Abstract

The synthesis and pharmacological activities of a new series of piperazinyl quinazolin-4-(3H)-one derivatives acting toward the α2δ-1 subunit of voltage-gated calcium channels (Cavα2δ-1) are reported. Different positions of a micromolar HTS hit were explored, and best activities were obtained for compounds containing a small alkyl group in position 3 of the quinazolin-4-(3H)-one scaffold and a 3-methyl-piperazin-1-yl- or 3,5-dimethyl-piperazin-1-yl-butyl group in position 2. The activity was shown to reside in the R enantiomer of the chain in position 2, and several eutomers reached single digit nanomolar affinities. Final modification of the central scaffold to reduce lipophilicity provided the pyrido[4,3-d]pyrimidin-4(3H)-one 16RR, which showed high selectivity for Cavα2δ-1 versus Cavα2δ-2, probably linked to its improved analgesic efficacy-safety ratio in mice over pregabalin.
Keywords: Cavα2δ-1 subunit, voltage-gated calcium channels, selective, pain, neuropathy
Gabapentinoids, represented by pregabalin (1, Figure 1), are close analogues of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) and are postulated to exert their action by blocking the α2δ subunit of voltage-gated calcium channels (VGCC).1 This subunit is one of the four constituents of VGCC, namely, α1 (Cavα1), β (Cavβ), α2δ (Cavα2δ), and γ (Cavγ), and although it exerts an auxiliary function, it is key for increasing the expression of the central pore forming α1 subunits in the plasma membrane and modulating their function. This provides a reduction in the calcium-dependent release of multiple neurotransmitters and calcium upregulation in the spinal dorsal horn and dorsal root ganglia (DRG). As a result, gabapentinoids are useful drugs in the treatment of several neuropathies, such as neuropathic pain2 and fibromyalgia3 and other nonpain indications, such as epilepsy4 or restless leg syndrome.5
Figure 1.
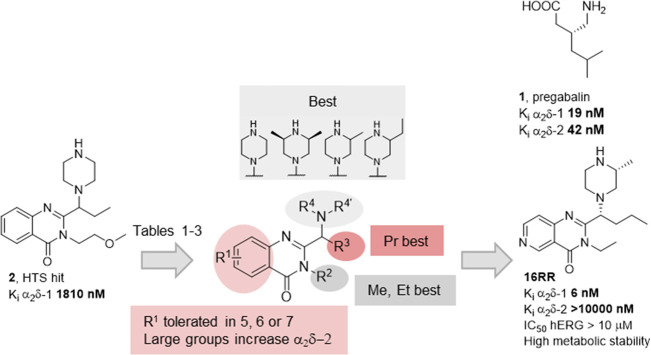
Structures of pregabalin (1) and HTS hit 2 and summary of SAR study leading to 16RR.
The overall function of the α2δ subunit is still a matter of intense research.6 The mechanism of calcium current increase is unknown, and it can only be partially explained by an increase in the trafficking of the channels to augment their amount on the cell surface. In addition, there is a pool of α2δ not associated with calcium channels but to other proteins that may account for some of its actions. These proteins include trombospondins, low-density lipoprotein receptor-related protein 1, large conductance potassium channels, and N-methyl-d-aspartate (NMDA) receptors.6,7 In addition, emerging roles of the α2δ subunit in synaptic connectivity8 or as key organizers of glutamatergic synapses9 are being identified and they will probably open the way to new therapeutic applications of Cavα2δ ligands.
Four Cavα2δ (Cavα2δ1–4) subunits of VGCC have been described with a differentiated distribution: while the Cavα2δ-1 subunit is quite ubiquitous, the Cavα2δ-2 and Cavα2δ-3 subunits are mainly concentrated in the brain and the Cavα2δ-4 subunit is largely non-neuronal. Pregabalin,7 gabapentin,10 and mirogabalin,11 the three marketed gabapentinoids, bind to both the Cavα2δ-1 and the Cavα2δ-2 subunits (but not to the Cavα2δ-3 and Cavα2δ-4 subunits).
