Abstract
Studies linking child maltreatment to abnormal neurophysiological responses to emotional stimuli and mental health impairment have not specifically explored these patterns in young children exposed to intimate partner violence (IPV). The present study examined two neurophysiological indicators, resting state EEG and an emotion event-related potential (ERP) in 21 IPV exposed and 30 non-exposed children ages 4–6 years recruited from the community and domestic violence shelters.. Frontal alpha asymmetry (FAA) was assessed while at rest. FAA is often associated with avoidant/withdrawn behavior and increased risk of IPV related mental health conditions (e.g., depression). Additionally, the late positive potential (LPP) ERP component, reflecting motivated attention, was acquired in the context of an age-appropriate affective oddball paradigm with low probability animal pictures as targets and human facial expressions (angry, happy, neutral) as distracters. Results demonstrated that IPV exposed children, compared to non-exposed children, exhibited lower left FAA during resting state and reduced LPPs to oddball targets and affective faces relative to neutral faces in the oddball task. . Together, these results suggest neural patterns associated with a blunted response to emotional stimuli and withdrawal tendencies, respectively, in young children exposed to IPV. Implications for emotional socialization in this vulnerable population are discussed.
Keywords: Intimate partner violence, child maltreatment, affective oddball, LPP, frontal asymmetry
Introduction
Childhood exposure to Intimate Partner Violence (IPV) is a prevalent and costly public health problem with immediate and long-term consequences for child development and mental health (Edleson, 1999; Hamby, Finkelhor, Turner, & Ormrod, 2011; Holt, Buckley, & Whelan, 2008; McTavish, MacGregor, Wathen, & MacMillan, 2016). IPV exposed children face disproportionate risk for developing a range of adverse outcomes that include posttraumatic stress, depression, anxiety, and problems with attention and disruptive behavior (Vu, Jouriles, McDonald, & Rosenfield, 2016; Wolfe, Crooks, Lee, McIntyre-Smith, & Jaffe, 2003). Considerable evidence now indicates that, for some children, these problems can emerge very early in development and have impacts that persist into adolescence and adulthood (Bogat, DeJonghe, Levendosky, Davidson, & Von Eye, 2006; Vu et al., 2016). Disruptions in affective processing may be one mechanism that explains the association between childhood violence exposure and psychopathology (Pollak, 2003). Identifying early indicators of disrupted emotion processing may help to determine the optimal timing and necessary targets for effective intervention to improve developmental outcomes in young children (Howell, 2011).
Studies of older maltreated children generally report disruptions in affective processing and threat responding, which may serve to explain intermediate pathways to emergent stress-related psychopathology (McLaughlin et al., 2016; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; Pollak, 2008; Teicher & Samson, 2016). In particular, maltreated children show a pattern of hyper-sensitivity to threatening stimuli and negative facial expressions (Dannlowski et al., 2012; Pollak, Klorman, Thatcher, & Cicchetti, 2001). Of note, much of what is known about the impact of maltreatment on neural structure and function comes from studies that have used f/MRI and EEG/ERP methods with older children and adolescents. With notable exceptions (Cicchetti & Curtis, 2005; Curtis & Cicchetti, 2011), the lower bound of psychophysiology research in maltreated youth has typically fallen around 8 years of age. Thus, relatively little is known about the effect of violence exposure on very young children.
While evidence for disrupted emotion processing in maltreated children is robust, relatively fewer studies have specifically examined emotion processing in children exposed to IPV. This is a notable gap in the literature. However, there are several reasons why existing studies of emotion processing in maltreated children may provide a blueprint for what to expect in IPV exposed children. Recent formulations of childhood adversity (McLaughlin, 2016) propose that maladaptive outcomes may depend on whether children are exposed to “threat” experiences that involve harm or threat of harm to self or other, in line with definitions of PTSD-qualifying events (American Psychiatric Association, 2013). Critically, threat is conceptualized as including not only physical abuse of the child, but also exposure to IPV. Given the conveyance of threat in both child physical abuse and IPV (e.g., threat to child vs. observed threat to another; McLaughlin, 2016), we hypothesized that IPV exposed children would exhibit a neurophysiologic profile that is similar to patterns observed in physically abused children. This is consistent with prior research demonstrating similar outcomes in IPV exposed and physically abused children. For example, in their meta-analysis, Kitzman et al. (2003) showed no statistically significant differences between IPV exposed and physically abused children in terms of internalizing or externalizing behavior problems. Moreover, there is converging evidence from behavioral, psychophysiological, and neural studies that children exposed to IPV and adult partner conflict are hypersensitive to a variety of threat indices, including affective behavioral/attentional biases (Davies, Thompson, Hentges, Coe, & Sturge-Apple, 2020), autonomic reactivity (El-Sheikh, 2005) and enhanced activation in threat-processing regions in fMRI when viewing angry faces (McCrory et al., 2011). Moreover, neural indicators of sensitivity to threats may emerge as early as infancy (Graham, Fisher, & Pfeifer, 2013).
One avenue for studying affective processing is with resting state electroencephalography (EEG). Specifically, frontal alpha asymmetry (FAA) is a resting EEG metric that has been used to study child abuse, neglect, and mental health problems in children. Unlike task-based neurophysiology measures that require children to comprehend instructions and sustain attention, FAA is measured at rest, an advantage when studying very young children. FAA measures the relative amount of brain activation in the right vs. left hemispheres and is associated with affective-behavioral (motivational) tendencies, such that greater left activation is associated with approach behavior and greater right activation is associated with avoidance (Calkins, Fox, & Marshall, 1996; Coan & Allen, 2003; Harmon-Jones, Gable, & Peterson, 2010). Similarly, lower left than right FAA has also been associated with higher trait negative emotionality and lower trait positive emotionality in adults and in young children (Goldstein et al., 2018; Tomarken, Davidson, Wheeler, & Doss, 1992). This is notable as higher negative emotionality and lower positive emotionality are considered to play a role in the development and course of IPV related mental health conditions such as PTSD and depression (Klein, Kotov, & Bufferd, 2011; Miller, 2003). Indeed, reduced left relative to right FAA, has been associated with depression (Goldstein & Klein, 2014; Thibodeau, Jorgensen, & Kim, 2006) and there is some evidence, albeit mixed, that FAA may be related to PTSD (Meyer et al., 2015). Overall, FAA has been associated with withdrawal tendencies, higher negative emotionality, but lower positive emotionality, depression, and to some extent PTSD all of which are relevant to the experiences of children exposed to IPV.
