Abstract
Lack of uricase leads to the high incidence of gout in humans and poultry, which is different from rodents. Therefore, chicken is considered to be one of the ideal animal models for the study of gout. Gout-related pain caused by the accumulation of urate in joints is one type of inflammatory pain, which causes damage to joint function. Our previous studies have demonstrated the crucial role of calcium-stimulated adenylyl cyclase subtype 1 (AC1) in inflammatory pain in rodents; however, there is no study in poultry. In the present study, we injected mono-sodium urate (MSU) into the left ankle joint of the chicken to establish a gouty arthritis model, and tested the effect of AC1 inhibitor NB001 on gouty arthritis in chickens. We found that MSU successfully induced spontaneous pain behaviors including sitting, standing on one leg, and limping after 1–3 h of injection into the left ankle of chickens. In addition, edema and mechanical pain hypersensitivity also occurred in the left ankle of chickens with gouty arthritis. After peroral administration of NB001 on chickens with gouty arthritis, both the spontaneous pain behaviors and the mechanical pain hypersensitivity were effectively relieved. The MSU-induced edema in the left ankle of chickens was not affected by NB001, suggesting a central effect of NB001. Our results provide a strong evidence that AC1 is involved in the regulation of inflammatory pain in poultry. A selective AC1 inhibitor NB001 produces an analgesic effect (not anti-inflammatory effect) on gouty pain and may be used for future treatment of gouty pain in both humans and poultry.
Keywords: Gout, arthritis, adenylyl cyclase 1, NB001, chicken, inflammatory pain, spontaneous pain
Introduction
Gouty pain is an inflammatory pain caused by the accumulation of urate in joints and tissue around joints.1,2 Poultry and humans have similar purine nucleotide metabolic pathways, and they have something in common in uric acid syntheses and metabolism. Particularly, both of them lack uricase.3,4 Different from the high cost of the uricase-deficient mouse model and the complexity of model construction, the establishment of the chicken model of gouty arthritis has advantages in the similarity of the uric acid metabolism pathway and the simplicity of operation.5,6 Therefore, chicken is an ideal animal for studying gouty arthritis.
Spontaneous pain, movement-induced pain, and joint allodynia are the main characteristics of gouty pain.7,8 This is related to the enhancement of the nociceptive input of the spinal cord and the excitability of cortices induced by the inflammatory response at the joint.9,10 Calcium-stimulated adenylyl cyclase 1 (AC1) is a downstream protein of glutamatergic N-methyl-D-aspartate receptor (NMDAR). High expression of AC1 in the spinal cord and pain-related cortices can cause long-term potentiation (LTP) of synaptic transmission and contribute to chronic pain.11–13 In addition, studies on neuropathic pain and inflammatory pain have shown that AC1 inhibitor (NB001) or AC1 gene knockout can alleviate pain and pain-related anxiety behaviors on chronic pain mice, such as NB001 alleviate CFA-induced arthritis-related pain.14–16 Moreover, when NB001 was used in animal models, no obvious side effects were observed. 17 At present, the treatment of gouty pain is mainly through non-steroidal anti-inflammatory drugs and glucocorticoids.18,19 Long-term use of these two treatment methods will cause serious side effects that cannot be ignored. Studying the regulatory effect of AC1 on gouty chickens can not only provide new ideas for the clinical intervention of gouty pain but also effectively promote the development of the poultry industry.
In the present work, we injected mono-sodium urate (MSU) into the left ankle joint of chickens to establish a gouty arthritis model and observed the changes of pain behaviors of chickens at different time points. We found that spontaneous pain behaviors (including sitting, standing on one leg, and limping (Table 1)), ankle edema, ankle stiffness, and the reduction of mechanical pain threshold occurred in the chickens after 1 h of MSU injection. Furthermore, peroral administration of the AC1 inhibitor (NB001, 10 mg/kg) to chickens with gouty arthritis effectively relieved the pain of chickens’ ankle joints but did not affect the ankle edema of chickens, which was consistent with our previous studies in mice. 14 Our results suggest that AC1 pathway is involved in the regulation of gouty pain, not by anti-inflammatory effect, which can provide a new idea for the future clinical intervention of gouty pain.
Table 1.
Behaviors recorded in the chickens with MSU-induced gouty arthritis.
| Behaviors | Description |
|---|---|
| Sitting a | Ventral side contact with the ground |
| Flinching a | Raising one leg and stand on the other leg alone |
| Limping a | Abnormal limb using and walking posture with weight distributed uneven on feet |
| Standing on both legs | Both feet touch the ground at the same time |
| Walking | Normal limb using and walking posture with weight distributed even on both feet |
| Scratching | Claws scratch the ground |
| Grooming | Preening feathers by beak |
| Pecking at food | Touching food by beak |
| Pecking environment | Touching cage or the outside of the cage by beak |
| Drinking | Touching water by beak |
| Phonating | Making any sound |
aSpontaneous pain behaviors.
Materials and methods
Animals
Male and female adult silky chickens aged 20 weeks were used in all experiments. All animals were randomly housed under an artificial light/dark cycle (lights on 7 am–7 pm) with food and water provided ad libitum. Animals acclimated to the laboratory environment for at least 1 week before the experiment begins. All experiment protocols were approved by the Ethics Committee of Xi’an Jiaotong University and the Ethics Committees of Qingdao International Academician Park.
Mono-sodium urate injection and NB001 administration
Mono-sodium urate (MSU, 0.2 mL, 40 mg/mL) was injected into the left ankle joint cavities of chickens to induce gouty arthritic pain of the ankle joint. The same volume of saline was injected into the left ankle joint cavities of the control chickens. Behavioral tests before MSU or saline injection were performed as the baseline. And the same behavioral tests were performed in the same environment at 1 h, 2 h, 3 h, and 4 h on the first day, and seventh day after MSU or saline injection, respectively. According to the pain response at different time after MSU injection, chickens were peroral administrated with NB001 (10 mg/kg) or saline at 1.5 h after MSU injection, and behavioral tests were performed at 2 h after MSU injection.
