups detailed in Table 2. With 0 JRP, the APC (equal to AAPC) of PCa was +1.54% (P-value < .05) (Figure 2) and significantly different than zero. JRP analysis (1 JRP model) identified 2 trends in the Lebanese ASRw of PCa: first, an average significant increase estimated at 7.28% per year for the period 2005–2009 (P-value < .05), with the ASRw reaching a peak in 2009 of 40.0 incident cases per 100 000 men; second, this was followed by a non-significant decrease in the ASRw of around .99% for 2009–2016, particularly between 2012 and 2016 (P-value > .05) (Figure 3, Table 2).
Table 2.
Trend analysis for prostate cancer age-specific rate (per 100 000) in men by age group per year in Lebanon from 2005 to 2016.
| Prostate cancer C61 | ASRw a | Age-specific rates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 40–44y | 45–49y | 50–54y | 55–59y | 60–64y | 65–69y | 70–74y | 75+y | ||
| 2005 | 29.1 | 2.5 | 5.1 | 17.0 | 63.9 | 127.4 | 230.4 | 273.5 | 379.1 |
| 2006 | 34.7 | 0.8 | 8.0 | 25.6 | 79.8 | 128.6 | 249.9 | 273.5 | 575.7 |
| 2007 | 34.1 | 2.8 | 7.9 | 38.9 | 79.8 | 163.2 | 228.7 | 354.8 | 386.3 |
| 2008 | 38.4 | 7.8 | 17.8 | 34.4 | 96.8 | 151.7 | 243.6 | 399.1 | 485.6 |
| 2009 | 40.0 | 2.9 | 11.7 | 32.5 | 74.7 | 157.7 | 302.6 | 397.3 | 553.7 |
| 2010 | 37.7 | 2.8 | 9.2 | 41.8 | 65.4 | 128.0 | 284.4 | 385.4 | 547.6 |
| 2011 | 39.0 | 0.9 | 14.7 | 30.2 | 89.3 | 124.5 | 286.1 | 376.8 | 592.9 |
| 2012 | 41.3 | 3.6 | 12.2 | 47.5 | 95.5 | 133.3 | 291.5 | 441.4 | 559.5 |
| 2013 | 39.8 | 0 | 7.0 | 28.4 | 64.6 | 160.4 | 347.3 | 351.4 | 573.0 |
| 2014 | 37.2 | 3.7 | 14.5 | 38.6 | 67.5 | 124.1 | 264.7 | 318.9 | 586.8 |
| 2015 | 37.6 | 0 | 8.8 | 28.6 | 90.0 | 135.7 | 265.2 | 353.1 | 578.9 |
| 2016 | 37.3 | 1.5 | 6.3 | 29.1 | 70.4 | 136.3 | 282.2 | 323.9 | 610.5 |
| Average | 37.2 | 2.4 | 10.3 | 32.7 | 78.1 | 139.2 | 273.1 | 354.1 | 535.8 |
| APC b | 1.54 b | 1.18 | 2.37 | .00 | −.33 | 1.93 | 1.08 | 3.25 b | |
Source: Lebanese Ministry of Public Health, Epidemiological Surveillance Program, National Cancer Registry, 2020 (Reference 20)
N.B. Data for the age groups below 40 years are available on the Lebanese ministry of public health website: https://www.moph.gov.lb/en/Pages/2/193/esu. We included data for the age groups 40 years and above as they are more relevant to the scope of the study.
aASRw: age-standardized rate (world).
bAPC significantly different from zero.
Figure 2.
Age-standardized incidence rates (ASRw) for prostate cancer in men, Lebanon 2005–2016 (0 joinpoints model).
Figure 3.
Age-standardized incidence rates (ASRw) for prostate cancer in men, Lebanon 2005–2016 (1 joinpoint model).
Projected ASRw of PCa in 2020 and 2025
Using log-linear regression model, the ASRw of PCa per 100 000 men was estimated at 32.8 cases in 2020 (95% confidence interval: 20.8, 51.7) and 30.0 cases in 2025 (95% confidence interval: 11.0, 81.0).
Comparison of Prostate Cancer Incidence Rates in Lebanon to Regional and Worldwide Countries
The ASRw of PCa among men in Lebanon was found to be higher than the average worldwide in 2008 (38.4 vs 28.5, respectively) and 2012 (41.3 vs 31.1, respectively).3,4 When compared to the ASRw of other countries for the year 2012: (a) Lebanon had a much higher ASRw than most MENA countries, but it was lower than Israeli Jews and almost equal to that of Israeli non-Jews, (b) the ASRw was also higher than that of different Asian countries like Thailand, South Korea, and Philippines, (c) yet, it was lower than the rates recorded in Australia, different European countries (e.g., Norway, Sweden, and Ireland) and America (e.g., Canada, USA, and Brazil) (Table 3). The ASRw of PCa in Lebanon was significantly lower than in countries with the highest rates of PCa in 2018, that is, Guadeloupe-France (ASRw: 189.1), Martinique-France (ASRw: 158.4), and Ireland (ASRw: 132.5). 2
Table 3.