In addition, existing research on FAA in abused and neglected children and adolescents finds relatively reduced left vs. right FAA compared to non-maltreated children (Curtis & Cicchetti, 2007; Lahat et al., 2018). Moreover, there is evidence that children with a history of abuse or neglect continue to exhibit a pattern of reduced left vs. right FAA that persists over time (McLaughlin, Fox, Zeanah, & Nelson, 2011; Miskovic, Schmidt, Georgiades, Boyle, & MacMillan, 2009). As we described above, the neurophysiology of abused and neglected children may serve as a blueprint for findings in IPV exposed children, suggesting that children with IPV may also exhibit relatively reduced left than right FAA compared to non-exposed children. However, we are not aware of any studies of FAA in children exposed to IPV.
Event-related brain potentials (ERPs) are another method for studying affective processing in children. ERPs represent a specific type of EEG signal that reflect the activity of a population of neurons in response to a stimulus. A key advantage of ERP methodology relative to magnetic resonance imaging is its fine temporal resolution, on the order of milliseconds. Two closely related ERP components are the late positive potential (LPP) and the P3. The LPP and P3 are thought to index emotional and motivational significance and, as a result, have received considerable interest in psychological research (Hajcak & Foti, 2020; Olofsson, Nordin, Sequeira, & Polich, 2008). The P3 begins at approximately 300 ms following stimulus onset. In contrast, the LPP has been defined over a longer time window, beginning as early as 300 ms and persisting for 1,000 ms or more. As such, the LPP and sometimes the P3 have been referred to as the positive slow wave (PSW). While the LPP scalp distribution in young children differs from that observed in adults (i.e., maximal over occipital/parietal sites in children, but frontocentral sites in adults), the LPP in children and adults are comparable in terms of latency and functional relevance to emotionally and motivationally salient stimuli (Kujawa, Klein, & Hajcak, 2012).
Recent theoretical discussions suggest that the P3 and LPP share similar functions and may be outputs of the same neurobiological generators (Hajcak & Foti, 2020). Additionally, the LPP and P3 are elicited by similar tasks, as reviewed below, and both are associated with symptoms and experiences relevant to trauma, child maltreatment, and potentially IPV. Despite this evidence, some studies have noted distinctions between these components on certain aspects of information processing (Matsuda & Nittono, 2015). Because our construct of interest is reflected in both P3 and LPP, we take the parsimonious approach of focusing the current hypotheses on LPP. However, we review relevant studies of P3 and LPP, referring to these components as the authors intended.
The P3 and LPP can be elicited in a variety of paradigms, such as passive viewing of affectively valenced and neutral pictures, as well as in oddball paradigms, in which these components have been found to be enhanced in response to less probable or “oddball” target stimuli relative to distractor stimuli. The affective oddball paradigm is a variant of the classic oddball paradigm in which both affective and non-affective stimuli serve as distractors. This enables one to examine the P3 and LPP in response to affective stimuli (e.g., angry or happy faces) relative to oddball/target stimuli (e.g., animals and non-affective distractors, such as neutral faces). Meta-analytic findings of affective oddball studies have demonstrated larger P3 responses to threat-related distractors in adults with PTSD vs. those without (Karl, Malta, & Maercker, 2006). Recent research has similarly linked greater PTSD symptom severity in adults with larger LPP amplitudes elicited from gruesome images (Lobo et al., 2014). Other studies have contradictorily observed blunted LPP amplitudes to threatening stimuli, relative to non-threatening stimuli, in adults with combat exposure (Digangi et al., 2017; MacNamara, Post, Kennedy, Rabinak, & Phan, 2013). Still others find a quadratic pattern, where those with the lowest and highest PTSD symptoms exhibit larger LPPs to angry faces, but those with more moderate symptom levels showed blunting (Macatee et al., 2020).
Mixed findings have emerged from the few available studies of children. One study reported enhanced LPPs to threatening relative to non-threatening faces in youth with anxiety disorders compared to those without anxiety disorders (Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015), whereas another study reported comparable LPPs to threat-related, relative to positive or neutral, pictures in youth with PTSD relative to healthy controls (Grasso & Simons, 2012). Furthermore, in children, there is mixed evidence that the P3 (P3a and P3b) and LPP may be related to child abuse and intimate partner violence. For instance, some studies have demonstrated smaller amplitude or blunted responses in the PSW when maltreated children viewed angry faces (Cicchetti & Curtis, 2005; Curtis & Cicchetti, 2011). In contrast, another study found enhanced P3b responses to angry faces in maltreated children compared to controls (Pollak et al., 2001). Similarly, one small study reported that older children exposed to more severe/frequent IPV exhibited greater P3s in response to images of couples displaying anger, relative to couples displaying happy or neutral affect, than children from low conflict homes (Schermerhorn, Bates, Puce, & Molfese, 2015). These findings suggest that P3 and LPP responses to threat-related images, relative to non-threatening images, may index violence-associated differences in early emotion processing in children; however, there are few studies in this research area and findings are sometimes in the opposite direction (e.g., blunted in one study, enhanced in another). Given that previous results are contradictory and there have been virtually no studies of young children exposed to IPV, additional research is needed.
To this end, the current study presents initial data examining FAA and the LPP in 4- to 6-year-old children with and without exposure to physical IPV. FAA and the LPP are virtually unexamined in IPV exposed children, despite evidence that both may be linked to threatening childhood experiences and related psychopathology. In the current study, we hypothesized that FAA would differentiate those with vs. without IPV exposure, such that children with IPV exposure would exhibit relatively reduced left than right brain activity compared to non-exposed children. Additionally, we elicited the LPP using an affective oddball paradigm that included faces of animals as the oddball target along with angry, happy, and neutral faces. First, we hypothesized that all children would exhibit larger LPPs in response to animal pictures, followed by affective faces and lastly, neutral faces. Second, we hypothesized that IPV-exposed children, relative to non-exposed children, would exhibit larger LPPs to angry relative to neutral faces compared to happy relative to neutral faces. However, we advanced this hypothesis tentatively given that blunted LPPs to threatening, relative to non-threatening, stimuli have been observed in some samples of maltreated children. As the LPP and FAA have been virtually unexamined in young children with IPV exposure, the current study represents a new line of research in this early developmental period.
Methods
Participants
The current paper is based on data from the Adaptation and Resilience in Childhood Study (ARCS), an ongoing longitudinal study examining exposure to violence, multi-level assessments of threat processing, and psychopathology in young children ages 4–6 years. Participants were recruited from domestic violence shelters and the general community. Children and their mothers were considered eligible if they were English-speaking. This study was approved by the institutional review boards of the [Withheld for review] and all mothers completed informed consent.
