Abstract
Background:
The aim of this study was to evaluate the efficacy and safety of gemcitabine plus nab-paclitaxel (GnP) as second-line chemotherapy following first-line FOLFIRINOX treatment failure in advanced pancreatic cancer.
Methods:
This was a multicenter, single-arm, open-label, phase 2 trial done at three tertiary centers in South Korea from May 2018 to December 2019. Eligible patients were aged 20 years or older, had histologically confirmed advanced pancreatic ductal adenocarcinoma, and disease progression after receiving first-line FOLFIRINOX. Patients received a second-line GnP regimen as intravenous nab-paclitaxel at a dose of 125 mg/m2 and gemcitabine at a dose of 1000 mg/m2, on days 1, 8, and 15 every 4 weeks until disease progression or unacceptable toxicity. The primary outcome was survival rate at 6 months and the secondary outcomes were median progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and adverse events. This study is registered with Clinicaltrials.gov. (NCT03401827)
Results:
Forty patients were enrolled in the study. The survival rate at 6 months was 72.5% [95% confidence interval (CI), 59.9–87.7], achieving superiority over prespecified assumed 6-month OS rate of 20% for best supportive care only (p < 0.001). The median PFS and OS were 5.8 months (95% CI, 4.3–8.7) and 9.9 months (95% CI, 7.5–12.4), respectively. DCR was 87.5% with six partial responses and 29 stable diseases. Grade 3 or higher treatment-related adverse events occurred in 25 (62.5%) patients with the most common being thrombocytopenia, anemia, neutropenia, peripheral neuropathy, and peripheral edema.
Conclusion:
GnP demonstrated favorable efficacy with acceptable toxicity in patients with advanced pancreatic ductal adenocarcinoma after FOLFIRINOX failure.
Keywords: gemcitabine, nab-paclitaxel, pancreatic cancer, second-line chemotherapy
Introduction
Pancreatic cancer is a lethal disease characterized by an extremely low overall survival; a 5-year survival rate of 9%. 1 It is currently the third leading cause of cancer-related deaths in the United States and the seventh leading cause of cancer-related deaths worldwide.1,2
So far, surgical resection is the only potentially curative treatment. However, only less than 20% of patients are eligible for curative resection at initial diagnosis, and chemotherapy remains the mainstay of treatment for both unresectable advanced and metastatic pancreatic cancer. 3 Currently, the standard first-line chemotherapy regimens for patients with good performance status are combinations of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), and gemcitabine plus nab-paclitaxel (GnP). 4 Based on the pivotal phase 3 randomized clinical trials (PRODIGE and MPACT trials) results, the median overall survival (OS) of patients with metastatic pancreatic cancer who received first-line FOLFIRINOX and GnP are 11.1 and 8.5 months, respectively.5,6 While there are no head-to-head clinical trials comparing the two regimens this far, real-world data showed that their treatment efficacy was comparable, 7 and both have generally been recommended for patients with metastatic pancreatic cancer. 4
However, second-line chemotherapy is not yet well established. While there are several clinical trials regarding second-line chemotherapy, most of them investigated gemcitabine-refractory patients,8–14 and data on second-line therapy following FOLFIRINOX failure are limited. Gemcitabine-based monotherapy or combination therapy has been suggested as potential second-line therapy following FOLFIRINOX failure. However, despite the encouraging results of MPACT study, 5 only a few prospective studies have evaluated GnP in a second-line setting.15,16 Nonetheless, based on the limited evidence, the current guidelines recommend gemcitabine-based regimens such as gemcitabine monotherapy, GnP, gemcitabine plus erlotinib, and gemcitabine plus cisplatin, following FOLFIRINOX failure, depending on the patient’s performance status and level of tolerability.4,17
This study aimed to evaluate the efficacy and safety of GnP as a second-line chemotherapy drug following FOLFIRINOX treatment failure in patients with advanced pancreatic cancer.
