Abstract
Background:
A considerable number of patients with myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) will experience a relapse, but the effect of maintenance therapies on re-attack rates is currently unknown.
Objective:
To investigate the efficacy and safety of immunosuppressive therapy for preventing disease relapses in patients with MOGAD, including rituximab (RTX), mycophenolate mofetil (MMF), and azathioprine (AZA).
Methods:
English-language studies published prior to August 31, 2020, were searched in the NCBI (PubMed), ISI Web of Science, and the Cochrane Library databases. Patient characteristics, treatment regimens, outcome measures, and adverse effects were retrieved.
Results:
We enrolled 11 studies in the final meta-analysis, including 346 patients with MOGAD. RTX therapy was demonstrated to result in reduced mean annualized relapse rate (ARR) by 1.35 (95% confidence interval (CI): 0.85–1.85) and reduced mean Expanded Disability Status Scale score by 0.80 (95% CI: 0.53–1.08) in patients with MOGAD. MMF therapy was associated with the mean ARR decreasing by 0.83 (95% CI: 0.31–1.35), and AZA was related to the mean ARR decreasing by 1.71 (95% CI: 0.83–2.58). The reported discontinuation rates of RTX, MMF, and AZA therapy due to adverse effects were 3/197 (1.52%), 3/39 (7.69%), and 4/37 (10.81%), respectively.
Conclusion:
The study provided evidence to support the efficacy of RTX, MMF, and AZA on the preventive treatment in patients with MOGAD. However, large randomized controlled trials are still needed in the future.
Keywords: immunosuppressive therapy, myelin oligodendrocyte glycoprotein antibody–associated disease, relapses
Introduction
Myelin oligodendrocyte glycoprotein (MOG) is a transmembrane protein uniquely expressed on the surface of oligodendrocytes and the outermost surface of myelin sheath, making up less than 0.05% of the total central nervous system (CNS) myelin protein. 1 MOG was first identified 40 years ago as a target of demyelinating antibodies in guinea pigs. 2 Antibodies to MOG were originally thought to be involved in multiple sclerosis (MS), based on results from enzyme-linked immunosorbent assays employing linearized or denatured MOG peptides as antigen. Over the past few years, with the development of new-generation cell-based assays against full-length, conformationally intact human MOG, the role of antibodies to MOG in patients with inflammatory CNS demyelination has been revisited. 3
Myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) is increasingly recognized as a distinct clinical entity, with varying characteristics of phenotypes, disease courses, and response to treatment.4,5 Recent studies with a long period of follow-up showed that a considerable proportion of patients with MOGAD tended to relapse.6–8 Although the long-term outcome of motor and visual disability seemed better in MOGAD than that in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (NMOSD) and MS, disability was accumulated from relapses, suggesting attack prevention may be necessary in MOGAD.9,10
According to the result of an international questionnaire investigation from 86 invited neurologists, the most favorable first-choice maintenance therapies in MOGAD were azathioprine (AZA), mycophenolate mofetil (MMF), and rituximab (RTX). 11 Up until now, there have been were no randomized controlled clinical trials in the maintenance treatment of MOGAD. Evidence-based guideline or consensus on management is urgently needed. In this study, we aimed to perform a systematic review and meta-analysis to investigate the efficacy and safety of RTX, MMF, and AZA in patients with MOGAD based on some observational studies.
Materials and methods
Study selection
We followed the PRISMA Statement guidelines 12 and Meta-analysis of Observational Studies in Epidemiology guidelines (MOOSE) 13 for conducting a systematic review. The authors (QLL and YXZ) independently searched relevant articles in NCBI (PubMed), ISI Web of Science, and the Cochrane Library databases for the period prior to August 31, 2020. The search was limited to English-language studies of humans. The search terms included “myelin oligodendrocyte glycoprotein”, “MOG”, “myelin oligodendrocyte glycoprotein antibody associated encephalomyelitis”, “myelin oligodendrocyte glycoprotein antibody-associated disorders/disease”, and “therapy”, “treatment” “immunosuppressant”, “mycophenolate mofetil”, “azathioprine”, and “rituximab”. We retrieved all the articles and searched their reference lists to identify as many studies as possible.
Eligibility criteria
As no randomized clinical trials were identified, only observational studies were included in the meta-analysis. The studies were read in their entirety to assess the appropriateness for their inclusion in the analysis. Studies were included if they met the following criteria: (1) original data from clinical studies; (2) MOG antibody testing was performed by a cell-based assay; (3) the exposure to MMF, AZA, or RTX; (4) the efficacy of medication was assessed by annualized relapse rate (ARR) and/or Expanded Disability Status Scale (EDSS), reporting the mean with standard deviation (SD), median with range or interquartile range. Single-case reports and studies concerning a single patient were excluded from the meta-analysis. All analyses were based on previously published studies, thus no ethical approval and patient consents are required.
Data extraction and quality assessment
All the studies were evaluated and examined carefully by two authors (QLL and YXZ). Discrepancies were discussed and resolved by verification from a third reviewer (CHS). The following characteristics were retrieved for each study: authors, publication year, study design, region, sample, age, gender ratio, treatment regimens, ARRs, and EDSS scores before and after treatment, adverse effects, and follow-up duration. The quality of included studies was assessed by an 11-item checklist which was proposed by the Agency for Healthcare Research and Quality (AHRQ) and recommended to estimate the quality of cross-sectional/prevalence studies. 14 Article quality was identified as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8–11.
Data analysis
Two primary efficacy outcome measures were assessed, namely, differences in mean ARR and EDSS score before and after treatment, separately of RTX, MMF, and AZA. The secondary outcomes were discontinuation of treatment and manifestations of adverse effects. If ARR or EDSS was provided in the form of median with range or interquartile range, it would be converted into mean with SD as described by Wan et al. 15 If ARR or EDSS was presented by individual data, it would be calculated into mean with SD. The data of interest were analyzed by mean difference (MD) as effect measures. Statistical heterogeneity was assessed using I2 statistical; p < 0.10 or I2 > 0.50 was considered significant. If substantial heterogeneity was detected, the analysis would be performed on random-effect model with DerSimonian and Laird method. Otherwise, fixed-effect model would be used.
Sensitivity analysis was conducted by excluding each study individually and recalculating the combined estimates for the remaining studies to assess the influence of an individual result on the pooled estimates. Begg’s tests were performed to evaluate publication bias, p < 0.05 was considered as existence of significant publication bias. All the data analyses were performed using STATA SE12.0 (Stata, TX, College Station, USA).
Data availability
Data were available upon request. Interested researchers may contact the corresponding author.
Results
Study characteristics
Eleven studies16–26 were included in the final meta-analysis, as seen in Figure 1. All the studies collectively included 346 patients diagnosed with MOGAD, who were receiving RTX (n = 231), MMF (n = 59), or AZA therapy (n = 56). The median age of patients ranged from 5.4 to 37.9 years, and the female to male ratio ranged from 1:1 to 3:1. The median disease duration before RTX, MMF, or AZA therapy ranged from 0.41 to 22.3 years. The median follow-up duration ranged from 0.92 to 7.8 years. Two of these studies23,26 were prospective observational studies, while the remaining nine being retrospective studies.16–22,24,25 Three of these studies18,24,26 focused on adults, three studies19,20,23 on children, and the remaining five studies16,17,21,22,25 on both adults and children. The quality score of each study were assessed by AHRQ checklist, indicating all included studies were identified as being high quality as shown in Table 1.
Figure 1.
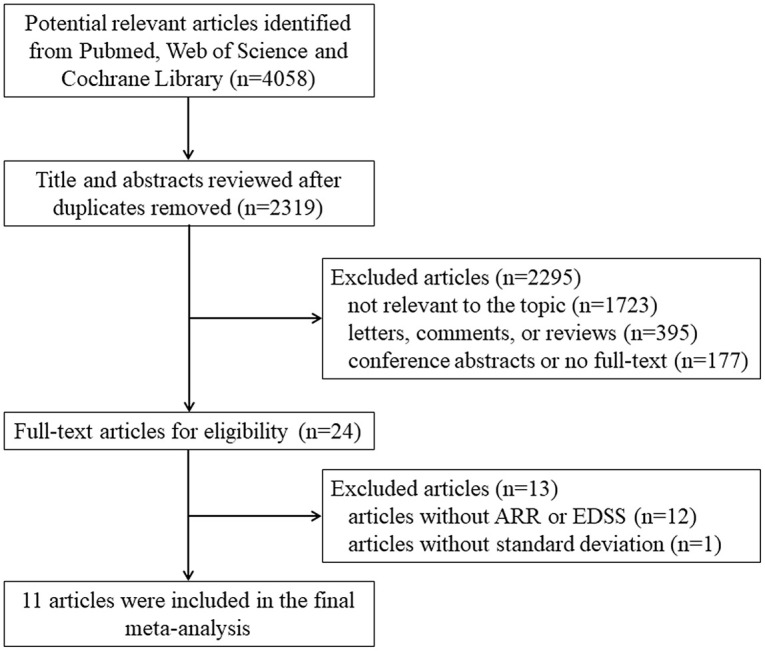
Study selection flow diagram.
Table 1.
Characteristics of the studies included in the final meta-analysis.
| Study (authors, year) | Study design | Region | Groups | No. of cases | Median or mean age | Female:male ratio | Clinical phenotype | Pre-treatment ARR, median (range) | Disease duration before treatment, years (median) |
|---|---|---|---|---|---|---|---|---|---|
| Montcuquet et al. 16 | Retrospective | France | Adults and children | 5 | 37.9 | 2.9:1 | NMO: 60% TM: 40% |
1 (1–1) | NA |
| Ramanathan et al. 17 | Retrospective | Australia | Adults and children | 22 | 12 | 2.11:1 | ADEM: 20% ON: 54% Others: 26% |
MMF 1.83 (0.47–6) RTX 1.65 (0.67–2) |
MMF 0.67 RTX 1.25 |
| Zhou et al. 20 | Retrospective | China | Children | 14 | 5.4 | 1.3:1 | NMOSD: 47.8% ADEM: 21.7% Others: 30.4% |
1.17 (0.25–7.67) | RTX 1.05 MMF 1.5 AZA 2.91 |
| Pedapati et al. 25 | Retrospective | India | Adults and children | 18 | 30.5 | 3:1 | ON: 75% TM: 15% NMO: 5% Others: 5% |
AZA 3.24 ± 3.13
a
MMF 0 ± 0 a |
NA |
| Tzartos et al. 24 | Retrospective | Greece | Adults | 7 | 34 | 1.33:1 | ON: 57.1% NMO: 14.3% Others: 28.6% |
0.33 (0–5) | AZA 17 MMF 22.3 |
| Mao et al. 19 | Retrospective | China | Children | 5 | 6.6 | 1.08:1 | NMOSD: 60% ADEM: 40% |
2.4 (0.67–8) | 0.92 |
| Cobo-Calvo et al. 18 | Retrospective | France and Spain | Adults | 48 | 34.1 | 1.23:1 | ON: 65.6% TM: 20% NMO: 7.2% Others: 7.2% |
0.79 ± 0.91 a | AZA 2.4 MMF 1.7 RTX 3.3 |
| Albassam et al. 23 | Prospective | Canada | Children | 12 | 10 | 2:1 | NMOSD: 33.3% ON: 16.7% ADEM: 50% |
NA | 0.41 |
| Durozard et al. 26 | Prospective | France | Adults | 16 | 33.5 | 1:1 | NA | 2 (1–10) | 0.65 |
| Chen et al. 22 | Retrospective | America | Adults and children | 70 | 29 | 1.38:1 | ON: 47% TM: 11% NMO: 10% ADEM: 31% |
1.6 (0–9.7) | NA |
| Whittam et al. 21 | