Abstract
Objective:
Spinal cord ependymomas account for 3–6% of all central nervous system tumors and around 60% of all intramedullary tumors. The aim of this study was to analyze the neurological outcome after surgery and to determine prognostic factors for functional outcome.
Patients and Methods:
Patients treated surgically due to a spinal cord ependymoma between 1990 and 2018 were retrospectively included. Demographics, neurological symptoms, radiological parameters, histopathology, and neurological outcome (using McCormick Score [MCS]) were analyzed. Possible prognostic factors for neurological outcome were evaluated.
Results:
In total, 148 patients were included (76 males, 51.4%). The mean age was 46.7 ± 15.3 years. The median follow-up period was 6.8 ± 5.4 years. The prevalence was mostly in the lumbar spine (45.9%), followed by the thoracic spine (28.4%) and cervical spine (25.7%). Gross-total resection was achieved in 129 patients (87.2%). The recurrence rate was 8.1% and depended on the extent of tumor resection (p = 0.001). Postoperative temporary neurological deterioration was observed in 63.2% of patients with ependymomas of the cervical spine, 50.0% of patients with ependymomas of the thoracic spine, and 7.4% of patients with ependymomas of the lumbosacral region. MCS 1–2 was detected in nearly two-thirds of patients with cervical and thoracic spinal cord ependymoma 36 months after surgery. Neurological recovery was superior in thoracic spine ependymomas compared with cervical spine ependymomas. Poor preoperative functional condition (MCS >2), cervical and thoracic spine location, and tumor extension >2 vertebrae were independent predictors of poor neurological outcome.
Conclusion:
Neurological deterioration was seen in the majority of cervical and thoracic spine ependymomas. Postoperative improvement was less in thoracic cervical spine ependymomas compared with thoracic spine ependymomas. Poor preoperative status and especially tumor extension >2 vertebrae are predictors of poor neurological outcome (MCS >2).
Keywords: neurological outcome, outcome prediction, predictors, spinal ependymoma, surgery
Introduction
Spinal cord ependymomas are usually slow-growing tumors arising from ependymal cells of the central canal of the spinal cord. 1 Ependymomas account for 3–6% of all central nervous system tumors and are the most frequent spinal cord neoplasm in adults, presenting 60% of all intramedullary tumors.2–5
Symptom presentation is related to the tumor location and can include radicular or local pain, motor weakness of the extremities, hypoesthesia, gait disturbance, and sphincter or sexual dysfunction.6–8 Non-specificity of symptoms can lead to adaptation to symptoms and late diagnosis. Cervical tumors can present symptoms of upper or lower extremities, due to the corticospinal tract or dorsal column being affected. 9 Symptom duration depends on tumor location and symptom characteristics, with back pain being the most common symptom. The average symptom duration described in the literature is around 2 years.10–12 In rare cases, an acute deterioration of the symptoms can be provoked by intratumoral hemorrhage.13–15
The World Health Organization (WHO) grading system includes three ependymoma subtypes: WHO grade I: the myxopapillary ependymoma and the subependymoma; WHO grade II: ‘classic’ ependymoma including papillary, clear cell, and tanycytic subtypes; and WHO grade III: anaplastic ependymoma. 16
The ‘classic’ ependymoma is the most common in the spinal cord with a frequency of 55–75%.9,13 Benign ependymomas (WHO grade I) and semi-benign ependymomas (WHO grade II) have well-defined margins that allow microsurgical tumor removal without damaging the spinal cord tissue. In contrast, anaplastic ependymomas (WHO III) are infiltrative and only subtotal resection is possible.13,17,18 The prognosis based on WHO grading alone is difficult due to the heterogeneity of ependymomas and its tumor characteristics.1,6,13
The long-term survival and prognostic factors for tumor-free survival in spinal cord ependymomas have been thoroughly investigated.
According to current gold standard, surgical resection remains the therapy of choice in spinal cord ependymomas, especially for patients presenting with neurological impairments.
Postoperative neurological deterioration remains a major problem that might be reduced further as surgical techniques continue to advance. 19 Despite the well-known neurological deterioration after surgery, studies focusing on predictors of neurological outcome are scarce because of the small sample size.6,9,20,21
This study is one of the largest single-center studies of a European neurosurgical center and aims to describe the tumor entity and the surgical course. In addition, we focused on factors causing postoperative functional deterioration and tried to identify predictive factors with impact on the postoperative neurological outcome.
Patients and methods
Study population
A retrospective analysis of the electronic database ‘spinal neoplasm’ evaluating the clinical and radiological data and operative reports of patients suffering from a spinal ependymoma who attended to our department between 1990 and 2018 was performed. Only patients suffering from primary spinal cord ependymoma were included in the analysis. Patients with primary cerebral ependymoma and secondary spinal cord metastasis or leptomeningeal metastasis were excluded.
Evaluated parameters
The demographics, symptom duration until surgery, neurological symptoms such as pain, sensory deficits, motor deficits (monoparesis/hemiparesis and paraparesis), gait disturbance, and bladder dysfunction for each case were noted. Radiological parameters including tumor location (intramedullary and extramedullary, cervical, thoracic, and lumbar), tumor size in terms of size expanding over the number of vertebrae, and volume (cm3) according to preoperative magnetic resonance imaging (MRI) were evaluated. Gross-total resection (GTR) was defined as complete tumor removal, showing no tumor remnants in the early postoperative MRI with contrast. Subtotal tumor resection (STR) was present if the early postoperative MRI showed tumor remnants.
Patients’ neurological status was evaluated using the McCormick Score (MCS I: neurologically normal; mild focal deficit not significantly affecting the function of the involved limb; mild spasticity or reflex abnormality; normal gait; MCS II: presence of sensorimotor deficit affecting the function of the involved limb; mild to moderate gait difficulty; severe pain or dysesthetic syndrome impairing patient’s quality of life; still functions and ambulates independently; MCS III: more severe neurological deficit; requires cane/brace for ambulation or significant bilateral upper extremity impairment; may or may not function independently; MCS IV: severe deficit; requires wheelchair or cane/brace with bilateral upper extremity impairment; usually not independent). 22 The MCS was retrospectively derived from clinical data at the beginning of the observational study. Later, MCS was routinely used in clinical practice. The MCS was modified according to the current literature20,23 to allow a more valuable discrimination in postoperative neurological outcome: ‘good’ was defined as MCS I + II and ‘poor’ was defined as MCS III + IV. A cut-off value of MCS >2 was chosen because patients suffer from moderate neurological deficits with limitations in function. Furthermore, external aid may be needed. Patients with MCS ⩽2 do not show severe neurological deficits and do not need external help.