Prostate cancer age-standardized incidence rates (ASRw) and age-specific rates per 100 000 men in Lebanon compared to MENA and non-MENA countries.
| Country | Year(s) | ASRw | 40–44y | 45–49y | 50–54y | 55–59y | 60–64y | 65–69y | 70–74y | 75+y | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MENA countries | Lebanon 20 | 2012 | 41.3 | 3.6 | 12.2 | 47.5 | 95.5 | 133.3 | 291.5 | 441.4 | 559.5 |
| Israeli non-Jews 26 | 2012 | 41.9 | 1.7 | 1.9 | 49.6 | 118.8 | 144.9 | 297.5 | 488.1 | 498.6 | |
| Israeli Jews 26 | 2012 | 55.8 | 1.7 | 9.4 | 59.8 | 136.1 | 283.2 | 480.5 | 601.2 | 443.7 | |
| Jordan 28 | 2012 | 12.7 | 0 | 3.7 | 4.9 | 20.0 | 27.5 | 94.6 | 170.0 | 205.7 | |
| Egypt, Minia Governorate 29 | 2009 | 5.2 | 1.0 | - | 1.3 | 10.2 | 16.2 | 28.5 | 51.1 | 105.3 | |
| Egypt, Aswan Governorate 29 | 2008 | 9.2 | - | - | 6.6 | - | 28.4 | 37.9 | 85.4 | 237.4 | |
| Egypt, Damietta Governorate 29 | 2012 | 6.8 | - | - | 7.6 | 5.0 | 26.3 | 28.9 | 77.9 | 136.8 | |
| Kuwait: Kuwaiti 26 | 2012 | 22.4 | 3.3 | 4 | 26.7 | 14.2 | 114.6 | 89.5 | 241.1 | 398.6 | |
| Bahrain: Bahraini 26 | 2012 | 16.3 | - | 5.9 | - | 16.7 | 49.1 | 115.6 | 199.1 | 290.7 | |
| Saudi Arabia: Saudi 30 | 2013 | 5.5 | 0.2 | 0.2 | 3.8 | 8.6 | 21.7 | 35.4 | 49.5 | 100.4 | |
| Algeria, Setif 27 | 2008-2011 | 7.7 | - | 2.6 | 8.1 | 8.1 | 28.3 | 62.9 | 80.2 | 110.4 | |
| Algeria, Batna 27 | 2008-2012 | 6.5 | - | 0.7 | 2.7 | 2.3 | 29.9 | 39.3 | 60.4 | 129.8 | |
| Iran, Golestan 27 | 2008-2011 | 11.8 | 0.5 | 0.6 | 9.0 | 17.2 | 29.1 | 77.1 | 130.4 | 221.1 | |
| Non-MENA countries | Cyprus 26 | 2012 | 65.7 | - | 14.7 | 46.4 | 158 | 206.6 | 510.9 | 798.8 | 692.8 |
| Turkey (2 registries) 26 | 2012 | 40.7 | 0.4 | 7.9 | 30.2 | 103.8 | 183.4 | 354.3 | 393.3 | 443.6 | |
| USA, SEER (9 registries) 26 | 2012 | 81.3 | 41.2 | 121.4 | 257.7 | 438.3 | 681.8 | 693.4 | 583.7 | 488.6 | |
| Denmark 31 | 2012 | 78.6 | 3.6 | 16.2 | 63.1 | 177.1 | 405.6 | 657.5 | 794.4 | 765.3 | |
| Norway 26 | 2012 | 111.0 | 4.1 | 29.5 | 99.1 | 303.9 | 590.9 | 918.6 | 1054.5 | 965.4 | |
| Estonia 26 | 2012 | 107.6 | 4.4 | 33.1 | 99.6 | 302.7 | 537.8 | 1003.5 | 1018.6 | 836.0 | |
| Sweden 31 | 2012 | 92.5 | 3.4 | 21.1 | 112.6 | 268.0 | 511.7 | 763.6 | 826.1 | 736.7 | |
| UK, England 26 | 2012 | 72.9 | 3.6 | 17 | 64.3 | 175.4 | 340.3 | 578.5 | 703.3 | 812.61 | |
| Ireland 26 | 2012 | 107.9 | 8.3 | 44.4 | 145.4 | 337.2 | 647.9 | 918.2 | 890.8 | 637.52 | |
| Spain (9 registries) 26 | 2010 | 70.2 | 2.3 | 16.9 | 50.4 | 206 | 394 | 577.3 | 706.5 | 547.23 | |