Methods
Study design, participants, and ethical considerations
This study was a multicenter, single-arm, open-label, phase 2 trial aimed at evaluating the efficacy and safety of GnP as a second-line chemotherapy drug after FOLFIRINOX treatment failure, for patients with advanced pancreatic cancer. Patients were enrolled from three tertiary medical centers in South Korea (Seoul National University Hospital, Severance Hospital, and Chungnam National University Hospital) from 3 May 2018 to 17 December 2019. Patient eligibility criterion was as follows: patients aged at least 20 years, histologically confirmed locally advanced or distant metastasis pancreatic ductal adenocarcinoma, Eastern Cooperative Oncology Group performance status of less than or equal to 2, FOLFIRINOX treatment failure, and confirmation of disease progression via any imaging modalities. We included patients who received standard or modified dose of first-line FOLFIRINOX. Dose reduction at the first or later cycles by 10–20% of one or more drug or schedule adjustment was performed in consideration of individual patient’s general condition and toxicities at the discretion of the treating physician. Patients were excluded if they had multiple organ failure, severe comorbidities with an expected survival period of less than 1 month, allergic to the test drug, or were participating in any other clinical trial. The study was approved by the institutional review boards of all the participating institutions (Seoul National University Hospital IRB no. 1710-067-894; Severance Hospital IRB no. 4-2019-0169; Chungnam National University Hospital IRB no. 2019-06-066) and all patients provided written informed consent. This trial was conducted according to the Declaration of Helsinki and was registered with Clinicaltrials.gov on 17 January 2018 (NCT03401827). The first patient was enrolled on 3 May 2018.
Treatments and evaluation
The patients were treated with intravenous nab-paclitaxel at a dose of 125 mg/m2 followed by gemcitabine at a dose of 1000 mg/m2, on days 1, 8, and 15, every 4 weeks. Dose modifications for the first dose or later cycles were permitted at the discretion of the attending physician. Sequential dose reduction to 60–80% of the GnP standard dose was allowed. Treatment was continued until disease progression, or unacceptable toxicity were confirmed. Tumor assessment was performed with computed tomography at baseline and every 8 weeks until the confirmation of disease progression or treatment discontinuation. Tumor response was assessed by local investigators every 8 weeks according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. 18
Study outcomes
The primary outcome was survival rate at 6 months after the initiation of second-line GnP. The secondary outcomes were median OS, progression-free survival (PFS), disease control rate (DCR), and adverse events. OS was defined as the duration from the first day of GnP administration until a patient’s death from any cause. PFS was defined as the duration from the first day of GnP administration until the confirmation of disease progression or a patient’s death from any cause. The best overall response was defined as the best response recorded after the start of the GnP treatment until the end (according to RECIST, version 1.1). 18 DCR was defined as the proportion of patients with complete response, partial response, or stable disease. Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Event, version 4.0. In case of oxaliplatin-induced neuropathy at enrollment, the evaluation of neuropathy was based on new onset neuropathy symptoms different from the existing features or worsening of the existing symptoms.
Statistical analysis
The sample size was calculated based on the exponential maximum likelihood estimation test for cube root transformed hazard ratio, in consultation with the Medical Research Collaboration Center of Seoul National University Hospital. On the basis of historical data, we assumed a 6-month survival rate of 20% for patients who were treated with the best supportive care only. 19 Assuming a 6-month survival rate of 40% for the GnP group, 36 patients were required to achieve 90% power to achieve a difference in 6-month survival rate at a two-sided type I error rate of 5%.15,20 Considering the dropout rate of 10%, we required 40 patients for enrollment. Efficacy endpoints were evaluated in the intention-to-treat population. The safety profile was evaluated in patients who received at least one single administration of GnP. The data cut-off date for these analyses was 17 December 2020. The primary (survival rate at 6 months) and secondary (OS, PFS) efficacy endpoints were estimated using the Kaplan–Meier method. Two-sided 95% exact confidence intervals (CIs) for objective response and DCR were estimated using the Clopper–Pearson method. 21 Statistical analyses were performed using R Studio version 3.6.3 statistical software package.