The data for the current study represent were obtained prior to our temporary lab closure due to the Covid-19 pandemic. In total, 71 mother-child pairs were able to attend an in-person lab visit of which 27% had reported IPV and/or received some domestic violence related services. Of the initial 71 participants, 2 children and/or their mothers did not consent to EEG and 6 children were unable to tolerate wearing the cap long enough to collect data, leaving a pool of 63 children. Three participants’ data were lost due to technical issues. Additionally, we required artifact free data for at least 6 of the trials in each of the four Affective Oddball conditions. Twelve children had less than 6 valid trials and one child was removed after inspecting data for outliers (e.g., an LPP magnitude more than 1 SD away from the next closest participants value). For the resting EEG, participants were required to have at least 20 segments of artifact free data (N = 44) and must have kept their eyes open for the majority of the resting period as determined by a trained research assistant (5 closed their eyes for prolonged periods). These requirements yielded a final sample of N = 47 and N = 39 for the Affective Oddball and resting FAA analyses, respectively. To summarize, of the original 71 children, 35 had both affective oddball and resting, 12 had only oddball data, 4 had only resting data, and 20 had neither (71.8% of the initial sample had some useable physiological data). There were no significant demographic differences comparing those in the final analytic sample to those excluded from analyses with regard to sex, race/ethnicity, age, or IPV-exposure (ps from .30 to .71).
In the final analytic sample (N = 51), children had a mean age of 5.51 years (SD = 0.82). The sample was 55% female (female N = 28). The majority of the mothers in the sample identified their children as a racial or ethnic minority. Specifically, 29 subjects identified as Latinx (3 preferred not to disclose). Additionally, 15 identified as Black/African American, 15 as White/Caucasian, 1 Native Hawaiian/Pacific Islander, 4 as multi-racial, 1 as Asian, 14 identified by their Latinx ethnicity and did not identify race, and 1 did not report either race or ethnicity.
Measures
Family Stress Interview-R
(FSI-R 2.0; Briggs-Gowan et al., 2019). The FSI-R 2.0 is a comprehensive, semi-structured interview that includes detailed assessment of family violence. This measure was adapted from an earlier studies (Dodge, Pettit, & Bates, 1994; O’Dor et al., 2017). The interview begins with mothers first describing their household composition (e.g., current and past partners, additional adults or children in the home). The mother is asked about the types of conflict between current and former partners during the child’s lifetime, which are assessed across two time periods, the past 12-months and from the time the child was born until 12 months ago. The interviewer is trained to use probes to get at specific examples and to determine the mother’s and her partner’s usual and most severe conflict. Physical IPV is rated on a 5 point ordinal scale: 0 = None, 1 = Mild, e.g., slamming doors, stamping feet without physical contact, 2 = Moderate, e.g., pushing, single hit, threatening posture, 3 = Severe, e.g., hit in face, hit with object, likely causing injury, 4 = Severe with harm, egregious acts of violence, injuries requiring hospitalization, sexual violence. Prior research has supported the validity of IPV assessed with the FSI coding in relation to widely-used, validated self-report measures of partner violence and trauma-related psychopathology (Briggs-Gowan et al., 2019). For the purposes of this study, IPV exposure was coded dichotomously to compare children without exposure (code = 0) to those with exposure (code ≥1). Inter-rater reliability, assessed by independent coding of 35% (N= 25) of interviews, was excellent (Kappa = 0.90). Of the 21 children with IPV exposure, 8 were mild, 2 were moderate, 6 were severe and 5 were severe with harm. With regard to when the IPV occurred, 10 mothers reported the IPV as occurring more than 12 months ago (but within their child’s lifetime), 2 reported the IPV as occurring in the past 12 months, and 9 reported that IPV occurred during both periods of time.
Affective Oddball Task: “The Zoo Game”
The LPP was elicited by an affective oddball task coded in E-Prime (Figure 1). Children were first told a story about how animals had escaped from the zoo and that it would be their job to identify whether the pictures they would see were the faces of animals or people. This introduction was given over the course of several slides that were designed to resemble a children’s story book. Children were shown two response buttons and instructed that one button should be pressed for people faces and the other for animal faces. The child was given an opportunity to practice the game and was given feedback on their performance. However, the task story and button presses were primarily included to increase task engagement for the children and to increase the salience of the animal faces as the intended oddball target. The task included four stimulus types: angry, happy, neutral, and animal faces. There were 32 trials of each stimulus type. The faces were taken from the NIMSTIM (Tottenham et al., 2009) database and balanced for sex and each affective condition had the same proportion of Black, White, and Latinx faces. For a given trial, children first saw a fixation cross for 1,000 ms. This was followed by presentation of the face image for 1,000 ms, which was then followed by a blank screen for 3,000 ms.
Figure 1.
Affective Oddball Task
Note that the proportion of the slide that is occupied by the face is smaller for the actual task, but was increased in size here to make the example easier to view.
EEG Recording
Continuous EEG data were collected from 32 channels based on the 10/20 system with Biosemi (ActiveTwo). The Biosemi ActiveTwo system uses Ag/AgCl electrodes that have minimal impedance output and electrode offsets where monitored throughout data collection (kept between +/−50 μV). Children wore a Lycra cap to which electrodes were attached. Electrodes were also placed on the right and left mastoids, to be used as references during offline processing. Four electrodes were placed to capture eye blinks, with an electrode placed near the corner of each eye and on the right and left upper cheek. Additional physiological measures were collected, but are not reported in the current study. EEG data were sampled at a rate of 512 Hz and data were acquired without an online filter. Resting EEG was recorded before the Affective Oddball task.
Data were further processed offline using EMSE (Cortech INC). The resting EEG and ERP data were cleaned in several steps. A polynomial detrend was used to remove signal drift. An upper and lower bandpass filter was applied with 0.1 and 30 Hz cutoffs. Data were referenced to linked mastoids. Eye blinks and artifact free data were identified and labeled as such through visual inspection. Then, eye blinks and artifact free data were used as inputs for a spatial filtering algorithm to automatically detect and remove the effects of blinks on EEG data (Pflieger, 2001).