| France (9 registries) 26 | 2011 | 98.4 | 2.6 | 14.8 | 111.3 | 313.8 | 549.9 | 864 | 878.6 | 677.82 | |
| Italy (8 registries) 26 | 2010 | 68.2 | 1.5 | 11.9 | 70.9 | 176.4 | 376.1 | 584.9 | 715.2 | 487.09 | |
| Belgium 32 | 2012 | 80.4 | 2.77 | 16.76 | 100.3 | 232.3 | 414.8 | 662.24 | 729.12 | 693.0 | |
| Germany (2 registries) 26 | 2012 | 59.6 | 2.9 | 13.3 | 45.2 | 166.2 | 303.9 | 486.4 | 610.2 | 518.34 | |
| Switzerland 26 | 2012 | 81.0 | 12.3 | 86.7 | 245.8 | 402.6 | 681.3 | 764.4 | 745.8 | 696.5 | |
| China (5 registries) 26 | 2012 | 17.5 | 0.2 | 1.6 | 7.1 | 18.9 | 54 | 133.3 | 229.6 | 275.13 | |
| Thailand (4 registries) 26 | 2012 | 6.6 | - | 0.5 | 2.5 | 11.3 | 25.8 | 35.7 | 82.9 | 113.3 | |
| Korea (5 registries) 26 | 2012 | 28.7 | 0.7 | 3.1 | 12.8 | 43.1 | 122 | 226.1 | 307.8 | 406.66 | |
| Japan (4 registries) 26 | 2010 | 38.0 | 0.4 | 4.1 | 17.0 | 57.0 | 149.3 | 300.0 | 467.4 | 513.3 | |
| Philippines, Manila 26 | 2012 | 22.6 | 0.5 | 1.3 | 5.1 | 19.5 | 73.8 | 91.8 | 325.2 | 459.2 | |
| Canada 26 (excl. Nunavut, Quebec and Yukon) | 2012 | 73.0 | 7 | 26.9 | 99 | 225.6 | 397.3 | 583.6 | 627.4 | 550.7 | |
| Australia 26 | 2012 | 112.3 | 12.3 | 59.7 | 152.7 | 364.6 | 612.9 | 896.0 | 900.5 | 808.3 | |
| Brazil, Goiania 26 | 2012 | 112.5 | 2.2 | 48.1 | 126.2 | 254.4 | 552.7 | 841.2 | 1124.9 | 1123.7 | |
| France, Martinique 26 | 2012 | 162.0 | - | 110.3 | 253.6 | 533.9 | 869.4 | 1272.2 | 1502.9 | 912.6 |
Abbreviations: ASR(w), age-standardized incidence rates (World Population); MENA, Middle East and North Africa; N.B, we included data for the age groups 40 years and above as they are more relevant to the scope of the study.
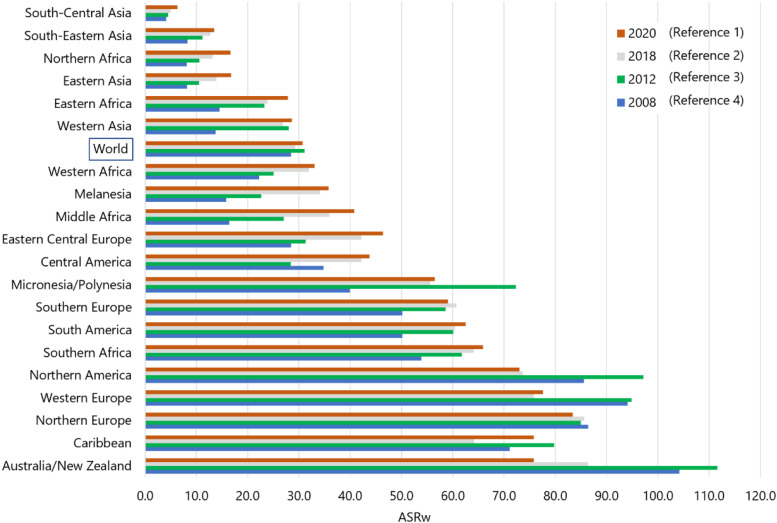
Projections from the published reports of the International Agency for Research on Cancer/Global Cancer Statistics (IARC/Globocan) revealed that the ASRw of PCa in Western countries was lower in 2020 than in 2012 following the 2012 USPSTF recommendation against the routine use of PSA testing (figure 4). 10 The 2018 USPTF recommendation does not seem to have a measurable effect yet. 11 As per Globocan 2020, Lebanon still has the highest ASRw (28.5) in MENA region after Israeli Jews and non-Jews (figure 5), although the Globocan projection figure for Lebanon is lower than our projection based on the NCR data (ASRw: 33.1, 95% confidence interval: 20.7, 45.4).
Figure 4.
Projected age-standardized incidence rates (ASRw) for prostate cancer by regions based on GLOBOCAN 2008, 2012, 2018, and 2020.