According to our clinical standard of care, neurological examination was performed routinely preoperatively, postoperatively on the last day at the hospital, and 3 months, 6 months, 12 months, 24 months, and 36 months after surgery. Furthermore, postoperative status including MCS was assessed 3, 5, and 10 years after surgery. Postoperative MRI was performed within 72 h after surgery. Further MRI controls were performed every 3–6 months.
Surgical treatment
The surgery was performed in microsurgical fashion using a standard dorsal approach in prone position for lesions located at the thoracal and lumbar spine. Semi-sitting position was favored in cervical spine tumors. Laminoplasty was routinely performed, whereas laminectomy was performed in cases where refixation of the laminae was deemed unfavorable (distinct osteoporosis or vertebral deformity). Hemilaminectomy was indicated in lateral located tumors and usually in lumbar filum terminal ependymomas. Surgical removal of the ependymoma was performed with the aid of intraoperative monitoring (somatosensory evoked potentials and motor evoked potentials). The tumor was removed piecemeal-like, beginning from the center to the well-defined margins and the surrounding spinal cord tissue (Figures 1–3). Patients were mobilized after a bed rest of 3 days to avoid postoperative cerebrospinal fluid fistula. Tumor analysis was performed at the Department of Neuropathology of the University Hospital Essen.
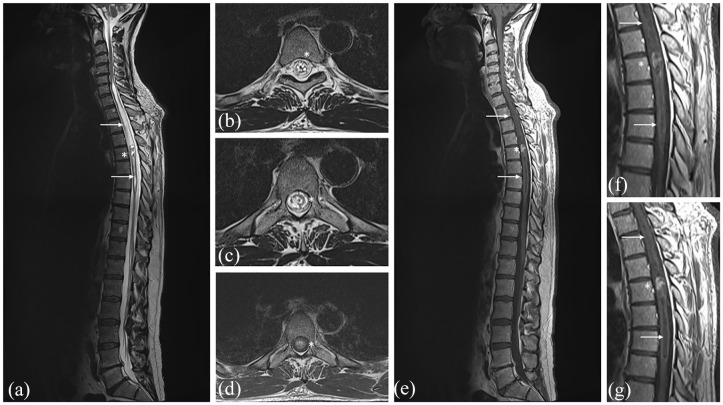
Figure 1.
Preoperative T2-weighted MRI showing the intramedullary ependymoma (*) at Th 5 with intertumoral hemorrhage and edema of the spinal cord (→) from C7-Th7 (a-c). T1-weighted MRI with contrast showing the contrast enhancement of the ependymoma (d–g).
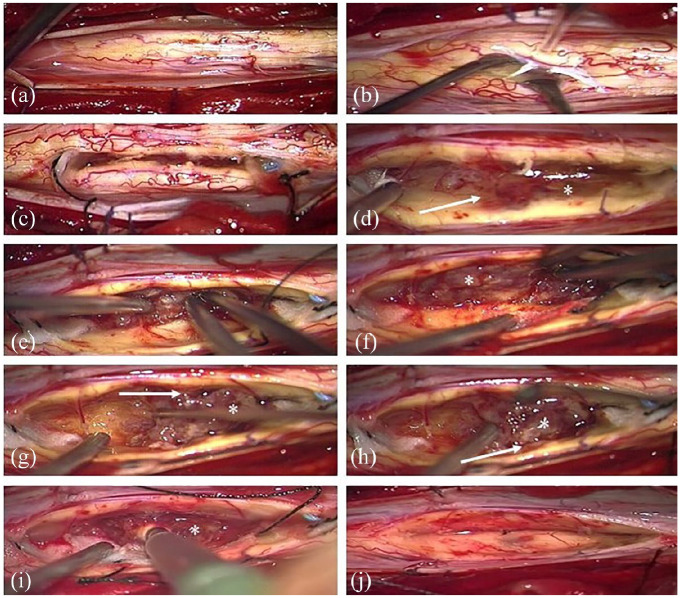
Figure 2.
The spinal cord is exposed after dura opening and bulged due to the intramedullary tumor (a). Myelotomy performed medially (b). The cranial and caudal boundary (see tidal flats) of the tumor is prepared (c). The margins (→) of the grayish tumor (*) are well defined (d). Debulking of the tumor and piecemeal removal using a CUSA with preservation of the surrounding spinal cord tissue (e–i). Spinal cord after complete tumor removal (j).
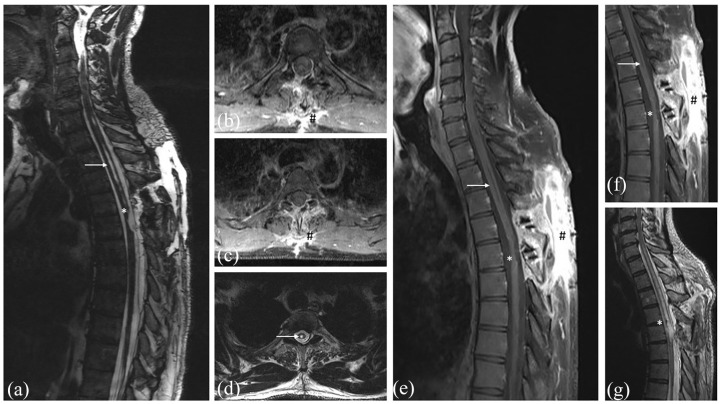
Figure 3.
Early postoperative T2-weighted MRI without contrast (a + d) and T1-weighted MRI with contrast (b + c) 24 h after surgery with completely removed ependymoma. T1-weighted MRI with contrast (e + f) 6 months after surgery showing no tumor recurrence (*) despite the normal contrast enhancement at the dorsal approach (#). T2-weighted MRI without contrast showing the postoperative changes of the spinal cord (*) and the smaller edema of the spinal cord (→) (g).
Statistics
Data were analyzed using SPSS 25.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA) for Windows. Metric data were described by mean and standard deviation and nominal data by frequency and valid percentage. P values <0.05 in two-sided testing were considered significant.
Demographic, clinical, and radiographic parameters were analyzed in a univariate way regarding their association or correlation with preoperative and postoperative McCormick Score. Therefore, Pearson’s χ2 statistics or Fisher’s exact test was used for dichotomous variables. For non-normally distributed data, the Kendall’s tau-b was assessed for continuous and ordinal, Spearman’s rho for continuous and dichotomous, and Mann–Whitney U test for ordinal and continuous variables. Significant parameters selected through univariate analysis and parameters with p values <0.1 were subsequently evaluated using multivariate analysis.
The neurological outcome was analyzed based on the tumor location: cervical, thoracic, and lumbar. Patients who were lost to follow-up were not included in statistical analysis at those time points.
Results
Clinical characteristics
Over a period of 28 years, 148 patients [72 females (48.6%) and 76 males (51.4%)] suffering from spinal cord ependymoma underwent surgery in our institute. The mean age was 46.7 ± 15.3 years, ranging from 9 to 83 years. Four patients were 16 years and younger. The mean follow-up was 6.8 ± 5.4 years (up to 27 years). However, 13 patients (8.8%) were lost to follow-up 12 months after surgery and 23.0% (34 patients) 36 months after surgery.
The most frequently involved localization was the lumbar-sacral region (45.9%), followed by the thoracic (28.4%) and cervical region (25.7%). The tumor was located intramedullary in 59.5% (Table 1).
Table 1.
Demographic, surgical, and tumor characteristics.
| Patients’ characteristic | P value | |
|---|---|---|
| Number of patients | 148 | |
| Age (years) | 46.7 ± 15.3 | |
| Sex (female) | 72 (48.6%) | |
| Duration of symptoms (months) | 29.4 ± 57.3 | |
| Tumor characteristic | P value | |
| Tumor location | ||
| Intramedullary | 88 (59.5%) | |
| Extramedullary | 60 (40.5%) | |
| Tumor location | ||
| Cervical | 38 (25.7%) | |
| Thoracic | 42 (28.4%) | |
| Lumbar | 68 (45.9%) | |
| WHO grade I | 70 (47.3%) | |
| WHO grade II | 74 (50.0%) | |
| WHO grade III | 4 (2.7%) | |
| Cervical: WHO grade (I/II/III) | (7/30/1)/38 | p = 0.0001 |
| Thoracic: WHO grade (I/II/III) | (17/24/1)/42 | |
| Lumbar: WHO grade (I/II/III) | (46/20/2)/68 | |
| Tumor recurrence | P value | |
| Total tumor recurrence | 12 (8.1%) | p = 0.024 |
| Cervical spine | 0/38 (0%) | |
| Thoracic spine | 7/42 (16.7%) | |
| Lumbar spine | 5/68 (7.4%) | |
| Tumor recurrence after GTR | 5/129 (6.0%) | p = 0.0001 |
| Tumor recurrence after STR | 7/19 (36.8%) | |
| Surgical characteristics | P value | |
| Surgical approach | ||
| Laminoplasty | 76 (51.4%) | |
| Laminectomy | 70 (47.3%) | |
| Hemilaminectomy | 2 (1.4%) | |
| GTR | 129/148 (87.2%) | |
| Cervical Spine | 33/38 (86.8%) | p = 0.100 |
| Thoracic Spine | 33/42 (78.6%) | |
| Lumbar Spine | 63/68 (92.6%) |
GTR, gross-total resection; STR, subtotal tumor resection; WHO, World Health Organization.
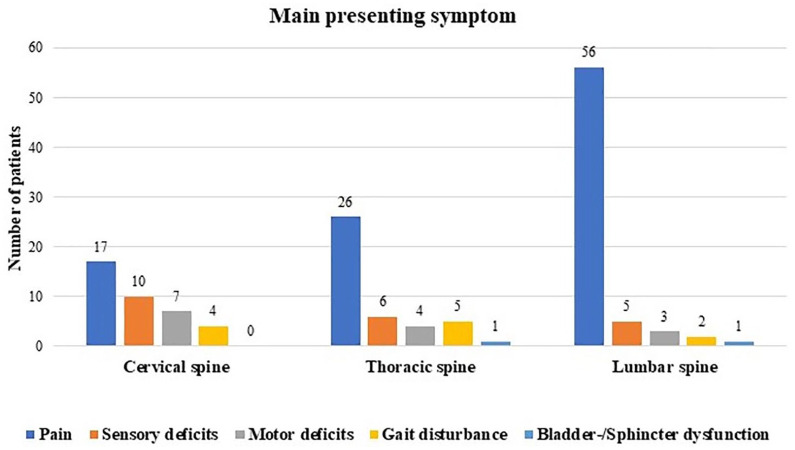
The average duration of symptoms until surgical treatment was 29.4 ± 57.3 months. The presenting symptom was pain in 67.6%. Radiating pain was presented in the majority of the cases (53.5%), while back pain was described in 34.3%. In 12.1%, the symptom could not be specified by the patients. Sensory deficits and motor deficits were described in 14.2% and 9.5%. Gait disturbances were complained in 7.4%. Only two patients (1.4%) reported sphincter and bladder dysfunction. The main presenting symptom depended on tumor location. Pain was more often seen in patients with ependymomas located in the lumbar spine (83.8%). Sensory deficits (26.3%) and motor deficits (18.4%) are more common in cervical spine ependymomas. Gait disturbance was detected in cervical and thoracic spine ependymomas, whereas sphincter and bladder dysfunction were observed in thoracic and lumbar spine tumors (Graph 1).
Graph 1.
Main presenting symptoms according to tumor location.
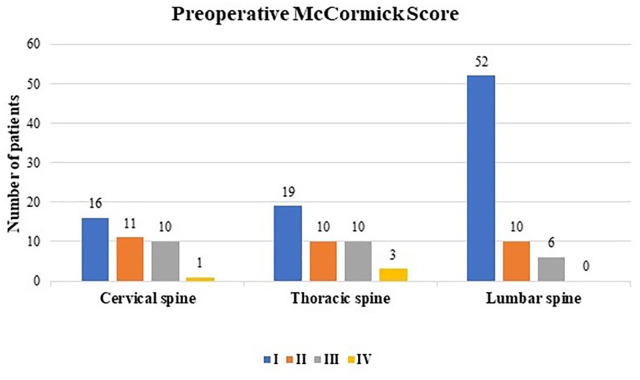
The preoperative functional status according to MCS was generally good (MCS I: 58.8% and MCS II: 20.9%). Only a few patients presented with severe neurological impairments (MCS III: 17.6% and MCS grade IV: 2.7%) prior to surgery. Worse preoperative MCS (III + IV) was seen more often in patients with cervical and thoracic ependymomas (Graph 2).
Graph 2.
Preoperative McCormick Score (I–IV) in relation to the affected spine level.
Surgery
Laminectomy was chosen in 47.3% of the cases and mainly performed in the 1990s and the early 2000s. Laminoplasty was the preferred approach in the later phase of the observation period. 24 The GTR was achieved in 129 of cases (87.2%), and the STR was performed in 19 cases (12.8%). Lumbar spine ependymomas were most commonly resected via GTR (92.6%) followed by cervical spine ependymomas (86.8%) and thoracic spine ependymomas (78.6%) (Table 1).
Surgical complications
A cerebrospinal fluid fistula prolonged the wound healing in 9 (6.1%) of 148 patients. There was no relation to the operative approach. Laminectomy was used in five cases and laminoplasty in four cases. The tumor was located in the cervical spine in two cases, in the thoracic spine in one case, in the thoracic-lumbar region in three cases, and in the lumbar spine in three cases. However, none of these patients required surgery and the fistula healed out completely with conservative treatment.
Histopathology
Ependymomas (WHO grade I) were diagnosed in 47.3% and ependymomas (WHO grade II) in 50.0%. Anaplastic ependymoma was detected in 2.7%. Ependymomas (WHO grade I) were more commonly located in the lumbar region, whereas tumors (WHO grade II) were more often diagnosed in cervical spine (Table 1).
Recurrence
Tumor recurrence was detected in 12 patients (8.1%) after a mean follow-up of 21.8 months (range, 1 month–4.6 years). One patient developed a spinal ependymoma 20 years after surgery at a completely different spinal location. A tumor recurrence occurred in 3.9% (5/129 cases) after GTR and in 36.8% after STR (7/119 cases), showing significantly higher rates of recurrence than after GTR (p = 0.0001). Histological examination confirmed a benign tumor (WHO grade I) in five cases, a semi-benign tumor (WHO grade II) in two cases and anaplastic ependymoma (WHO grade III) in five patients. All patients with recurrent tumor, except one, who were treated using radiotherapy after biopsy underwent a second surgery (Table 1).
Adjuvant therapy
Each case was discussed at the interdisciplinary tumor board. Postoperative radiotherapy was routinely offered to all patients with ependymomas WHO grades II and III. Of these patients, 15.6% of the patients with a grade II ependymoma (10/64 patients) and 100% of the patients with a grade III ependymoma underwent postoperative radiotherapy (Table 1).
Neurological outcome according to the spine level
Cervical spine
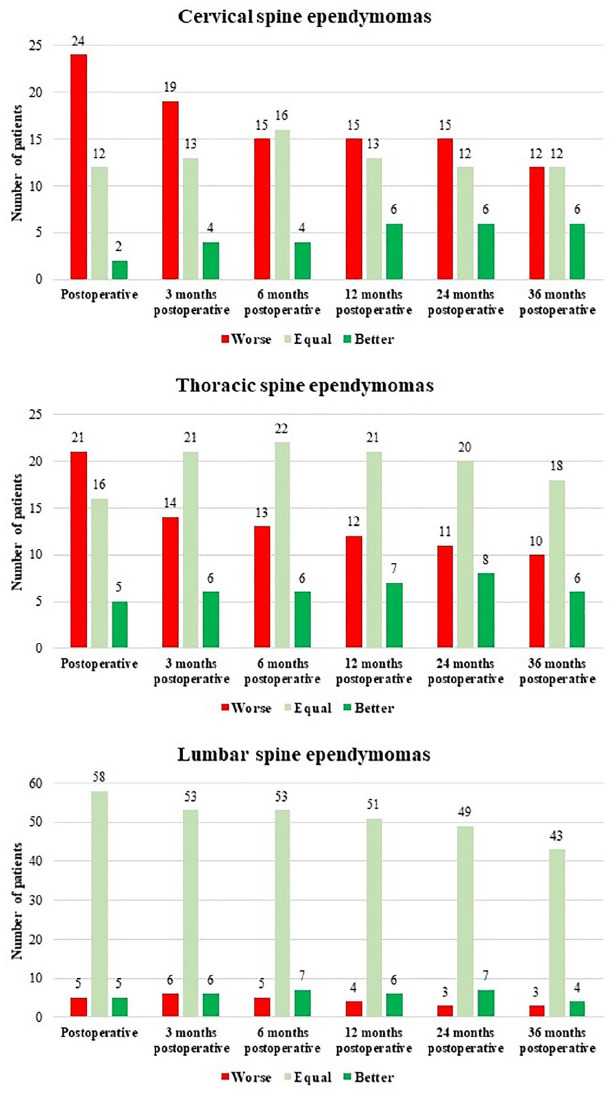
Preoperative MCS was ‘good’ in 71.1% and ‘poor’ in 28.91% (Table 2). Postoperative neurological deterioration was seen in the majority of the patients (63.2%). Of these, 40% did not reach preoperative neurological status (Graph 3). However, almost all patients (63.3%) recovered to the previous ‘good’ preoperative status. The ‘poor’ MCS consecutively decreased from 63.2% postoperatively to 48.6% 6 months and to 36.7% 36 months after surgery (Table 2).
Table 2.
