Abstract
Aberrant kinase signaling that involves platelet-derived growth factor receptor (PDGFR) α/β, colony stimulating factor 1 receptor (CSF1R), and stem cell factor receptor (c-KIT) pathways may be responsible for vascular remodeling in pulmonary arterial hypertension. Targeting these specific pathways may potentially reverse the pathological inflammation, cellular proliferation, and fibrosis associated with pulmonary arterial hypertension progression. Seralutinib (formerly known as GB002) is a novel, potent, clinical stage inhibitor of PDGFRα/β, CSF1R, and c-KIT delivered via inhalation that is being developed for patients with pulmonary arterial hypertension. Here, we report on an ongoing Phase 2 randomized, double-blind, placebo-controlled trial (NCT04456998) evaluating the efficacy and safety of seralutinib in subjects with World Health Organization Group 1 Pulmonary Hypertension who are classified as Functional Class II or III. A total of 80 subjects will be enrolled and randomized to receive either study drug or placebo for 24 weeks followed by an optional 72-week open-label extension study. The primary endpoint is the change from baseline to Week 24 in pulmonary vascular resistance by right heart catheterization. The secondary endpoint is the change in distance from baseline to Week 24 achieved in the 6-min walk test. A computerized tomography sub-study will examine the effect of seralutinib on pulmonary vascular remodelling. A separate heart rate monitoring sub-study will examine the effect of seralutinib on cardiac effort during the 6-min walk test.
Keywords: pulmonary vascular remodeling, PDGFR, CSF1R, c-KIT, BMPR2, GB002
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by increased proliferation of pulmonary artery smooth muscle cells (PASMCs), endothelial cells, and myofibroblasts.1–3 This pathologic vascular remodeling is driven, in part, by aberrant kinase signaling that involves multiple pathways and leads to increased pulmonary vascular resistance (PVR).4,5 Currently approved treatment options do not reverse this aberrant kinase signaling.
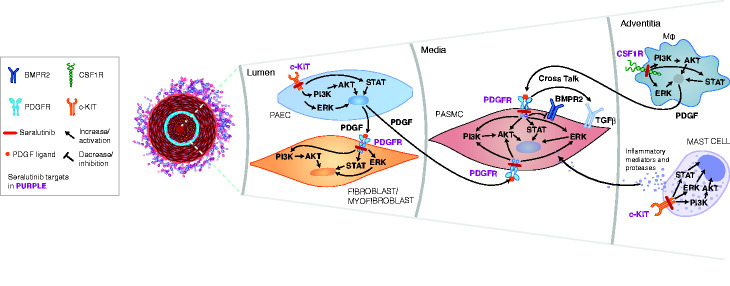
Identifying novel therapeutic targets involved in PAH pathogenesis may potentially improve the outcome for patients with this disease. Several studies have shown that PDGFRα/β, colony stimulating factor 1 receptor (CSF1R), and c-KIT play key roles in cellular overgrowth in the pulmonary arteriolar lesions associated with PAH.2,6–8 Aberrant PDGFR signaling drives proliferation of smooth muscle cells (in the vessel media) and myofibroblasts (in the vessel lumen) leading to pulmonary arteriolar medial hypertrophy, neointimal lesions, and fibrosis. c-KIT is expressed on endothelial progenitor cells and mast cells, potentially contributing to perivascular inflammation and vascular remodeling.7,9,10 Increased infiltration of c-KIT-positive cells has been observed in pulmonary arterial plexiform lesions in PAH patient lungs. 7 CSF1R is expressed on monocytes and macrophages. 11 CSF1R-positive perivascular/adventitial macrophages secrete PDGF ligands and pro-inflammatory cytokines, contributing to pathological remodeling in PAH.12–14 PDGF activation decreases bone morphogenetic protein receptor type 2 (BMPR2) and can also activate transforming growth factor beta (TGFβ), which further drives PASMC proliferation in the media of the pulmonary arterioles.15,16 Effects of CSF1R, PDGFR, and c-KIT activation can be mediated by multiple intracellular kinases including PI3K, AKT, ERK, and STATs. Fig. 1 highlights the interactions between CSF1R, PDGFR, and c-KIT signaling as well as PDGF modulation of BMPR2, which lead to abnormal proliferation of PASMCs, and myofibroblasts within the pulmonary arterioles, thereby leading to elevated PVR, a hallmark of PAH. Targeting these specific pathways (PDGFRα/β, CSF1R, and c-KIT) may potentially reverse the pathological inflammation, cellular proliferation, and fibrosis associated with PAH. Thus, there is a compelling rationale to target PDGFR, CSF1R, and c-KIT as a treatment for this disease.
Fig. 1.
Aberrant PDGFR signaling drives proliferation of smooth muscle cells in the pulmonary arteriolar media and myofibroblasts within the vessel lumen, leading to pulmonary arteriolar medial hypertrophy, neointimal lesions, and fibrosis. c-KIT is expressed on endothelial progenitor cells and mast cells, potentially contributing to perivascular inflammation and vascular remodeling. PDGF can mediate a decrease in BMPR2 and activation of TGFβ , which activates proliferative signaling. Perivascular/adventitial CSF1R-positive macrophages secrete PDGF ligands thereby activating PDGF receptors on PASMCs and myofibroblasts and abnormal proliferation of these cells within the diseased pulmonary arterioles. Effects of CSF1R, PDGFR, and c-KIT activation are mediated by multiple intracellular kinases including PI3K, AKT, ERK, and STATs. The interactions between CSF1R, PDGFR, and c-KIT signaling as well as PDGF-mediated decrease in BMPR2 lead to abnormal proliferation of PASMCs, and myofibroblasts within the pulmonary arterioles, and thereby to elevated PVR, a hallmark of pulmonary arterial hypertension (PAH). Seralutinib, by targeting these pathways, has the potential to reverse the abnormal pulmonary vascular remodeling characteristic of PAH.
AKT: AK strain transforming (protein kinase B); BMPR2: bone morphogenetic protein receptor type 2; c-KIT: stem cell factor receptor; CSF1R: colony stimulating factor 1 receptor; ERK: extracellular signal-regulated kinase; PAEC: pulmonary arterial endothelial cells; PASMC: pulmonary arterial smooth muscle cells; PDGF: platelet-derived growth factor; PDGFR: platelet-derived growth factor receptor; PI3K: phosphoinositide 3-kinase; STATs: signal transducer and activator of transcription; TGFβ: transforming growth factor beta.
Seralutinib (formerly known as GB002) is a small-molecule, selective kinase inhibitor that targets PDGFRα/β, CSF1R, and c-KIT, and increases BMPR2. This novel chemical entity has been specifically developed to target these pathways involved in the pathogenesis of PAH (Fig. 1). Seralutinib is administered by dry power inhalation to potentially maximize the therapeutic index of this kinase inhibitor by directly targeting diseased pulmonary arterioles, minimizing systemic exposure, and decreasing the potential for off-target adverse effects. The results of preclinical studies are consistent with an inhaled lung deposition profile such that a significantly higher lung tissue exposure compared to plasma exposure was observed. 17
Seralutinib was evaluated in two preclinical animal models: the monocrotaline/pneumonectomy (MCTPN) model and the Sugen5416 hypoxia (SU5416/H) model. These models were chosen because they replicate key features of human PAH, including the development of neointimal proliferative lesions in the pulmonary arterioles and severe pulmonary hypertension. In these models, seralutinib reversed pulmonary vascular remodeling, improved hemodynamics by significantly decreasing right ventricular systolic pressure, reducing circulating levels of N-terminal pro b-type natriuretic peptide (NT-proBNP), and increasing lung BMPR2 protein expression compared to controls.17,18
Two Phase 1a randomized, double-blind, placebo-controlled studies in healthy volunteers were conducted to determine the safety and tolerability of a range of doses of seralutinib. 19 These single- and multiple-ascending dose studies found that exposure to seralutinib increased in a dose-proportional manner. Seralutinib was rapidly absorbed into and cleared from the systemic circulation. Doses of seralutinib up to 90 mg (the highest dose tested) twice daily were well tolerated. Preliminary results from a Phase 1b, randomized, double-blind, placebo-controlled multicenter trial (NCT03926793) that assessed seralutinib doses of 45 and 90 mg twice daily in patients with PAH showed similar results. Doses were selected based on allometric scaling and PK/PD modeling. A Phase 2 trial evaluating the efficacy and safety of seralutinib in subjects with WHO Group 1 PH (PAH) (TORREY, NCT04456998) is ongoing and described herein.