Figure 5.
Projected age-standardized incidence rates (ASRw) for prostate cancer in MENA countries-GLOBOCAN 2020 (Reference 1).
The ASRw of PCa in Lebanon increased by 7.5% between 2008 (38.4) and 2012 (41.3), mirroring a global increase in the ASRw by 9.1% between 2008 (28.5) and 2012 (31.1).3,4 The ASRw of PCa in Lebanon decreased by 9.7% between 2012 (41.3) and 2016 (37.3), concomitant with a global decrease by 6.1% between 2012 (31.1) and 2018 (29.3).2,3 Overall, the ASRw of PCa in Lebanon increased by 7.5% over the decade between 2006 (34.7) and 2016 (37.3), while it increased globally by 2.8% for the decade between 2008 (28.5) and 2018 (29.3) (the decades are not identical and are based on currently available NCR data and Globocan, respectively).2,4
Discussion
PCa is the most common cancer among men in Lebanon, like many countries worldwide.2,3 In 2018, PCa was the most common incident cancer among men in more than 50% of the countries worldwide (105 out of 185). 2 The ASRw of PCa in Lebanon was among the second highest in the MENA region after the Israeli Jews (almost equal to Israeli non-Jews), and higher than many countries in Asia, but much lower than the rates recorded in Australia/New Zealand, and countries in Northern and Western Europe and Northern America.2,3
The increase in the incidence of PCa and the variation in ASRw among different countries can be attributed to several factors including genetic factors, differences in population size and age structure, prevalence of risk factors, PCa screening recommendations and access to diagnostic and health care services.2,33,34 Despite the relatively moderate to high incidence rates of PCa in Lebanon, little research has been done to study this disease. Establishing epidemiological studies and statistics on PCa, along with a better understanding of its etiology and causative risk factors, are essential for creating the necessary infrastructure needed for the primary prevention and/or early detection of this disease. This infrastructure will provide strategies to identify at-risk men and to support the development of effective screening and prevention methods. In the following section, we will discuss various factors that may have contributed to the increased incidence of PCa in Lebanon.
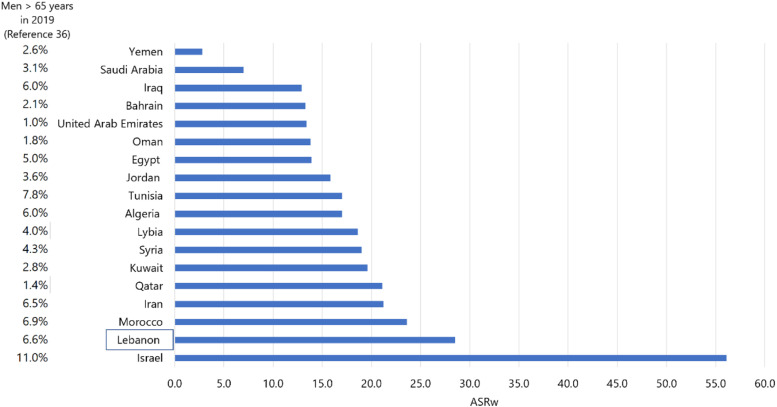
Sociological factors : The population in Lebanon increased by a staggering 43.7% between 2008 and 2018, mostly due to an influx of refugees and displaced people (42.8% increase in population between 2005 and 2016). 35 In 2019, the proportion of elderly men aged 65 years or more in Lebanon was estimated at 7%, a figure which is among the highest in the MENA countries excluding Israel (figure 5), yet much lower than Western countries (e.g., 15% in the United States, 16% in Norway, 17% in the United Kingdom, and 13% in Ireland). 36 However, the demographic shifts that Lebanon has experienced over the last few decades indicate that it has one of the fastest growing older adult populations in the region; between 2008 and 2018, Lebanon’s older adult population (age 65 years or more) ranked fifth in growth rate in the MENA region, after Qatar, United Arab Emirates, Kuwait, Bahrain, and Jordan. 36 The proportion of this elderly population in Lebanon increased by around 52.4% between 2005 and 2016. 36 This shift in the age structure/distribution has been attributed to 3 major factors: first, a decline in the fertility rate in Lebanon to around 1.9 37 ; second, a longer life expectancy at birth (76.3 years in general compared to 69.1 years in the WHO Eastern Mediterranean Region in 2016), 38 and third, high rates of youth emigration out of Lebanon. 39 These population changes in Lebanon are likely to have contributed to the rise in the incidence of PCa, similarly to what happened globally where 21% of the increase in the global incidence of PCa can be attributed to the shift in the population’s age structure, and another 13% to a change in the population size. 33
Screening recommendations and early detection: The significant increase in PCa incidence globally was linked to screening recommendations and the introduction of PSA test around 4 decades ago. This increase started initially in the USA followed by Europe, Australia, and Canada within a few years. 2 PCa rates are on the rise in countries where PSA testing became more widely used at a later stage, including Japan, Brazil, Costa Rica, and Thailand. 2
