Abstract
We have synthesized series of 2-prenylated benzopyrans as analogues of the natural polycerasoidol, a dual PPARα/γ agonist with anti-inflammatory effects. The prenylated side chain consists of five or nine carbons with an α-alkoxy-α,β-unsaturated ester moiety. Prenylation was introduced via the Grignard reaction, followed by Johnson–Claisen rearrangement, and the α-alkoxy-α,β-unsaturated ester moiety was introduced by the Horner–Wadsworth–Emmons reaction. Synthetic derivatives showed high efficacy to activate both hPPARα and hPPARγ as dual PPARα/γ agonists. These prenylated benzopyrans emerge as lead compounds potentially useful for preventing cardiometabolic diseases.
Keywords: Prenylated benzopyrans, Horner−Wadsworth−Emmons reaction, PPARα/γ activity
Among the nuclear receptor family, peroxisome proliferator activated receptors (PPARs) are transcription factors activated by ligands, which are implicated in numerous cell functions including glucose and lipid metabolism and inflammation.1 Dual PPARα/γ activators can improve atherogenic dyslipidemia and insulin resistance.1 Polycerasoidol is a trans-δ-tocotrienolic acid analogue2 isolated from Polyalthia cerasoides (Annonaceae).3,4 Polycerasoidol, containing a 6-chromanol nucleus and a 2-prenylated side chain, is biosynthesized from homogentisate and geranylgeranyl pyrophosphate via the shikimate pathway and the mevalonate pathway, respectively (Figure 1). Pharmacologically, it displays dual PPARα/γ agonist activity and ameliorates inflammation of dysfunctional endothelium.5 In structural terms, this natural benzopyran possesses a benzopyran nucleus (lipophilic tail), a prenylated chain (flexible linker), and a carboxylic acid (polar head), as do other natural and synthetic PPARα and/or PPARγ agonists.6 The structure–activity relationships (SARs) of polycerasoidol and semisynthetic analogues revealed that the 6-oxygenated dihydrobenzopyran core and the linker hydrocarbon chain of at least a five-carbon length are essential moieties to activate PPARα and/or PPARγ.7 It is noteworthy that the chroman-6-ol pharmacophore is present in troglitazone, the first PPARγ agonist belonging to the class of thiazolidinediones, which was approved as an antidiabetic agent.8,9 Currently, rosiglitazone and pioglitazone are selective PPARγ agonists used to manage hyperglycemia in type 2 diabetes, while saroglitazar and lobeglitazone are PPARα/γ activators (agonists) approved against diabetes in India and South Korea, respectively.9 In order to find new active and safer PPAR activators, we describe the synthesis and PPAR activity of 2-prenylated benzopyrans that bear an α-alkyloxy-α,β-unsaturated ester as a trioxygenated polar head on the prenylated side chain.
Figure 1.
Bioactive prenylated benzopyrans.