Phase 2 TORREY study
The TORREY study is a randomized, double-blind, placebo-controlled trial (NCT04456998) designed to examine the efficacy and safety of inhaled seralutinib in subjects with PAH over a 24-week course of treatment. Rather than being an acronym, TORREY was named for the rare, critically endangered Torrey pine tree (Pinus torreyana) that is native only to San Diego County and immediate environs in California. This open-crowned pine tree creates and emits oxygen and as such, is symbolic of a life-sustaining resource.
The study will include investigational sites in United States, Canada, Europe, Israel, and Australia. Prior to study initiation, the study protocol, amendments, informed consent forms, and other relevant documents will be reviewed by the institutional review board or independent ethics committee for each site. The study will be conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation-Good Clinical Practice guidelines.
The primary objectives of the TORREY study are to determine the effect of inhaled seralutinib on pulmonary hemodynamics and to assess safety in subjects with WHO Group 1 PH (PAH) who are classified as Functional Class (FC) II or III. The secondary objective is to determine the effect of seralutinib on exercise capacity in this population.
Eighty subjects will be enrolled. Key inclusion and exclusion criteria are summarized in Table 1. Eligible subjects must have an established diagnosis of WHO group 1 PH (PAH) receiving standard of care background therapies (on stable doses for at least four weeks) and documentation of right heart catheterization (RHC) consistent with a PAH diagnosis, including a mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest, PVR ≥400 dyne · s/cm5, and pulmonary capillary wedge pressure (PCWP) or left ventricular end diastolic pressure (LVEDP) ≤12 mmHg if PVR is ≥400 but <500 dyne · s/cm5 or PCWP or LVEDP ≤15 mmHg if PVR ≥500 dyne · s/cm5.
Table 1.
Key inclusion and exclusion criteria for the TORREY study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Current diagnosis of symptomatic PAH classified by one of the following: – IPAH, HPAH, PAH-CTD– PAH associated with anorexigen or use of methamphetamine– Congenital heart disease with simple systemic to pulmonary shunt at least 1 year after surgical repair• WHO FC II or III• 6MWD ≥150 m and ≤550 m at screening• Treatment with standard of care PAH background therapies; prostacyclins allowed• Documentation of RHC consistent with PAH diagnosis, and meeting the following criteriaa:– mPAP ≥25 mmHg (at rest), AND– PVR ≥400 dyne · s/cm5, AND – PCWP or LVEDP ≤12 mm Hg if PVR ≥400 to <500 dyne · s/cm5– OR– PCWP or LVEDP ≤15 mmHg if PVR ≥500 dyne · s/cm5• PFTs and DLCO meeting the following criteriab:– Forced expiratory volume in 1 second (FEV1) ≥60% (predicted),– DLCO ≥40% predicted except for subjects with PAH associated with systemic sclerosis (SSc-APAH) where DLCO ≥30% is required. | • Evidence of chronic thromboembolic disease or acute pulmonary embolism• WHO Pulmonary Hypertension Group 2–5• HIV-associated PAH• History of left-sided heart disease and/or clinically significant cardiac disease• Inhaled prostanoids• Use of anticoagulants at randomization; If on coumadin or NOAC, these drugs can be withdrawn, if clinically appropriate, during the screening period and should have normal coagulation parameters prior to randomization• History of intracranial hemorrhage |
6MWD: 6 minute walk distance; DLCO: carbon monoxide diffusing capacity; FC: functional class; FEV1: forced expiratory volume in 1 s; HPAH: heritable pulmonary arterial hypertension; HIV: human immunodeficiency virus; IPAH: idiopathic PAH; LVEDP: left ventricular-end diastolic pressure; mPAP: mean pulmonary arterial pressure; NOAC: novel oral anticoagulant; PAH: pulmonary arterial hypertension; PAH-CTD: PAH with connective tissue diseases; PCWP: pulmonary capillary wedge pressure; PFT: pulmonary function test; PVR: pulmonary vascular resistance; RHC: right heart catheterization; SSc-APAH: PAH associated with systemic sclerosis; WHO: World Health Organization.