The Cavα2δ-1 subunit is present in many neuronal cell types,12 including DRG neurons, where it is upregulated in neuropathic pain conditions13,14 and modulates spinal hyperexcitability.15 Point mutations on the binding site on Cavα2δ-1 in a transgenic mouse line diminished binding and antihyperalgesic activity of gabapentinoids,15,16 and recent findings suggest that injury-induced maladaptation of the Cavα2δ-1 subunit is responsible for the chronic development of neuropathic pain.17 In addition, the Cavα2δ-1 subunit is the one forming heterodimeric complexes with NMDA receptors to potentiate their synaptic trafficking and excitatory synaptic transmission. Interrupting this interaction reverses the synaptic activity associated with neuropathic pain.18
On the other hand, the Cavα2δ2 expression is not upregulated in neuropathic pain models13,14 and is restricted to some areas, such as the cerebellum, a key player in coordinating voluntary movements.12 In fact, the deletion of the Cavα2δ2 subunit causes cerebellar ataxia and absence seizures in mice and humans,19,20 via a mechanism that probably does not involve calcium channels.21
The previous findings, among others, led to the hypothesis that the Cavα2δ-1 contributes to the analgesic effects of pregabalin and gabapentin, whereas the Cavα2δ-2 contributes to their central nervous system (CNS) side effects (such as sedation, dizziness, ataxia and fatigue). In the case of mirogabalin, its slower dissociation rate from Cavα2δ1 versus Cavα2δ2 is postulated to be the reason behind its wider safety margin in relation to pregabalin, which shows the same dissociation rate for both of them.11
In concordance with the previously mentioned distribution, behavioral, and neurochemical studies, we set up a program devoted to the finding of compounds binding to the Cavα2δ-1 with selectivity toward the Cavα2δ-2 subunit. The final aim was to find safer drugs to be used as analgesic agents or in the treatment of other neuropathies. A high throughput screening (HTS) with our internal library (ca. 80 000 compounds) provided compound 2 (Figure 1, Ki Cavα2δ-1 = 1810 ± 82 nM) as an unprecedented scaffold in the field. It is worth mentioning that most of the described Cavα2δ-1 ligands are amino acidic structures closely related to 1, with only a few non-amino acidic scaffolds published. These have been summarized elsewhere22 and in our recent work describing a new family of bicyclic diazepinones as dual ligands of the Cavα2δ-1 and the norepinephrine transporter.23
We describe here the structure–activity relationship (SAR) study that led from the HTS hit 2 to the identification of the highly selective derivative 16RR, through a process summarized in Figure 1 and outlined below.
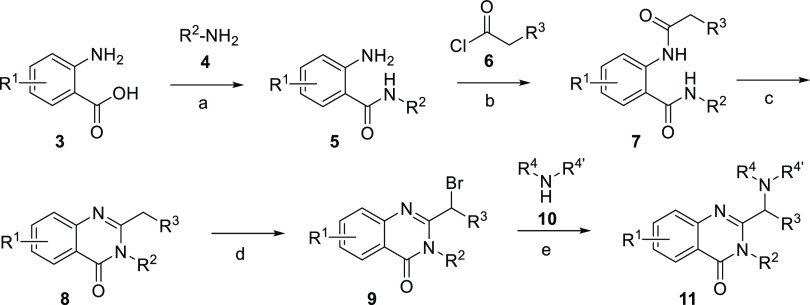
The synthesis of the compounds was accomplished as depicted in Schemes 1 and 2.24 Anthranilic acids 3 were coupled to amines 4 using 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxid hexafluorophosphate (HATU) and the resulting amides were reacted with acyl chlorides 6 in DCM in the presence of TEA. Cyclization of diamides 7 using iodine and hexamethyldisilazane (HMDS) provided quinazolin-4-(3H)-one derivatives 8 in good overall yields (around 65% for the three steps). Bromination of the α position of the alkyl chain in position 2 was accomplished in good yield (ca. 80%) with bromine in acetic acid. Finally, displacement of the bromide leaving group of 9 with the corresponding amines 10 provided compounds 11 in good yields (60–80%). Most of the amines 10 contained a second free NH group, but no protection was used. Instead, an excess of amine directly provided the target compounds. The potential dimeric derivatives were not observed at all or were detected in the crude mixtures in very small proportion and never isolated. In the case of unsymmetrical amines, addition always occurred by the less sterically hindered nitrogen, and a decrease in reactivity was observed when α-substituted amines were used. All the compounds 11a–x were prepared following this process, except 11n, 11p, and 11r that were obtained from the corresponding bromo derivatives 11m and 11q. As described in the experimental part, compound 11m was Boc-protected and treated with Zn(CN)2 under catalysis of Pd(PPh3)4 or with pyridin-4-ylboronic acid under Suzuki conditions, followed in both cases by deprotection with trifluoroacetic acid in DCM, to provide 11n and 11p, respectively. Analogously, 11r was obtained from 11q.
Scheme 1.
Reagents and conditions: (a) DMF, TEA, HATU, 0 °C to rt, overnight; (b) DCM, TEA, 0 °C to rt, overnight; (c) I2, HMDS, DCM, 0 °C to rt, overnight; (d) Br2, AcOH, NaOAc, 50 °C, 3 h; (e) ACN, TEA, KI, 90 °C, overnight.
Scheme 2.
Reagents and conditions: (a) H2SO4, NaNO2, 0 °C to rt, 16 h; (b) HATU, TEA, DCM, rt, 16 h; (c) I2, HMDS, DCM, 50 °C, 16 h; (d) TBAF, THF, 0 °C, 30 min; (e) 2,6-lutidine, Tf2O, DCM, −78 °C, 2 h; (f) DMF/DCM, −78 °C to rt, 4 h (; (g) MsCl, DIPEA, DCM, −78 °C, 5 min; (h) ACN, 80 °C, 24 h.
The final compounds contain an asymmetric center in the alkyl chain of position 2 and were obtained as racemic mixtures, except when indicated with the suffix R or S, corresponding to the R or S configuration, respectively. Several enantiomers were separated by chiral preparative HPLC, as exemplified here by compound 11m (Table 2). The activity was always shown to reside in one of the enantiomers, while the other exhibited only residual affinity. The four individual diastereoisomers of compound 11x were obtained by reaction with 2-methylpiperazine with the bromo derivative 9a followed by chiral HPLC preparative separation.
Table 2. Modification of R1 over Compound 11k.

| compd | R1 | Cavα2δ-1 (Ki, nM)a | CYP3A4 inhibition (%)b |
|---|---|---|---|
| 11m | 6-Br | 16 ± 28 | 95 |
| 11mR | 6-Br | 8 ± 2 | 94 |
| 11mS | 6-Br | 2903 ± 703 | 88 |
| 11n | 6-CN | 288 ± 139 | 70 |
| 11o | 6-NHEt | 44 ± 23 | 69 |
| 11p | 6-(4-pyridinyl) | 38 ± 13 | 92 |
| 11q | 7-Br | 15 ± 24 | 97 |
| 11r | 7-(4-pyridinyl) | 18 ± 24 | 97 |
| 11s | 5-Br | 66 ± 16 | 95 |
| 11t | 8-Br | >1000c | NDd |
Binding affinity (Ki, nM) in human α2δ-1 enriched membranes from hamster tumor CHO-K1 cells (Human Cav2.2/β3/α2δ1 Calcium Channel Cell Line, ChanTest) using [3H]-gabapentin as the radioligand. Each value is the mean ± SD of two determinations.
Percentage of inhibition after incubation at 1 μM with recombinant human CYP3A4 using BFC (7-benzyloxy-4-trifluoromethylcoumarin) as the CYP probe substrate and fluorescence detection.
Less than 50% inhibition at 1 μM.
Not determined.
The pyrido[4,3-d]pyrimidin-4(3H)-one derivatives 16 (Table 4) were synthesized in an analogous way to that described in Scheme 1, but using alternative conditions. As described in the experimental part, the initial 4-aminonicotinic acid was converted to 4-aminonicotinoyl chloride and reacted with ethylamine (instead of HATU-coupling), followed by reaction with pentanoyl chloride and cyclization with TMSCl (instead of iodine). Bromination was performed with NBS in acetonitrile and final displacement with (R)- or (S)-2-methylpiperazine provided the two pairs of diastereoisomers, that were separated using chiral HPLC.
Table 4. Diastereoisomers of Compound 16.
| compd | Cavα2δ-1 (Ki, nM)a | CYP3A4 inhibition (%)b |
|---|---|---|
| 16RR | 6 ± 9 | 46 |
| 16RS | 27 ± 8 | 56 |
| 16SR | >1000c | 15 |
| 16SS | >1000c | 44 |
Binding affinity (Ki, nM) in human α2δ-1 enriched membranes from hamster tumor CHO-K1 cells (Human Cav2.2/β3/α2δ1 Calcium Channel Cell Line, ChanTest) using [3H]-gabapentin as the radioligand. Each value is the mean ± SD of two determinations.
Percentage of inhibition after incubation at 1 μM with recombinant human CYP3A4 using BFC (7-benzyloxy-4-trifluoromethylcoumarin) as the CYP probe substrate and fluorescence detection.
Less than 50% inhibition at 1 μM.
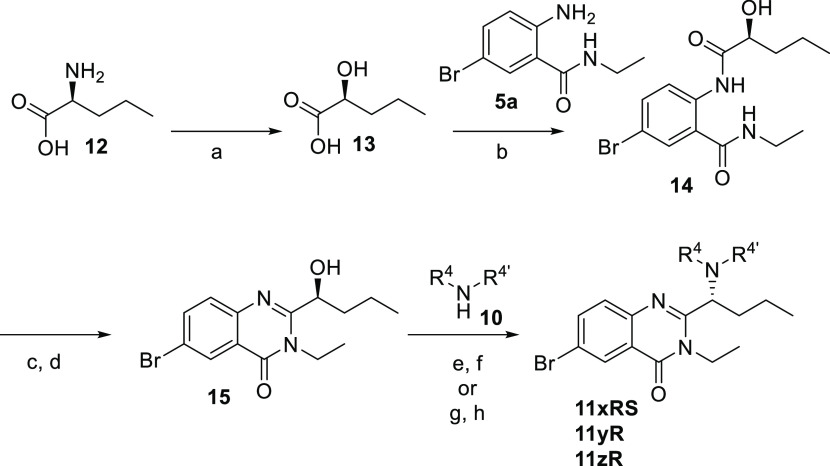
The eutomers of compounds 11 were assigned to the R configuration, based on the enantioselective route applied to different compounds and exemplified in Scheme 2 for the 6-bromo derivative. The route involved the formation of compound 14 by reaction of 5a with (S)-2-hydroxypentanoic acid 13, that was obtained from L-norvaline 12 using a described method that proceeds with retention of configuration.25 Cyclization of 14 in the conditions described above provided a mixture of compound 15 with some trimethylsilyl protected alcohol, which was fully deprotected using tetrabutylammonium fluoride (TBAF). Transformation of the hydroxyl group to a triflate and its displacement with the corresponding amines 10 occurred with inversion of configuration, to provide the final derivatives 11xRS and 11zR. Alternatively, conversion of the hydroxyl group to a mesylate and subsequent displacement provided the final derivative 11yR. This allowed the assignment of the R configuration to the eutomers, since the three compounds were identical to the corresponding active isomers isolated by chiral preparative HPLC from the racemic mixtures obtained using Scheme 1. The enantiomeric ratios were good in all cases, as determined by chiral HPLC analysis (enantiomeric ratios of 97.0(+):3.0(−), 96.5(+):3.5(−), and 95.7(+):4.3(−) for 11xRS, 11yR, and 11zR, respectively). The configuration of the pyridyl derivatives 16 was assigned by analogy to the 6-bromo analogues, with the R enantiomer as the eutomer.
