Abstract
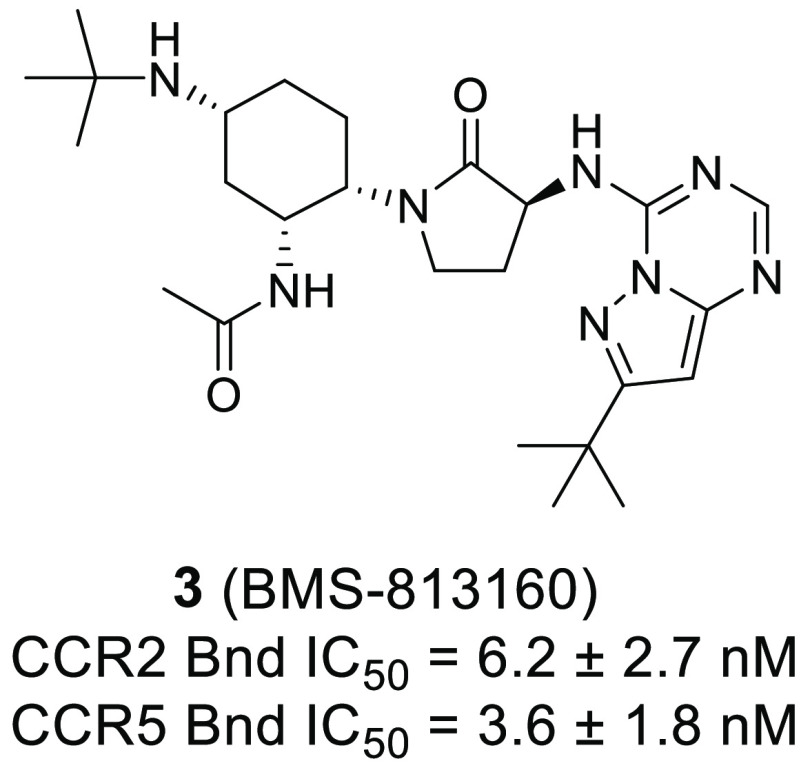
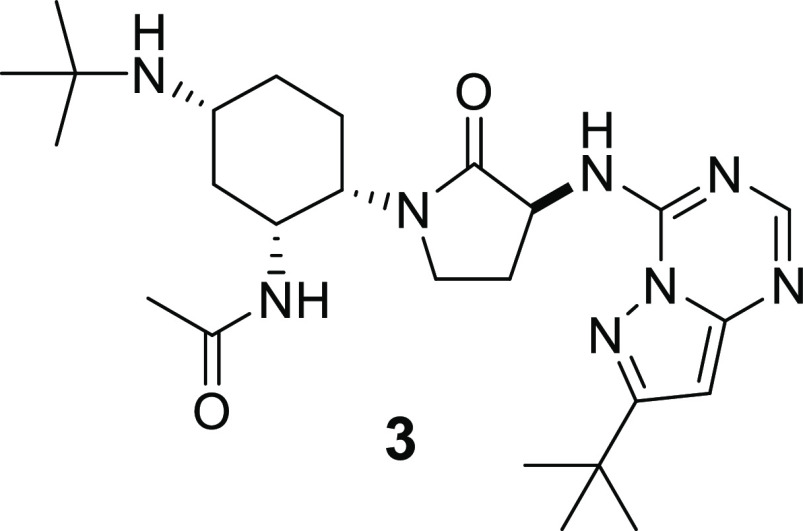
BMS-813160 (compound 3) was identified as a potent and selective CCR2/5 dual antagonist. Compound 3 displayed good permeability at pH = 7.4 in PAMPA experiments and demonstrated excellent human liver microsome stability. Pharmacokinetic studies established that 3 had excellent oral bioavailability and exhibited low clearance in dog and cyno. Compound 3 was also studied in the mouse thioglycollate-induced peritonitis model, which confirmed its ability to inhibit the migration of inflammatory monocytes and macrophages. As a result of this profile, compound 3 was selected as a clinical candidate.
