Abstract
Background
Metabolic Syndrome (MS) is associated to vascular damage, increased arterial stiffness, and impaired myocardial perfusion. Subendocardial viability ratio (SEVR) is a noninvasive estimation of myocardial workload, oxygen supply, and perfusion. The aim of the study was to describe the relation between arterial stiffness, SEVR, and cardio-metabolic risk factors.
Methods
A cohort of 55 patients, aged 59.9 ± 10.8 years, was studied; 28 subjects (50.9%) had metabolic syndrome. All patients underwent a clinical evaluation and blood venous sampling, to assess glico-lipid profile. Applanation tonometry was performed, to obtain pulse wave analysis and SEVR values.
Results
In the overall study population, SEVR showed negative associations with mean (r = −0.301; p = 0.026) and systolic (borderline relation, r = −0.257; p = 0.058) arterial pressure. Metabolic syndrome patients presented lower level of SEVR (p = 0.012), even after adjusting for age, sex, and mean arterial pressure (p = 0.040). Subdividing the study population by the number of metabolic syndrome components, SEVR significantly decreased as the number of Metabolic Syndrome components increased (p for trend 0.005). In a logistic backward regression analysis, both metabolic syndrome and mean arterial pressure resulted significant predictors of SEVR, accounting for 18% of variance.
Conclusion
The reduced SEVR in metabolic syndrome patients could be an important pathophysiological determinant of the increased cardiovascular risk.
Keywords: Arterial stiffness, cardiovascular risk factors, metabolic syndrome, SEVR, subendocardial perfusion
Introduction
Metabolic syndrome (MS) is a cluster of abnormalities leading to increased cardiovascular risk. MS is strongly associated to both evident and subclinical vascular damage 1 as well as increased arterial stiffness,2–4 but the possible implication on myocardial perfusion should be further analyzed. Pulse wave analysis (PWA) and subendocardial viability ratio (SEVR) estimation are interesting methods aimed to determine the burden of MS on arterial and myocardial function.
PWA is a noninvasive method which provides hemodynamic parameters and information concerning subendocardial perfusion; it is considered a valid instrument in cardiovascular risk assessment. 5 SEVR is a noninvasive estimation of myocardial workload, oxygen supply, and perfusion, 6 and it can be obtained by applanation tonometry. First defined by Buckberg in 1972, 7 it represents the ratio between diastolic pressure time index (DPTI) and systolic pressure time index (SPTI), also known as tension–time index (TTI or Sarnoff Index). 8 DPTI, which is the area under the diastolic phase in the aortic profile, estimates myocardial oxygen supply, whereas SPTI (the area under the systolic phase) represents cardiac tissue oxygen consumption. 9 Therefore, low values of SEVR indicate an impaired subendocardial perfusion.7,8
Several studies described the possible relations between SEVR and many pathological conditions. SEVR reduction can mirror an impaired coronary flow reserve in hypertensive patients, 10 and it is related to peripheral arterial disease.11,12 Moreover, SEVR impairment is a frequent finding in aortic stenosis, described both in animal models13,14 and human subjects15,16; it shows a positive relation with the disease severity, and a significant improvement after aortic valve replacement. 16 Low SEVR values have been related to albuminuria, 6 reduced hemoglobin level, 17 and they predict cardiovascular events in patients with chronic kidney disease. 18 Aging itself, is known to be an independent predictor of SEVR, 19 but the relation is not completely defined and it is not possible to draw cause-effect conclusions.
Just a few previous studies demonstrate that SEVR is reduced in patients affected by metabolic syndrome,20,21 or with single metabolic abnormalities as obesity, dyslipidemia, and hyperglycemia. 19
The aim of the present study was to further describe the relation between metabolic syndrome, arterial stiffness, and SEVR.
Materials and methods
Subjects
Consecutive patients who were referred to the outpatient clinic of the Geriatric Department of Verona University Hospital, for Clinical Nutrition or Cardiovascular Risk Factors or Hypertension evaluation, were included in the present study. A detailed clinical history and physical examination were recorded for each patient. Inclusion criteria were age >40 years and life expectancy >2 years. Exclusion criteria were: (I) limb amputation or history of surgical treatment to aorta, carotids, or femoral arteries; (II) severe peripheral arterial disease or proximal arterial stenosis; (III) body mass index (BMI) >40 kg/m2; (IV) atrial fibrillation or other major arrhythmias. None of the subjects was affected by heart failure or ischemic heart disease. The study was approved by the Ethical Committee of the University of Verona. All participants gave informed consent to be involved in the research study.