aPCWP and PVR inclusion criteria are based on revised criteria utilized in the AMBITION study. 20
bIf the subject uses continuous oxygen therapy, they must be able to complete PFTs without oxygen. Subjects who are unable to complete PFTs without oxygen therapy are not eligible for the study.
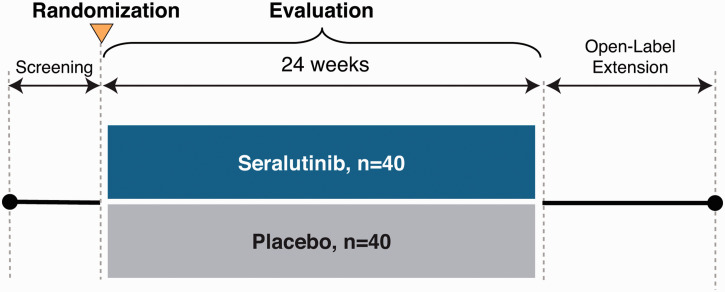
The TORREY study schema is shown in Fig. 2. Following informed consent, subjects will be assessed for eligibility during a screening period of up to five weeks in duration. Subjects will be maintained on previously prescribed PAH background therapies. Prostacyclins will be allowed except for those administered by inhalation. Medication regimens should remain stable for the four weeks prior to consent and throughout the screening period. As needed diuretics for intermittent weight gain and/or edema will be allowed.
Fig. 2.
TORREY study schema.
Eligible subjects will be randomized 1:1 to one of two treatment groups to receive seralutinib twice per day (BID) or matched placebo by dry powder inhaler (Plastiape RS01L, Model 7, Osnago, Italy). Randomization will be stratified by PVR (< and ≥800 dyne · s/cm5). Subjects will have periodic study visits to monitor safety, tolerability and for efficacy assessments. Accommodations and other interventions may be implemented as needed due to the COVID-19 pandemic.
The primary and secondary endpoints of the TORREY study are summarized in Table 2. The primary endpoint is the change from baseline to Week 24 in PVR determined by RHC. The secondary endpoint is the change in distance from baseline to Week 24 achieved on the 6-min walk test (6MWT). 21 Safety will be evaluated throughout the study by determining the incidence of treatment-emergent adverse events, changes in laboratory values, and vital signs. Other exploratory studies may include whole blood gene expression, proteomics, and epigenetic analyses.
Table 2.
TORREY study: objectives and endpoints.
| Objectives | Endpoints |
|---|---|
| • Primary: Determine the effect of inhaled seralutinib on pulmonary hemodynamics in subjects with WHO Group 1 PAH• Secondary: Determine the effect of seralutinib on exercise capacity in this population | • Primary: Change from baseline to Week 24 in pulmonary vascular resistance by right heart catheterization• Secondary: Change from baseline to Week 24 in distance achieved on the six-minute walk test• Safety: Incidence of treatment-emergent adverse events |
In light of serious events of subdural hematoma reported with oral imatinib in the IMPRES trial, 22 the TORREY study will exclude subjects receiving anticoagulation therapy (Table 3). In addition, subjects with any history of intracranial hemorrhage will be excluded. An Independent Data Monitoring Committee comprised of external expert physicians and an external biostatistician will regularly monitor emerging efficacy and overall safety data, including potential for bleeding-related events and other adverse events, as well as general aspects of study conduct, to ensure that the benefits and risks to subjects of study participation remain acceptable.