Assessment of ankle joint edema
To quantify the effects of NB001 on joint inflammation, a flexible ruler was used to evaluate the circumference of the chicken ankle at different time points before and after injection of MSU, NB001, or saline.
Evaluation of spontaneous pain behaviors
Spontaneous pain is a behavioral phenotype of joint pain that has been used to evaluate arthritis. 20 Paw flinching and sitting down are two indicators of spontaneous pain. Flinches were calculated as the number and time of chickens raised their left paws. Sits were calculated as the number and time of chickens sat down on the ground without supporting their body on their legs. In order to detect flinching and sitting behaviors, chicken was placed in a cage with a length of 70 cm, a width of 50 cm, and a height of 60 cm for 30 min acclimation. The number and time of spontaneous flinch and sit were recorded during 5 min.
As previously reported, limb use was measured on a scale of 5 to 0 during spontaneous movement.21,22 Chickens were placed in a cage with the length of 70 cm, the width of 50 cm and the height of 60 cm for 30 min acclimation. Limb use was detected during 5 min. According to previous reports,21,22 limb use was scored: 5 = normal; 4 = partial limp, but not prominent; 3 = prominent limp; 2 = partial non-use of left limb; 1 = vast non-use of left limb; and 0 = complete non-use of left limb.
Measurement of mechanical withdrawal threshold
The chickens were placed in cages to acclimate for 30 min before the experiment. Mechanical withdrawal threshold was measured with Von Frey filaments, a series of filaments (10, 15, 26, 60, 100, 180, and 300 g) was applied to the left ankle cavity and evaluated by left limb responsiveness to different stimulation. If the animal did not respond to the maximum filament (300 g), 300 g would be used as the final mechanical threshold. The filaments were applied vertically to the surface center of the joint cavity with enough force to cause a slight bend to remain for 6 s. According to the up-down strategy, a mechanical withdrawal threshold was detected 5 times with the stimulation interval of 10 min. Positive reactions include pecking, and swift withdrawal of the paw.
Measurement of ankle joint stiffness
Methods for examining joint stiffness scores have been reported as previous. 14 One experimenter immobilized the chicken and another experimenter bent and extended the left ankle (once in each direction). The standards for stiffness scores are as follows: score 2, there were restrictions of joint movement in both bending and extension; score 1, there was a restriction of joint movement only in one direction (bending or extension); and score 0, there was no restriction in two directions, normal bending, or extension joint movement.
Data analysis
The data were presented as means ± SEM. Statistical comparisons between two groups were performed to identify significant differences. When the data were normally distributed and the variance was homogeneous, a two-tail unpaired Student’s t-test was used. When the distribution was not normal or the variance was not homogeneous, the Mann–Whitney U test was used. * p < 0.05 was considered statistically significant.
Results
Mono-sodium urate–induced spontaneous pain behaviors
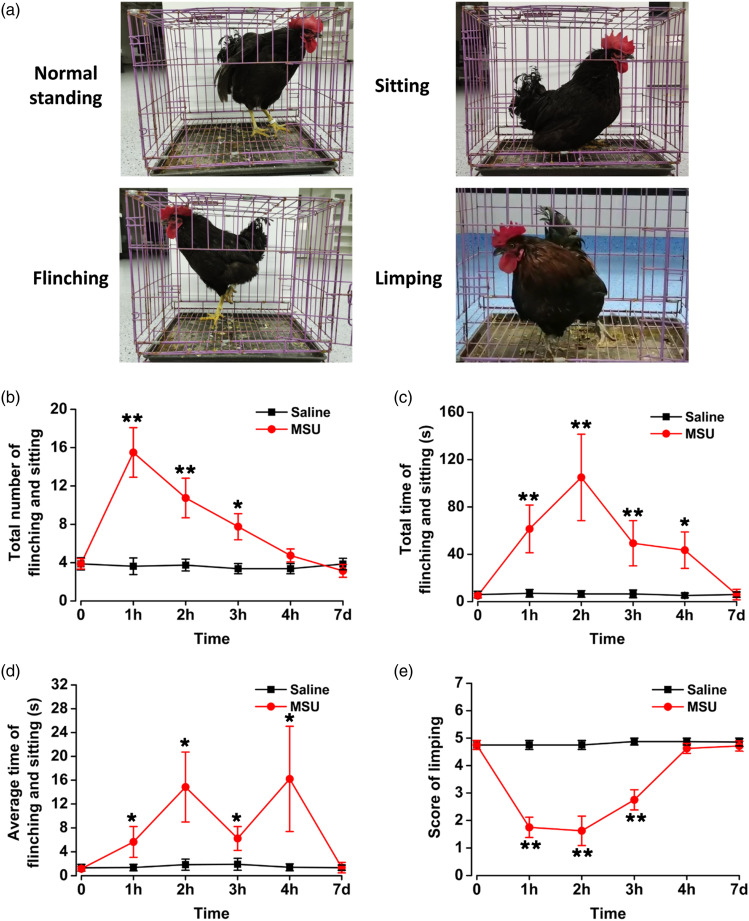
An MSU-induced gouty arthritic pain model was used to assess arthritic pain of ankle joints in chickens. After injection of MSU into the ankle cavity, various behaviors of the chickens were observed and recorded (Table 1). Flinching, sitting, and limping were measured as three important indicators of spontaneous pain (Figure 1(a)). After MSU injection, the number of flinching and sitting behaviors of gouty chickens increased significantly and reached the peak at 1 h (saline: 3.6 ± 0.9; MSU: 15.5 ± 2.6; p < 0.01; Figure 1(b)). Compared with saline group, the number of flinching and sitting were still increased at 2 h (saline: 3.8 ± 0.6; MSU: 10.8 ± 2.1; p < 0.01; Figure 1(b)) and 3 h (saline: 3.4 ± 0.5; MSU: 7.8 ± 1.4; p < 0.05; Figure 1(b)) after MSU injection in chickens. Finally, the number of flinching and sitting were basically restored to the level of saline at 4 h after MSU injection (saline: 3.4 ± 0.5; MSU: 4.8 ± 0.7; p > 0.05; Figure 1(b)). To observe the effect of MSU on spontaneous pain more comprehensively, in addition to counting the number of flinching and sitting, we also measured the total duration time and average duration time of these behaviors. The total time of flinching and sitting in chickens reached 105.0 ± 36.5 s during 5 min testing period at 2 h after MSU injection, which was significantly increased compared with 6.5 ± 2.7 s in saline-injected chickens (p < 0.01; Figure 1(c)). Compared with the saline group, the average time of flinching and sitting were significantly increased at 1 h (saline: 1.4 ± 0.5 s; MSU: 5.6 ± 2.6 s; p < 0.05; Figure 1(d)), 2 h (saline: 1.9 ± 0.9 s; MSU: 14.8 ± 5.9 s; p < 0.05; Figure 1(d)), 3 h (saline: 1.9 ± 1.0 s; MSU: 6.2 ± 2.0 s; p < 0.05; Figure 1(d)), and 4 h (saline: 1.4 ± 0.6 s; MSU: 16.2 ± 8.8 s; p < 0.05; Figure 1(d)) after MSU injeciton in chickens. These results suggest that injection of MSU into chicken ankles induces spontaneous pain behaviors.
Figure 1.
Mono-sodium urate–induced spontaneous pain behaviors in chickens of gouty arthritis. (a) Pictures of chickens standing normally before MSU injection; sitting, flinching, and limping after MSU injection. (b) Injection of MSU increased the number of spontaneous pain behaviors in chickens of gouty arthritis at 1 h after MSU injection and disappeared at 4 h after injection. (c), (d) 1 h after MSU injection, the total time in 5 min and average time of each flinching and sitting of chickens suffering gouty arthritis increased and disappeared after 4 h. (e) After MSU injection, the limping behaviors of chickens with gouty arthritis increased significantly and disappeared at 4 h after injection. *p < 0.05, **p < 0.01, MSU (n = 8) vs. saline (n = 8).
To observe the behavior of limping in chickens after MSU injection, the uses of limb were observed and measured in free movement chickens after MSU injection, and limping was rated on a scale of 5 to 0. Subsequent to MSU injection into left ankle cavity, the score of limping decreased rapidly and reached the peak at 2 h (saline: 4.8 ± 0.2; MSU: 1.6 ± 0.5; p < 0.01; Figure 1(e)), and basically returned to the level of saline group at 4 h (saline: 4.9 ± 0.1; MSU: 4.6 ± 0.2; p > 0.05; Figure 1(e)). These results indicate that injection of MSU into the chicken ankles induces limping and recovers 4 h later.
Mono-sodium urate decreased mechanical withdrawal threshold
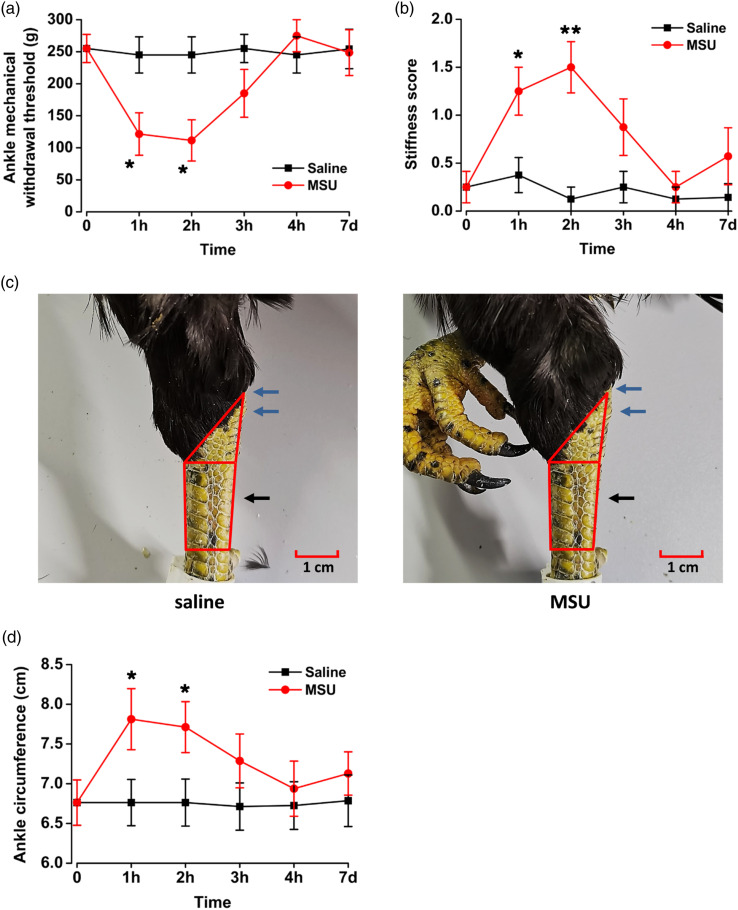
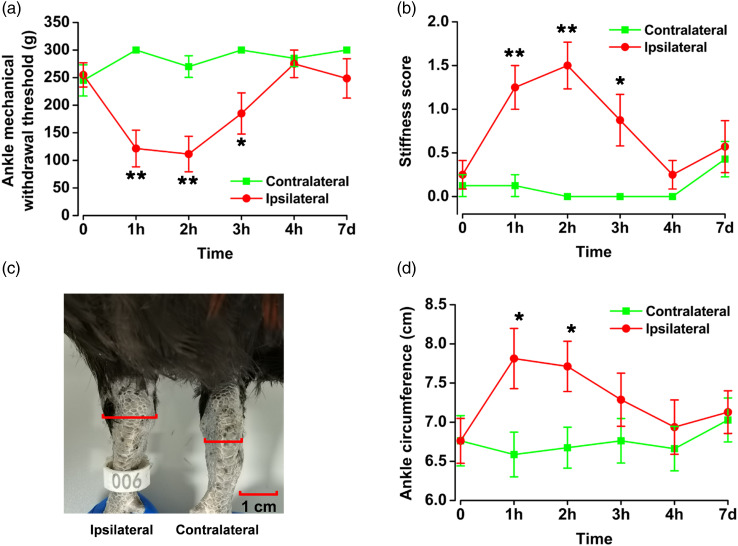
To further detect other pain-related behaviors in arthralgia, we measured the mechanical withdrawal threshold in the MSU-induced arthralgia model of chickens. The mechanical withdrawal threshold in left ankle joint of chickens decreased significantly at 1 h (saline: 245.0 ± 28.2 g; MSU: 121.5 ± 33.2 g; p < 0.05; Figure 2(a)) and 2 h (saline: 245.0 ± 28.2 g; MSU: 111.5 ± 32.2 g; p < 0.05; Figure 2(a)) after the injection with MSU into the left ankle cavity. In the MSU model group, the mechanical withdrawal threshold of the ipsilateral ankle with an injection of MSU at 1 h or 2 h was 121.5 ± 33.2 g and 111.5 ± 32.2 g respectively, which were significantly lower than 300.0 ± 0.0 g and 270.0 ± 19.6 g in that of the contralateral ankle (1 h: p < 0.01; 2 h: p < 0.01; Figure 3(a)). These results indicate that MSU injection into the chicken ankles reduces the mechanical withdrawal threshold of the ipsilateral ankle.
Figure 2.
Mono-sodium urate–induced mechanical withdrawal threshold decreased and edema of left ankle in chickens of gouty arthritis. (a) 1 h after MSU injection, the left ankle of the chicken mechanical withdrawal threshold decreased. (b–d) After MSU injection, the stiffness score and circumference of the left ankle in chickens suffering gouty arthritis showed significant differences compared with the saline group. In the schematic diagram of ankle swelling (c) of the same scale (red area), after injection of MSU, the black arrow indicates that there is no edema, and the blue arrow indicates that there is edema. *p < 0.05, **p < 0.01, MSU (n = 8) vs. saline (n = 8).
Figure 3.
Differences in mechanical withdrawal threshold and inflammatory symptoms of ipsilateral and contralateral ankles in chickens of MSU-induced gouty arthritis. (a) Compared with the contralateral ankle, the ipsilateral ankle of chickens decreased the mechanical withdrawal threshold at 1 h after MSU injection (b) After MSU injection, the stiffness score of ipsilateral ankle of chickens with gouty arthritis showed significant differences compared with contralateral ankle. (c, d) After MSU injection, the circumference of ipsilateral ankle of chickens with gouty arthritis showed significant differences compared with contralateral ankle. *p < 0.05, **p < 0.01, ipsilateral (n = 8) vs. contralateral (n = 8).
MSU-induced joint inflammatory symptoms
After the injection of the MSU inflammatory substance, the chicken ankles experienced some inflammatory reactions, such as stiffness and swelling. To quantify the inflammatory response in the MSU-induced arthralgia model in chickens, the stiffness and circumference of the ankle joint were measured. Joint stiffness, as an indicator of arthritis, was assessed by the bending and extension test. 23 Before MSU injection, both ankles of all chickens were no restrictions in bending and extending. Compared with the saline group, the bending and extension of the MSU-injected ankle were significantly limited after injection of MSU, showing significant differences at 1 h (saline: 0.4 ± 0.2; MSU: 1.3 ± 0.3; p < 0.05; Figure 2(b)) and 2 h (saline: 0.1 ± 0.1; MSU: 1.5 ± 0.3; p < 0.01; Figure 2(b)), and basically recovered to the level of baseline at 4 h (saline: 0.1 ± 0.1; MSU: 0.3 ± 0.2; p > 0.05; Figure 2(b)). Compared with contralateral group, the stiffness scores of the ipsilateral ankles also showed significant differences at 1 h (contralateral: 0.1 ± 0.1; ipsilateral: 1.3 ± 0.3; p < 0.01; Figure 3(b)) and 2 h (contralateral: 0.0 ± 0.0; ipsilateral: 1.5 ± 0.3; p < 0.01; Figure 3(b)) after MSU injection to the left ankles. Taken together, our results suggest that injection of MSU increases ipsilateral limb stiffness in chickens.
To detect the swelling of the ankle joint, one experimenter held the chicken in place while the other used a soft ruler to measure the circumference of the ankle joint cavity. Significant swelling of the left ankles appeared at 1 h (saline: 6.8 ± 0.3 cm; MSU: 7.8 ± 0.4 cm; p < 0.05; Figures 2(c) and (d)) and 2 h (saline: 6.8 ± 0.3 cm; MSU: 7.7 ± 0.3 cm; p < 0.05; Figure 2(c)) after MSU injection, and gradually recovered. After MSU was injected into the left ankle joint, there were significant differences in ipsilateral and contralateral ankle swelling at 1 h (contralateral: 6.6 ± 0.3 cm; ipsilateral: 7.8 ± 0.4 cm; p < 0.05; Figure 3(d)) and 2 h after MSU injection (contralateral: 6.7 ± 0.3 cm; ipsilateral: 7.7 ± 0.3 cm; p < 0.05; Figure 3(d)). These results show that injection of MSU into chicken induces inflammatory swelling of ipsilateral ankle.