Anthropometric variables
With the subject barefoot and wearing light indoor clothing, body weight was measured to the nearest 0.1 kg (Salus scale, Milan, Italy), and height to the nearest 0.5 cm using a stadiometer (Salus stadiometer, Milan, Italy). BMI was calculated as body weight adjusted by stature (kg/m2). Waist circumference was obtained with a measuring tape at the level of the narrowest part of the torso as viewed anteriorly.
Blood pressure and arterial stiffness measurements
Brachial blood pressure (BP) was measured thrice in a time frame of 15 min using a mercury sphygmomanometer (Heine Optothechnik, Gliching, Germany) in the left arm of the subject, in the supine position. The average of three readings was considered as the subject’s BP. Mean arterial pressure (MAP) was then derived by the formula:
Pulse pressure (PP), strongly related to cardiovascular risk and coronary heart disease,22–24 was derived as the difference between SBP and DBP.
The BP was recorded immediately prior to tonometric recording. The PWA was performed noninvasively using a small portable device called PulsePen® (Diatecne, Milan) 25 and its software, WPulsePen 2.0.1 (Diatecne, Milan). Also, pulse pressure amplification (PPa%) was calculated; it represents the increment of peripheral PP as compared to the central PP, and it relates to age, sex, and body composition. 26 PPa% is described as the ratio between the difference of brachial PP (pPP) and ascending aorta PP (cPP):
Subendocardial viability ratio
PulsePen® Software, by PWA traces analysis, provides SEVR measurement, which represents an indirect estimation of myocardial perfusion, relative to left ventricle workload. 27 SEVR is obtained by the following formula: SEVR = DPTI/SPTI. DPTI, which is the area under the diastolic phase in the aortic profile, estimates myocardial oxygen supply, and it is defined by the formula: DPTI = [(mean diastolic aortic pressure−mean diastolic left ventricular pressure) × diastolic time]; whereas SPTI (the area under the systolic phase) represents cardiac tissue oxygen consumption, defined as SPTI = mean systolic aortic pressure (corresponding to left ventricular mean systolic pressure) ×left-ventricular ejection time. 9 Since SEVR is described as DPTI/SPTI ratio, it indirectly reflects the adequacy of subendocardial perfusion.
A critical value for SEVR of 0.5 has been suggested,13,28–30 lower values may represent insufficient subendocardial perfusion, as indicated by a corresponding reduction of the ratio of subendocardial/subepicardial flow per gram of left ventricular myocardium. 9
Metabolic syndrome criteria
MS diagnoses was valued according to the NCEP classification. 31 NCEP, modified in 2005, 32 establishes that the metabolic syndrome is diagnosed if at least 3 among the following criteria are exceeded: waist circumference greater than 102 cm for men and greater than 88 cm for women; triglycerides, 150 mg/dL or greater; high-density lipoprotein (HDL) cholesterol, less than 40 mg/dL for men and less than 50 mg/dL for women (or patients having HDL-lowering treatment); systolic blood pressure, 130 mmHg or higher, and diastolic blood pressure, 85 mmHg or higher (or treatment of previously diagnosed hypertension); and fasting glucose, 100 mg/dL or higher (or previously diagnosed diabetes).
Biochemical analyses
Venous blood samples for all metabolic assessments were obtained after the subjects fasted overnight. Plasma glucose was measured with a glucose analyzer (Beckman Instruments Inc, Palo Alto, CA). Cholesterol and triacylglycerol concentrations were determined with an automated enzymatic method (Autoanalyzer; Technicon, Tarrytown, NY). High-density lipoprotein (HDL) cholesterol was measured by using the method of Warnick and Albers. LDL cholesterol was calculated using the Friedwald formula. 33
Statistical analyses
Results are shown as mean value ± standard deviation (SD). Variables not normally distributed were log-transformed before analysis. Pearson correlation analyses were used to test association between SEVR and other variables. Independent Samples t-tests were used to compare baseline characteristics of male and female population and of the subgroups with and without MS. ANOVA and ANCOVA analyses were performed to study SEVR and numbers of MS components, even after age, sex, and glucose level adjustment. Multiple backward regression analysis valued the combine effects of age, sex, MAP, and MS presence on SEVR.