For the Affective Oddball tasks, data were segmented into 1200 ms windows, with 200 ms prior to stimulus (emotional human or animal face picture) onset and 1000ms after stimulus onset. Data were baseline corrected using the 200 ms prior to stimulus onset. EEG signal changes of +/− 140 μV within a trial segment were rejected. The average number of useable trials per participant was 24.43 (SD = 6.77) for Angry, 23.17 (SD = 7.33) for Happy, 22.83 (SD = 7.40) for Neutral, and 22.13 (SD = 7.07) for Animal. The LPP was formed by taking the mean amplitude from 400 ms – 1000 ms after stimulus onset. The LPP was based on pooled amplitudes from PO4, PO3, Pz, O1, O2, and Oz in order to reduce noise from a single electrode source. It is common practice to examine ERPs by forming differences waves between two of the within-subjects experimental conditions using either subtraction (e.g., LPP to Angry-Neutral) or to use a residual based difference score. Specifically, the later approach is conducted by running a regression in which the LPP in one condition (Angry) is predicted by second condition (Neutral) and the unstandardized residual is saved for subsequent analysis. Given recommendations to use the residual based method (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017), we created three residual scores by having the LPP from the Neutral condition predict Angry, Happy, and Animal LPPs. Because neutral is used as the reference condition, residual scores reflect a contrast between conditions with higher significance (e.g., oddball animal trials or affective faces) and lower significance (neutral faces). Using a traditional difference score (e.g., animal minus neutral) produced a pattern of results that was virtually identical.
For the resting FA data, the first 18 participants were recorded at rest for 60 seconds. In order to increase the number of accepted segments, the resting data for the remaining 21 participants were recorded for 120 seconds. Children recorded for 60 seconds or 120 seconds did not significantly differ in their FAA scores or on any important demographic variables including, sex, ethnicity, race, age, or IPV exposure. Children were instructed to rest comfortably with eyes open. For FA, data were epoched (segmented) into 2 second windows. EEG signal changes of +/− 140 μV within a trial segment were rejected. Segments were set extracted with a 50% overlapping Hanning window. Fast Fourier transformation was used to compute power spectra. Alpha power was defined by 6–10 Hz band as recommended for children (Marshall, Bar-Haim, & Fox, 2002). Those included in the final analysis were required to have at least 20 epochs of clean data, the average number of accepted epochs was 66.36 per participant. Alpha data was extracted from frontal electrode sites at F3 and F4. Alpha is believed to index the inverse of activity, such that a higher alpha value indicates less activation, this is based on findings that alpha is lower when there is greater cortical activity (Coan & Allen, 2004). The F3 and F4 electrode pair was selected because this pairing may be most sensitive for relating FAA to individual differences in young children (e.g., Goldstein et al., 2016). Following common practices, the data were naturally log transformed and asymmetry scores were formed by taking the difference between homologous electrode pairs such that asymmetry at F4 and F3 = Ln(F4)-Ln(F3). When difference scores are calculated in this way, a positive score indicates greater relative left brain activation, scores close to zero indicate relatively balanced left and right activity, and negative scores indicate lower relative left activity (Coan & Allen, 2004).
Data Analytic Plan
We first report the means and standard deviations (SD) for the LPP generated by each condition of the ERP task, the residual LPP scores with animal, happy, and angry regressed on neutral, and mean alpha power at F3 and F4 for those with vs. those without exposure to IPV. To examine the effect of violence exposure on frontal asymmetry, we conducted a t-test where the F4-F3 asymmetry difference score was the dependent variable and children’s violence exposure was the independent variable. To analyze the Affective Oddball LPP data we conducted a mixed ANOVA with a three-level within subject variable for each of the three face types (Angry, Happy, and Animal) and the binary categorical between subject variable indicating children with vs. without IPV exposure. The dependent variable in this model was the LPP unstandardized residual score for the Angry, Happy, and Animal faces after having used Neutral faces as the predictor in the regression model. Follow-up post hoc t-tests were planned only after first confirming the significance of main effects of condition, violence exposure, or their interaction.
Results
Violence exposure characteristics
Twenty-one mothers (41%) reported physical IPV and 30 did not (59%) as indicated by the FSI-R. The average IPV exposure was of moderate severity (M = 2.38), with 52% (n = 11) reporting severe IPV involving being hit in the face, hit with an object, sexual violence, or violence resulting in medical treatment. The children with IPV exposure did not differ significantly from those without IPV exposure with regard to any demographic variables, p > .05; thus, these variables were not included as covariates in the models.
Violence exposure and FAA difference score
Table 1 presents the means and SDs for the alpha at F4 and F3. To examine FAA, we conducted a t-test comparing children with vs. without IPV exposure as the independent variable and Ln(F4) – Ln(F3) FAA difference score as the dependent variable. We found a significant difference, t = 2.94, p = .006, such that children with IPV exposure exhibited significantly lower relative left than right FAA (M = −0.07) compared to children without exposure, who had a pattern of greater relative left than right FAA (M = 0.05).
Table 1.
Descriptive data for children with vs. without domestic violence exposure
| Non-exposed N =30 | Violence exposed N =21 | |
|---|---|---|
| Child Sex (%, Female) | 53.3% | 57.1% |
| Child Age (Mean and SD) | 5.63 (0.76) | 5.35 (0.89) |
| Child Race (N) | ||
| Black | 7 | 8 |
| White | 9 | 6 |
| Multiracial | 2 | 2 |
| Other/Prefer not to say1 | 12 | 5 |
| Child Latinx (N) | 19 | 10 |
| Annual Family Income (N) | ||
| $0–25,000 | 15 | 7 |
| $25,000–50,000 | 7 | 6 |
| $50,000–80,000 | 4 | 4 |
| $80,000+ | 3 | 4 |
| Maternal Education (N) | ||
| Less than HS | 6 | 0 |
| High School Graduate | 4 | 3 |
| Trade School/Associate degree | 3 | 6 |
| Some college | 8 | 8 |
| Bachelors Degree | 3 | 3 |
| Graduate Degree | 6 | 1 |
|
| ||
| Mean (SD) | Mean (SD) | |
|
| ||
| FSI Physical IPV | 0.00 (0.00) | 2.38 (1.24) |
|
| ||
| N = 29 | N = 18 | |
| Angry LPP | 7.59 (7.40) | 6.21 (6.05) |
| Happy LPP | 8.59 (6.60) | 6.79 (6.89) |
| Neutral LPP | 6.30 (6.83) | 8.61 (7.69) |
| Animal LPP | 12.21 (8.45) | 9.18 (8.64) |
| Angryres neutral | 0.96 (5.87) | −1.54 (5.88) |
| Happyres neutral | 1.12 (5.20) | −1.80 (6.19) |
| Animalres neutral | 1.79 (6.75) | −2.88 (6.28) |
|
| ||
| N = 23 | N = 16 | |
| Left Alpha Power Ln(F3) | 2.33 (0.29) | 2.77 (0.57) |
| Right Alpha Power Ln(F4) | 2.39 (0.34) | 2.70 (0.57) |
| FAA | 0.06 (0.13) | −0.07 (0.13) |
Note. FSI IPV = Family Stress Interview Intimate Partner Violence; LPP = Late Positive Potential; FAA = Frontal Alpha Asymmetry. The residual scores were formed by regressing Animal, Happy, and Angry LPPs on the Neutral LPP.