Keywords: CCR2 antagonist, CCR5 antagonist, dual antagonist, chemokine, G protein-coupled receptor
Chemokine receptors are G protein-coupled receptor (GPCR) family members involved in the activation and migration of leukocytes.1−3 Two chemokine receptors that are often implicated in inflammatory conditions are CC chemokine receptor 2 (CCR2)4 and CC chemokine receptor 5 (CCR5).5 The primary ligand for CCR2 is CC chemokine ligand 2 (CCL2);6 however CCR2 also functions via binding with CCL7, CCL8, CCL13, and CCL16. CCR5 has multiple chemokine ligands,5 including CCL3, CCL4, CCL5, CCL8, CCL13, and CCL16. CCR2 is the main chemokine receptor expressed on monocytes, but it is also expressed on T cells, immature dendritic cells, and endothelial cells. CCR5 is expressed on monocytes, macrophages, T cells, natural killer cells, and dendritic cells. CCR2 activation initiates the migration of monocytes from the circulation to areas of inflammation within tissues. Overproduction of CCR2 and CCR5 along with their respective ligands is associated with many inflammatory conditions, for example: rheumatoid arthritis,7,8 multiple sclerosis,9−11 diabetic nephropathy,12,13 and fibrosis.14,15 As such, there has been a tremendous effort over the years to identify antagonists of CCR2 and CCR5.16−21 Recent findings have also implicated CCR2 and CCR5 in tumor associated immunosuppression22−24 and the migration of immunosuppressive cells25−28 to the tumor environment. As a result of these findings, we initiated a program to identify CCR2/5 dual antagonists. Herein we report the results of this effort, which culminated in the identification of BMS-813160 as a clinical candidate.
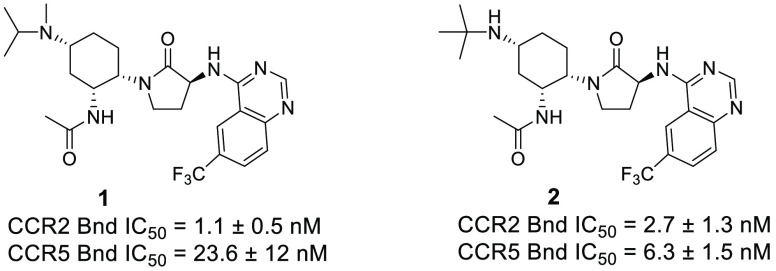
As shown in Figure 1, our previous efforts in this area were focused on CCR2 selective antagonists and yielded compounds 1(29) and 2.30 Compound 1 was 21-fold more selective for CCR2 compared to CCR5 as measured by our binding assay.31 Surprisingly, replacement of the isopropylmethylamine of 1 with a tert-butyl amine group to provide 2 afforded improved CCR5 affinity. In fact, compound 2 was only 2-fold selective for CCR2 over CCR5 in binding affinity. As we initiated our search for CCR2/5 dual antagonists, we continued to incorporate the tert-butyl amine group as a way to ensure CCR5 activity. The tert-butyl amine group also conferred superior metabolic stability when compared to the isopropylmethylamine group, which suffered from lower metabolic stability due to a demethylation. However, compound 2 was not an optimized CCR2/5 dual antagonist as a result of its activity in our CD11b human whole blood assay.31 As shown in Table 1, compound 2 had a CCR5 CD11b human whole blood IC50 = 34.7 nM. We viewed the CCR5 CD11b human whole blood assay (and the CCR2 version) as our most physiologically relevant assay and sought to optimize compound 2 for CD11b assay activity. Hoping for improved potency in this assay, we examined replacement of the right-hand side quinazoline moiety by a number of other heterocycles. This effort yielded the tert-butyl pyrazolotriazine compound 3, which displayed excellent binding affinity for both CCR2 and CCR5. In fact, 3 had 2-fold better binding affinity for CCR5 over CCR2; however, it was equipotent in the functional chemotaxis assay dependent on either CCR5 or CCR2. Importantly, compound 3 had excellent activity in the CCR2 CD11b human whole blood assay and the CCR5 CD11b human whole blood assay, making it a potent dual antagonist worthy of additional profiling.
Figure 1.
Our previously reported CCR2 antagonists.
Table 1. Comparison of Compound 3 to Prior Lead Compounds 1 and 2a.
| CCR2 Bnd | CCR2 CTX | CCR2 CD11b | CCR5 Bnd | CCR5 CTX | CCR5 CD11b | |
|---|---|---|---|---|---|---|
| no. | IC50 (nM) | IC50 (nM) | IC50 (nM) | IC50 (nM) | IC50 (nM) | IC50 (nM) |
| 1 | 1.1 ± 0.5 | 0.7 ± 0.2 | NT | 23.6 ± 12 | NT | NT |
| 2 | 2.7 ± 1.3 | 0.8 ± 0.5 | 2.6 ± 2.2 | 6.3 ± 1.5 | 1.1 ± 0.7 | 34.7 ± 11 |
| 3 | 6.2 ± 2.7 | 0.8 ± 0.8 | 4.8 ± 2.7 | 3.6 ± 1.8 | 1.1 ± 0.6 | 5.7 ± 2.4 |
All assays are reported as means plus or minus standard deviation from two or more determinations. The CCR2 binding (Bnd) assay was performed in human peripheral blood mononuclear cells using labeled 125I-CCL2 as the ligand. CCR5 binding (Bnd) and chemotaxis (CTX) assays were performed using human peripheral T cells using MIP-1β as the ligand (125l-MIP-1β for Bnd). CCR2 chemotaxis was performed using labeled human THP-1 cells and CCL2 as the ligand. CCR2 CD11b and CCR5 CD11b upregulation assays used human whole blood with CCL2 and MIP-1β as the ligands, respectively. For additional details and references on assays, see the Supporting Information. NT = not tested.
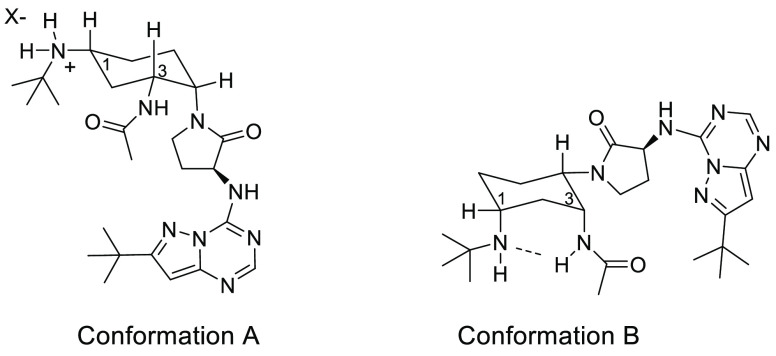
As shown in Table 2, selectivity profiling was performed on compound 3, and it proved to be selective against chemokine family member assays we had in-house: CCR1, CCR4, and CXCR2 (3 was also inactive in a broad GPCR panel—data not shown). As was the case with many of our antagonists, the human CCR2 activity did not translate to mouse, as the binding affinity in mouse was moderate (mouse CCR2 binding IC50 = 45 nM). Compound 3 had excellent human liver microsome stability, which translated well into other species. Although ion channel liabilities are known to be problematic for chemokine antagonists,32 compound 3 was not potent in a hERG patch clamp assay. Our cyclohexylamine antagonist class has historically had high plasma free fraction, and compound 3 continued this trend. Also inherent in this class is pH-dependent permeability that is controlled by two conformations.29 As shown in Figure 2, at low pH the 1,3-diequatorial conformation A predominates, which has poor permeability as reflected in the PAMPA Pc of 19 nm/s at pH = 5.5. Conformation A is also the bioactive conformation observed in a CCR2 crystal structure of the close analogue BMS-687681.33 However, at higher pH (as in pH = 7.4) some of the free base is available which will predominately have the 1,3-diaxial conformation B stabilized by the hydrogen bond between the free tert-butyl amine and the “NH” of the acetamide. This 1,3-diaxial conformation shields the polar groups beneath the cyclohexane and increases the cLogP.29 The result is excellent permeability for the free base (Pc 336 nm/s), as the 1,3-diaxial conformation shuttles the compound through the membrane.
Table 2. Compound 3 Profilea.
| assay | result |
|---|---|
| CCR2 Bnd IC50 | 6.2 ± 2.7 nM |
| CCR5 Bnd IC50 | 3.6 ± 1.8 nM |
| CCR1 Bnd IC50 | >25 μM |
| CCR4 Bnd IC50 | >40 μM |
| CXCR2 Bnd IC50 | >40 μM |
| mouse CCR2 Bnd IC50 | 45 ± 22 nM |
| LM t1/2: h, m, r (min) | 146/>200/>200 |
| hERG PC %inhib @ 30 μM | 9 |
| protein binding % free h/m/r | 71/58/69 |
| PAMPA Pc pH = 5.5 (nm/s) | 19 |
| PAMPA Pc pH = 7.4 (nm/s) | 336 |
Liver microsomes (LM) t1/2 reported for human (h), mouse (m), rat (r). Protein binding by equilibrium dialysis with 1 μM conc at 37 °C. For additional details on assays and references see the Supporting Information.
Figure 2.
Conformations of compound 3.
As shown in Table 3, compound 3 had excellent oral bioavailability across the four species studied. This confirms the excellent permeability observed for 3 as predicted by the in vitro PAMPA measurements. Compound 3 displayed mixed clearances in vivo, significantly higher in rodents, whereas dog and cyno displayed low clearances. The oral 24-h AUC was very good across all four species. This excellent oral bioavailability prompted us to study compound 3 in a mouse model of inflammatory cellular recruitment.
Table 3. Pharmacokinetic Data for Compound 3a.
| species | dose (mpk) iv/po | CLiv (mL/min/kg) | F% | oral AUC (nM·h) |
|---|---|---|---|---|
| mouse | 2/10 | 85 | 100% | 5406 |
| rat | 2/10 | 50 | 94% | 6500 |
| dog | 1/1 | 17 | 61% | 1230 |
| monkey | 1/1 | 16 | 63% | 1300 |
Values are means obtained from three or more animals.
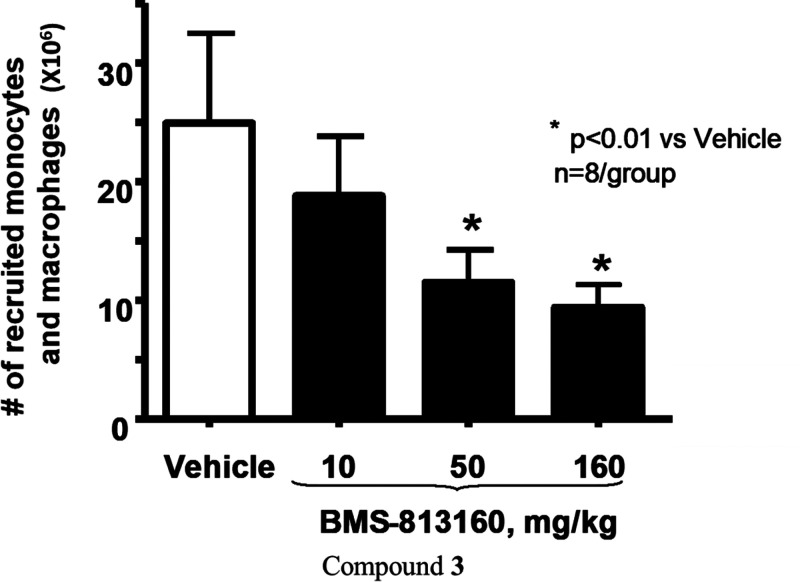
As mentioned above, CCR2 and CCR5 play an important role in the migration of inflammatory monocytes and macrophages, and this can be modeled in the 48 h mouse thioglycollate (TG) induced peritonitis model.34 However, since compound 3 only has moderate mouse activity, we performed this study in a human-CCR2 knock-in mouse.35 As shown in Figure 3, compound 3 was dosed orally BID, because of its high clearance in mouse, over 48 h at three different doses (10, 50, and 160 mg/kg). The thioglycollate challenge was administered only once, which was 1 h after the first dose of compound 3 (preventative mode). The results show that compound 3 significantly reduced inflammatory monocyte and macrophage infiltration in the peritoneum in a dose-dependent fashion.
Figure 3.
Oral efficacy of compound 3 in the 48 h peritonitis mouse model.
Compound 3 was synthesized starting from 3-(tert-butyl)-1H-pyrazol-5-amine 4 as shown in Scheme 1. Compound 4 was treated with ethoxycarbonyl isothiocyanate overnight to yield the thiourea 5.36 Base treatment of 5 initiated a cyclization to give compound 6. Desulfurization of 6 was accomplished with Raney nickel (Ra-Ni) and afforded compound 7. Treatment of 7 with refluxing POCl3 gave compound 8, which was used without purification. In the final step, our previously reported amine 9(30) was coupled to compound 8 and provided the desired compound 3.
Scheme 1. Synthesis of Compound 3.
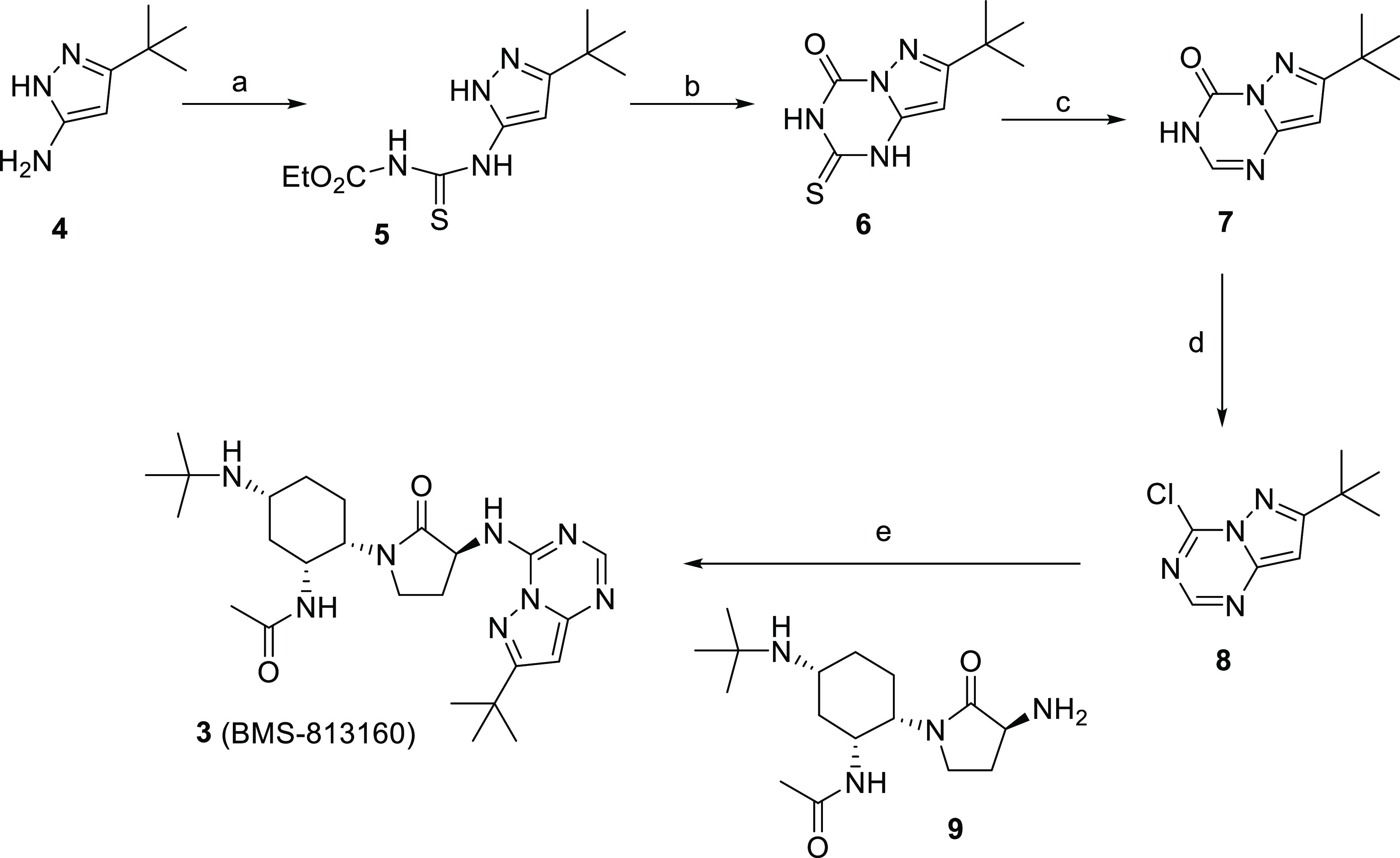
Reagents and conditions: (a) EtO2C-NCS, EtOAc, benzene, 90%; (b) NaOH, 95%; (c) Ra-Ni, NH4OH, MeOH, 100 °C, 50%; (d) POCl3, Δ; (e) 9, Et3N, iPrOH, 15%.