A significance threshold level of 0.05 was used throughout the study. All statistical analyses were performed using SPSS 23.0 version for Windows (IBM, Armonk, NY).
Results
A cohort of 55 patients, aged 59.9 ± 10.8 years, 63% female (n = 35) was studied. The main characteristics of the study population are explained in Table 1.
Table 1.
Study population characteristics. BMI = body mass index; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; MAP = mean arterial pressure; SEVR = subendocardial viability ratio; PPa% = pulse pressure amplification.
| Total (n = 55) | |
|---|---|
| Age (years) | 59.91 ± 10.84 |
| Body weight (kg) | 83.64 ± 14.18 |
| Height (cm) | 164.38 ± 9.75 |
| BMI (kg/m2) | 30.92 ± 4.58 |
| Waist circumference (cm) | 105.09 ± 14.07 |
| Blood glucose (mg/dL) | 95.39 ± 15.98 |
| Total cholesterol (mg/dL) | 186.21 ± 36.88 |
| HDL cholesterol (mg/dL) | 54.35 ± 14.69 |
| LDL cholesterol (mg/dL) | 110.53 ± 30.32 |
| TG (mg/dL) | 114.41 ± 51.91 |
| Creatinine (mg/dL) | 0.86 ± 0.16 |
| SBP (mmHg) | 140.67 ± 13.32 |
| DBP (mmHg) | 88.16 ± 8.07 |
| PP (mmHg) | 52.51 ± 10.68 |
| MAP (mmHg) | 109.29 ± 11.59 |
| SEVR | 1.19 ± 0.27 |
| PPa (%) | 25.78 ± 10.80 |
A subgroup of 28 subjects (50.9%) had MS, according to NCEP-ATPIII criteria. 31 A large percentage of the study population was composed by obese patients (81.8%, n = 45) and forty-one subjects (74.5%) were affected by arterial hypertension.
Looking at the biochemical profile, 30.9% (n = 17) had low HDL cholesterol level, 20% (n = 11) had high triglycerides, and 5.5% (n = 3) presented impaired fasting glucose tolerance (IFG).
Total cholesterol and HDL cholesterol were significantly higher in female population (p value 0.033 and 0.001 respectively).
Female population also presented higher DBP (p = 0.029) and PP (p = 0.027) values.
No significant difference was observed between male and female population for SEVR values (p = 0.419).
Univariate analysis between SEVR and main study variables was performed. A significant negative relation was found between SEVR and MAP (r = −0.301 and p = 0.026). SEVR was not related to age neither to other metabolic parameters.
The subpopulation of patients with MS (n = 28, 50.9%) was further analyzed (Table 2): it was composed by older subjects (64.2 ± 10.4 years vs 55.4 ± 9.5 years; p = 0.002), with larger waist circumference (108.54 ± 15.04 cm vs 101.50 ± 12.29 cm, p = 0.079) and higher blood glucose level (103.07 ± 18.44 mg/dL vs 88.31 ± 8.87 mg/dL; p = 0.001). HDL cholesterol was significantly lower (49.92 ± 11.64 mg/dL vs 58.96 ± 16.28 mg/dL, p = 0.028)
Table 2.
Difference between subjects with and without metabolic syndrome. BW = body weight; BMI = body mass index; WC = waist circumference; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; PP = pulse pressure; MAP = mean arterial pressure.
| n = 55 | Metabolic syndrome (N = 28) | Not metabolic syndrome (N = 27) | p value |
|---|---|---|---|
| Age | 64.25 ± 10.41 | 55.41 ± 9.50 | 0.002 |
| BW (kg) | 85.69 ± 14.84 | 81.51 ± 13.93 | 0.277 |
| Height (cm) | 164.46 ± 9.31 | 164.30 ± 10.37 | 0.95 |
| BMI (kg/m2) | 31.62 ± 4.50 | 30.19 ± 4.63 | 0.251 |
| WC (cm) | 108.54 ± 15.04 | 101.50 ± 12.29 | 0.079 |
| Blood glucose (mg/dL) | 103.07 ± 18.44 | 88.31 ± 8.87 | 0.001 |
| Total cholesterol (mg/dL) | 187.12 ± 39.35 | 185.31 ± 34.99 | 0.862 |
| HDL cholesterol (mg/dL) | 49.92 ± 11.64 | 58.96 ± 16.28 | 0.028 |
| LDL cholesterol (mg/dL) | 109.00 ± 32.51 | 112.12 ± 28.44 | 0.717 |
| TG (mg/dL) | 139.54 ± 54.42 | 88.28 ± 33.67 | <0.001 |
| Creatinine (mg/dL) | 0.84 ± 0.20 | 0.88 ± 0.13 | 0.529 |
| SBP (mmHg) | 143.32 ± 13.12 | 137.93 ± 13.19 | 0.134 |
| DBP (mmHg) | 88.25 ± 7.90 | 88.07 ± 9.62 | 0.941 |
| PP (mmHg) | 55.07 ± 12.43 | 49.85 ± 7.89 | 0.068 |
| MAP (mmHg) | 111.04 ± 11.06 | 107.48 ± 12.04 | 0.26 |
| SEVR | 1.10 ± 0.20 | 1.28 ± 0.30 | 0.012 |
| PPa (%) | 24.67 ± 12.13 | 26.81 ± 9.55 | 0.494 |
Blood pressure parameters did not significantly differ among groups.
The MS subgroup showed lower level of SEVR (1.10 ± 0.20 vs 1.28 ± 0.30, p = 0.012), even after adjustment for age, sex, and MAP (p = 0.040).
As concerns single MS components, SEVR values were higher in normoglycemic subjects than IFG or diabetic patients (p = 0.07). No significant relation was found between BMI, waist circumference, HDL levels, Triglycerides levels, presence of hypertension, and SEVR.
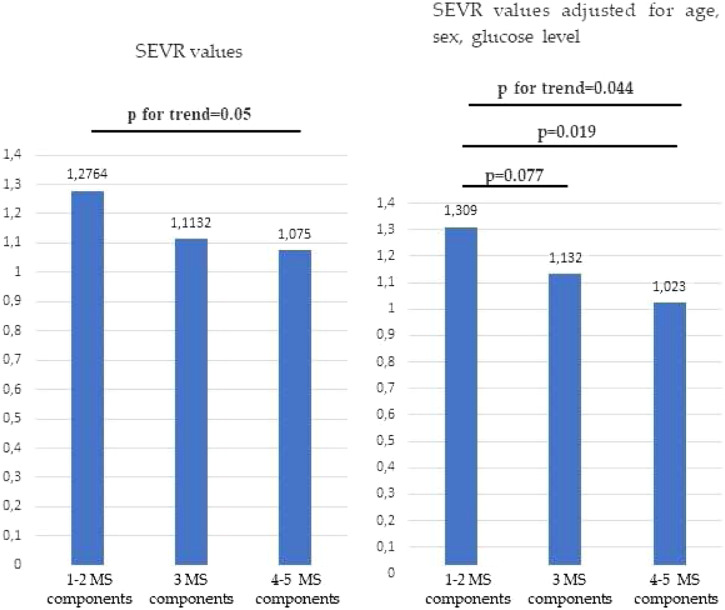
Furthermore, we observed that as the number of MS components increased (Figure 1), SEVR significantly decreased (p = 0.05), even after adjustment for age, sex and glucose level (p = 0.044).
Figure 1.
SEVR values in the study population stratified accordingly to MS components before and after adjustment for age, sex and glucose levels.
A logistic backward regression analysis (Table 3) in which SEVR was considered the dependent variable, and age, sex, MAP and the presence of MS were covariates was performed. MS and blood pressure were predictors of SEVR, explaining about 18% of variance.
Table 3.
Multiple backward regression analysis considering SEVR as dependent variable and age, sex, and MS as covariates. MS = metabolic syndrome, MAP = mean arterial pressure.
| n=55 | β Coefficient | p value | R 2 |
|---|---|---|---|
| Age | 0 | 0.99 | |
| Sex | −0.11 | 0.37 | |
| MS | 0.29 | 0.04 | |
| MAP | −0.26 | 0.05 | 0.19 |
| Sex | −0.11 | 0.38 | |
| MS | 0.29 | 0.02 | |
| MAP | −0.26 | 0.05 | 0.19 |
| MS | 0.3 | 0.02 | |
| MAP | −0.25 | 0.05 | 0.18 |
Discussion
The present study shows that SEVR is significantly reduced in patients affected by MS. Moreover, SEVR decreases as the number of MS criteria increases and finally MS and MAP resulted as predictors of SEVR, justifying 18% of variance.