However, global PCa incidence declined in the past few years, particularly in the USA and most Nordic countries since the USPSTF (in 2008 and again in 2012), the Canadian Task Force of Preventive Services (in 2014), and United Kingdom (UK) (in 2015) issued recommendations against screening.2,10,40,41 USPSTF updated their recommendations in 2018 and reconfirmed those of the American Urological Association (AUA) calling for screening men in age group 55–69 years based on shared decision, that is, asking patient approval after explaining the benefits and harms of screening.11,42 USPSTF and AUA do not recommend screening men aged 70 years and above or those between 40 and 54 years with average risk for PCa,11,42 namely, because of high overdiagnosis rates (23% to 42%) and common serious side effects associated with treatment. 43 Screening men in the age group 40–54 years with high risk for PCa should be individualized; high risk include men of African American race, and those with a family history of metastatic or lethal adenocarcinomas (e.g., prostate, male and female breast cancer, ovarian, and pancreatic) crossing multiple generations, affecting multiple first-degree relatives, and that developed at younger ages.11,42 More research is being conducted currently on more effective ways to screen for PCa, identify its fatal forms, detect men at higher risk and use of chemoprophylaxis such as aspirin and 5α-reductase inhibitors. 44
In Lebanon, the increased utilization of PSA testing, widely available over the counter, may have also partially accounted for some of the increasing trend in PCA incidence till 2012. National public awareness campaigns promoting screening for PCa were initiated since 1994. 45 These campaigns were either supported by the MoPH or the Lebanese Urological Society or by academic medical centers. However, the 2012 recommendation against the routine use of PSA testing by the USPSTF have probably influenced the practice of primary care physicians, resulting in a decline in the ASRw of PCa in Lebanon after that. In fact, only 29% of family physicians in Lebanon reported to systematically screen men aged above 50 years for PCa. 46 With the recent change in the recommendations by the USPSTF, the rates of screening might rise again leading to higher incidence rates in the future. It is worth noting that 25% of patients in Lebanon present with metastatic prostate disease, 47 which increases the need to enhance awareness among men namely those at increased risk.
Family history and genetic factors : Family history is an established PCa risk factor. 8 Men who have a first-degree relative with PCa have around 2.5 higher risk of disease development.12,44 PCa is a multifactorial disease where polymorphisms of genes involved in carcinogen metabolism such as CYP1A1 and CYP2E1 could play a key role. To date, several genetic mutations/polymorphisms and more than 100 single nucleotide polymorphisms (SNPs) associated with increased PCa risk were identified. 48 The allele CYP1A1*2A was found among the genetic risk factors that contribute to PCa in Lebanon, with individuals carrying at least one CYP1A1*2A allele being at increased risk of PCa by 2.7-fold. 49 Another study demonstrated the association of some SNPs in the VDR gene and the SRD5A2 gene with the increased risk of PCa among Lebanese men. 50
Lifestyle factors : Potentially modifiable lifestyle factors, namely, cigarette smoking and excess body weight increase the risk of advanced and fatal PCa, while physical activity decreases the risk of fatal PCa (Table 1).6,8,12-17 Paradoxically, greater leisure-time physical activity was associated with a higher risk of PCa in one meta-analysis, 51 but the effect of screening bias was not clear.
In Lebanon, risky lifestyles (unhealthy diet and physical inactivity), obesity, and smoking are becoming more conspicuous.52-54 Physical inactivity is prominent among adults, with 61% of those aged 18-69 years report having low levels of physical activity, 55 and 34.8% of adults (40.9% of men) report spending more than 12 sedentary hours per day. 52 Obesity is also reaching an alarming level, 54 with 27% of adults aged 18-69 being obese, and 37.9% being overweight. 55 Tobacco smoking is also common among male adults, with a prevalence increasing from 43% in 2009 to 48% in 2016.52,55 Adult Lebanese consume high number of cigarette packs, reaching 12.4 packs per person per month. 56 Moreover, unsafe smoking-related indoor air pollution levels in several public places, 57 puts people at risk of passive smoking.
The association of alcohol with PCa is not conclusive. 8 Based on a recent meta-analysis, this association may vary with the type of alcohol; for example, the risk of non-aggressive PCa was associated with liquor (linearly) and beer (non-linearly), but not with wine or total alcohol intake. Moreover, the risk of aggressive PCa was higher with low liquor or heavy wine intake. 58 Alcohol consumption in Lebanon is the highest in the Arab region, 59 with 51.9% of adult men reporting drinking alcohol. 52
Our study is based on the cancer data published by the MoPH on its official website. Despite the improvements done in data collection for the NCR, information on collected cases is still incomplete lacking in-situ conditions, risk factors, as well as mortality and survival rates. Since our results are based on PCa rates for the years 2005 through 2016, caution is to be exerted when interpreting these results beyond the study period.