Many participants identified by their Latinx identity, but did not provide a racial identity.
Violence exposure and LPP during the Affective Oddball
Figure 2 shows the ERP waveforms for each condition and separately by violence exposure. Figure 3 presents the corresponding scalp distributions. Table 1 includes the means and SD of the LPPs generated by each condition in the Affective Oddball Task for IPV-exposed and non-exposed children. Table 1 also includes the LPP residual scores with neutral serving as the reference condition for angry, happy and animal faces.
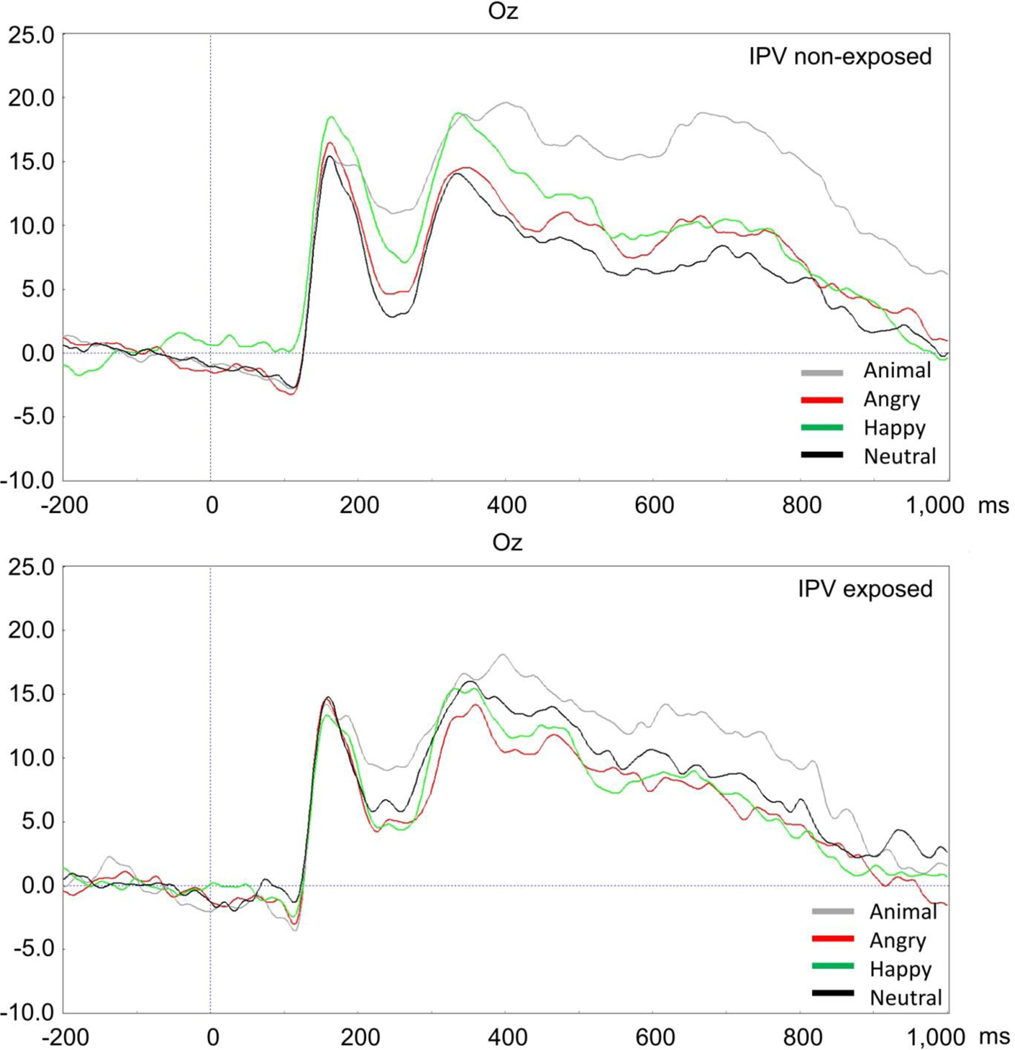
Figure 2.
Affective Oddball ERP waveforms for children with vs. without IPV exposure
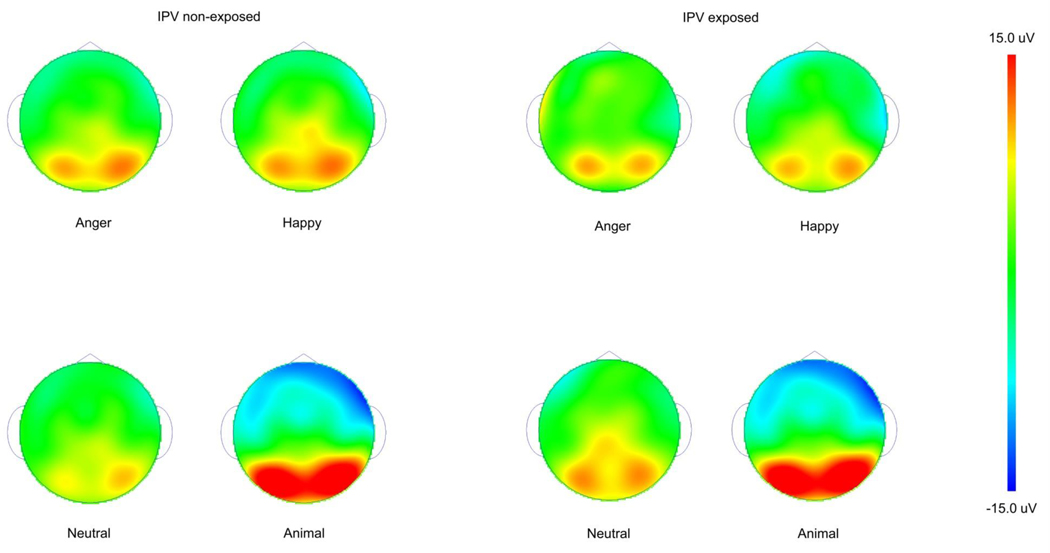
Figure 3.
Affective Oddball ERP scalp distribution for children with vs. without IPV exposure
The depicted scalp distributions are for 400–1000ms after picture onset. All scalp distributions are on the same scale ranging from +/−15 micro volts.