SEVR reduction describes a subendocardial perfusion impairment. Vascular damage is a frequent and early finding in MS patients, and low SEVR values, along with higher intima-media thickness and higher augmentation pressure have been previously described. 20 Our data confirm these previous observations, showing a significant and strong reduction of SEVR values in patients with MS, even if mean SEVR values exceed the critical threshold level of 0.5 (probably also because of the small size of our study population).
In our study, SEVR values decrease as MS components increase and the relation remained still significant after adjustment for age, sex, and glucose level. Although, single MS components such as HDL cholesterol levels, triglycerides, obesity, and hypertension did not show any significant relation with SEVR reduction. Since SEVR estimates subendocardial perfusion, we can hypothesize that subendocardial perfusion impairment is a consequence not properly of each single MS component, but mostly, it may be related to the comprehensive burden of MS. Therefore, SEVR may be considered an epiphenomenon of MS, reflecting the complexity and inter-relation of several components, with effects on endothelial dysfunction, arterial stiffness and, consequently, on subendocardial perfusion. Anyway, the small size of our cohort did not allow to draw definite conclusions about this pathophysiological hypothesis.
As expected, in our study, we observed a significant negative relation between SEVR and MAP (r = −0.301, p = 0.026), and a borderline negative relation with SBP (r = −0.257, p = 0.058). In line with previous studies,11,20,34 our data support the hypothesis that arterial hypertension is strongly related to arterial stiffness and impaired subendocardial perfusion. The underlying mechanism is not completely clarified. Arterial stiffening is associated to earlier pulse wave reflection and increased pulse pressure. Thus, in case of hypertension, with augmented pulse pressure (therefore higher SBP and lower DPB values), left ventricular afterload would be increased, leading to a major myocardial oxygen demand. At the same time, the reduced DBP levels compromise coronary blood flow. Consequently, the mismatch between increased oxygen demand and reduced blood flow affects, particularly, subendocardial perfusion. Among different blood pressure parameters, MAP may better represent the arterial hypertension burden. Our data showed in a multiple backward regression analysis that MAP and presence of MS were the main predictors of SEVR, explaining 18% of variance.
Furthermore, we found a borderline relation between reduced SEVR and diabetes: diabetic and IFG patients had lower SEVR (although with borderline significance) as compared to normoglycemic subjects.
As previously demonstrated, 35 diabetes is associated to increased arterial stiffness, due to Advanced glycation end products (AGE) exposure. It is widely known that AGE induce cross-linking of collagen binding molecules of arterial walls; therefore, reduced arterial elasticity and compliance may represent a possible mechanism of impaired subendocardial perfusion in diabetic subjects, explaining the reduced SEVR values. Moreover, as previously described, diabetes is associated to endothelial dysfunction, due to vasoactive substances impairment, including NO, prostacyclin (PGI2), and endothelium-derived hyperpolarizing factors (EDHF). 36 The lack of NO and EDHF is a relevant cause of microcirculation dysfunction, and it may contribute to subendocardial perfusion impairment. Another possible mechanism could be played by the higher inflammation of MS diabetic patients. The presence of visceral adipose tissue, in these subjects, contributes to higher levels of IL-6, plasminogen activator inhibitor, TNFα, and leptin. All these cytokines negatively affect endothelium homeostasis. Moreover, these patients have lower levels of adiponectin, an adipokine with known protective effect over endothelium. 37 Further studies may better explain the relation between endothelium homeostasis impairment, vascular inflammation and SEVR reduction.
Finally, our data could not define a significant relation between age and SEVR and previous researches show contrasting results.10,19 Our conclusions are in line with findings by Tsiachris et al., 10 who studied the relationship between coronary flow reserve (CFR) and SEVR in a population of 36 patients, with non-treated hypertension. In this study, SEVR and age did not show any correlation. On the other hand, in another study 19 lead on 178 Japanese subjects, age and heart rate were both significant predictors of SEVR. Even if aging is strongly related to arterial stiffness, 37 further research is needed to better define whether and how it affects coronary and subendocardial perfusion.