Results
The baseline characteristics of study patients
A total of 40 patients were enrolled from 3 May 2018 to 17 December 2019. The baseline characteristics of all the patients are summarized in Table 1. The median age for the patients was 62 years (interquartile range (IQR), 41–82). Twenty-two (55%) were male patients. Thirty-five (87.5%) patients had metastatic disease and five (12.5%) patients had locally advanced disease at the time of enrollment. The median number of cycles of the first-line FOLFIRINOX was 15 (IQR, 7–20). Ten (25%) patients experienced dose reduction during the first-line FOLFIRINOX. Eighteen (45.0%) patients suffered from chronic oxaliplatin-induced peripheral neuropathy, mostly sensory neuropathy that was accompanied by symptoms such as numbness, tingling, or pain.
Table 1.
Baseline characteristics of all the patients (N = 40).
| Variable | N (%) or median (IQR) |
|---|---|
| Age, years | 62 (58, 70) |
| Sex | |
| Male | 22 (55.0%) |
| Female | 18 (45.0%) |
| ECOG Performance status | |
| 0 | 26 (65.0%) |
| 1 | 10 (25.0%) |
| 2 | 4 (10.0%) |
| Primary tumor location | |
| Head | 14 (35.0%) |
| Body | 14 (35.0%) |
| Tail | 12 (30.0%) |
| Disease stage | |
| Locally advanced | 5 (12.5%) |
| Metastatic | 35 (87.5%) |
| Site of metastasis | |
| Liver | 22 (55.0%) |
| Peritoneum | 14 (35.0%) |
| Lung | 4 (10.0%) |
| Baseline laboratory findings | |
| CEA—U/mL | 6.5 (4.2, 19.5) |
| CA 19-9—U/mL | 2155 (194, 7996) |
| Time on FOLFIRINOX (months) | 8.2 (4.3, 15.5) |
| Number of FOLFIRINOX cycle | 15 (7, 20) |
| Reason for FOLFIRINOX discontinuation | |
| Radiographic progression | 40 (100%) |
| Oxaliplatin neurotoxicity at enrollment | 18 (45.0%) |
CA 19-9, Carbohydrate antigen 19-9; CEA, Carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Detailed information of second-line chemotherapy with GnP
At the time of the study period (17 December 2020), 39 patients discontinued GnP except for one patient. Reasons for discontinuation of GnP were as follows: disease progression in 24 (60.0%) patients, death in 4 (10.0%) patients, unacceptable toxicity in 4 (10.0%) patients, conversion to surgery in 3 (7.5%) patients, lost to follow-up in 3 (7.5%) patients and patient withdrawal in 1 (2.5%) patient (Figure 1). Nine (22.5%) patients were treated with a lower dose during the first cycle of GnP. Dose reduction at the second or later cycles occurred in 20 (50.0%) patients, the reasons were as follows: neuropathy in 16 (40.0%) patients, neutropenia in 2 (5.0%) patients, peripheral edema in one (2.5%) patient, and asthenia in one (2.5%) patient. The median number of treatment cycles of the second-line GnP was 5 (IQR, 4–8), and the median duration of treatment with second-line GnP was 19 weeks (IQR, 15–33).
Figure 1.