Conclusion
PCa is a significant health problem in Lebanon, affecting mainly older men. Changes in population age structure, screening and early detection practices, and prevalence of genetic and risky lifestyle factors could have increased the incidence rate of this disease. Despite that PCa is a slowly growing tumor, early detection and treatment can still improve the quality of life in men. To date, there is no evidence whether the introduction of a population screening program would reduce mortality without significant numbers of men being overtreated. Recommendations in this regard are still controversial. Countries are implementing different screening programs for addressing PCa burden. For example, United Kingdom implemented an informed choice program for PSA testing in asymptomatic men, using a tool that promotes patient-centered care and involves patients and physicians in weighing the benefits and harms of PSA testing.60,61
Given the high incidence rate of PCa in Lebanon, it is important to increase the awareness among men on primary prevention through adopting healthier lifestyle, and to strengthen healthy lifestyle education programs at the primary care level. Moreover, it is advised to follow the USPSTF latest recommendations regarding PSA testing, that is, shared decision testing in asymptomatic men aged 55–69 years and individualized testing in high-risk men aged 40–54 years.
Footnotes
Abbreviations: APC Annual Percent Change
AAPC Average Annual Percent Change
ASRw age-standardized incidence adjusted to the world population
AUA American Urological Association
C15 Plus Cancer Incidence in 5 Continents Time Trends
C15 XI Cancer Incidence in 5 Continents Volume XI
HDI Human Development Index
IARC International Agency for Research on Cancer
ICD10 International Classification of Diseases, 10th revision
IGF-I Insulin-like growth factor-I
JRP Joinpoint Regression Program
MENA Middle East and North Africa
MoPH Ministry of Public Health
NCR National Cancer Registry
PCa prostate cancer
PSA prostate-specific antigen
SNP single nucleotide polymorphism
UK United Kingdom
USA United States of America
USPSTF United States Preventive Services Taskforce
WHO World Health Organization
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Mona H. Osman https://orcid.org/0000-0002-6364-6541
References
- 1.Ferlay J, Ervik M, Lam F, et al. GLOBOCAN 2020. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. https://gco.iarc.fr/today. Accessed January 18, 2021. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi:10.1002/ijc.25516. PMID: 21351269. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Development Programme . Human Development Reports. Human Development Indices and Indicators. 2018 Statistical Update. http://hdr.undp.org/en/content/human-development-indices-indicators-2018-statistical-update. http://hdr.undp.org/en/content/human-development-indices-indicators-2018-statistical-update.Accessed January 18, 2021. [Google Scholar]
- 6.Rawla P. Epidemiology of Prostate Cancer. World J Oncol. 2019;10(2):63-89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med. 2018;8(12):a030361. doi: 10.1101/cshperspect.a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and prostate cancer. https://www.wcrf.org/dietandcancer/prostate-cancer. https://www.wcrf.org/dietandcancer/prostate-cancer.Accessed December 12, 2020
- 9.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology. prostate cancer early detection: version 2. 2017. www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf.Accessed December 17, 2020
- 10.Moyer VA. On behalf of the U.S. preventive services task force. Screening for prostate cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2012;157:120-134. [DOI] [PubMed] [Google Scholar]
- 11.US Preventive Services Task Force . Screening for prostate cancer: US preventive services task force recommendation statement. J Am Med Assoc. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Cornago A, Key TJ, Allen NE, et al. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117(10):1562-1571. doi: 10.1038/bjc.2017.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabir A, Zendehdel R, Tayefeh-Rahimian R. Dioxin Exposure in the Manufacture of Pesticide Production as a Risk Factor for Death from Prostate Cancer: A Meta-analysis. Iran J Public Health. 2018;47(2):148-155. [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser GE, Jacobsen BK, Knutsen SF, Mashchak A, Lloren JI. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2. Cancer Causes Control. 2020;31(4):341-351. doi: 10.1007/s10552-020-01279-z. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer (IARC) . Personal Habits and Indoor Combustions; Tobacco Smoking, Volume100E. IARC Monographs; 2012. http://publications.iarc.fr/122. Accessed December 10, 2020. [Google Scholar]