The biological activity was evaluated at 10 μM in human α2δ-1 and α2δ-2 enriched membranes using [3H]-gabapentin as the radioligand. For those compounds showing inhibition higher than 50%, the Ki (nM) was calculated. The results are shown in Tables 1-4, where pregabalin (1) and the HTS hit 2 are displayed for comparison purposes. Most of the compounds described were devoid of Cavα2δ-2 activity. Therefore, these results are not tabulated and they are only described in the text in the few cases where some affinity was found.
Table 1. Initial Modifications of Hit Compound 2.
| compd | sc | R3 | R2 | Cavα2δ-1 (Ki, nM)a | CYP3A4 inhibition (%)b |
|---|---|---|---|---|---|
| 1 | 19 ± 12 | 43 | |||
| 2 | A | Et | 2-methoxyethyl | 1810 ± 82 | 37 |
| 11a | A | Pr | 2-methoxyethyl | 638 ± 345 | 67 |
| 11b | A | Me | 2-methoxyethyl | 4896 ± 1307 | 0 |
| 11c | A | H | 2-methoxyethyl | >10 000c | 6 |
| 11d | B | Pr | 2-methoxyethyl | 241 ± 246 | 94 |
| 11e | B | Et | 2-methoxyethyl | 761 ± 235 | 86 |
| 11f | B | Bu | 2-methoxyethyl | 1340 ± 856 | 97 |
| 11g | B | CyPrMe | 2-methoxyethyl | 413 ± 188 | 94 |
| 11h | A | Et | 3-methoxypropyl | >10 000c | NDd |
| 11i | B | Pr | benzyl | 4816 ± 45 | 98 |
| 11j | B | Pr | 2-furylmethyl | 230 ± 101 | 99 |
| 11k | B | Pr | ethyl | 36 ± 28 | 85 |
| 11l | B | Pr | methyl | 73 ± 20 | 80 |
Binding affinity (Ki, nM) in human α2δ-1 enriched membranes from hamster tumor CHO-K1 cells (Human Cav2.2/β3/α2δ1 Calcium Channel Cell Line, ChanTest) using [3H]-gabapentin as the radioligand. Each value is the mean ± SD of two determinations.
Percentage of inhibition after incubation at 1 μM with recombinant human CYP3A4 using BFC (7-benzyloxy-4-trifluoromethylcoumarin) as the CYP probe substrate and fluorescence detection.
Less than 50% inhibition at 10 μM.
Not determined.
No functional activity of the Cavα2δ-1 ligands identified was evaluated at this stage, since there is no assay available for screening purposes. Extensive studies are needed to determine suitable approaches (culture conditions, subunit expression, etc.) to provide meaningful information on downstream signaling for each Cavα2δ-1 ligand, as shown in the literature for gabapentin.26,27
To identify from the beginning compounds with good potential regarding their absorption, distribution, metabolism, and excretion (ADME) properties, the active compounds were tested in two additional tests. Metabolic stability was evaluated in human, mouse, and rat liver microsomes,28 and all the compounds tested showed acceptable clearances (generally below 15 μL/min/mg protein). Inhibition in recombinant human cytochrome P450 isoforms (rhCYP1A2, 2C9, 2C19, 2D6, and 3A4) was evaluated to identify potential drug–drug interaction issues.29 In this case, it was found that several derivatives inhibited CYP3A4, and their inhibition values (% at 1 μM) are reported in Tables 1–4.
Initial modification of hit compound 2 involved exploration of positions R2 and R3 and the amine moiety NR4R4’. Over the parent compound (R1 = H, R2 = methoxyethyl, R3 = ethyl), the amine was changed and only the (3S,5R)-3,5-dimethylpiperazin-1-yl derivative 11d provided Cavα2δ-1 activity, improving that of 2. Many other amines were explored (such as the methylated analogues of 11d and 11k, the piperidin-1-yl, 4-aminopiperidin-1-yl, 2-oxopiperazin-1-yl, or the linear 2-methylaminoethyl derivatives, results not shown), and all of them were devoid of activity. A change of R3 over the piperazin-1-yl or (3S,5R)-3,5-dimethylpiperazin-1-yl derivatives (Table 1) indicated that propyl was the optimal length in both cases (11a and 11d), with shorter (11b–e), longer (11f), or ramified (11g) derivatives being less active. A change of the methoxyethyl substituent in R2 showed that increasing length or bulkiness was detrimental (11h and 11i). Activity was somehow restored by introduction of 2-furylmethyl instead of benzyl (11j), but a substantial improvement was seen when reducing the chain length to provide the ethyl or methyl derivatives, 11k and 11l, respectively.
The nature of R1 was explored while keeping the remaining substituents of 11k (R2 = ethyl, R3 = propyl, NR4R4’ = (3S,5R)-3,5-dimethylpiperazin-1-yl). Many different analogues were prepared24 and only a handful of them are shown in Table 2. Introduction of bromine in position 6 or 7 provided 11m and 11q with a 2-fold improvement in affinity versus the parent hydrogenated, 11k. Isolation of the two enantiomers of 11m showed that there was clearly an eutomer, 11mR, assigned to the R configuration via the enantioselective synthesis described above. The distomer 11mS retained a residual affinity. Changing the bromine by cyano in 11n provided a loss in affinity, while the ethylamino derivative 11o retained affinity, as did other electron-donating groups explored (results not shown). Larger groups were also tolerated but led to a worsening of other properties. For instance, compounds 11p and 11r showed 53% and 95% inhibition of Cavα2δ-2 at 10 μM, respectively. Finally, substitution in position 5 led to a small impairment in affinity versus the parent hydrogen (see 11s versus 11k), while the 8-bromo derivative 11t was much less active.
An additional round of exploration of the amine substituent was carried out over the 6-bromo-2-butyl-3-ethyl derivatives (Table 3). The naked piperazin-1-yl derivative, 11u, was shown to be less active than the dimethylated 11m, confirming the results of the hydrogenated counterparts. The methylaminopiperidine 11v and bicyclic compound 11w displayed a diminished activity, but the 2-methylpiperazin-4-yl derivatives 11x were more interesting. In this case, the individual diastereoisomers were isolated as described above and both the 11xRS and 11xRR derivatives were highly active. The corresponding enantiomers 11xSR and 11xSS were poorly active, as expected. The (S)-2-ethyl and 2,2-dimethyl derivatives 11yR and 11zR, prepared only via the enantioselective route described in Scheme 2, showed a diminished affinity versus the monomethyl 11xRS.
Table 3. Modification of Amine over 6-Bromo-2-butyl-3-ethyl Derivatives.

| compd | NR4R4’ | Cavα2δ-1 (Ki, nM)a | CYP3A4 inhibition (%)b |
|---|---|---|---|
| 11u | piperazin-1-yl | 76 ± 20 | 65 |
| 11v | 4-methylaminopiperidin-1-yl | 436 ± 60 | 83 |
| 11w | 3,8-diazabicyclo[3.2.1]octan-1-yle | 397 ± 161 | 74 |
| 11xRS | (S)-3-methylpiperazin-1-yl | 91 ± 15 | 73 |
| 11xRR | (R)-3-methylpiperazin-1-yl | 16 ± 15 | 51 |
| 11xSS | (S)-3-methylpiperazin-1-yl | 479 ± 324 | 33 |
| 11xSR | (R)-3-methylpiperazin-1-yl | >1000c | 21 |
| 11yR | (R)-3-ethylpiperazin-1-yl | 123 ± 340 | 97 |
| 11zR | 3,3-dimethylpiperazin-1-yl | 1578 ± 1998 | 72 |
Binding affinity (Ki, nM) in human α2δ-1 enriched membranes from hamster tumor CHO-K1 cells (Human Cav2.2/β3/α2δ1 Calcium Channel Cell Line, ChanTest) using [3H]-gabapentin as the radioligand. Each value is the mean ± SD of two determinations.
Percentage of inhibition after incubation at 1 μM with recombinant human CYP3A4 using BFC (7-benzyloxy-4-trifluoromethylcoumarin) as the CYP probe substrate and fluorescence detection.
Less than 50% inhibition at 1 μM.
Derivative with 6-H instead of 6-Br.
Among the best compounds identified so far, derivatives 11mR and 11q showed high CYP3A4 inhibition. The methylpiperazines 11xRS and 11xRR were better in this regard, but they showed additional activities when tested versus a small panel of targets related to pain (σ1R, 5-HT1A receptor, 5-HT2B receptor, α1A and α2A adrenoreceptors, dopamine transporter, μ-, δ-, and κ-opioid receptors, histamine H1 receptor, norepinephrine transporter and serotonin transporter).30 In fact, the compound 11xRS showed a Ki of 157 ± 67 nM for the μ-opioid receptor and was selected as a lead for a program aimed at the finding of dual compounds with Cavα2δ-1 and μ-opioid receptor activity, that will be reported in due course.
The introduction of nitrogen atoms in the central scaffold was then anticipated to decrease the cLogP, which could improve ADME properties and selectivity, since lipophilicity is a well-known parameter associated with suboptimal druglike properties31 and promiscuity.32 Several derivatives were prepared, but only the best set of diastereoisomers identified is shown in Table 4. Compound 16RR is the most potent derivative versus the Cavα2δ-1 identified so far in this compound class, while its diastereoisomer 16RS is also quite potent. As expected, the other enantiomeric pair (16SR and 16SS) is much less active.