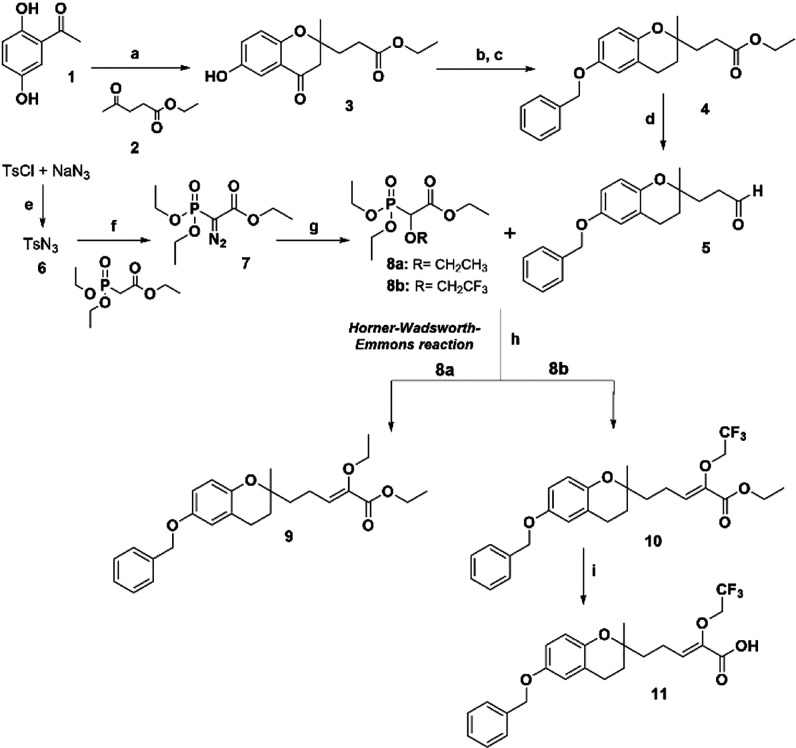
We first prepared the benzopyran-4-one nucleus (γ-benzopyrone 3) by conventional methods.10 The reaction mechanism consists of an aldol condensation between the enolate from an ortho-hydroxyacetophenone and the carbonyl group from an alkyl ketone. This is followed by an intramolecular cyclization via Michael addition, which is promoted by the ortho-phenol group.11 Thus, 2,5-dihydroxyacetophenone (1) and ethyl levulinate (2) in the presence of pyrrolidine gave the chroman-4-one 3 as a racemic mixture in a single step with a good yield (80%) (Figure 2).7,10 γ-Benzopyrone 3 was reduced under Clemmensen conditions, and the phenol hydroxyl group at 6-position was protected by O-benzylation to give compound 4 with a good overall yield. The controlled reduction of the ester function by DIBAL-H at −78 °C gave the aldehyde 5 with a 92% yield. In a first approach, we synthesized five-carbon side chain alkoxylated prenylated benzopyrans (series 1) from aldehyde intermediate 5 by Horner–Wadsworth–Emmons olefination. For this purpose, appropriate alkylphosphonates, e.g., ethyl 2-(diethoxyphosphoryl)-2-ethoxyacetate (8a) and ethyl 2-(diethoxyphosphoryl)-2-(2,2,2-trifluoroethoxy)acetate (8b), were previously prepared from (ethoxyacetate) diethoxyphosphorane and TsN3 (6) in the presence of NaH.12 Then, aldehyde intermediate 5 was reacted with phosphonate 8a or 8b to afford the α-alkyloxy-α,β-unsaturated esters of prenylated benzopyrans 9 (20%, Z/E = 60:40) or 10 (84%, Z/E = 35:65), respectively. The saponification of ethyl ester (10) quantitatively afforded carboxylic acid (11, Z/E = 40:60) (Scheme 1).
Figure 2.
hPPARα and hPPARγ transactivation assays. Synthesized benzopyrans were tested at 10 μM, and WY-14,643 (10 μM) and rosiglitazone (1 μM) are reference compounds for α and γ, respectively.
Scheme 1. Synthesis of Prenylated Benzopyrans 9–11 (Series 1).
Reaction conditions: (a) Pyrrolidine, EtOH, 60 °C, molecular sieve 3 Å, 24 h, 80%; (b) Zn/HCl, AcOH-H2O, rt, 2 h; (c) ClCH2C6H5, K2CO3, EtOH, 60 °C, 5 h, 90%; (d) DIBAL-H, CH2Cl2, −78 °C, N2, 20 min, 92%; (e) 0 °C, acetone, 2 h, 81%; (f) 60% NaH, THF, 0 °C, N2, 16 h, 65%; (g) EtOH, Rh(OAc)2, toluene, 45 °C, overnight, 8a (R = CH2CH3), 40.2% or 8b (R = CH2CF3), 51%; (h) phosphonate 8a or 8b, THF, NaH, 0 °C, N2, 1 h + 5, THF, rt, overnight: 9, 20% or 10, 84%; (i) 20% KOH, reflux, 5 h, 99%.