- 16.Pardo LA, Beane Freeman LE, Lerro CC, et al. Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ Health. 2020;19(1):30. doi: 10.1186/s12940-020-00583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shephard RJ. Physical activity and prostate cancer: an updated review. Sports Med. 2017;47:1055-1073. DOI: 10.1007/s40279-016-0648-0. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) . Global Health Observatory Data Repository. WHO Eastern Mediterranean Region: Lebanon. https://www.who.int/countries/lbn/. Accessed February 23, 2021. [Google Scholar]
- 19.World Health Organization . Cancer Country Profiles; 2014. Lebanon https://www.who.int/cancer/country-profiles/lbn_en.pdf. Accessed November 11, 2020. [Google Scholar]
- 20.Adib S, Daniel J. Cancer in Lebanon 2004 with an Update of Cancer 2003: Ministry of Public Health, National Cancer Registry; 2008. https://www.moph.gov.lb/DynamicPages/download_file/571. Accessed December 11, 2020. [Google Scholar]
- 21.National Cancer Registry . Lebanon. Ministry of Public Health Web Site; 2020. https://www.moph.gov.lb/en/Pages/2/7164/national-cancer-registry#/en/Pages/2/193/esu. Accessed November 1, 2020. [Google Scholar]
- 22.Bray F, Ferlay J. Age Standardization (chapter 7). In: Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, et al. , eds Cancer Incidence in Five Continents, Vol. XI. Lyon, France: International Agency for Research on Cancer (IARC); 2017. (electronic version) http://ci5.iarc.fr/CI5-XI/Pages/Chapter7.aspx. Accessed November 11, 2020. [Google Scholar]
- 23.United States Cancer Statistics (USCS) . Centers for Disease Control and Prevention (CDC) Website. https://www.cdc.gov/cancer/npcr/uscs/technical_notes/stat_methods/rates.htm. Accessed November 11, 2020. [Google Scholar]
- 24.Joinpoint Help System 4.7.0.0 (online)- Surveillance Research Program. National Cancer Institute (NCI), Division of cancer control and population sciences, USA. Accessed November 11, 2020. https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab.
- 25.Dyba T, Hakulinen T. Comparison of different approaches to incidence prediction based on simple interpolation techniques. Stat Med. 2000;19(13):1741-1752. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents Time Trends (CI5plus): IARC CancerBase No. 9 [Internet]: International Agency for Research on Cancer (IARC); 2018. http://ci5.iarc.fr/CI5plus/Pages/table4_sel.aspx. Accessed November 11, 2020. [Google Scholar]
- 27.Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents, Vol. XI (CI5 XI) [electronic Version]. Lyon, France: International Agency for Research on Cancer (IARC); 2017. http://ci5.iarc.fr/CI5-XI/Pages/registry_summary.aspx. Accessed November 31, 2020. [Google Scholar]
- 28.Jordan Cancer Registry . Cancer Incidence in Jordan 2012. http://www.moh.gov.jo/Echobusv3.0/SystemAssets/a05a084b-3781-4979-a217-2184d5d57ede.pdf. Accessed November 11, 2020. [Google Scholar]
- 29.National Cancer Registry Program of Egypt (NCRPE) . https://mcit.gov.eg/en/Publication/Publication_Summary/554. Accessed November 11, 2020.
- 30.Kingdom of Saudi Arabia Saudi Health Council Saudi Cancer Registry . Cancer Incidence Report Saudi Arabia 2013. https://nhic.gov.sa/eServices/Documents/2013.pdf. Accessed November 11, 2020. [Google Scholar]
- 31.The NORDCAN project . https://www-dep.iarc.fr/nordcan/english/frame.asp. Accessed November 11, 2020.
- 32.European Cancer Information System (ECIS) . https://ecis.jrc.ec.europa.eu/index.php. Accessed November 11, 2020.
- 33.Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taitt HE. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am J Men's Health. 2018;12(6):1807-1823. doi: 10.1177/1557988318798279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Bank . The World Bank Annual Report 2019: Ending Poverty, Investing in Opportunity. Washington, DC: World Bank. © World Bank. License: CC BY-NC-ND 3.0 IGO; 2019. https://openknowledge.worldbank.org/handle/10986/32333. Accessed January 19, 2021. [Google Scholar]
- 36.World Bank . Population ages 65 and above, male (% of male population). 2019. https://data.worldbank.org/indicator/SP.POP.65UP.MA.ZS?name_desc=false. Accessed January 19, 2021.