To examine the LPP during the affective oddball paradigm, we conducted a mixed repeated-measures ANOVA in which there was a within subject variable with three levels representing angry, happy, and animal residual scores on neutral and a between subject dichotomous variable for those with vs. without IPV exposure. The main within subject effect of condition was not significant (F = 0.31, p = .97), suggesting that after accounting for the effect of the neutral faces, there were no significant differences in the LPP magnitudes for angry, happy, and animal faces for the sample as a whole. Additionally, the interaction between IPV exposure group and condition was not significant (F = 0.57, p = .57). However, the main effect of IPV exposure group was significant, F = 6.53, p = .01. Specifically, those without IPV exposure showed significantly larger LPPs to angry, happy, and animal faces relative to neutral (M = 0.96 angry, 1.12 happy, and 1.79 animal), whereas those with IPV exposure had smaller residual LPPs (M = −1.54 angry, −1.80 happy, and −2.88 animal).
Discussion
Our preliminary results from this sample of young children from domestic violence shelters and community settings reveal notable neurophysiological distinctions in both the FAA and LPP in IPV exposed compared to non-exposed children. The patterns of FAA observed in IPV exposed children were consistent with patterns previously associated with temperamental withdrawal, negative emotionality, and mood and anxiety psychopathology(e.g., Calkins et al., 1996; Goldstein et al., 2018, 2016; Meyer et al., 2015). Additionally, the IPV exposed children exhibited a comparatively blunted LPP response to affective and task-relevant stimuli. Together, these findings suggest specific neurophysiological patterns that may be associated with early life risk in young children who have experienced IPV. Further research is needed to replicate these patterns in a larger sample and explore directional effects between neurophysiological patterns and psychosocial impairment over early development.
The FAA analysis revealed differences in left vs. right hemisphere activation in children with vs. without IPV exposure. As hypothesized, children with IPV exposure exhibited lower relative left than right FAA compared to those without IPV exposure, who exhibited greater relative left than right FAA. This pattern aligns with previous results for early life adversity. For example, the same pattern of FAA observed in our IPV group has been reported in children from neglectful early rearing environments (e.g., eastern European orphanages; McLaughlin et al., 2011). Our results may fit with these findings as the caregiving capacity or monitoring of children may be limited in homes where IPV occurs. Additionally, lower relative left than right FAA has been observed across a range of maltreatment types, including physical abuse (Curtis & Cicchetti, 2007). The convergence of our findings with those from other studies reflects McLaughlin’s (2016) proposal that adverse events sharing phenomenological features (e.g., threat conveyed by witnessing IPV and direct forms of child abuse) impose similar perturbations on neurobiological stress systems.
The significance of the FAA patterns observed in IPV exposed children in our sample is further underscored by associations between lower relative left than right FAA (as observed in our IPV-exposed children) and withdrawal-related behavior (Calkins et al., 1996), lower trait positive emotionality, greater negative emotionality (Goldstein et al., 2018), and risk for developing depression (Goldstein et al., 2016), and to some extent risk for developing PTSD (Meyer et al., 2015). Overall, our findings are consistent with previous studies examining maltreated and emotionally vulnerable children (Curtis & Cicchetti, 2007; McLaughlin et al., 2011). Replication in other samples of young children will be important.
For the LPP results, we found evidence of differences in the LPPs of children with vs. without IPV exposure. When interpreting these residual LPPs, it can be helpful to think of them as similar to a difference score. In our case, this means that the magnitude of the LPP to angry faces should be understood as reflecting emotion specific related activity as activity for neutral faces is being “subtracted” out. The non-exposed children exhibited the expected pattern of LPPs in that they exhibited larger LPPs to emotional faces and oddball targets (animals) relative to neutral faces. This pattern of LPP responses in the non-exposed children is consistent with the vast majority of LPP findings that associate larger responses with more salient/emotional content relative to neutral, non-affective stimuli (Dennis & Hajcak, 2009; Hajcak & Foti, 2020). This suggests that our affective oddball task is functioning as expected in eliciting the LPP in young children without IPV exposure.
In contrast, we found that children with IPV exposure exhibited an LPP pattern suggesting less neurophysiological differentiation between salient and non-salient stimuli regardless of whether those stimuli were of affective (angry or happy) or contextual significance (animal oddball targets). These results add to a small and already mixed body of research suggesting differences in P3 or LPP in youth exposed to maltreatment or IPV. Specifically, some studies have found evidence of relatively enhanced Pb3 to angry faces in maltreated children ( Pollak et al., 2001) and enhanced P3 to images of angry adult dyads in IPV exposed children (Schermerhorn et al., 2015). While our patterns generally align with evidence of blunted LPP in IPV exposed children, they differ in that we observed a global blunting of the LPP to angry, happy, and motivationally significant animal faces, whereas others seemed to suggest more specific group differences related to angry faces in particular (Cicchetti & Curtis, 2005; Pollak et al., 2001). One explanation for the apparent blunted responding to all salient stimuli (angry, happy, animal) in the affective oddball task is that there is heterogeneity within our IPV exposed group. For example, there may be subgroups of children who manifest different patterns of emotional responding (e.g., some relatively enhanced for angry but others relatively blunted), perhaps influenced by unexamined individual differences or environmental factors. This may also help to explain inconsistent findings in previous studies of children and adults. For instance, some studies of adults associate larger LPPs with greater PTSD symptom severity (Lobo et al., 2014). Others suggest that multiple/chronic interpersonal trauma exposure may be linked to hypo-responsivity, which is noteworthy given that IPV is inherently of an interpersonal nature (McTeague et al., 2010). Alternatively, there is evidence that maltreated children often have difficulty differentiating threatening cues and types of facial expressions (McLaughlin et al., 2016; Pollak & Kistler, 2002) and this is compounded by the fact that the neutral faces have a different functional meaning for children (Herba & Phillips, 2004). Thus, it is possible that neutral faces were also deemed to be emotionally salient by IPV exposed children, leading to an LPP to neutral faces that compares in magnitude to the other conditions - whereas non-exposed children may have more appropriately processed neutral faces as less emotionally salient. As this is the first paper to examine the LPP in young children exposed to physical IPV, additional research is needed to replicate these findings.