Long-term effects of MSU-induced gouty arthritis on behaviors
To test whether the MSU-induced arthralgia model would transform into long-term chronic pain, all behavioral tests were performed again on the seventh day after the injection of MSU or saline in the left ankle cavity. Compared with the saline group, the MSU group showed no significant differences in spontaneous pain, mechanical withdrawal threshold, and inflammatory response on the seventh day after injection (Figures 1–3). These results indicate that the MSU-induced gouty arthritis model did not transform into chronic pain, which returned to normal condition after 4 h.
Effects of NB001 on spontaneous pain behaviors
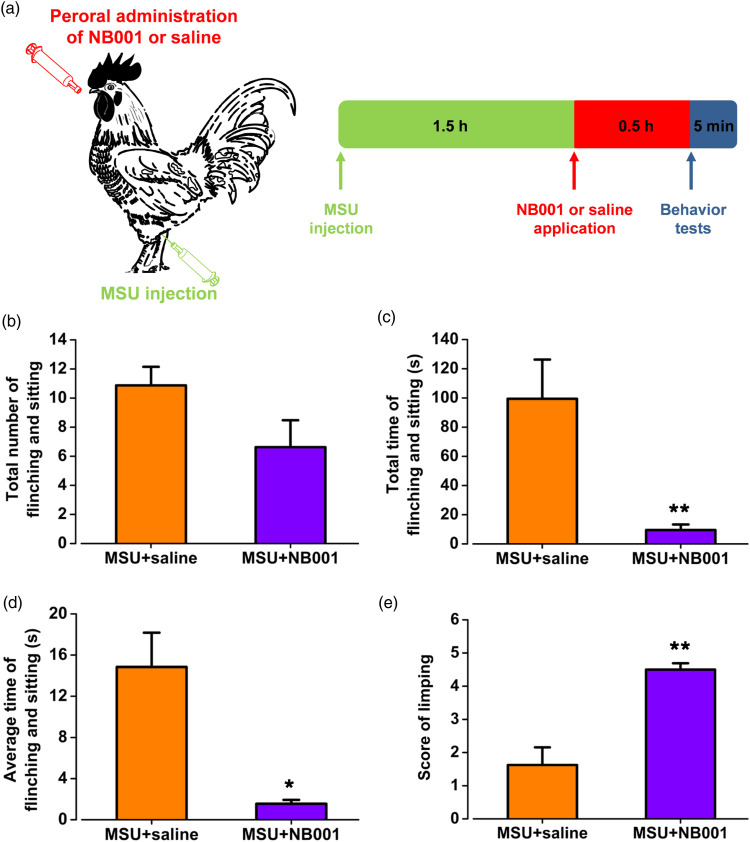
To test the effect of NB001 (AC1 inhibitor) on spontaneous pain in the MSU-induced arthritis pain model in chickens, based on the behavioral results of the previous model, 1.5 h after MSU injection into the left ankle, peroral administration of NB001 (10 mg/kg) or saline was performed and a series of behavioral experiments were tested at 0.5 h after NB001 or saline application (Figure 4(a)). Compared with the saline group, the numbers of flinching and sitting in spontaneous pain did not show a statistically significant difference after peroral administration of NB001 (MSU + saline: 10.9 ± 1.3; MSU + NB001: 6.6 ± 1.9; p > 0.05; Figure 4(b)). However, the total time during 5 min testing period (MSU + saline: 99.4 ± 26.9 s; MSU + NB001: 9.5 ± 3.8 s; p < 0.01; Figure 4(c)) and average time of each reaction (MSU + saline: 14.8 ± 3.3 s; MSU + NB001: 1.6 ± 0.4 s; p < 0.05; Figure 4(d)) were significantly decreased. After peroral administration of NB001, the score of limping increased significantly and the limb using of chickens was more normal (MSU + saline: 1.6 ± 0.5; MSU + NB001: 4.5 ± 0.2; p < 0.01; Figure 4(e)). The results indicate that NB001 administration obviously relieves gout-related spontaneous pain, which has a certain analgesic effect on the spontaneous pain of MSU-induced ankle arthritic pain in chickens.
Figure 4.
The effect of NB001 on spontaneous pain behaviors in chickens of gouty arthritis. (a) The schematic figures of administration route and time-line for peroral administration of NB001 or saline into chickens of gouty arthritis. (b) NB001 didn’t affect the number of spontaneous pain behavior on chickens with gouty arthritis. (c, d) NB001 decreased the total time in 5 min and the average time of each flinching and sitting in chickens with gouty arthritis. (e) NB001 significantly improved the limping behavior of chickens suffering gouty arthritis. *p < 0.05, **p < 0.01, MSU + saline (n = 8) vs. MSU + NB001 (n = 8).