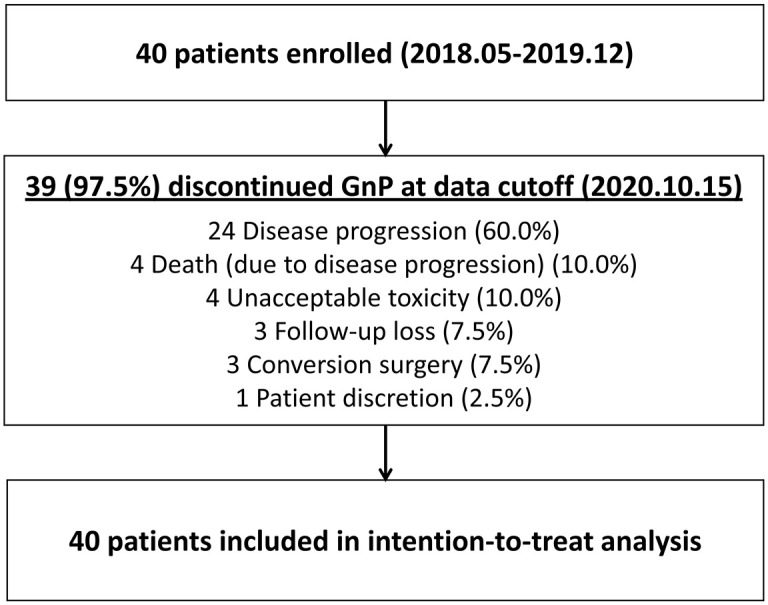
Trial profile.
Efficacy of second-line GnP
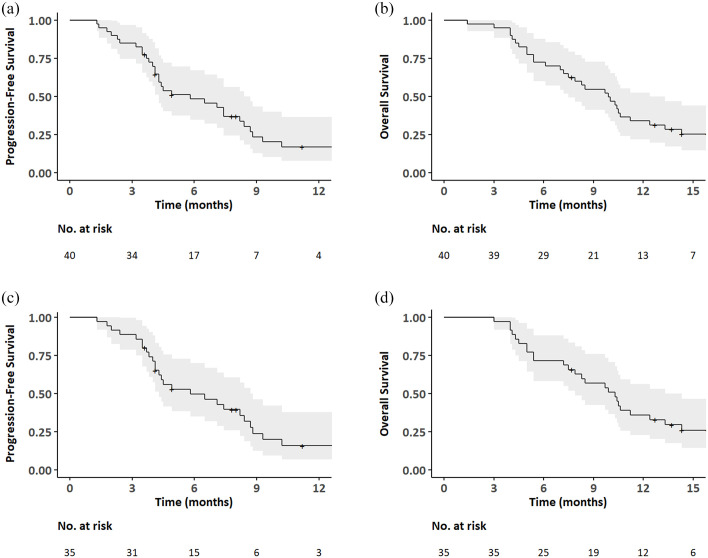
The median follow-up period by the reverse Kaplan-Meier estimates for patients in the ITT population was 16.3 months (95% CI, from 14.3 to not reached). Survival rate at 6 months by Kaplan–Meier estimates was 72.5% (95% CI, 59.9–87.7), achieving superiority over the prespecified assumed 6-month OS rate of 20% for the best supportive care only (p < 0.001). Median PFS was 5.8 months (95% CI, 4.3–8.7) (Figure 2(a)) and median OS was 9.9 months (95% CI, 7.5–12.4) (Figure 2(b)). The best overall responses included a partial response in 6 (15.0%) patients, stable disease in 29 (72.5%) patients, and progressive disease in 5 (12.5%) patients, resulting in an object response rate of 15.0% (95% CI, 5.7–29.8), and DCR of 87.5% (73.2–95.8) (Table 2). Three patients (7.5%; two in the locally advanced and one in the metastatic group) underwent conversion surgery after treatment with the second-line GnP. Treatment duration with the second-line GnP for these patients was 208, 305, and 342 days, respectively. All the three patients developed recurrence 29, 201, and 175 days, respectively, after surgical resection and subsequently received palliative chemotherapy.
Figure 2.
Kaplan–Meier estimates for progression-free survival (a) and the overall survival (b) in the intention-to-treat population, progression-free survival (c) and the overall survival (d) in patients with metastatic pancreatic cancer (post hoc analysis).
Table 2.