In a second approach, we synthesized the nine-carbon side chain O-alkoxylated prenylated benzopyrans (series 2) 15, 16, and 17 from aldehyde intermediate 5. The prenylated side chain at the 2-position of the dihydrobenzopyran nucleus was elongated with a sequence of Grignard reaction, Johnson–Claisen rearrangement and Horner–Wadsworth–Emmons olefination (Scheme 2). The aldehyde synthon 5 was treated with isoprenylmagnesium bromide as the vinyl Grignard reagent, followed by Johnson–Claisen rearrangement of allylic alcohol 12 using ethyl orthoacetate to produce unsaturated ester 13 with a 50% yield in the last two steps. Ester 13 was subjected to a controlled reduction using DIBAL-H at −78 °C to give the aldehyde intermediate 14 in 89% yield. The α-alkoxy-α,β-unsaturated ester on the prenylated side chain was introduced by a Horner–Wadsworth–Emmons reaction.13 Thus, aldehyde 14 was treated with phosphonates 8a and 8b to afford esters 15 (85%) and 16 (82%), respectively.14 It is noteworthy that ester 15 was obtained as a Z-alkene isomer exclusively. Once again, the saponification of ethyl ester 15 quantitatively yielded carboxylic acid 17 (Scheme 2). In addition to O-benzyloxy benzopyrans bearing a nine-carbon prenylated alkoxy side chain (series 2), we accomplished the synthesis of its O-propyloxy and O-p-fluorobenzyloxy benzopyran analogues. According to the second approach followed to prepare ester 15, but starting from the chroman-4-one 3, we synthesized O-propyloxy ester 18 and O-p-fluorobenzyloxy ester 19 (Scheme 3).
Scheme 2. Synthesis of Prenylated Benzopyrans 15–17 (Series 2).
Reaction conditions: (a) CH3C(MgBr)=CH2, THF, −78 °C, N2, 1 h, 84%; (b) MeC(OEt)3, isobutyric acid, 140 °C, 2 h, 50%; (c) DIBAL-H, CH2Cl2, −78 °C, N2, 20 min, 89%; (d) phosphonate 8a or 8b, THF, NaH, 0 °C, N2, 1 h + 14 in THF, rt, overnight: 15 (R = CH2CH3), 85% or 16 (R = CH2CF3), 82%; (e) 20% KOH, reflux, 5 h, 99%.
Scheme 3. Synthesis of Prenylated Benzopyrans 18 and 19.
Reaction conditions: (a) See reagents and conditions described in Scheme 1 for the synthesis of 5, and Scheme 2 for the synthesis of 15 and 16.
The transactivation studies15 of the synthesized benzopyrans were carried out and compared to the maximal efficacy of WY-14,643 (at 10 μM) or rosiglitazone (at 1 μM) as hPPARα and hPPARγ reference compounds, respectively. At the 10 μM dose, compounds 10, 15, 16, and 18 were moderate hPPAR activators for both receptors while compounds 11, 17, and 19 showed high efficacy as dual hPPARα/γ agonists. Indeed, compound 11 showed higher efficacy to activate hPPARα than PPARγ did (α/γ ratio = 1.73), and 17 displayed slight selectivity toward hPPARγ (α/γ ratio = 0.64). Therefore, the elongation of the side chain from five to nine carbons is beneficial to activate hPPARγ. In agreement with a previous docking analysis of polycerasoidol, the carboxylic moiety at the C-9′ position of 17 plays a key role as an anchoring point to bind the PPARγ receptor.5
In conclusion, we efficiently prepared new series of the 2-prenylated O-alkoxylated benzopyrans possessing the α-alkoxy-α,β-unsaturated moiety on the prenylated chain by the Horner–Wadsworth–Emmons reaction. Synthetic derivatives were efficient in activating both hPPARα and hPPARγ as dual PPARα/γ agonists. These prenylated benzopyrans emerge as lead compounds that might be potentially useful for preventing cardiometabolic diseases.
Acknowledgments
We are grateful for the financial support from Generalitat Valencia (APOTIP/2020/011), Carlos III Health Institute (ISCIII), and the European Regional Development Fund (CP15/00150 and PI18/01450) and from Agence Nationale pour la Recherche ANR-10 LABX-0046 and an ERC Avanced Grant (694717). N.C. is a “Miguel Servet” program researcher (CP15/00150, CPII20/00010) of the ISCIII cofunded by the European Social Fund. C.V.-V. is thankful for the PFIS grant (FI19/00153) of ISCIII.