Table 3.
Open-label extension study: objectives and endpoints.
| Objectives | Endpoints |
|---|---|
| • Primary: Evaluate the long-term safety and tolerability of orally inhaled seralutinib• Secondary: Evaluate the long-term effect of orally inhaled seralutinib on exercise capacity | • Primary: Incidence of treatment-emergent adverse events• Secondary: Change in distance achieved on the six-minute walk test (6MWT) |
Assuming a mean (SD) treatment effect of 300 (340) dyne · s/cm5in the seralutinib group vs. a treatment effect of 79 (270) dyne · s/cm5 in the placebo group 23 , a sample size of 40 subjects per treatment group has approximately 90% power to detect a statistically significant difference between seralutinib and placebo with α = 0.05, two-sided.
Open-label extension study
Subjects may be eligible to participate in a 72-week open-label extension (OLE) study provided they have completed study treatment and all associated assessments of the blinded parent study (TORREY) or another seralutinib study. The objectives of this OLE are to investigate the long-term safety/tolerability and efficacy of inhaled seralutinib in the described study population (Table 3). Subjects will receive seralutinib for up to 18 months. Safety data will be collected from subjects entering the OLE from a blinded parent study (e.g., TORREY) periodically through Week 72. Right heart catheterization will be performed at Week 48 to evaluate ongoing efficacy on PVR.
TORREY sub-studies
Two sub-studies are planned in the TORREY protocol and subjects at select sites will be offered the option to participate. These sub-studies will explore novel endpoints relevant to the underlying pathophysiology of the disease. In the functional respiratory imaging study, CT imaging will evaluate the effect of seralutinib on pulmonary vascular remodeling by quantifying changes in pulmonary arterial blood volume. In the heart rate monitoring sub-study, cardiac effort will be measured during the 6MWT. 24
Discussion
Patients with PAH experience high levels of disease burden resulting in severe impairment of functional status and associated quality of life.25,26 The goals of PAH therapy are to improve exercise capacity, decrease the frequency of hospitalization, improve quality of life, and ultimately, prolong survival by reversing disease pathology.27,28 Current therapies target three established pathways contributing to pathologic vasoconstriction in PAH: the nitric oxide/cGMP pathway, the endothelin pathway, and the prostacyclin pathway. However, these therapies do not adequately reverse the underlying pathological remodeling and cellular overgrowth that characterize PAH.
PAH progression involves multiple pathways of aberrant kinase signaling, including PDGFR, CSF1R, c-KIT as well as BMPR2 deficiency.2,6–8 Imatinib is an antiproliferative agent developed to target BCR-ABL tyrosine kinase in patients with chronic myeloid leukemia; however, inhibitory effects of imatinib on PDGFRα/β and c-KIT suggested that it might be efficacious in PAH. In the IMPRES study, improvements in exercise capacity and cardiopulmonary hemodynamics were observed in patients treated with imatinib, but serious adverse events limited further development in PAH. 22
Seralutinib is an inhaled small molecule PDGFR/CSF1R/c-Kit kinase inhibitor that was developed to target these key pathways and, in so doing, may impact the underlying pathological remodeling and cellular overgrowth that characterize PAH. Preclinical models of PAH demonstrated that seralutinib reversed pulmonary vascular remodeling, improved hemodynamic parameters, increased lung BMPR2, and reduced circulating NT-proBNP.17,18 In early clinical trials in healthy volunteers and subjects with PAH, seralutinib was well tolerated and demonstrated a pharmacokinetic profile characteristic of an inhaled product.