NB001 increased the mechanical withdrawal threshold
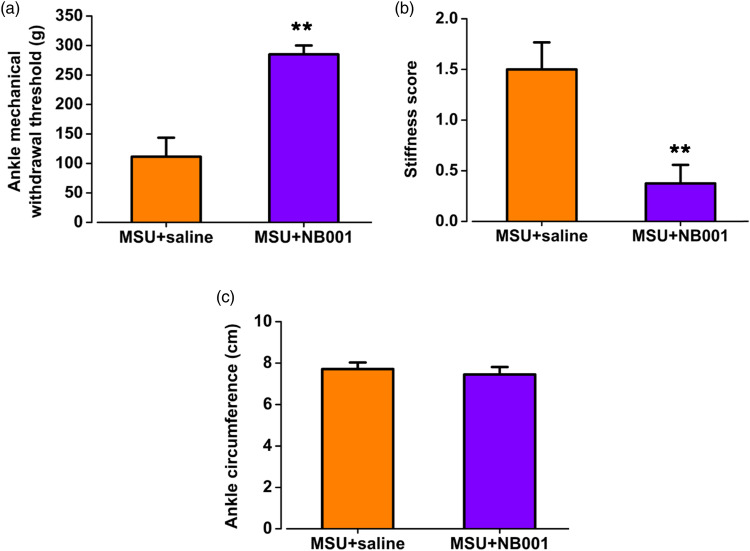
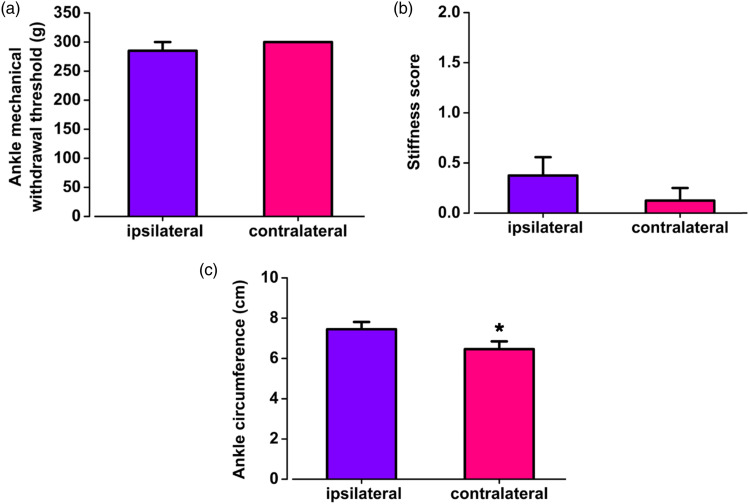
To detect the effect of NB001 on mechanical withdrawal threshold of arthritis pain model in chickens, peroral administration of NB001 or saline was performed on MSU-induced arthritis animals, respectively. Compared with the saline group, the mechanical withdrawal threshold was significantly increased after NB001 administration (MSU + saline: 111.5 ± 32.2 g; MSU + NB001: 285.0 ± 15.0 g; p < 0.01; Figure 5(a)). There was no significant difference in the mechanical withdrawal threshold between the ipsilateral ankle and the contralateral ankle in the MSU-induced arthritis pain chickens that were treated by NB001 (contralateral: 300.0 ± 0.0 g; ipsilateral: 285.0 ± 15.0 g; p > 0.05; Figure 6(a)). These results suggest that NB001 restored the mechanical pain threshold of the limb and reduced gouty pain in the animals suffering gouty arthritis.
Figure 5.
Effects of NB001 on mechanical withdrawal threshold and edema in the ipsilateral ankle of chickens suffering gouty arthritis. (a, b) NB001 significantly improved the mechanical pain threshold and stiffness score of the ipsilateral ankle in chickens with gouty arthritis. (c) NB001 didn’t alleviate the inflammatory edema of the ipsilateral ankle in chickens with gouty arthritis. **p < 0.01, MSU + saline (n = 8) vs. MSU + NB001 (n = 8).
Figure 6.
Effects of NB001 on mechanical withdrawal threshold and inflammatory symptoms of ipsilateral and contralateral ankles in chickens of MSU-induced gouty arthritis. (a, b) After NB001 application, there were no significant differences between ipsilateral and contralateral ankles in the mechanical pain threshold and stiffness score of gouty arthritis chickens. (c) After NB001 application, inflammatory edema of the ipsilateral ankle was also significantly higher than that of the contralateral in the chickens with gouty arthritis. *p < 0.05, ipsilateral (n = 8) vs. contralateral (n = 8).
NB001 exerted no effect on inflammatory edema but relieved stiffness
To test the effect of NB001 on the inflammatory symptoms of the MSU-induced arthritis model, the stiffness and swelling tests of animals suffering the MSU-induced arthritis were performed after NB001 or saline administration, respectively. For stiffness experiments, NB001 was found to reduce stiffness scoring in chickens with MSU-induced arthritis, which means it lowered the limits of bending and extending (MSU + saline: 1.5 ± 0.3; MSU + NB001: 0.4 ± 0.2; p < 0.01; Figure 5(b)). There was no significant difference in bending and extension between the ipsilateral and contralateral ankles after NB001 application in MSU-injected chickens (contralateral: 0.1 ± 0.1; ipsilateral: 0.4 ± 0.2; p > 0.05; Figure 6(b)). In the swelling experiment, NB001 had no effect on the circumference of the ankle, and the ankle was still swollen after the application of NB001 to the chickens with arthritis pain (MSU + saline: 7.7 ± 0.3 cm; MSU + NB001: 7.5 ± 0.4 cm; p > 0.05; Figure 5(c)). And the ipsilateral ankle was noticeably swollen compared to the contralateral (contralateral: 6.5 ± 0.4 cm; ipsilateral: 7.5 ± 0.4 cm; p < 0.05; Figure 6(c)). The results suggest that NB001 does not affect the inflammatory swelling of MSU-induced arthritis pain in chickens but reduces stiffness.
Discussion
Gouty pain is seriously affecting human health and the development of the poultry industry. However, there is a lack of effective and low side effect medicine for the treatment of gouty pain in both humans and poultry.2,18,19 Chicken is considered to be one of the ideal animal models of gout due to lack of uricase.3,4 In the present work, we injected MSU into the left ankle joint of chickens to establish a gouty arthritis model. We found that the ankle joint of chickens generated spontaneous pain behaviors for several hours by injection of MSU, and the left ankle joint was showed pain hypersensitivity for mechanical stimulus. The ankle edema and stiffness were also observed in the chicken model of gouty arthritis. Finally, peroral administration of AC1 inhibitor effectively relieved ankle pain but had no significant effect on inflammatory symptoms of chickens with gouty arthritis. Our results provide evidence for the first time that AC1 regulates gouty pain in chickens.