In summary, we have identified compound 3 (BMS-813160) as a potent and selective CCR2/5 dual antagonist. In vitro experiments indicated that 3 had good permeability at pH = 7.4 and excellent metabolic stability. These measurements were confirmed in vivo, as compound 3 displayed excellent oral bioavailability and had low clearance in dog and cyno. Compound 3 proved to be efficacious in the mouse TG-induced peritonitis model, which validated its ability to inhibit the migration of inflammatory monocytes and macrophages. As a result of the above profile, compound 3 was selected as a clinical candidate and was advanced into clinical trials.
Acknowledgments
We would like to thank our colleagues in the Department of Discovery Synthesis at the Biocon-BMS R&D Center (Bengaluru, India) for the synthesis of intermediate 9. We also thank Dr. Joseph A. Tino and Dr. Robert M. Borzilleri for a critical review of the manuscript.
Glossary
Abbreviations
- AUC
area under the curve
- BID
twice a day
- BMS
Bristol Myers Squibb
- Bnd
binding
- BSA
bovine serum albumin
- C
concentration
- CCL2
CC chemokine ligand 2
- CCR2
CC chemokine receptor 2
- CCR5
CC chemokine receptor 5
- CL
clearance
- CTX
chemotaxis
- EDTA
ethylenediaminetetraacetic acid
- F
bioavailability
- GPCR
G protein-coupled receptors
- h
hour
- hERG
human ether-a-go-go-related gene
- hWB
human whole blood
- iv
intravenous
- LM
liver microsome
- M
molar
- NADPH
dihydronicotinamide-adenine dinucleotide phosphate
- ND
not determined
- NMP
N-methylpyrrolidinone
- PC
patch clamp
- PD
pharmacodynamics
- PK
pharmacokinetic
- po
per os, oral dose
- qd
once a day
- Ra-Ni
Raney nickel
- SAR
structure activity relationship
- SFC
supercritical fluid chromatography
- TG
thioglycollate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00373.
Compound characterization data and additional assay details/references (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- D’Agostino G.; Garcia-Cuesta E. M.; Gomariz R. P.; Rodriguez-Frade J. M.; Mellado M. The multilayered complexity of the chemokine receptor system. Biochem. Biophys. Res. Commun. 2020, 528, 347–358. 10.1016/j.bbrc.2020.02.120. [DOI] [PubMed] [Google Scholar]
- Hughes C. E.; Nibbs R. J. B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. W.; Sokol C. L.; Luster A. D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- Feria M.; Diaz-Gonzalez F. The CCR2 receptor as a therapeutic target. Expert Opin. Ther. Pat. 2006, 16, 49–57. 10.1517/13543776.16.1.49. [DOI] [Google Scholar]
- Vangelista L.; Vento S. The expanding therapeutic perspective of CCR5 blockade. Front. Immunol. 2018, 8, 1–7. 10.3389/fimmu.2017.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi V.; Sahebkar A.; Atkin S. L.; Pirro M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. 10.1097/MOH.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Vergunst C. E.; Tak P. P. Chemokines: Their role in rheumatoid arthritis. Curr. Rheumatol. Rep. 2005, 7, 382–388. 10.1007/s11926-005-0026-7. [DOI] [PubMed] [Google Scholar]
- Quinones M. P.; Estrada C. A.; Kalkonde Y.; Ahuja S. K.; Kuziel W. A.; Mack M.; Ahuja S. S. The complex role of the chemokine receptor CCR2 in collagen-induced arthritis: implications for therapeutic targeting of CCR2 in rheumatoid arthritis. J. Mol. Med. (Heidelberg, Ger.) 2005, 83, 672–681. 10.1007/s00109-005-0637-5. [DOI] [PubMed] [Google Scholar]