Treatment responses based on RECIST version 1.1.
| Best response | Intention-to-treat population (N = 40) | Metastatic pancreatic cancer (N = 35) |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 6 (15.0%) | 6 (17.1%) |
| Stable disease | 29 (72.5%) | 26 (74.3%) |
| Progressive disease | 5 (12.5%) | 3 (8.6%) |
| Response rate | 6 (15.0%) | 6 (17.1%) |
| Disease control rate | 35 (87.5%) | 32 (91.4%) |
RECIST, Response Evaluation Criteria in Solid Tumors.
Efficacy of second-line GnP in metastatic pancreatic cancer
A post hoc subgroup analysis of 35 metastatic disease patients showed that the median PFS and OS were 5.8 months (95% CI, 4.3–8.7) (Figure 2(c)) and 10.3 months (95% CI, 7.9–13.3) (Figure 2(d)), respectively. The best overall responses included a partial response in 6 (17.1%) patients, stable disease in 26 (74.3%) patients, and progressive disease in 3 (8.6%) patients, resulting in an object response rate of 17.1% (95% CI, 6.6–33.7) and DCR of 91.4% (76.9–98.2) (Table 2).
Third-line treatment after second-line GnP failure
Third-line treatment following second-line GnP failure was administered in 21 (52.5%) patients. Third-line chemotherapy was given to 18 (45.0%) patients as follows; nanoliposomal irinotecan plus 5-fluorouracil/folinic acid in nine (22.5%) patients, tegafur/gimeracil/oteracil potassium (TS-1) in eight (20.0%) patients, and 5-fluorouracil plus cisplatin in one (2.5%) patient. One (2.5%) of the patients received concurrent chemoradiation therapy with capecitabine. Two (5.0%) patients received palliative radiation therapy.
Safety analysis
All patients who received at least one dose of treatment were included in the safety analysis (Table 3). Thirty-nine patients (97.5%) received at least one cycle of treatment. Treatment-related grade 3 and 4 adverse events occurred in 25 patients (62.5%). The most common treatment-related grade 3 or higher adverse events were thrombocytopenia (37.5%), anemia (32.5%), neutropenia (27.5%), peripheral neuropathy (10.0%), and peripheral edema (7.5%). All treatment-related grade 4 adverse events were hematologic, and there were no grade 5 adverse events. Four patients (10.0%) discontinued GnP because of treatment-related adverse events; grade 3 peripheral neuropathy and edema in one patient, grade 3 peripheral neuropathy in two patients, and grade 3 peripheral edema in one patient. Twenty patients (50.0%) required dose reduction following peripheral neuropathy in 16 (40.0%) patients, neutropenia in 2 (5.0%) patients, peripheral edema in 1 (2.5%) patient, and asthenia in 1 (2.5%) patient. The last dose of nab-paclitaxel was 100 mg/m2 (80% of full dose) in 12 (30.0%) patients and 85 mg/m2 (68% of full dose) in 3 (7.5%) patients.
Table 3.
Grade 3 or higher treatment-related adverse events.
| Grades, No. (%) (N = 40) | |||
|---|---|---|---|
| 3 | 4 | ⩾3 | |
| Hematologic | |||
| Thrombocytopenia | 11 (27.5%) | 4 (10.0%) | 15 (37.5%) |
| Anemia | 13 (32.5%) | 0 | 13 (32.5%) |
| Neutropenia | 4 (10.0%) | 7 (17.5%) | 11 (27.5%) |
| Leukopenia | 7 (17.5%) | 2 (5.0%) | 9 (22.5%) |
| Febrile neutropenia | 1 (2.5%) | 0 | 1 (2.5%) |
| Non-hematologic | |||
| Peripheral neuropathy | 4 (10.0%) | 0 | 4 (10.0%) |
| Peripheral edema | 3 (7.5%) | N/A | 3 (7.5%) |
| Liver infection | 2 (5.0%) | N/A | 2 (5.0%) |
| Lung infection | 2 (5.0%) | N/A | 2 (5.0%) |
| Peritonitis | 2 (5.0%) | N/A | 2 (5.0%) |
| Asthenia | 1 (2.5%) | 0 | 1 (2.5%) |
N/A, not applicable.