- 37.Central Administration of Statistics . Population and Housing Characteristics in Lebanon, Statistics in Focus (SIF), Beirut, Lebanon, Issue Number 2; 2012. http://www.cas.gov.lb/images/PDFs/SIF/CAS_Population_and_housing_In_Lebanon_SIF2.pdf. Accessed December 11, 2020. [Google Scholar]
- 38.World Health Organization . Life Expectancy and Healthy Life Expectancy Data by WHO Region. https://apps.who.int/gho/data/view.main.SDG2016LEXREGv?lang=en. Accessed January 20, 2021. [Google Scholar]
- 39.Abdulrahim S, Ajrouch KJ, Antonucci TC. Aging in Lebanon: Challenges and Opportunities. Gerontol. 2015;55(4):511-518. doi: 10.1093/geront/gnu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297-316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- 41.Canadian Task Force on Preventive Health Care . Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ (Can Med Assoc J). 2014;186:1225-1234. https://canadiantaskforce.ca/canadian-task-force-recommends-against-screening-for-prostate-cancer/. Accessed December 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of Prostate Cancer: AUA Guideline 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed December 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009. Mar 18;101(6):374-383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014. Oct;15(11):e484-92. doi: 10.1016/S1470-2045(14)70211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shamseddine AI, Musallam KM. Cancer Epidemiology in Lebanon. Middle East Journal of Cancer. 2010;1(1):41-44. [Google Scholar]
- 46.Ghayad T, Mekhael M, Gebara N, et al. Lebanese physicians' attitude towards prostate cancer screening]. Bull Cancer. 2020. Nov;107(11):1199-1201. French. doi: 10.1016/j.bulcan.2020.07.010. Epub 2020 Sep 22. PMID: 32977939. [DOI] [PubMed] [Google Scholar]
- 47.Mukherji D, Abed El Massih S, Daher M, et al. Prostate cancer stage at diagnosis: First data from a Middle Eastern cohort. J Clin Oncol. 2017;35(6_suppl):e552-e. [Google Scholar]
- 48.Al Olama AA, Kote-Jarai Z, Berndt SI, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014. Oct;46(10):1103-1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdel-Razzak Z, Houssein Al-Attrache H, Rammal G. Association of CYP1A1 and CYP2E1 gene polymorphisms with prostate cancer in a Lebanese population. International Research Journal of Public and Environmental Health. October. 2015;2(10):135-143. doi: 10.15739/irjpeh.031. [DOI] [Google Scholar]
- 50.El Ezzi AA, Boyko VG, Baker MT, et al. Association of Some Polymorphisms in the VDR Gene, CYP17 Gene and SRD5A2 Gene and Prostate Cancer among Lebanese Men. Asian Pac J Cancer Prev. 2017;18(1):93-100. doi: 10.22034/APJCP.2017.18.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816-825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sibai A, Tohme RA, Mahfoud Z, Chaaya M, Hwalla N. Noncommunicable Diseases and Behavioral Risk Factor Survey Comparison of Estimates Based on Cell Phone Interviews with Face-to-Face Interviews: American University of Beirut; 2009. https://www.moph.gov.lb/DynamicPages/download_file/563. Accessed December 10, 2020. [Google Scholar]
- 53.Nasreddine L, Hwalla N, Sibai A, Hamzé M, Parent-Massin D. Food consumption patterns in an adult urban population in Beirut, Lebanon. Publ Health Nutr. 2006;9:194-203. [DOI] [PubMed] [Google Scholar]
- 54.Nasreddine L, Naja F, Chamieh MC, Adra N, Sibai AM, Hwalla N. Trends in overweight and obesity in Lebanon: evidence from two national cross-sectional surveys (1997 and 2009). BMC Publ Health. 2012;12:798. doi: 10.1186/1471-2458-12-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization, Ministry of Public Health . Lebanon, 2016-2017. WHO Stepwise Approach for Non-Communicable Diseases Risk Factor Surveillance. https://www.who.int/ncds/surveillance/steps/Lebanon_STEPS_report_2016-2017.pdf?ua=1. Accessed December 28, 2020. [Google Scholar]
- 56.Salti N, Chaaban J, Naamani N. The economics of tobacco in Lebanon: an estimation of the social costs of tobacco consumption. Subst Use Misuse. 2014;49(6):735-742. doi: 10.3109/10826084.2013.863937. [DOI] [PubMed] [Google Scholar]
- 57.Saade G, Seidenberg AB, Ree VW, Otrock Z, Connolly GN. Indoor secondhand tobacco smoke emission levels in six Lebanese cities. Tobac Control. 2010;19:138e142. doi: 10.1136/tc.2009.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong S, Khil H, Lee DH, Keum N, Giovannucci EL. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients. 2020;12(8):2188. doi: 10.3390/nu12082188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghandour L, Chalak A, El-Aily A, et al. Alcohol consumption in the Arab region: What do we know, why does it matter, and what are the policy implications for youth harm reduction? Int J Drug Policy. 2016;28:10-33. doi: 10.1016/j.drugpo.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Louie KS. UKNSC Screening for Prostate Cancer Review 2015 Update. UK National Screening Committee; 2016. Accessed 19 January 19, 2021. [Google Scholar]
- 61.Public Health England . Prostate Cancer Risk Management Programme (PCRMP): Benefits and Risks of PSA Testing 2016. https://www.gov.uk/government/publications/prostate-cancer-risk-management-programme-psa-test-benefits-and-risks/prostate-cancer-risk-management-programme-pcrmp-benefits-and-risks-of-psa-testing#fn:3. Accessed January 19, 2021. [Google Scholar]