Postoperative functional outcome according to the MCS.
| MCS | Preoperative n = 148 | Postoperative n = 148 |
3 months postoperatively n = 142 |
6 months postoperatively n = 141 |
12 months postoperatively n = 135 |
24 months postoperatively n = 131 |
36 months postoperatively n = 114 |
|
|---|---|---|---|---|---|---|---|---|
| Cervical | MCS I + II | 27 (71.1%) | 14 (36.8%) | 17 (47.2%) | 18 (51.4%) | 21 (61.7%) | 20 (60.6%) | 19 (63.3%) |
| MCS III + IV | 11 (28.9%) | 24 (63.2%) | 19 (52.8%) | 17 (48.6%) | 13 (38.3%) | 13 (39.4%) | 11 (36.7%) | |
| Thoracic | MCS I + II | 29 (69.0%) | 23 (54.7%) | 24 (58.5%) | 24 (58.5%) | 25 (62.5%) | 24 (61.5%) | 21 (61.8%) |
| MCS III + IV | 13 (31.0%) | 19 (45.3%) | 17 (41.5%) | 17 (41.5%) | 15 (37.5%) | 15 (38.5%) | 13 (38.2%) | |
| Lumbar | MCS I + II | 62 (91.2%) | 63 (92.6%) | 60 (92.3%) | 60 (92.3%) | 57 (93.4%) | 55 (93.2%) | 46 (92.0%) |
| MCS III + IV | 6 (8.8%) | 5 (7.4%) | 5 (7.7%) | 5 (7.7%) | 4 (6.6%) | 4 (6.8%) | 4 (8.0%) |
MCS, McCormick Score.
Graph 3.
Postoperative neurological status according to different spine levels.
Thoracic spine
Patients with thoracic spine ependymoma presented with ‘good’ preoperative MCS in 69.0%. The ‘good’ MCS decreased to 54.7% postoperatively and increased to 61.8% 36 months after surgery (Table 2). Neurological deterioration was seen in 50.0% postoperatively, while 38.1% remained stable and 11.9% reported direct postoperative improvement in neurological deficits. However, 36 months after surgery neurological deterioration was still present in 29.4%. Preoperative status was reached in 53.0%, while neurological improvement compared with the preoperative status was detected in 17.6% (Graph 3).
Lumbar spine
In contrast to patients suffering from cervical spine and thoracic spine ependymomas, preoperative MCS was ‘good’ in general (91.29%). A ‘poor’ preoperative MCS was only detected in 8.8%. Postoperative MCS remained ‘good’ in the majority of the cases (92.6%). A ‘good’ MCS was seen in 92.0% 36 months after surgery (Table 2).
Postoperative deterioration was only seen in 7.4%, while 85.2% showed stable neurological status or improvement in status after surgery (7.4%). Neurological deterioration was observed in only 6% 36 months after surgery, while 86.0% remained unchanged or improved their neurological status (8.0%) (Graph 3).
Analysis of possible predictors of neurological outcome
Complete cohort
Univariate analysis of the total cohort revealed that poor preoperative functional condition (MCS > 2), WHO grades II and III, tumor volume (cm3), spine segment (cervical and thoracic spine), tumor extension >2 vertebras, and STR had a high significant impact on poor neurological outcome (p < 0.05).
In addition, neurological symptoms (pain, paresis, and ataxia) at onset of the disease were negatively associated with postoperative outcome (p < 0.05), whereas sensory disorders had no effect (p > 0.05) on patients’ outcome. Symptom duration also showed no significant correlation with poor neurological outcome (p > 0.05) (Supplementary Table 3).
Multivariate analysis confirmed that preoperative status (MSC >2) was a negative predictor of neurological outcome at all analyzed time points. Tumor extension >2 vertebrae was a negative predictor until 24 months after surgery. In addition, STR was also a negative predictor 36 months after surgery (p < 0.05) (Supplementary Table 3).
Cervical and thoracal spine ependymomas
The univariate analysis demonstrated that preoperative MCS >2 and tumor extension (>2 vertebrae) had significant association with poor neurological outcome at every evaluated time points (Supplementary Table 4).
The multivariate analysis showed an important impact of tumor extension >2 vertebrae on a poor functional outcome (postoperatively: 24 months after surgery (Supplementary Table 4).
Lumbar ependymomas
The univariate analysis of the lumbar ependymomas revealed preoperative MCS >2, pain, tumor extension (>2 vertebrae), and ataxia as potential predictive factors of poor neurological outcome (p < 0.05) (Supplementary Table 5).
Multivariate analysis revealed that preoperative MCS was a predictive factor postoperatively (p < 0.05). However, quality of this subgroup was limited due to the low number of evaluated patients and lost to follow-up 24 and 36 months after surgery (Supplementary Table 5).
Discussion
The surgical treatment of spinal ependymomas remains challenging, as postoperative neurological deterioration plays a key role in prognosis. Up to now, several authors presented their surgical experience and their recommendation for surgical tumor removal or mass reduction.2,4,7,25,26
The known postoperative neurological deterioration of the majority of patients makes it necessary to define possible prognostic factors for functional outcome. Therefore, we tried to present our single-center experience on the surgical treatment of spinal cord ependymomas and evaluated possible prognostic factors for neurological deterioration. This single cohort outnumbers cohorts published in literature.
A mild predominance of males (52.4%) was detected in our study. The frequency of males ranged from 62% to 82% in other series.20,22,25 Ependymomas were mostly located in the lumbar region (45.9%). Lumbar ependymomas were described in 2.9–29.1% of the cases in the literature.27,28
Symptoms caused by the spinal ependymoma are unspecific. The most common symptom was pain in 67.6% of the cases, followed by sensory deficits in 14.2% and motor weakness in 9.5%. These results are similar to previously published reports.6,18,29 Symptom duration until surgery was, on average, 29.4 months. Late diagnosis is caused by the non-specificity of symptoms.29,30 Furthermore, authors described a misinterpretation of symptoms such as back pain or slow deterioration of other neurological symptoms with resulting lack of differential diagnosis, including a slow-growing spinal tumor. 31 In addition, patients can adopt the slow worsening of neurological deficits at the early stage or comorbidities can cover these symptoms. Nevertheless, at the time of diagnosis, a considerable number of patients showed severe neurological deficits (MCS III = 17.6%) or were not able to walk (MCS IV = 2.7%). Boström et al. 18 reported about 2% and Klekamp 6 reported about 11.2% of patients who were unable to walk preoperatively. Li et al. 25 reported about 26.2% of patients with MCS III and 6.2% of patients with MCS IV in a series of 210 patients suffering from a spinal cord ependymoma.