Adenylyl cyclase 1 signaling pathway regulates gouty pain in chickens
Adenylyl cyclase 1 is a functional protein widely distributed in neurons of mammalian and birds.12,24–26 Previous studies in rodents have shown that AC1 plays a crucial role in the generation of chronic pain. Genetic knockout or pharmacological inhibition of AC1 reduces pain behavioral responses of neuropathic pain and inflammatory pain.11,14,17,27,28 Our present study found that AC1 is also involved in the regulation of gouty pain in chickens, although we did not explore the upstream and downstream molecules of the AC1 signaling pathway involved in the regulation of gouty pain in chickens. We found that selective inhibition of the AC1 by NB001 in gouty chickens significantly alleviated spontaneous pain and mechanical hypersensitivity. Rodent studies have shown that calcium-stimulated AC1 is essential for central synaptic plasticity, such as NMDAR-dependent LTP, which is a key cellar mechanism for chronic pain.11,12 Our unpublished data also show that there is LTP in the dorsolateral corticoid area of chicken, which is similar to the functional area of anterior cingulate cortex in rodents and humans.29,30 Taken together, these results indicate that AC1 signaling pathway regulates gouty pain in chickens potentially possessed the same mechanism as in rodents. In addition, a few studies on pain in chickens show that the increase of Ca2+ in neurons also leads to pain behaviors and inhibition of transient receptor potential A1 (TRPA1) significantly reduces such pain-related behaviors.31,32 Combined with the previous researches, we propose NMDAR-AC1-TRPA1 pathway may also participate in the regulation of gouty pain in chickens, but the exact mechanism needs to be further explored.
Anti-AC1 has analgesic but not anti-inflammatory effects on gouty pain
Gouty pain is one major form of inflammatory pain. Studies have shown that gouty pain in chickens is mainly transmitted by C fibers, which is similar to rodent studies. 10 In addition, the results of chicken neural circuits have shown that there are similar functional and anatomical pain neural circuits between chickens and rodents.29,30,33 In the rodent model of arthritis, Tian et al. reported that NB001 has an analgesic effect, but it does not relieve the inflammatory symptoms in the joints of mice with arthritis. 14 This result is consistent with our present data in chickens that although NB001 had analgesic effect on the gouty pain chickens, the edema of the ankle joint had not been improved by NB001. This is because AC1 is mainly located in the central neurons of rodents and chickens, and the analgesic effect of AC1 inhibitor may be induced by inhibiting the central synaptic plasticity. These results indicate that central synaptic plasticity may also contribute to MSU-induced gouty pain.
NB001 may be a new strategy for gouty pain treatment in the clinic and poultry industry
Previous studies have shown that intraperitoneal, intracerebral injection, or peroral administration of NB001 reduces pain-related behavioral responses in rodents with neuropathic or inflammatory pain.17,34,35 In our present study, peroral administration of NB001 in chickens also significantly reduced spontaneous pain behaviors and mechanical hypersensitivity in gouty pain chickens. These data show that the peroral administration of NB001 has a good therapeutic effect on gouty pain. In addition, we did not find that NB001 had obvious side effects on gouty chickens. After administration of NB001, the spontaneous pain behaviors, the mechanical withdrawal threshold, and the motor behaviors of contralateral ankle joints were not significantly changed. These data are consistent with that the data of animal experiments 17 and our unpublished clinical trials, indicating that NB001 has no obvious side effects on animals and humans. Furthermore, we also found that NB001 had an analgesic effect on both male and female chickens, which is consistent with our one recent study in that we found NB001 showed an analgesic effect in female mice. 35
In nature, both humans and poultry are the high incidence groups of gout. 36 Gouty pain significantly reduces the quality of life for poultry and affects the economic benefits of the poultry industry. 36 Currently, non-steroidal anti-inflammatory drugs and glucocorticoids are mainly used in the treatment of gout.18,19 Long-term use of these two treatments has serious side effects on humans. The two drugs have not been used in the poultry industry due to the cost and food health concerns. Our current results show that NB001 has an analgesic effect on gouty joint pain in chickens. Combined with the previous research results of NB001, NB001 may become a new analgesic medicine for gouty pain in the poultry industry.
Acknowledgments
The authors would like to thank Xiuxiu Duan for her contribution to the experiment.
Footnotes
Author contributions: R.H.L., X.H.L., and M.Z. designed the experiments. R.H.L., W.S., and Y.X.Z. performed experiments and analyzed data. R.H.L., W.S., X.H.L., and M.Z. drafted the manuscript and finished the final version of the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by M.Z. is in part supported by grants from the Canadian Institute for Health Research (CIHR) project grants (PJT-148648 and 419286). X.H.L. is supported by grants from China Postdoctoral Science Foundation (2019M663669) and Basic Research Program of Natural Science in Shaanxi Province (2020JQ-085).