Compound 16RR showed acceptable experimental lipophilicity (log P = 2.22, log D7.4 = 0.49) and pKa (9.1 and 3.4) values and a good in vitro profile. It was devoid of CYP3A4 or 1A2, 2C9, 2C19, 2D6 inhibition (<50% at 1 μM), showed good metabolic stability in human, rat, and mouse liver microsomes (Clint = 2.6, 3.9, and 4.1 μL/min/mg protein, respectively), was highly selective versus the pain panel mentioned before, and was devoid of hERG inhibition (IC50 > 10 μM). The only drawback identified in the ADME profile of compound 16RR was its suboptimal permeability in Caco-2 cells (43 nm/s) and elevated efflux ratio (5.4), that could be indicative of it being substrate of P-glycoprotein (P-gp).
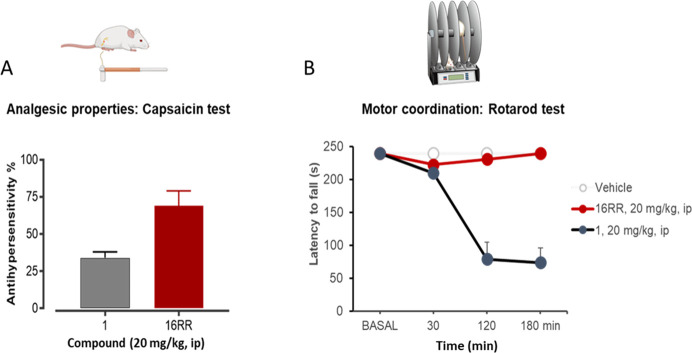
To have a first indication of the series in vivo behavior, compound 16RR was evaluated in two mouse efficacy and safety models. Considering its expected poor oral absorption and brain distribution, it was administered using the ip route at the same dose in both models. Pregabalin was used for comparison in the same experimental paradigm. The analgesic activity was evaluated in the capsaicin test in CD1 male mice (n = 8–12 per group). After ip administration at 20 mg/kg, 16RR showed a 69% antiallodynic effect, that was superior to that shown by pregabalin (1, 34%) (Figure 2A). The plasma levels of 16RR were high at this dose (Cmax 3071 ng/mL and AUC0–t 8052 ng·h/mL). When tested in the rotarod test at 20 mg/kg ip, 16RR did not provide any sign of motor discoordination (Figure 2B), as no changes in the latency to fall-down at any time point were observed. In contrast, the same doses of pregabalin induced a marked motor impairment, suggesting that 16RR could have a superior safety margin compared to 1 in pain treatment.
Figure 2.
(A) Effects of pregabalin (1) and 16RR on mechanical hypersensitivity (allodynia) induced by intraplantar injection of capsaicin (1 μg) to mouse hind paw. The results represent the percentage reduction in capsaicin-induced mechanical hypersensitivity. (B) Effect of 1 and 16RR on motor coordination using the rotarod test. The results represent the latencies to fall-down from the elevated rotating drum. Rotarod latencies were measured at 30, 120, and 180 min after administration of vehicle or compounds 1 and 16RR at 20 mg/kg ip.
In summary, exploration of the different positions of a micromolar piperazinyl quinazolin-4-(3H)-one HTS hit provided a series of derivatives with single digit nanomolar affinities versus the Cavα2δ-1 subunit of VGCC. Modification of the central quinazolinone to reduce lipophilicity, provided the pyrido[4,3-d]pyrimidin-4(3H)-one lead compound 16RR, an unprecedented scaffold in the field, that shows an excellent selectivity over the Cavα2δ-2, the subunit postulated to be linked to the undesired effects of gabapentinoids. Compound 16RR showed a good ADME profile, except for its moderate permeability in Caco-2 cells and high efflux ratio, properties that have been addressed in a subsequent lead optimization program that will be reported in due course. As an initial indication of the in vivo behavior of the series, compound 16RR assessed in the rotarod model in mice at a dose eliciting analgesia (i.e., capsaicin induced mechanical allodynia) did not induce motor discoordination. In contrast, pregabalin did produce motor discoordination with evident overlapping between the motor impairment and the antiallodynic effect. These results suggest that selectively targeting the Cavα2δ-1 in relation to the Cavα2δ-2 may provide an interesting approach for the treatment of pain and other neuropathies. Compound 16RR may also serve as pharmacological tool to provide further insight on the roles played by the two Cavα2δ-1/2 subunits, in view of the novel functions that are being identified for these proteins, both dependent and independent of calcium channels.
Acknowledgments
We thank Magda Bordas, Adriana Port, Joan Andreu Morató, Mónica Carro, Edmundo Ortega, Manuel Merlos, Pilar Pérez, Javier Burgueño, Marta Pujol, Enrique Hernández, Javier Farré, Inés Álvarez, Xavier Monroy, Maite Serafini, Eva Ayet, Sandra Yeste for their expert contribution to analytical and biological studies and Carlos Pérez and Eduardo Villarroel for their contribution to compound management. This work was a part of activities in R&D Projects IDI20130944 and IDI20150916 supported by the Spanish Ministerio de Economía y Competitividad (MINECO), through the Centro para el Desarrollo Tecnológico Industrial (CDTI), cofinanced by the European Union through the European Regional Development Fund (ERDF; Fondo Europeo de Desarrollo Regional, FEDER).