Glossary
Abbreviations
- PPARs
peroxisome proliferator-activated receptors
- SAR
structure–activity relationships
- DIBAL-H
diisobutylaluminum hydride
- TsN3
tosyl azide
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters virtual special issue “Medicinal Chemistry in Portugal and Spain: A Strong Iberian Alliance”.
References
- Villarroel-Vicente C.; Gutiérrez-Palomo S.; Ferri J.; Cortes D.; Cabedo N. Natural products and analogs as preventive agents for metabolic syndrome via peroxisome proliferator-activated receptors: An overview. Eur. J. Med. Chem. 2021, 221, 113535. 10.1016/j.ejmech.2021.113535. [DOI] [PubMed] [Google Scholar]
- Azzi A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. 10.1016/j.redox.2019.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M. C.; Serrano A.; Zafra-Polo M. C.; Cortes D.; Rao K. S. Polycerasoidin and polycerasoidol, two new prenylated benzopyran derivatives from Polyalthia cerasoides. J. Nat. Prod. 1995, 58, 1278–1284. 10.1021/np50122a022. [DOI] [Google Scholar]
- Zafra-Polo M. C.; González M. C.; Tormo J. R.; Estornell E.; Cortes D. Polyalthidin: new prenylated benzopyran inhibitor of the mammalian mitochondrial respiratory chain. J. Nat. Prod. 1996, 59, 913–916. 10.1021/np960492m. [DOI] [PubMed] [Google Scholar]
- Bermejo A.; Collado A.; Barrachina I.; Marques P.; El Aouad N.; Franck X.; Garibotto F.; Dacquet C.; Caignard D.-H.; Suvire F.; Enriz R. D.; Piqueras L.; Figadère B.; Sanz M. J.; Cabedo N.; Cortes D. Polycerasoidol, a natural prenylated benzopyran with a dual PPARα/PPARγ agonist activity and anti- inflammatory effect. J. Nat. Prod. 2019, 82, 1802–1812. 10.1021/acs.jnatprod.9b00003. [DOI] [PubMed] [Google Scholar]
- Mirza A. Z.; Althagafi I. I.; Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Bermejo A.; Barrachina I.; El Aouad N.; Franck X.; Chahboune N.; Andreu I.; Figadère B.; Vila L.; Hennuyer N.; Staels B.; Dacquet C.; Caignard D.-H.; Sanz D M. J.; Cortes D.; Cabedo N. Synthesis of benzopyran derivatives as PPARα and/or PPARγ activators. Bioorg. Med. Chem. 2019, 27, 115162. 10.1016/j.bmc.2019.115162. [DOI] [PubMed] [Google Scholar]
- Tafazoli S.; Wright J. S.; O'Brien P. J. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem. Res. Toxicol. 2005, 18, 1567–1574. 10.1021/tx0500575. [DOI] [PubMed] [Google Scholar]
- Yasmin S.; Jayaprakash V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: From past to present. Eur. J. Med. Chem. 2017, 126, 879–893. 10.1016/j.ejmech.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Pearce B. C.; Parker R. A.; Deason M. E.; Dischino D. D.; Gillespie E.; Qureshi A. A.; Volk K.; Wright J. J. K. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. J. Med. Chem. 1994, 37, 526–541. 10.1021/jm00030a012. [DOI] [PubMed] [Google Scholar]
- Xiong W.; Wang X.; Shen X.; Hu C.; Wang X.; Wang F.; Zhang G.; Wang C. Synthesis of flavonols via pyrrolidine catalysis: Origins of the selectivity for flavonol versus aurone. J. Org. Chem. 2020, 85, 13160–13176. 10.1021/acs.joc.0c01869. [DOI] [PubMed] [Google Scholar]
- General procedure for the synthesis of alkylphosphonates: Synthesis of ethyl 2-(diethoxyphosphoryl)-2-ethoxyacetate (8a). Ethyl 2-diazo-2-(diethoxyphosphoryl) acetate (7, 379 mg, 1.503 mmol) in ethanol (900 μL) was stirred, and rhodium acetate (II) (6.66 mg, 0.015 mmol) in toluene (8 mL) was added to this solution. The mixture was stirred at 45 °C overnight. Usual workup followed by column chromatography purification (ethyl ether/ EtOAc 98:2) gave 162 mg of 8a (40.2%).