Among the 22 patients without oxaliplatin neurotoxicity, new onset of peripheral neuropathy occurred in 15 patients, including two patients with grade 3 peripheral neuropathy. Among 18 patients with oxaliplatin neurotoxicity, seven patients suffered from worsening or new onset of peripheral neuropathy, including two patients with grade 3 peripheral neuropathy.
Discussion
This study showed promising efficacy of GnP as a second-line chemotherapy agent following FOLFIRINOX failure in advanced pancreatic cancer. GnP achieved its primary objective, with a 6-month survival rate of 72.5%, supporting the hypothesis of survival benefit with second-line GnP over best supportive care only. At a median follow-up of 16.3 months (95% CI, from 14.3 to not reached), median PFS and OS were 5.8 months (95% CI, 4.3–8.7) and 9.9 months (95% CI, 7.5–12.4), respectively. The objective response rate and disease control rate were 15.0% and 87.5%, respectively. Treatment-related grade 3 or higher adverse events occurred in 25 (62.5%) patients.
Second-line chemotherapy in advanced pancreatic cancer has not been established yet. Based on the MPACT trial findings, GnP was proposed as a second-line treatment following FOLFIRINOX failure in advanced pancreatic cancer. Similarly, a response rate of 15.0% and median OS of 9.9 months in this study are consistent with the previous studies and suggest promising efficacy of second-line GnP after FOLFIRINOX failure. A French prospective multicenter cohort study showed a median OS of 8.8 months with second-line GnP in patients with 57 metastatic pancreatic cancer after FOLFIRINOX failure. 15 Recently, a Japanese prospective multicenter phase 2 study evaluated the efficacy of second-line GnP in 30 patients with locally advanced or metastatic pancreatic cancer and reported a response rate of 13.3%, a median PFS of 3.8 months, and a median OS of 7.6 months. 16 A recent systematic review evaluating gemcitabine-based chemotherapy after FOLFIRINOX failure in patients with advanced pancreatic cancer showed that GnP was associated with a superior objective response rate (14.4% vs 8.4%; p = 0.038) and disease control rate (53.5% vs 30.5%; p < 0.001) when compared with gemcitabine monotherapy, 22 and similar results were reported in multicenter large retrospective study including 445 patients with metastatic pancreatic cancer. 23 However, these findings should be interpreted cautiously because apart from one study, all the other studies included in the meta-analysis were retrospective studies. A comparative, multicenter, randomized, open-label, phase 3 trial (NCT03943667) is ongoing to evaluate the superiority of GnP over gemcitabine monotherapy in regards to the OS in metastatic pancreatic cancer after FOLFIRINOX failure or intolerance. 24
The median cycles of first-line FOLFIRINOX in our study was 15, which was relatively higher than those from previous studies (12 in the French study and 8 in the Japanese study),15,16 but similar to those from recent Korean studies.25,26 Given that patient characteristics and disease features were similar to previous studies, it is unclear where the difference stemmed from. It might have resulted from ethnic differences or different dose reduction protocols or schedule adjustment of FOLFIRINOX between studies. Another possible explanation is that good medical infrastructure in Korea, such as broad medical coverage and good accessibility to tertiary medical center for proper biliary decompression and supportive care, might have contributed to maintain better performance status of patients, resulting in longer duration of chemotherapy and favorable survival outcomes.