Glossary
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- BFC
7-benzyloxy-4-trifluoromethylcoumarin
- Cavα2δ-1
α2δ-1 subunit of voltage-gated calcium channels
- Cavα2δ-2
α2δ-2 subunit of voltage-gated calcium channels
- CNS
central nervous system
- DRG
dorsal root ganglia
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium3-oxid hexafluorophosphate
- HMDS
hexamethyldisilazane
- hERG
human ether-a-go-go-related gene
- NMDA
N-methyl-d-aspartate
- P-gp
P-glycoprotein
- rhCYP
recombinant human cytochrome P450
- SAR
structure–activity relationships
- VGCC
voltage-gated calcium channels
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00416.
Detailed synthetic procedures of representative compounds; experimental procedures for the determination of α2δ-1 and α2δ-2 activity and CYP3A4 inhibition; experimental procedures for determination of metabolic stability of 16RR; experimental procedures for in vivo testing: plasma levels determination and efficacy and safety testing in mice; analytical data of final compounds (purity, 1H NMR, HRMS); 13C NMR data for selected final compounds; enantiomeric ratios by chiral HPLC and optical rotation of enantiopure compounds; 1H NMR characterization of intermediates; HPLC traces of final compounds (PDF)
Author Contributions
The manuscript was written by C.A. and contributions of the rest of the authors, mainly M.G. and J.L.D. A.F., C.A., and J.L.D. contributed to the design of the new compounds. A.F., A.L., and S.R. synthesized the compounds, R.E. analyzed the compounds, A.D. did the binding experiments, E.P.-S. and M. P. did the in vivo pharmacology experiments, R.F.R. did the ADME and pharmacokinetics experiments, B.F. and M.G. coordinated part of the work, and J.M.V. supervised the work. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters virtual special issue “Medicinal Chemistry in Portugal and Spain: A Strong Iberian Alliance”.
Supplementary Material
References
- Zamponi G. W.; Striessnig J.; Koschak A.; Dolphin A. C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret D.; Luo Z. D. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 2009, 6, 679–692. 10.1016/j.nurt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry S.; Cording M.; Wiffen P. J.; Law S.; Phillips T.; Moore R. A. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst. Rev. 2016, 29, CD011790. 10.1002/14651858.CD011790.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. P.; Angelotti T.; Fauman E. Pharmacology and mechanism of action of pregabalin: The calcium channel alpha2-selta subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007, 73, 137–150. 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Faulkner M. A. Use of α2δ ligands for restless legs syndrome/Willis Ekbom Disease. CNS Drugs 2018, 32, 149–159. 10.1007/s40263-018-0502-z. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C. Voltage-gated calcium channel α2δ subunits: an assessment of proposed novel roles. F1000Research 2018, 7, 1830. 10.12688/f1000research.16104.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles S. R. A.; Cain S. M.; Snutch T. P. Pregabalin as a pain therapeutic: Beyond calcium channels. Front. Cell. Neurosci. 2020, 14, 83. 10.3389/fncel.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher W. C.; Eroglu C. Emerging roles for α2δ subunits in calcium channel function and synaptic connectivity. Curr. Opin. Neurobiol. 2020, 63, 162–169. 10.1016/j.conb.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf C. L.; Ablinger C.; Geisler S. M.; Stanika R. I.; Campiglio M.; Kaufmann W. A.; Nimmervoll B.; Schlick B.; Brockhaus J.; Missler M.; Shigemoto R.; Obermair G. J. Presynaptic α2δ subunits are key organizers of glutamatergic synapses. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e1920827118 10.1073/pnas.1920827118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffen P. J.; Derry S.; Bell R. F.; Rice A. S.; Tölle T. R.; Phillips T.; Moore R. A. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2020, CD007938. 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajączkowska R.; Mika J.; Leppert W.; Kocot-Kępska M.; Malec-Milewska M.; Wordliczek J. Mirogabalin-A novel selective ligand for the α2δ calcium channel subunit. Pharmaceuticals 2021, 14, 112. 10.3390/ph14020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. L.; Lechner S. L.; Williams M. E.; Prodanovich P.; Bleicher L.; Varney M. A.; Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J. Comp. Neurol. 2005, 491, 246–269. 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Newton R. A.; Bingham S.; Case P. C.; Sanger G. S.; Lawson S. N. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Mol. Brain Res. 2001, 95, 1–8. 10.1016/S0169-328X(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Bauer C. S.; Nieto-Rostro M.; Rahman W.; Tran-Van-Minh A.; Ferron L.; Douglas L.; Kadurin I.; Ranjan Y. S.; Fernandez-Alacid L.; Millar N. S.; Dickenson A. H.; Lujan R.; Dolphin A. C. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009, 29, 4076–88. 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y.; Zhang X. L.; Matthews E. A.; Li K. W.; Kurwa A.; Boroujerdi A.; Gross J.; Gold M. S.; Dickenson A. H.; Feng G.; Luo Z. H. Calcium channel α2–δ–1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006, 125, 20–34. 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. J.; Cox P. J.; Stott E.; Melrose H.; Offord J.; Su T. Z.; Bramwell S.; Corradini L.; England S.; Winks J.; Kinloch R. A.; Hendrich J.; Dolphin A. C.; Webb T.; Williams D. Identification of the α2–δ–1 subunit of voltage dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 17537–17542. 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N.; Park J.; Luo Z. D. Injury-induced maladaptation and dysregulation of calcium channel α2δ subunit proteins and its contribution to neuropathic pain development. Br. J. Pharmacol. 2018, 175, 2231–2243. 10.1111/bph.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li L.; Chen S. R.; Chen H.; Xie J. D.; Sirrieh R. E.; MacLean D. M.; Zhang Y.; Zhou M. H.; Jayaraman V.; Pan H. L. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018, 22, 2307–2321. 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson K. A.; Beeson R.; Westbrook G. L.; Schnell E. α2δ-2 protein controls structure and function at the cerebellar climbing fiber synapse. J. Neurosci. 2020, 40, 2403–2415. 10.1523/JNEUROSCI.1514-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J.; Klocke R.; Paul D.; Boison D.; Gouder N.; Klugbauer N.; Hofmann F.; Becker C. M.; Becker K. Entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J. Biol. Chem. 2004, 279, 7322–7330. 10.1074/jbc.M308778200. [DOI] [PubMed] [Google Scholar]
- Celli R.; Santolini I.; Guiducci M.; van Luijtelaar G.; Parisi P.; Striano P.; Gradini R.; Battaglia G.; Ngomba R. T.; Nicoletti F. The α2δ subunit and absence epilepsy: beyond calcium channels?. Curr. Neuropharmacol. 2017, 15, 918–925. 10.2174/1570159X15666170309105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. J.; Li Z.; Schwarz J. B. Ca2+ channel alpha2-delta ligands for the treatment of neuropathic pain. J. Med. Chem. 2007, 50, 2569–2575. 10.1021/jm060650z. [DOI] [PubMed] [Google Scholar]
- Díaz J. L.; Cuevas F.; Pazos G.; Álvarez-Bercedo P.; Oliva A. I.; Sarmentero M. A.; Font D.; Jiménez-Aquino A.; Morón M.; Port A.; Pascual R.; Dordal A.; Portillo-Salido E.; Reinoso R. F.; Vela J. M.; Almansa C. Bicyclic diazepinones as dual ligands of the α2δ-1 subunit of voltage-gated calcium channels and the norepinephrine transporter. J. Med. Chem. 2021, 64, 2167–2185. 10.1021/acs.jmedchem.0c01867. [DOI] [PubMed] [Google Scholar]
- Fernández A.; Díaz J. L.; Almansa C.; Lorente A.. Piperazinyl and Piperidinyl Quinazolin-4-(3H)-one Derivatives Having Activity Against Pain. PCT Int. Appl. WO2020089400, 2020.
- Mori K.; Takaishi H. Synthesis of monocerin, an antifungal, insecticidal and phytotoxic heptaketide metabolite of exserohilum monoceras. Tetrahedron 1989, 45, 1639–1646. 10.1016/S0040-4020(01)80027-2. [DOI] [Google Scholar]
- Martin D. J.; McClelland D.; Herd M. B.; Sutton K. G.; Hall M. D.; Lee K.; Pinnock R. D.; Scott R. H. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology 2002, 42, 353–66. 10.1016/S0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- Heblich F.; Tran Van Minh A.; Hendrich J.; Watschinger K.; Dolphin A. C. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels 2008, 2, 4–9. 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- Obach R. S.; Baxter J. G.; Liston T. E.; Silber B. M.; Jones B. C.; MacIntyre F.; Rance D. J.; Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther. 1997, 283, 46–58. [PubMed] [Google Scholar]
- Stresser D. M.High-throughput Screening of Human Cytochrome P450 inhibitors Using Fluorometric Substrates. Methodology for 25 Enzyme/Substrate Pairs. In Optimization in Drug Discovery. In vitro Methods; Yan Z., Caldwell G. W.; Eds.; Humana Press: Totowa, NJ, 2004; pp 215–230. [Google Scholar]
- García M.; Virgili M.; Alonso M.; Alegret C.; Farran J.; Fernández B.; Bordas M.; Pascual R.; Burgueño J.; Vidal-Torres A.; Rodriguez-Fernández de Henestrosa A.; Ayet E.; Merlos M.; Vela J. M.; Plata-Salamán C. R.; Almansa C. Discovery of EST73502, a dual C-opioid receptor agonist and o1 receptor antagonist clinical candidate for the treatment of pain. J. Med. Chem. 2020, 63, 15508–15526. 10.1021/acs.jmedchem.0c01127. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Lipophilicity in drug discovery. Expert Opin. Drug Discovery 2010, 5, 235–248. 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
- Tarcsay A.; Keserü G. M. Contributions of molecular properties to drug promiscuity. J. Med. Chem. 2013, 56, 1789–1795. 10.1021/jm301514n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.