Surgical treatment and tumor recurrence
In our series, complete resection of spinal cord ependymomas was seen in the early postoperative MRI in 87.2%, showing comparable results to the literature.2,5,20,25,32 However, GTR was most commonly achieved after tumor removal within the lumbar spine followed by the cervical spine. Rate of GTR was lowest in thoracic spine ependymomas. This might be caused by the anatomical differences between the spine segments. In the current literature, GTR, encapsulated tumors, and postoperative radiotherapy are reported as the most important prognostic factors for progression-free survival.2,5–7,15,29,33 Tumor recurrence was detected in 8.1% of the patients after a median of 21.8 months, leading to consecutive impairment of the spinal cord. This was also reported by Samii and Klekamp, who described higher rates of neurological deterioration in STR. The regrowth of tumor remnants was proposed as a possible explanation. 34 However, the only significant predictor of recurrence-free survival is the degree of resection. 18 In addition, it has been well established that an early start of adjuvant treatment after non-total resection prolongs progression-free survival. 35
Functional outcome
Change in surgical approach (laminectomy at the beginning of the period versus laminoplasty 24 from the early 2000s onward) during the treatment period had no impact on neurological outcome. 5 Neurological deterioration after surgery was most common in cervical and thoracic spine ependymomas due to the anatomical differences between the lumbar spine. Neurological deterioration after surgery was present in 63.2% of the cervical spine ependymomas and in 50.0% of the thoracic spine ependymomas compared with a worsening of 7.4% of the lumbar spine ependymomas. However, direct postoperative improvement of the neurological function was detected in 5.2%, 11.9%, and 7.4%, respectively. Neurological improvement after rehabilitation 36 months after surgery was seen especially in thoracic spine ependymomas. Of those, 53% reached the preoperative status, while 17.6% were better than preoperative status. Thoracic spine ependymomas showed poor MCS after 36 months in 61.8%. Some authors propose that the anatomy of the spinal cord (smaller volume of the thoracic spinal cord, compared with the cervical spinal cord) is reasonable for the worse recovery. 6 However, neurological improvement might be also influenced by tumor recurrence at the time of follow-up. In our analysis, tumor recurrence was observed after 21.8 months. Nevertheless, good MCS was observed in the majority of lumbar spine ependymomas and in 63.3% of the cervical and 61.8% of the thoracic spine ependymomas 36 months after surgery. Cervical spine ependymomas presented less improvement than thoracic spine ependymomas. This is comparable with the findings of Klekamp, 6 who described neurological deterioration of 67.5% of the patients with intramedullary ependymomas and of 16.6% of the patients with filum terminale ependymomas. 27 Transient neurological deterioration was evaluated in 40% and 8.3%, respectively.6,27 Similar to our study, Klekamp 6 found a higher rate of postoperative permanent morbidity in patients with tumors of thoracic spine.
Predictors of poor neurological outcome
However, there are some factors with potential risk for permanent neurological deficits. In our series as well as in others, the preoperative neurological status bears an increased risk of poor neurological outcome.6,7,18,20,36,37 Preoperative neurological deficit reflects the damage of the spinal cord caused by the tumor, and the operative procedure increases this damage. In addition, regeneration of the neurological function is limited, especially if the preoperative poor neurological status persists over a longer time. Early diagnosis and referral to specialized surgical centers might also improve the postoperative outcome. Epstein et al. also call for early surgery based on their findings.
Another predictive factor for poor neurological outcome until follow-up of 24 months is tumor extension >2 vertebrae. The extent of tumor resection and the consecutive injury of the spinal cord may be reasonable. Prokopienko et al. 20 showed in their study that neurological outcome is worse in tumors extending over three spinal levels. Wang et al. 38 evaluated that tumor size is a predictive factor for worse neurological outcome if the tumor is larger than 4 cm.
Tumor location was also a predictor of poor neurological outcome. Tumors located in the cervical and thoracic spine were less likely to achieve good neurological outcome compared with lumbar spine ependymomas. These findings are similar to the current literature. 39 For example, Samuel et al. 19 showed similar results analyzing treatment of 63 patients with intramedullary spinal cord tumors. Interestingly, Wostrack et al. 21 evaluated that cervically located ependymomas causing transient deficits were more frequent but failed to demonstrate that cervical tumor location is a predictor of permanent neurological deficits.
Finally, STR showed a tendency to be a predictor of neurological outcome 12 and 24 months after surgery. It was detected as a negative predictor of neurological outcome 36 months after surgery. The majority of tumor recurrence occurred in patients with STR after a mean follow-up of 21.8 months. In those cases, second surgery was offered to the patients and a second neurological deterioration was detected.
Study limitations
Various limitations must be addressed. First, this is a retrospective, non-randomized study with its associated inherent bias. Second, data were analyzed from our retrospective electronic database ‘spinal neoplasm’, in which the patient’s electronic data, surgical reports, and radiological data were collected. Nevertheless, incomplete data bear an additional limitation and risk of selection bias. Univariant and multivariant analysis was not useful due to the ongoing lost to follow-up 36 months after surgery, caused by the retrospective character of the study. In addition, the study encompassed a long epoch in time, in which patients were treated by different neurosurgeons creating another confounding factor. Furthermore, some surgical techniques have changed over these years in terms of minimally invasive approaches or the use of intraoperative electrophysiology. In addition, lower image quality of MRI during the beginning of the observational study has to be acknowledged and might have influenced the quality of the data.
Conclusion
Spinal cord ependymomas present different clinical features according to their location. The surgical treatment of these tumors is associated with a considerable risk of postoperative neurological deterioration, which is most common in cervical and thoracal spine ependymomas. However, postoperative improvement is likely in half of these patients.