Seralutinib addresses critical cellular and molecular mechanisms driving vascular remodeling. By targeting inflammatory, proliferative, and fibrotic pathways implicated in PAH, seralutinib has the potential to be a paradigm-shifting, disease-modifying therapy in PAH. The primary endpoint of the Phase 2 TORREY study is change in PVR from baseline to Week 24. This physiologic measure is directly relevant to demonstrating a significant effect of seralutinib on the course of disease. The secondary endpoint, change in 6MWD from baseline to Week 24, constitutes an accepted measure of functional improvement. Although the study is not powered to show a significant effect of seralutinib on 6MWD, a trend or directionality of a favorable response could be demonstrated. Other exploratory endpoints are relevant to understanding the potential effect of seralutinib on right heart function, quality of life measures, hospitalization for PAH, time to clinical worsening, and risk score. Other exploratory studies may include whole blood gene expression, proteomics, and epigenetic analyses. The planned sub-studies are designed to explore novel endpoints relevant to pulmonary vascular remodeling and cardiac performance. The Phase 2 TORREY study (NCT04456998) is now enrolling patients with WHO Group 1 PH, FC II or III currently receiving standard of care background therapies.
Acknowledgments
The authors gratefully acknowledge Andrea R. Gwosdow, Ph.D. and Jill Luer, PharmD, CMPP, Gossamer Bio, Inc., for medical writing and editorial assistance.
Footnotes
Author contributions: All authors made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data; drafted the article or revised it critically for important intellectual content; approved the version to be published; and participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RPF has received consulting fees from Gossamer Bio, Inc., Bayer (to his institution) and Janssen, Liquidia (personal) and speaker fees from United Therapeutics (to his institution), Janssen (personal). RLB has received research grants (to his institution) from Gossamer Bio, Inc., Aria, Abbott, United Therapeutics, Janssen, and Cereno Therapeutics and consulting fees from Gossamer Bio, Inc., United Therapeutics, Cereno Therapeutics, and Respira Therapeutics. RNC received consulting fees from Gossamer Bio, Inc., Bayer, United Therapeutics, Janssen, and Third Pole Therapeutics, and speaker fees from Janssen and Bayer. KC has received research grants (to her institution) from Altavant, Gossamer Bio, Inc., Janssen, and Pfizer, consulting fees from Altavant, Gossamer Bio, Inc., Janssen, United Therapeutics, and UCSD (via a grant from Bayer), travel (meeting) support from Actelion, and fees for work as an associate editor from the American Heart Association. LSH has received a research grant (to his institution) from Janssen, lecture fees from Janssen, MSD, travel (meeting) support from Janssen, and consulting fees from Janssen, GlaxoSmithKline, MSD, and Gossamer Bio, Inc. VVM has received research grants (to her institution) from Acceleron, Actelion, Altavant, Gilead, SonoVie, Reata, and United Therapeutics, consulting fees from Acceleron, Actelion, Caremark, CiVi Biopharma, Gossamer Bio, Inc., Liquidia, and United Therapeutics. OS has received research/educational grants (to his institution) from Acceleron, Janssen, GlaxoSmithKline, and MSD, consulting fees from Acceleron, Janssen, MSD and Gossamer Bio, Inc., speaker fees from Janssen, MSD, Ferrer, and AOP Orphan, and travel support (conferences) from Janssen and MSD. RTZ has received consulting fees from Morphogen-IX, Vivus, Pfizer and Aria and holds a patent for FK506. ARH has received research grants (to her institution) from the National Institutes of Health/National Heart, Lung, and Blood Institute and the Cardiovascular Medical Research and Education Fund, consulting fees from Bayer, United Therapeutics, Gossamer Bio, Inc., and Janssen, and owns stock from Tenax Therapeutics. HAG has received consulting fees from Gossamer Bio, Inc.
Guarantor: Lawrence S. Zisman is responsible for the content of this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The TORREY study is sponsored and supported by Gossamer Bio, Inc.