- Wadsworth W. S. Jr Synthetic Applications of Phosphoryl-Stabilized Anions. Org. React. 1977, 25, 73–253. 10.1002/0471264180.or025.02. [DOI] [Google Scholar]
- General procedure for Horner–Wadsworth–Emmons reaction. Synthesis of 6-benzyloxy-2-((8′-ethoxy-4′-methyl)ethylnona-3′E, 7′Z-dienoate)-2-methyl-dihydrobenzopyran (15): Ethyl 2-(diethoxyphosphoryl)-2-ethoxyacetate (8a) (0.156 mmol) in anhydrous THF (4 mL) at 0 °C under N2 atmosphere was treated with 60% NaH (10.6 mg, 0.265 mmol), and the mixture was stirred for 1 h at 0 °C. Then, a solution of aldehyde (59 mg, 0.156 mmol) in anhydrous THF (5 mL) was added and the mixture was stirred overnight at room temperature. Usual workup followed by column chromatography purification (hexane/ EtOAc 80:20) yielded 64.5 mg of 15 (85%) as a colorless oil: 1H NMR (300 MHz, CDCl3): δ 7.36 (m, 5H, H-2″ to 6″), 6.75 (dd, J = 7.1, 2.3 Hz, 1H, H-7), 6.72 (d, J = 7.1 Hz, 1H, H-8), 6.69 (d, J = 2.3 Hz, 1H, H-5), 6.23 (t, J = 7.4 Hz, 1H, H-7′), 5.16 (m, 1H, H-3′), 4.99 (s, 2H, OCH2Ar), 4.21 (q, J = 7 Hz, 2H, COOCH2CH3), 3.85 (q, J = 7 Hz, 2H, OCH2CH3), 2.73 (t, J = 6.8 Hz, 2H, CH2-4), 2.34 (q, J = 7.4 Hz, 2H, CH2-6′), 2.09 (m, 4H, CH2-2′, CH2-5′), 1.81 (m, 2H, CH2-3), 1.62 (m, 2H, CH2-1′), 1.60 (s, 3H, CH3-10′), 1.30, 132 (2t, J = 7 Hz, 3H, OCH2CH3, COOCH2CH3), 1.27 (s, 3H, CH3–11′). 13C NMR (75 MHz, CDCl3): δ 164.6 (CO), 152.5 (C-6), 148.5 (C-8a), 145.4 (C-8′), 137.9 (C-1″), 134.5 (C-4′), 129.1 (CH-7′), 128.9, 128.2, 127.9 (CH-2″ to CH-6″), 125.4 (CH-3′), 122.0 (C-4a), 116.4 (CH-7), 115.5 (CH-5), 114.7 (CH-8), 76.0 (C-2), 71.0 (OCH2Ar), 68.4 (OCH2CH3), 61.1, (COOCH2CH3), 40.3 (CH2-5′), 39.7 (CH2–1′), 31.1 (CH2-3), 25.4. (CH2-6′), 24.5 (CH3-11′), 22.8 (CH2-4), 22.6 (CH2-2′), 16.2 (CH3-10′), 15.8 (COOCH2CH3), 14.9 (OCH2CH3). HREIMS m/z 492.2869 [M]+ (492.2875 calcd. for C31H40O5) (100%).
- Amans D.; Bellosta V.; Dacquet C.; Ktorza A.; Hennuyer N.; Staels B.; Caignard D.-H.; Cossy J. Synthesis and evaluation of new polyenic compounds as potential PPARs modulators. Org. Biomol. Chem. 2012, 10, 6169–6185. 10.1039/c2ob25593f. [DOI] [PubMed] [Google Scholar]