ORCID iD
Xu-Hui Li https://orcid.org/0000-0003-4376-6252
References
- 1.Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis 2017; 70: 422–439. DOI: 10.1053/j.ajkd.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers 2019; 5: 69. DOI: 10.1038/s41572-019-0115-y. [DOI] [PubMed] [Google Scholar]
- 3.Simoyi MF, Falkenstein E, Van Dyke K, et al. Allantoin, the oxidation product of uric acid is present in chicken and turkey plasma. Comp Biochem Physiol B Biochem Mol Biol 2003; 135: 325–335. DOI: 10.1016/s1096-4959(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 4.Nanda P, Babu PE. Isolation, screening and production studies of uricase producing bacteria from poultry sources. Prep Biochem Biotechnol 2014; 44: 811–821. DOI: 10.1080/10826068.2013.867875. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Hou X, Yuan X, et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int 2018; 93: 69–80. DOI: 10.1016/j.kint.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Li H, Liu Z, et al. Impaired intestinal barrier function in a mouse model of hyperuricemia. Mol Med Rep 2019; 20: 3292–3300. DOI: 10.3892/mmr.2019.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer SPM, Brusco I, Camponogara C, et al. Arctium minus crude extract presents antinociceptive effect in a mice acute gout attack model. Inflammopharmacology 2018; 26: 505–519. DOI: 10.1007/s10787-017-0384-6. [DOI] [PubMed] [Google Scholar]
- 8.Marcotti A, Miralles A, Dominguez E, et al. Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan. Pain 2018; 159: 739–748. DOI: 10.1097/j.pain.0000000000001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunam CA, Gentle MJ. Substance P immunoreactive nerve fibres in the domestic chick ankle joint before and after acute urate arthritis. Neurosci Lett 2004; 354: 87–90. DOI: 10.1016/s0304-3940(03)00575-5. [DOI] [PubMed] [Google Scholar]
- 10.Gentle MJ. Sodium urate arthritis: effects on the sensory properties of articular afferents in the chicken. Pain 1997; 70: 245–251. DOI: 10.1016/s0304-3959(97)03324-6. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. DOI: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 12.Li XH, Chen QY, Zhuo M. Neuronal Adenylyl Cyclase targeting central plasticity for the treatment of chronic pain. Neurotherapeutics 2020; 17: 861–873. DOI: 10.1007/s13311-020-00927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei F, Vadakkan KI, Toyoda H, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci 2006; 26: 851–861. DOI: 10.1523/jneurosci.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Z, Wang DS, Wang XS, et al. Analgesic effects of NB001 on mouse models of arthralgia. Mol Brain 2015; 8: 60. DOI: 10.1186/s13041-015-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang MM, Liu SB, Chen T, et al. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol Brain 2014; 7: 47. DOI: 10.1186/1756-6606-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga K, Descalzi G, Chen T, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015; 85: 377–389. DOI: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Xu H, Wu LJ, et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 2011; 3: 65ra63. DOI: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- 18.Hainer BL, Matheson E, Wilkes RT. Diagnosis, treatment, and prevention of gout. Am Family Phys 2014; 90: 831–836. [PubMed] [Google Scholar]
- 19.Zavodovsky BV, Sivordova LE. Cardiovascular safety of non-steroidal anti-inflammatory drugs in chronic inflammatory rheumatic diseases. Ter Arkh 2018; 90: 101–106. DOI: 10.26442/terarkh2018908101-106. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 2012; 14: R101. DOI: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honore P, Luger NM, Sabino MA, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000; 6: 521–528. DOI: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 22.Luger NM, Sabino MAC, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002; 99: 397–406. DOI: 10.1016/s0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 23.Nagakura Y, Okada M, Kohara A, et al. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther 2003; 306: 490–497. DOI: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- 24.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 2000; 279: F400–F416. DOI: 10.1152/ajprenal.2000.279.3.F400 [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann S, Böhme J, Kube C, et al. Breed-related differences in age-dependent down-regulation of the β1-adrenoceptor and adenylate cyclase activity in atrial and ventricular myocardium of Cröllwitzer (“wild-type”) turkeys. Poult Sci 2018; 97: 1041–1049. DOI: 10.3382/ps/pex362. [DOI] [PubMed] [Google Scholar]
- 26.Chaurasia SS, Haque R, Pozdeyev N, et al. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J Neurochem 2006; 99: 1142–1150. DOI: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei F, Qiu CS, Kim SJ, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002; 36: 713–726. DOI: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu SB, Wang XS, Yue J, et al. Cyclic AMP-dependent positive feedback signaling pathways in the cortex contributes to visceral pain. J Neurochem 2020; 153(2): 252–263. DOI: 10.1111/jnc.14903. [DOI] [PubMed] [Google Scholar]
- 29.Xin Q, Ogura Y, Uno L, et al. Selective contribution of the telencephalic arcopallium to the social facilitation of foraging efforts in the domestic chick. Eur J Neurosci 2017; 45: 365–380. DOI: 10.1111/ejn.13475. [DOI] [PubMed] [Google Scholar]
- 30.Montagnese CM, Zachar G, Bálint E, et al. Afferent connections of septal nuclei of the domestic chick (Gallus domesticus): a retrograde pathway tracing study. J Comp Neurol 2008; 511: 109–150. DOI: 10.1002/cne.21837. [DOI] [PubMed] [Google Scholar]
- 31.Majikina A, Takahashi K, Saito S, et al. Involvement of nociceptive transient receptor potential channels in repellent action of pulegone. Biochem Pharmacol 2018; 151: 89–95. DOI: 10.1016/j.bcp.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Banzawa N, Saito S, Imagawa T, et al. Molecular basis determining inhibition/activation of nociceptive receptor TRPA1 protein: a single amino acid dictates species-specific actions of the most potent mammalian TRPA1 antagonist. J Biol Chem 2014; 289: 31927–31939. DOI: 10.1074/jbc.M114.586891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montagnese CM, Mezey SE, Csillag A. Efferent connections of the dorsomedial thalamic nuclei of the domestic chick (Gallus domesticus). J Comp Neurol 2003; 459: 301–326. DOI: 10.1002/cne.10612. [DOI] [PubMed] [Google Scholar]
- 34.Griggs RB, Santos DF, Laird DE, et al. Methylglyoxal and a spinal TRPA1-AC1-Epac cascade facilitate pain in the db/db mouse model of type 2 diabetes. Neurobiol Dis 2019; 127: 76–86. DOI: 10.1016/j.nbd.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Shi W, Fan K, et al. Inhibition of calcium-stimulated adenylyl cyclase subtype 1 (AC1) for the treatment of neuropathic and inflammatory pain in adult female mice. Mol Pain 2021; 17: 17448069211021698. DOI: 10.1177/17448069211021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong F, Zheng A, Xu P, et al. High-protein diet induces hyperuricemia in a new animal model for studying human gout. Int J Mol Sci 2020; 21: 2147. DOI: 10.3390/ijms21062147. [DOI] [PMC free article] [PubMed] [Google Scholar]