This study has several strengths including the use of two electrophysiological measures of emotion, recruitment of high-risk families, and comprehensive interview-based assessment ratings of IPV. It also makes a contribution by establishing that our novel child-friendly affective oddball paradigm is effective for eliciting LPPs and that the LPP is modulated by salient stimuli, as expected for non-exposed children. However, this study also has limitations. First, disruptions caused by the COVID-19 pandemic resulted in a small sample, which limits our ability to explore more complex analytic models that could have provided more insight into the neurophysiological functioning of IPV exposed children. Rather than dismiss these data, we consider them to serve as a foundation for future research. Future studies will need to examine the role the pandemic may have on these processes given it’s likely to impact not only experiences of IPV within families (Boserup, McKenney, & Elkbuli, 2020) but also children’s socio-emotional development (Cardenas, Bustos, & Chakraborty, 2020). Second, we were underpowered to examine children’s psychopathology or child abuse history, which may also influence results. Finally, several participants could not be included as a result of poor quality EEG/ERP data; although this is often a problem in electrophysiological studies of young children.
Overall, this study makes a valuable contribution to the literature by providing preliminary data on two electrophysiological measures of emotion in children with and without IPV. Although IPV exposure is prevalent in families with young children (Hamby et al., 2011; McTavish et al., 2016), it is often overshadowed by child abuse and other forms of child maltreatment, which receive the lion’s share of research attention. However, our results clearly indicated pervasive differences in the LPP and FAA for children with IPV exposure. Moreover, our results point toward several promising future avenues of research to further elucidate how IPV exposure in young children may influence the physiology of emotions and the emergence of psychopathology beginning early in development.
Supplementary Material
Acknowledgements:
This study was supported by ARCS-U01MHMH113390 (MPI Grasso & Briggs-Gowan). We wish to thank our scientific collaborators, Lauren Wakschlag, PhD, Daniel Pine, MD, and Lisa McTeague, PhD, for their valuable input and support of the larger NIMH-funded study from which this manuscript draws. We also extend our sincere appreciation to recruitment specialist, Ms. Mayra Pino, lab coordinator, Ms. Miranda Marris, administrators, and the many students and interns who have devoted their time and energy to the success of this study. Lastly, we are indebted to the many mothers and “kid scientists” who shared their time and energy to help us learn about children’s development.
Footnotes
Data Availability Statement: Data from this project are being made available to researchers through the NIMH Data Archive (NDA). Data for participants who consented to release their data to the NDA will be posted at the following location: DOI:10.15154/1521332.
Conflicts of Interest Statement: The authors report no conflicts of interest.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Pub. [Google Scholar]
- Bogat GA, DeJonghe E, Levendosky AA, Davidson WS, & Von Eye A. (2006). Trauma symptoms among infants exposed to intimate partner violence. Child Abuse & Neglect, 30(2), 109–125. [DOI] [PubMed] [Google Scholar]
- Boserup B, McKenney M. & Elkbuli A. (2020). Alarming trends in US domestic violence during the COVID-19 pandemic. American Journal of Emergency Medicine, 38(12), 2753–2755. 10.1016/j.ajem.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Goldstein BL, Forbes D, O’Dor S, Grasso DJ, Wakschlag LS, Bates J. (2019). The Family Socialization Interview-Revised Version 2.0: A method for assessing harsh parenting, conflict and stress within families. Farmington, CT: University of Connecticut School of Medicine. [Google Scholar]
- Calkins SD, Fox NA, & Marshall TR (1996). Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development, 67(2), 523–540. 10.1111/j.1467-8624.1996.tb01749.x [DOI] [PubMed] [Google Scholar]
- Cardenas MC, Bustos SS & Chakraborty R. (2020). A ‘parallel pandemic’: The psychosocial burden of COVID‐19 in children and adolescents. Acta Paediatrica, 109(11), 2187–2188. 10.1111/apa.15536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Curtis WJ (2005). An event-related potential study of the processing of affective facial expressions in young children who experienced maltreatment during the first year of life. Development and Psychopathology, 17(03), 641–677. 10.1017/S0954579405050315 [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106–114. 10.1111/1469-8986.00011 [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1), 7–50. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Curtis WJ, & Cicchetti D. (2007). Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Development and Psychopathology, 19(3), 811–840. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, & Cicchetti D. (2011). Affective facial expression processing in young children who have experienced maltreatment during the first year of life: An event-related potential study. Development and Psychopathology, 23(2), 373–395. 10.1017/S0954579411000125 [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H. (2012). Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Davies PT, Thompson MJ, Hentges RF, Coe JL, & Sturge-Apple ML (2020). Children’s Attentional Biases to Emotions as Sources of Variability in Their Vulnerability to Interparental Conflict. Developmental Psychology, 56(7), 1343–1359. 10.1037/dev0000994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA & Hajcak G. (2009). The late positive potential: A neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 10.1111/j.1469-7610.2009.02168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digangi JA, Burkhouse KL, Aase DM, Babione JM, Schroth C, Kennedy AE, … Phan KL (2017). An electrocortical investigation of emotional face processing in military-related posttraumatic stress disorder. Journal of Psychiatric Research, 92, 132–138. 10.1016/j.jpsychires.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, & Bates JE (1994). Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Development, 65(2), 649–665. [PubMed] [Google Scholar]
- Edleson JL (1999). Children’s witnessing of adult domestic violence. Journal of Interpersonal Violence, 14(8), 839–870. 10.1177/088626099014008004 [DOI] [Google Scholar]
- El-Sheikh M. (2005). The role of emotional responses and physiological reactivity in the marital conflict-child functioning link. Journal of Child Psychology and Psychiatry and Allied Disciplines, 46(11), 1191–1199. 10.1111/j.1469-7610.2005.00418.x [DOI] [PubMed] [Google Scholar]
- Goldstein BL, & Klein DN (2014). A review of selected candidate endophenotypes for depression. Clinical Psychology Review, 34, 417–427. 10.1016/j.cpr.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Shankman SA, Kujawa A, Torpey-Newman DC, Dyson MW, Olino TM & Klein DN (2018). Positive and Negative Emotionality at Age 3 Predicts Change in Frontal EEG Asymmetry across Early Childhood. Journal of Abnormal Child Psychology, 1–11. 10.1007/s10802-018-0433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Shankman SA, Kujawa A, Torpey-Newman DC, Olino TM, & Klein DN (2016). Developmental changes in electroencephalographic frontal asymmetry in young children at risk for depression. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(9), 1075–1082. 10.1111/jcpp.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Fisher PA, & Pfeifer JH (2013). What Sleeping Babies Hear: A Functional MRI Study of Interparental Conflict and Infants’ Emotion Processing. Psychological Science, 24(5), 782–789. 10.1177/0956797612458803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, & Simons RF (2012). Electrophysiological responses to threat in youth with and without Posttraumatic Stress Disorder. Biological Psychology, 90(1), 88–96. 10.1016/j.biopsycho.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, & Foti D. (2020). Significance?... Sgnificance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), 1–15. 10.1111/psyp.13570 [DOI] [PubMed] [Google Scholar]