In our opinion, SEVR provides an interesting evaluation of myocardial workload, oxygen supply and perfusion, 6 also because of its feasibility by the mean of a noninvasive technique, such as applanation tonometry and it represents DPTI on SPTI ratio. 8 DPTI, which is the area under the diastolic phase in the aortic profile, estimates myocardial oxygen supply, whereas SPTI (the area under the systolic phase) represents cardiac tissue oxygen consumption. 9 As previously described in patients with different hypertension phenotypes, 34 we suggest that SEVR may be considered a valid alternative to invasive assessment of microvascular coronary perfusion. Although, the relative lack of studies comparing SEVR values to other estimations of endocardial perfusion (by other cardiac imaging techniques) represents a possible limitation to the systematic use of SEVR, and further studies are needed.
Our study had some limitations. First, it was based on a small population, and this could have influenced statistical significance of some relationships. Second, we could not provide information about inflammation indexes such as IL-1, IL-6, and TNFα, all chronic inflammation mediators that weigh on vascular damage, which is a possible link between chronic inflammation and SEVR reduction. Glycosylated hemoglobin could have better described the effects of glycemic control on SEVR. Unfortunately it was not available for our study population, and therefore we based our analysis on fasting glucose level, as suggested by MS definition criteria. Moreover, our study was cross-sectional, and due to its own structural characteristics, it did not allow conclusions regarding causality of arterial stiffness, MS, DM on SEVR variations.
In conclusion, SEVR can be considered a valid noninvasive estimation of subendocardial coronary perfusion that mostly occurs during the diastolic phase. Subendocardial perfusion impairment, as described by SEVR reduction, may contribute to the burden of increased cardiovascular risk in patients with MS. Thus, we suggest that considering subendocardial perfusion pattern in patients with MS may provide additional information aimed to comprehensive evaluation of cardio-metabolic risk assessment.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Francesco Fantin https://orcid.org/0000-0003-1763-6024
References
- 1.Fantin F, Di Francesco V, Rossi A, et al. Abdominal obesity and subclinical vascular damage in the elderly. J Hypertens 2010; 28: 333–339. [DOI] [PubMed] [Google Scholar]
- 2.Yue M, Liu H, He M, et al. Gender-specific association of metabolic syndrome and its components with arterial stiffness in the general Chinese population. PLoS One 2017; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes-Vicente WRP, Rodrigues S, Cepeda FX, et al. Arterial stiffness and its association with clustering of metabolic syndrome risk factors. Diabetol Metab Syndr 2017; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topouchian J, Labat C, Gautier S, et al. Effects of metabolic syndrome on arterial function in different age groups: the advanced approach to arterial stiffness study. J Hypertens 2018; 36: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. a position paper from the European society of cardiology working group on peripheral circulation. endorsed by the association for research into arterial structure and physiology (ARTERY). Atherosclerosis 2015; 241: 507–532. [DOI] [PubMed] [Google Scholar]
- 6.Ekart R, Bevc S, Hojs N, et al. Albuminuria is associated with subendocardial viability ratio in chronic kidney disease patients. Kid Blood Press Res 2015; 40: 565–574. [DOI] [PubMed] [Google Scholar]
- 7.Buckberg GD, Fixler DE, Archie JP, et al. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 1972; 30: 67–81. [DOI] [PubMed] [Google Scholar]
- 8.Sarnoff SJ, Braunwald E, Welch GH, et al. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol 1958; 192: 148–156. [DOI] [PubMed] [Google Scholar]
- 9.Salvi P, Revera M, Faini A, et al. Changes in subendocardial viability ratio with acute high-altitude exposure and protective role of acetazolamide. Hypertension 2013; 61: 793–799. [DOI] [PubMed] [Google Scholar]
- 10.Tsiachris D, Tsioufis C, Syrseloudis D, et al. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J Hum Hypertens 2012; 26: 64–70. [DOI] [PubMed] [Google Scholar]