The overall toxicity profile in our study was consistent with those in the previous studies of second-line GnP. Grade 3 or 4 adverse events occurred in 62.5% of all the patients and most of them were hematological adverse events such as thrombocytopenia (37.5%), anemia (32.5%), neutropenia (27.5%), and leukopenia (22.5%). Similarly, in a Japanese phase 2 clinical trial, grade 3 or higher adverse events occurred in 70% of all the patients who received second-line GnP and the majority of them were hematological; neutropenia (50.0%), thrombocytopenia (20.0%), and anemia (26.7%). 16 In a French prospective cohort study, grade 3 or higher adverse events occurred in 38% of patients and they included neutropenia (12.5%), thrombocytopenia (6.5%), and anemia (3.5%). 15 Differences in these hematologic adverse event profiles might have resulted from heterogeneity regarding the cumulative dose of FOLFIRINOX and/or second-line GnP delivered among studies. However, although the direct statistical comparison was not possible, the median number of FOLFIRINOX cycles in our study (15) was higher than those of the two aforementioned studies (12 in the French study; and 8 in the Japanese study).15,16 The median number of GnP cycles in our study was five while those were four for two previous studies.
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the main toxicity that hampers the patients’ quality of life and a reason for dose reduction or even discontinuation of chemotherapeutic agents. 27 CIPN is a major issue in second-line GnP after FOLFIRINOX failure because both oxaliplatin and nab-paclitaxel are well known to induce CIPN and oxaliplatin-induced neurotoxicity likely to worsen after cessation (coasting phenomenon).28,29 In this study, nearly half of the patients had peripheral neuropathy at enrollment due to oxaliplatin. However, worsening or new-onset neuropathy occurred in only a small number of these patients. Among all the patients, only 10% experienced grade 3 or higher peripheral neuropathy, these findings are similar to a Japanese study result (13.3%), where patients with grade 2 or higher oxaliplatin neuropathy were excluded. 16 Unfortunately, there are no preventive treatments for CIPN and there are no established predictive factors for the development of nab-paclitaxel-induced peripheral neuropathy. Nevertheless, considering the survival benefit of second-line GnP and acceptable risk of neuropathy, presence of oxaliplatin-related peripheral neuropathy itself should not be considered as a contraindication to second-line GnP at the time of disease progression after FOLFIRINOX.
The main strength of this study lies in the study design. It is a multicenter, phase II, clinical trial and was conducted with a prespecified statistically calculated sample size to explore the efficacy and safety of second-line GnP. Until now, there are only a few prospective studies and real-world data on the efficacy and safety of second-line GnP after FOLFIRINOX failure. Moreover, in contrast with previous prospective studies, we excluded patients with FOLFRINOX intolerance and included only those with disease progression after FOLFIRINOX. In this way, this offered the benefits of specifically evaluating the efficacy and safety of GnP as second-line chemotherapy in a homogeneous group of FOLFIRINOX-refractory pancreatic cancer patients. In addition, we confirmed the tolerability of second-line GnP after FOLFIRINOX failure even in patients with oxaliplatin-induced neuropathy.
There are several notable limitations of this study that should be addressed. First, it is a non-randomized, open-label, single-arm study design with relatively small sample size. In order to reduce the predictable selection bias, we choose a multicenter study and tried to evaluate superiority rather than non-inferiority compared with the existing data in the sample size calculation. Second, both patients with locally advanced disease and metastatic disease were included in this study. Post hoc analysis for metastatic disease patients showed similar survival outcomes. However, we were unable to investigate the efficacy of second-line GnP in a few participants with locally advanced disease. Third, about one-fifth of the patients received nanoliposomal irinotecan plus 5-fluorouracil/folinic acid as the third-line treatment, which showed efficacy in gemcitabine-refractory pancreatic cancer. 13 However, the survival benefit of this third-line therapy is not clear because all the patients in this study had pancreatic cancer that was refractory to first-line FOLFIRINOX, an irinotecan-containing regimen.
In conclusion, we showed the efficacy and safety of second-line GnP following FOLFIRINOX failure in patients with advanced pancreatic cancer. This study met its primary endpoint of demonstrating an increase in 6-month survival rate compared with a historical cohort of best supportive care only. In future, a randomized phase 3 trial will be needed for the confirmation of these promising findings in this study.
Acknowledgments
The authors express sincere gratitude to Dr Ki Ho Yang and essayist Myeong Hee Choi for their support in this study.