- Hamby S, Finkelhor D, Turner H, & Ormrod R. (2011). Children’s exposure to iIntimate partner violence and other family violence. Juvenile Justice Bulletin, 12. 10.1037/e725322011-001 [DOI] [Google Scholar]
- Harmon-Jones E, Gable PA, & Peterson CK (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology, 84(3), 451–462. 10.1016/j.biopsycho.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Herba CM, & Phillips M. (2004). Annotation: Development of facial expression recognition from childhood to adolescence: Behavioural and neurological perspectives. Journal of Child Psychology and Psychiatry and Allied Disciplines, 45(7), 1185–1198. 10.1111/j.1469-7610.2004.00316.x [DOI] [PubMed] [Google Scholar]
- Holt S, Buckley H, & Whelan S. (2008). The impact of exposure to domestic violence on children and young people: A review of the literature. Child Abuse & Neglect, 32, 797–810. 10.1016/j.chiabu.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Howell KH (2011). Resilience and psychopathology in children exposed to family violence. Aggression and Violent Behavior, 16(6), 562–569. 10.1016/j.avb.2011.09.001 [DOI] [Google Scholar]
- Karl A, Malta LS, & Maercker A. (2006). Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biological Psychology, 71(2), 123–147. 10.1016/j.biopsycho.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Klein DN, Kotov R. & Bufferd SJ (2011). Personality and depression: Explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7(1), 269–295. 10.1146/annurev-clinpsy-032210-104540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann KM, Gaylord NK, Holt AR, & Kenny ED (2003). Child witnesses to domestic violence: a meta-analytic review. Journal of Consulting and Clinical Psychology, 71(2), 339–352. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Hajcak G. (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2(4), 458–467. 10.1016/j.dcn.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Tang A, Tanaka M, Van Lieshout RJ, MacMillan HL, & Schmidt LA (2018). Longitudinal Associations Among Child Maltreatment, Resting Frontal Electroencephalogram Asymmetry, and Adolescent Shyness. Child Development, 89(3), 746–757. 10.1111/cdev.13060 [DOI] [PubMed] [Google Scholar]
- Lobo I, David IA, Figueira I, Campagnoli RR, Volchan E, Pereira MG, & Oliveira De L. (2014). Brain reactivity to unpleasant stimuli is associated with severity of posttraumatic stress symptoms . Biological Psychology, 103, 233–241. 10.1016/j.biopsycho.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Macatee RJ, Burkhouse KL, Afshar K, Schroth C, Aase DM, Greenstein JE, … Phan KL (2020). Nonlinear relations between post-traumatic stress symptoms and electrocortical reactivity during emotional face processing in combat-exposed veterans. Psychophysiology, 57(1), 1–11. 10.1111/psyp.13423 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, & Phan KL (2013). Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biological Psychology, 94(2), 441–449. 10.1016/j.biopsycho.2013.08.009 [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E. (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21(23), R947–R948. 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future Directions in Childhood Adversity and Youth Psychopathology. Journal of Clinical Child and Adolescent Psychology, 45(3), 361–382. 10.1080/15374416.2015.1110823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, & Nelson CA (2011). Adverse rearing environments and neural development in children: the development of frontal electroencephalogram asymmetry. Biological Psychiatry, 70(11), 1008–15. 10.1016/j.biopsych.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, … Pine DS (2016). Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology, 41(8), 1956–1964. 10.1038/npp.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish JR, MacGregor JCD, Wathen CN, & MacMillan HL (2016). Children’s exposure to intimate partner violence: an overview. International Review of Psychiatry, 28(5), 504–518. 10.1080/09540261.2016.1205001 [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, & Bradley MM (2010). Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry, 67(4), 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G. (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Quaedflieg CWEM, Smulders FTY, Meijer EH, & Merckelbach HLGJ (2015). The role of frontal EEG asymmetry in post-traumatic stress disorder. Biological Psychology. 10.1016/j.biopsycho.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Miller MW (2003). Personality and the etiology and expression of PTSD: A three-factor model perspective. Clinical Psychology: Science and Practice, 10(4), 373–393. 10.1093/clipsy/bpg040 [DOI] [Google Scholar]
- Miskovic V, Schmidt LA, Georgiades K, Boyle M, & MacMillan HL (2009). Stability of resting frontal electroencephalogram (EEG) asymmetry and cardiac vagal tone in adolescent females exposed to child maltreatment. Developmental Psychobiology, 51(6), 474–487. 10.1002/dev.20387 [DOI] [PubMed] [Google Scholar]
- O’Dor SL, Grasso DJ, Forbes D, Bates JE, McCarthy KJ, Wakschlag LS, & Briggs-Gowan MJ (2017). The Family Socialization Interview—Revised (FSI-R): a comprehensive assessment of parental disciplinary behaviors. Prevention Science, 18(3), 292–304. 10.1007/s11121-016-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J. (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD (2003). Experience-Dependent Affective Learning and Risk for Psychopathology in Children. In Annals of the New York Academy of Sciences (Vol. 1008, pp. 102–111). 10.1196/annals.1301.011 [DOI] [PubMed] [Google Scholar]
- Pollak SD (2008). Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science, 17(6), 370–375. 10.1111/j.1467-8721.2008.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, & Kistler DJ (2002). Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences of the United States of America, 99(13), 9072–9076. 10.1073/pnas.142165999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, & Cicchetti D. (2001). P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology, 38(2), 267–274. 10.1017/S0048577201990808 [DOI] [PubMed] [Google Scholar]
- Schermerhorn AC, Bates JE, Puce A, & Molfese DL (2015). Neurophysiological correlates of children’s processing of interparental conflict cues. Journal of Family Psychology, 29(4), 518–527. 10.1037/fam0000088 [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S. (2006). Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology, 115(4), 715–729. 10.1037/0021-843X.115.4.715 [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE & Doss RC (1992). Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology, 62(4), 676–687. 10.1037/0022-3514.62.4.676 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu NL, Jouriles EN, McDonald R, & Rosenfield D. (2016). Children’s exposure to intimate partner violence: A meta-analysis of longitudinal associations with child adjustment problems. Clinical Psychology Review, 46, 25–33. 10.1016/j.cpr.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Wolfe DA, Crooks CV, Lee V, McIntyre-Smith A, & Jaffe PG (2003). The effects of children’s exposure to domestic violence: A meta-analysis and critique. Clinical Child and Family Psychology Review. 10.1023/A:1024910416164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.