- 11.Scandale G, Dimitrov G, Recchia M, et al. Arterial stiffness and subendocardial viability ratio in patients with peripheral arterial disease. J Clin Hypertens 2018; 20: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosimann K, Jacomella V, Thalhammer C, et al. Severity of peripheral arterial disease is associated with aortic pressure augmentation and subendocardial viability ratio. J Clin Hypertens 2012; 14: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazier J, Cooper N, Buckberg G. The adequacy of subendocardial oxygen delivery: the interaction of determinants of flow, arterial oxygen content and myocardial oxygen need. Circulation 1974; 49: 968–977. [DOI] [PubMed] [Google Scholar]
- 14.Borkon AM, Jones M, Bell JH, et al. Regional myocardial blood flow in left ventricular hypertrophy. An experimental investigation in Newfoundland dogs with congenital subaortic stenosis. J Thorac Cardiovasc Surg 1982; 84: 876–885. [PubMed] [Google Scholar]
- 15.Buckberg G, Eber L, Herman M, et al. Ischemia in aortic stenosis: Hemodynamic prediction. Am J Cardiol 1975; 35: 778–784. [DOI] [PubMed] [Google Scholar]
- 16.Müller C, Goliasch G, Schachinger S, et al. Transcatheter aortic valve replacement (TAVR) leads to an increase in the subendocardial viability ratio assessed by pulse wave analysis. PLoS One 2018; 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekart R, Bevc S, Hojs N, et al. Relationship between subendocardial viability ratio and hemoglobin in patients with chronic kidney disease. Clin Nephrol 2017; 88: 22–26. [DOI] [PubMed] [Google Scholar]
- 18.Ekart R, Bevc S, Hojs N, et al. Derived subendocardial viability ratio and cardiovascular events in patients with chronic kidney disease. Cardio Renal Med 2019; 9: 41–50. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, Kasuya A. Relationship between the subendocardial viability ratio and risk factors for ischemic heart disease. Sangyo Eiseigaku Zasshi 2003; 45: 114–119. [DOI] [PubMed] [Google Scholar]
- 20.Di Pino A, Alagona C, Piro S, et al. Separate impact of metabolic syndrome and altered glucose tolerance on early markers of vascular injuries. Atherosclerosis 2012; 223: 458–462. [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan J, Swaminathan N, Pereira NM, et al. Acute changes in arterial stiffness following exercise in people with metabolic syndrome. Diabetes Metab Syndr Clin Res Rev 2017; 11: 237–243. [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990; 15(4): 339–347. [DOI] [PubMed] [Google Scholar]
- 23.Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: A predictor of long-term cardiovascular mortality in a french male population. Hypertension 1997; 30: 1410–1415. [DOI] [PubMed] [Google Scholar]
- 24.Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The framingham heart study. Circulation 1999; 100: 354–360. [DOI] [PubMed] [Google Scholar]
- 25.Salvi P, Lio G, Labat C, et al. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: The pulse pen device. J Hypertens 2004; 22: 2285–2293. [DOI] [PubMed] [Google Scholar]
- 26.Pichler G, Martinez F, Vicente A, et al. Pulse pressure amplification and its determinants. Blood Press 2016; 25: 21–27. [DOI] [PubMed] [Google Scholar]
- 27.Salvi P. Pulse Waves: How Vascular Hemodynamics Affects Blood Pressure, 2012. Epub ahead of print 2012. DOI: 10.1007/978-88-470-2439-7_1. [DOI] [Google Scholar]
- 28.Hoffman JIE, Buckberg GD. The myocardial supply: Demand ratio-a critical review. Am J Cardiol 1978; 41: 327–332. [DOI] [PubMed] [Google Scholar]
- 29.Barnard RJ, MacAlpin R, Kattus AA, et al. Ischemic response to sudden strenuous exercise in healthy men. Circulation 1973; 48: 936–942. [DOI] [PubMed] [Google Scholar]
- 30.Griggs DM, Chen CC. Coronary hemodynamics and regional myocardial metabolism in experimental aortic insufficiency. J Clin Invest 1974; 53: 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the national cholesterol (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc 2001; 285: 2486–2487. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 33.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 34.Anyfanti P, Gkaliagkousi E, Triantafyllou A, et al. Noninvasive assessment of myocardial perfusion in different blood pressure phenotypes and its association with arterial stiffness indices. Am J Hypertens 2019; 32: 557–563. [DOI] [PubMed] [Google Scholar]
- 35.Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care 1999; 22: 1722–1727. [DOI] [PubMed] [Google Scholar]
- 36.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ Res 2018; 122: 624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantin F, Disegna E, Manzato G, et al. Adipokines and arterial stiffness in the elderly. Vasc Heal Risk Manag 2020; 16: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]