Footnotes
Author contributions: G.H., H.S.L., J.H.C., S.H.L., W.H.P., J.K.R., Y.-T.K., S.B., and E.S.L. recruited patients for the study, collected, analyzed and interpreted the data. J.H.C. and S.H.L. contributed to the study design. G.H. and S.H.L. developed the first draft. All authors were involved in revising the manuscript. All authors have seen and approved the final manuscript. G.H., H.S.L., and J.H.C. contributed equally to this work.
Conflict of interest: S.H.L. report personal fees from Boryung Pharmaceutical and Celgene, outside the submitted work. Other authors declare no competing interests.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate: This study was approved by the institutional review board for every site and was conducted in conformity with the Declaration of Helsinki. All patients provided written informed consent.
Availability of data and materials: The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Contributor Information
Gunn Huh, Department of Internal Medicine, Liver Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Hee Seung Lee, Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea; Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
Jin Ho Choi, Department of Internal Medicine, Liver Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Sang Hyub Lee, Professor, Division of Gastroenterology, Department of Internal Medicine, Seoul National University College of Medicine, Liver Research Institute, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Woo Hyun Paik, Department of Internal Medicine, Liver Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Ji Kon Ryu, Department of Internal Medicine, Liver Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Yong-Tae Kim, Department of Internal Medicine, Liver Research Institute, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea.
Seungmin Bang, Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea; Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
Eaum Seok Lee, Pancreaticobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer, Seoul, Korea; Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 3. Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020; 395: 2008–2020. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, Version 2. 2021, www.nccn.org/patients [DOI] [PubMed]
- 5. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 7. Pusceddu S, Ghidini M, Torchio M, et al. Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: a systematic review and meta-analysis. Cancers (Basel) 2019; 11: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009; 101: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012; 69: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 10. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 11. Zaanan A, Trouilloud I, Markoutsaki T, et al. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer 2014; 14: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 13. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 14. Chung V, McDonough S, Philip PA, et al. Effect of selumetinib and MK-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: SWOG S1115 study randomized clinical trial. JAMA Oncol 2017; 3: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015; 113: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mita N, Iwashita T, Uemura S, et al. Second-line gemcitabine plus nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J Clin Med 2019; 8: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. Epub ahead of print 5 August 2020. DOI: 10.1200/JCO.20.01364. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 19. Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013; 24: 1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawless JF. Statistical models and methods for lifetime data. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc, 2003. [Google Scholar]
- 21. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–413. [Google Scholar]
- 22. de Jesus VHF, Camandaroba MPG, Calsavara VF, et al. Systematic review and meta-analysis of gemcitabine-based chemotherapy after FOLFIRINOX in advanced pancreatic cancer. Ther Adv Med Oncol 2020; 12: 1758835920905408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaibet S, Hautefeuille V, Auclin E, et al. PD-6 Gemcitabine + nab-paclitaxel or gemcitabine alone after FOLFIRINOX failure in patients with metastatic pancreatic adenocarcinoma: a population-based, multicenter AGEO study. Ann Oncol 2020; 31: S213–S214. [Google Scholar]
- 24. de la, Fouchardiere C, Hammel P, Launay S, et al. 1566TiP PRODIGE 65—UCGI 36—GEMPAX: a unicancer phase III randomized study evaluating gemcitabine and paclitaxel versus gemcitabine alone after FOLFIRINOX failure or intolerance in metastatic pancreatic ductal adenocarcinoma. Ann Oncol 2020; 31: S954–S955. [Google Scholar]
- 25. Chae H, Jeong H, Cheon J, et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis. Ther Adv Med Oncol 2020; 12: 1758835920923424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho IR, Kang H, Jo JH, et al. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: single-center cohort study. World J Gastrointest Oncol 2020; 12: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 2020; 38: 3325–3348. [DOI] [PubMed] [Google Scholar]
- 28. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth 2017; 119: 737–749. [DOI] [PubMed] [Google Scholar]
- 29. Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 2017; 81: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]