The preoperative neurological status, tumor location at the cervical spine, and STR are negative predictors of the postoperative MCS. Therefore, the surgical treatment of spinal cord ependymomas before further neurological deterioration is recommended.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211055694 for Surgical outcome and prognostic factors in spinal cord ependymoma: a single-center, long-term follow-up study by Oliver Gembruch, Mehdi Chihi, Merle Haarmann, Ahmet Parlak, Marvin Darkwah Oppong, Laurèl Rauschenbach, Anna Michel, Ramazan Jabbarli, Yahya Ahmadipour, Ulrich Sure, Philipp Dammann and Neriman Özkan in Therapeutic Advances in Neurological Disorders
Acknowledgments
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Footnotes
Author contributions: Oliver Gembruch was involved in conception and design of the study; drafting, analysis, and interpretation of data; drafting the article; and revision and final approval of the version to be submitted. Mehdi Chihi was involved in analysis and interpretation of data; statistics; and revision and final approval of the version to be submitted. Merle Haarmann was involved in drafting, analysis, and interpretation of data; and revision and final approval of the version to be submitted. Ahmet Parlak was involved in analysis and interpretation of data, and revision and final approval of the version to be submitted. Marvin Darkwah Oppong was involved in analysis and interpretation of data, and revision and final approval of the version to be submitted. Laurèl Rauschenbach was involved in analysis and interpretation of data, and revision and final approval of the version to be submitted. Anna Michel was involved in analysis and interpretation of data, and revision and final approval of the version to be submitted. Ramazan Jabbarli was involved in revising it critically for important intellectual content and final approval of the version to be submitted. Yahya Ahmadipour was involved in revising it critically for important intellectual content and final approval of the version to be submitted. Ulrich Sure was involved in revising it critically for important intellectual content and final approval of the version to be submitted. Philipp Dammann was involved in revising it critically for important intellectual content and final approval of the version to be submitted. Neriman Özkan was involved in conception and design of the study; analysis and interpretation of data; and revision and final approval of the version to be submitted.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines after the approval by the Institutional Review Board (Medical Faculty, University of Duisburg-Essen, Registration Number: 16-7178-BO), and followed The Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was not needed due to the retrospective character of the study and the approval of Institutional Review Board.
ORCID iDs: Oliver Gembruch  https://orcid.org/0000-0002-0054-1611
https://orcid.org/0000-0002-0054-1611
Marvin Darkwah Oppong  https://orcid.org/0000-0003-1021-5024
https://orcid.org/0000-0003-1021-5024
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Oliver Gembruch, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45122 Essen, Germany.
Mehdi Chihi, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Merle Haarmann, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Ahmet Parlak, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Marvin Darkwah Oppong, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Laurèl Rauschenbach, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Anna Michel, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Ramazan Jabbarli, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Yahya Ahmadipour, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Ulrich Sure, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Philipp Dammann, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
Neriman Özkan, Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
References
- 1. Benesch M, Frappaz D, Massimino M. Spinal cord ependymomas in children and adolescents. Childs Nerv Syst 2012; 28: 2017–2028. DOI: 10.1007/s00381-012-1908-4. [DOI] [PubMed] [Google Scholar]
- 2. Brotchi J, Fischer G. Spinal cord ependymomas. Neurosurg Focus 1998; 4: e2. DOI: 10.3171/foc.1998.4.5.5. [DOI] [PubMed] [Google Scholar]
- 3. Eroes CA, Zausinger S, Kreth FW, et al. Intramedullary low grade astrocytoma and ependymoma. Surgical results and predicting factors for clinical outcome. Acta Neurochir (Wien) 2010; 152: 611–618. DOI: 10.1007/s00701-009-0577-x. [DOI] [PubMed] [Google Scholar]
- 4. Ruda R, Gilbert M, Soffietti R. Ependymomas of the adult: molecular biology and treatment. Curr Opin Neurol 2008; 21: 754–761. DOI: 10.1097/WCO.0b013e328317efe8. [DOI] [PubMed] [Google Scholar]
- 5. Sandalcioglu IE, Gasser T, Asgari S, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord 2005; 43: 34–41. DOI: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 6. Klekamp J. Spinal ependymomas. Part 1: intramedullary ependymomas. Neurosurg Focus 2015; 39: E6. DOI: 10.3171/2015.5.FOCUS15161. [DOI] [PubMed] [Google Scholar]
- 7. Lee SH, Chung CK, Kim CH, et al. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: a retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro Oncol 2013; 15: 921–929. DOI: 10.1093/neuonc/not038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oh MC, Tarapore PE, Kim JM, et al. Spinal ependymomas: benefits of extent of resection for different histological grades. J Clin Neurosci 2013; 20: 1390–1397. DOI: 10.1016/j.jocn.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Celano E, Salehani A, Malcolm JG, et al. Spinal cord ependymoma: a review of the literature and case series of ten patients. J Neurooncol 2016; 128: 377–386. DOI: 10.1007/s11060-016-2135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang UK, Choe WJ, Chung SK, et al. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol 2002; 57: 133–139. DOI: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 11. Al-Habib A, Al-Radi OO, Shannon P, et al. Myxopapillary ependymoma: correlation of clinical and imaging features with surgical resectability in a series with long-term follow-up. Spinal Cord 2011; 49: 1073–1078. DOI: 10.1038/sc.2011.67. [DOI] [PubMed] [Google Scholar]