- Conductier G.; Blondeau N.; Guyon A.; Nahon J. L.; Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010, 224, 93–100. 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Stoolman J. S.; Duncker P. C.; Huber A. K.; Segal B. M. Site-specific chemokine expression regulates central nervous system inflammation and determines clinical phenotype in autoimmune encephalomyelitis. J. Immunol. 2014, 193, 564–570. 10.4049/jimmunol.1400825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen T.; Nielsen J.; Berkhof J.; Barkhof F.; Kamphorst W.; Bo L.; Ravid R.; Verweij C. L.; Huitinga I.; Polman C. H.; Uitdehaag B. M. CCL5 and CCR5 genotypes modify clinical, radiological and pathological features of multiple sclerosis. J. Neuroimmunol. 2007, 190, 157–164. 10.1016/j.jneuroim.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Kanamori H.; Matsubara T.; Mima A.; Sumi E.; Nagai K.; Takahashi T.; Abe H.; Iehara N.; Fukatsu A.; Okamoto H.; Kita T.; Doi T.; Arai H. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem. Biophys. Res. Commun. 2007, 360, 772–777. 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- Gale J. D.; Gilbert S.; Blumenthal S.; Elliott T.; Pergola P. E.; Goteti K.; Scheele W.; Perros-Huguet C. Effect of PF-04634817, an oral CCR2/5 chemokine receptor antagonist, on albuminuria in adults with overt diabetic nephropathy. Kidney Int. Rep. 2018, 3, 1316–1327. 10.1016/j.ekir.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefere S.; Devisscher L.; Tacke F. Targeting CCR2/5 in the treatment of nonalcoholic steatohepatitis (NASH) and fibrosis: opportunities and challenges. Expert Opin. Invest. Drugs 2020, 29, 89–92. 10.1080/13543784.2020.1718106. [DOI] [PubMed] [Google Scholar]
- Lefebvre E.; Moyle G.; Reshef R.; Richman L. P.; Thompson M.; Hong F.; Chou H. L.; Hashiguchi T.; Plato C.; Poulin D.; Richards T.; Yoneyama H.; Jenkins H.; Wolfgang G.; Friedman S. L. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One 2016, 11, e0158156. 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. H. Progress in the discovery of CC chemokine receptor 2 antagonists, 2009 – 2012. Expert Opin. Ther. Pat. 2013, 23, 549–568. 10.1517/13543776.2013.771168. [DOI] [PubMed] [Google Scholar]
- Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J. Leukocyte Biol. 2010, 88, 41–55. 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- Fantuzzi L.; Tagliamonte M.; Gauzzi M. C.; Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell. Mol. Life Sci. 2019, 76, 4869–4886. 10.1007/s00018-019-03255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A.; Kokornaczyk A. K.; Strunz A. K.; Wünsch B. Selective and dual targeting of CCR2 and CCR5 receptors: a current overview. Top. Med. Chem. 2014, 14, 187–241. 10.1007/7355_2014_40. [DOI] [Google Scholar]
- Hou C.; Sui Z. Chapter 12. CCR2 antagonists for the treatment of diseases associated with inflammation, in anti-inflammatory drug discovery; Levin J. I., Laufer S., Eds.; RSC Publishing: Cambridge, UK, 2012; pp 350–390. [Google Scholar]
- Lemoine R.; Wanner J. Small molecule antagonists of the chemokine receptor CCR5. Curr. Top. Med. Chem. 2010, 10, 1299–1338. 10.2174/156802610791561219. [DOI] [PubMed] [Google Scholar]
- Deci M. B.; Ferguson S. W.; Scatigno S. L.; Nguyen J. Modulating macrophage polarization through CCR2 inhibition and multivalent engagement. Mol. Pharmaceutics 2018, 15, 2721–2731. 10.1021/acs.molpharmaceut.8b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y.; Mai J.; Li X.; Mitchell-Flack M.; Zhang T.; Zhang L.; Chouchane L.; Ferrari M.; Shen H.; Ma X. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017, 77, 2857–2868. 10.1158/0008-5472.CAN-16-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X.; Nawab O.; Patel T.; Kossenkov A. V.; Halama N.; Jaeger D.; Pestell R. G. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res. 2019, 79, 4801–4807. 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin N.; Razon H. The role of CCR5 in directing the mobilization and biological function of CD11b(+)Gr1(+)Ly6C(low) polymorphonuclear myeloid cells in cancer. Cancer Immunol. Immunother. 2018, 67, 1949–1953. 10.1007/s00262-018-2245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman J. G.; Nywening T. M.; Belt B. A.; Panni R. Z.; Krasnick B. A.; DeNardo D. G.; Hawkins W. G.; Goedegebuure S. P.; Linehan D. C.; Fields R. C. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology 2018, 7, e1470729. 10.1080/2162402X.2018.1470729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford D. E.; Belt B. A.; Panni R. Z.; Mayer A. B.; Deshpande A. D.; Carpenter D.; Mitchem J. B.; Plambeck-Suess S.; Worley L. A.; Goetz B. D.; Wang-Gillam A.; Eberlein T. J.; DeNardo D. G.; Goedegebuure P.; Linehan D. C. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: A role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 2013, 19, 3404–3415. 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. H.; Garstka M. A.; Li Z. F. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol. Immunol. 2020, 117, 201–215. 10.1016/j.molimm.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Yang M. G.; Xiao Z.; Cherney R. J.; Tebben A. J.; Batt D. G.; Brown G. D.; Chen J.; Cvijic M. E.; Dabros M.; Duncia J. V.; Galella M.; Gardner D. S.; Khandelwal P.; Ko S. S.; Malley M. F.; Mo R.; Pang J.; Rose A. V.; Santella J. B. 3rd; Shi H.; Srivastava A.; Traeger S. C.; Wang B.; Xu S.; Zhao R.; Barrish J. C.; Mandlekar S.; Zhao Q.; Carter P. H. Use of a conformational-switching mechanism to modulate exposed polarity: discovery of CCR2 antagonist BMS-741672. ACS Med. Chem. Lett. 2019, 10, 300–305. 10.1021/acsmedchemlett.8b00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. G.; Xiao Z.; Zhao R.; Tebben A. J.; Wang B.; Cherney R. J.; Batt D. G.; Brown G. D.; Cvijic M. E.; Duncia J. V.; Gallela M. A.; Gardner D. S.; Khandelwal P.; Malley M. F.; Pang J.; Rose A. V.; Santella J. B.; Sarjeant A. A.; Xu S.; Mathur A.; Mandlekar S.; Vuppugalla R.; Zhao Q.; Carter P. H. Discovery of BMS-753426: A potent orally bioavailable antagonist of CC chemokine receptor 2. ACS Med. Chem. Lett. 2021, 12, 969–975. 10.1021/acsmedchemlett.1c00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. H.; Cherney R. J.. N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-a] [1,3,5] triazin-4-ylamino)- 2-oxopyrrolidin-1-yl)cyclohexyl) acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes. Patent no. US 8,618,101B2, December 31 2013.
- Shamovsky I.; Connolly S.; David L.; Ivanova S.; Norden B.; Springthorpe B.; Urbahns K. Overcoming undesirable hERG potency of chemokine receptor antagonists using baseline lipophilicity relationships. J. Med. Chem. 2008, 51, 1162–1178. 10.1021/jm070543k. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Qin L.; Zacarias N. V. O.; de Vries H.; Han G. W.; Gustavsson M.; Dabros M.; Zhao C.; Cherney R. J.; Carter P.; Stamos D.; Abagyan R.; Cherezov V.; Stevens R. C.; IJzerman A. P.; Heitman L. H.; Tebben A.; Kufareva I.; Handel T. M. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature 2016, 540, 458–461. 10.1038/nature20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnicoff M. J.; Horan P. K.; Morahan P. S. Kinetics of changes in peritoneal cell populations following acute inflammation. Cell. Immunol. 1989, 118, 178–191. 10.1016/0008-8749(89)90367-5. [DOI] [PubMed] [Google Scholar]
- Sullivan T.; Miao Z.; Dairaghi D. J.; Krasinski A.; Wang Y.; Zhao B. N.; Baumgart T.; Ertl L. S.; Pennell A.; Seitz L.; Powers J.; Zhao R.; Ungashe S.; Wei Z.; Boring L.; Tsou C. L.; Charo I.; Berahovich R. D.; Schall T. J.; Jaen J. C. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Renal Physiol. 2013, 305, F1288–F1297. 10.1152/ajprenal.00316.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe J.; Robins R. K.; O’Brien D. E. The synthesis and chemical reactions of certain pyrazolo[1,5-a]-1,3,5-triazines. J. Heterocycl. Chem. 1974, 11, 199–204. 10.1002/jhet.5570110217. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.