ORCID iD: Kelly Chin https://orcid.org/0000-0002-1214-6723
References
- 1.Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(12 Suppl S): 13S–24S. [DOI] [PubMed] [Google Scholar]
- 2.Yamamura A, Nayeem MDJ, Al Mamun A, et al. Platelet-derived growth factor up-regulates Ca2+-sensing receptors in idiopathic pulmonary arterial hypertension. FASEB J 2019; 33: 7363–7374. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Boehm M, Egemnazarov B, et al. Kinases as potential targets for treatment of pulmonary hypertension and right ventricular dysfunction. Br J Pharmacol 2020; 1–23. [DOI] [PubMed] [Google Scholar]
- 4.Lai YC, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014; 115: 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemnes AR, Humbert M. Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled. Eur Respir Rev. 2017; 26: 170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perros F, Montani D, Dorfmuller P, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 81–88. [DOI] [PubMed] [Google Scholar]
- 7.Montani D, Perros F, Gambaryan N, et al. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2011; 184: 116–123. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Franklin RA, Adler M, et al. Circuit design features of a stable two-cell system. Cell 2018; 172: 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonigk D, Golpon H, Bockmeyer CL, et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 2011; 179: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toshner M, Voswinckel R, Southwood M, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 2009; 180: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014; 6: a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abid S, Marcos E, Parpaleix A, et al. CCR2/CCR5-mediated macrophage-smooth muscle cell crosstalk in pulmonary hypertension. Eur Respir J 2019; 54: 1802308. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh AQ, Saddouk FZ, Ntokou A, et al. Cell autonomous and non-cell autonomous regulation of SMC progenitors in pulmonary hypertension. Cell Rep 2018; 23: 1152–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi N, Watanabe S, Verma R, et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur Respir J 2020; 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Cui X, Qian Z, et al. Multi-omics analysis reveals regulators of the response to PDGF-BB treatment in pulmonary artery smooth muscle cells. BMC Genomics 2016; 17: 781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porsch H, Mehić M, Olofsson B, et al. Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other's signaling and stability. J Biol Chem. 2014; 289:19747–19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galkin A, Clemons B, Garcia E, et al. GB002, a novel inhaled PDGFR kinase inhibitor, demonstrates efficacy in the SU5416 hypoxia rat model of PAH. Circulation 2019; 140: A11102. [Google Scholar]
- 18.Sitapara R, Slee D, Salter-Cid L, et al. In vivo efficacy of a novel, inhaled PDGFRα/β inhibitor, GB002, in the rat monocrotaline and pneumonectomy model of pulmonary arterial hypertension. Circulation 2019; 140: A12947.30592650 [Google Scholar]
- 19.Li J, Yamashita M, Cravets M, et al. Phase 1A randomized double-blind placebo-controlled single-ascending dose and multiple-ascending dose studies of orally inhaled GB002 in healthy adult subjects. Am J Respir Crit Care Med 2020; 201: A2907. [Google Scholar]
- 20.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 21. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 22.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 23.Ghofrani HA, Morrell NW, Hoeper MM, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 2010; 182(9): 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachant DJ, Light A, Offen M, et al. Heart rate monitoring improves clinical assessment during 6-min walk. Pulm Circ 2020; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathai SC, Suber T, Khair RM, et al. Health-related quality of life and survival in pulmonary arterial hypertension. Ann Am Thorac Soc 2016; 13: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes CJ, Martins BCS, Jardim CVP, et al. Quality of life as a prognostic marker in pulmonary arterial hypertension. Health Qual Life Outcomes 2014; 12: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Col Cardiol 2013; 62: D73–81. [DOI] [PubMed] [Google Scholar]
- 28.Weatherald J, Boucly A, Sahay S, et al. The low-risk profile in pulmonary arterial hypertension. Am J Respir Crit Care Med 2018; 197: 860–868. [DOI] [PubMed] [Google Scholar]