- 12. Patel P, Mehendiratta D, Bhambhu V, et al. Clinical outcome of intradural extramedullary spinal cord tumors: a single-center retrospective analytical study. Surg Neurol Int 2021; 12: 145. DOI: 10.25259/SNI_839_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hubner JM, Kool M, Pfister SM, et al. Epidemiology, molecular classification and WHO grading of ependymoma. J Neurosurg Sci 2018; 62: 46–50. DOI: 10.23736/S0390-5616.17.04152-2. [DOI] [PubMed] [Google Scholar]
- 14. Payne NS, II, McDonald JV. Rupture of spinal cord ependymoma. Case report. J Neurosurg 1973; 39: 662–665. DOI: 10.3171/jns.1973.39.5.0662. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz TH, McCormick PC. Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol 2000; 47: 211–218. DOI: 10.1023/a:1006414405305. [DOI] [PubMed] [Google Scholar]
- 16. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. DOI: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 17. Bostrom A, Kanther NC, Grote A, et al. Management and outcome in adult intramedullary spinal cord tumours: a 20-year single institution experience. BMC Res Notes 2014; 7: 908. DOI: 10.1186/1756-0500-7-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bostrom A, von Lehe M, Hartmann W, et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery 2011; 68: 302–308; discussion 309. DOI: 10.1227/NEU.0b013e3182004c1e. [DOI] [PubMed] [Google Scholar]
- 19. Samuel N, Tetreault L, Santaguida C, et al. Clinical and pathological outcomes after resection of intramedullary spinal cord tumors: a single-institution case series. Neurosurg Focus 2016; 41: E8. DOI: 10.3171/2016.5.FOCUS16147. [DOI] [PubMed] [Google Scholar]
- 20. Prokopienko M, Kunert P, Podgorska A, et al. Surgical treatment of intramedullary ependymomas. Neurol Neurochir Pol 2017; 51: 439–445. DOI: 10.1016/j.pjnns.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 21. Wostrack M, Ringel F, Eicker SO, et al. Spinal ependymoma in adults: a multicenter investigation of surgical outcome and progression-free survival. J Neurosurg Spine 2018; 28: 654–662. DOI: 10.3171/2017.9.SPINE17494. [DOI] [PubMed] [Google Scholar]
- 22. McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990; 72: 523–532. DOI: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 23. Svoboda N, Bradac O, de Lacy P, et al. Intramedullary ependymoma: long-term outcome after surgery. Acta Neurochir (Wien) 2018; 160: 439–447. DOI: 10.1007/s00701-017-3430-7. [DOI] [PubMed] [Google Scholar]
- 24. Wiedemayer H, Sandalcioglu IE, Aalders M, et al. Reconstruction of the laminar roof with miniplates for a posterior approach in intraspinal surgery: technical considerations and critical evaluation of follow-up results. Spine (Phila Pa 1976) 2004; 29: E333–342. DOI: 10.1097/01.brs.0000134592.07941.5e. [DOI] [PubMed] [Google Scholar]
- 25. Li TY, Chu JS, Xu YL, et al. Surgical strategies and outcomes of spinal ependymomas of different lengths: analysis of 210 patients: clinical article. J Neurosurg Spine 2014; 21: 249–259. DOI: 10.3171/2014.3.SPINE13481. [DOI] [PubMed] [Google Scholar]
- 26. Sun XY, Wang W, Zhang TT, et al. Factors associated with postoperative outcomes in patients with intramedullary Grade II ependymomas: a systematic review and meta-analysis. Medicine (Baltimore) 2019; 98: e16185. DOI: 10.1097/MD.0000000000016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klekamp J. Spinal ependymomas. Part 2: ependymomas of the filum terminale. Neurosurg Focus 2015; 39: E7. DOI: 10.3171/2015.5.FOCUS15151. [DOI] [PubMed] [Google Scholar]
- 28. Sun XY, Kong C, Lu SB, et al. Survival outcomes and prognostic factors of patients with intramedullary Grade II ependymomas after surgical treatments. J Clin Neurosci 2018; 57: 136–142. DOI: 10.1016/j.jocn.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 29. Engelhard HH, Villano JL, Porter KR, et al. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine 2010; 13: 67–77. DOI: 10.3171/2010.3.SPINE09430. [DOI] [PubMed] [Google Scholar]
- 30. Tarapore PE, Modera P, Naujokas A, et al. Pathology of spinal ependymomas: an institutional experience over 25 years in 134 patients. Neurosurgery 2013; 73: 247–255; discussion 255. DOI: 10.1227/01.neu.0000430764.02973.78. [DOI] [PubMed] [Google Scholar]
- 31. Pena M, Galasko CS, Barrie JL. Delay in diagnosis of intradural spinal tumors. Spine (Phila Pa 1976) 1992; 17: 1110–1116. DOI: 10.1097/00007632-199209000-00017. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi K, Ando K, Kato F, et al. Surgical outcomes of spinal cord and cauda equina ependymoma: postoperative motor status and recurrence for each WHO grade in a multicenter study. J Orthop Sci 2018; 23: 614–621. DOI: 10.1016/j.jos.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 33. Halvorsen CM, Kolstad F, Hald J, et al. Long-term outcome after resection of intraspinal ependymomas: report of 86 consecutive cases. Neurosurgery 2010; 67: 1622–1631; discussion 1631. DOI: 10.1227/NEU.0b013e3181f96d41. [DOI] [PubMed] [Google Scholar]
- 34. Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 1994; 35: 865–873; discussion 873. DOI: 10.1227/00006123-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 35. Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol 2005; 71: 205–210. DOI: 10.1007/s11060-004-1386-y. [DOI] [PubMed] [Google Scholar]
- 36. McCormick PC, Stein BM. Intramedullary tumors in adults. Neurosurg Clin N Am 1990; 1: 609–630. [PubMed] [Google Scholar]
- 37. Yang C, Sun J, Xie J, et al. Multisegmental versus monosegmental intramedullary spinal cord ependymomas: perioperative neurological functions and surgical outcomes. Neurosurg Rev. Epub ahead of print 1 November 2021. DOI: 10.1007/s10143-021-01567-5. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Gao J, Wang T, et al. The long-term outcome after resection of upper cervical spinal cord tumors: report of 51 consecutive cases. Sci Rep 2018; 8: 14831. DOI: 10.1038/s41598-018-33263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aghakhani N, Messerer M, David P, et al. [Intramedullary ependymomas: a French retrospective multicenter study of 221 cases]. Neurochirurgie 2017; 63: 391–397. DOI: 10.1016/j.neuchi.2016.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211055694 for Surgical outcome and prognostic factors in spinal cord ependymoma: a single-center, long-term follow-up study by Oliver Gembruch, Mehdi Chihi, Merle Haarmann, Ahmet Parlak, Marvin Darkwah Oppong, Laurèl Rauschenbach, Anna Michel, Ramazan Jabbarli, Yahya Ahmadipour, Ulrich Sure, Philipp Dammann and Neriman Özkan in Therapeutic Advances in Neurological Disorders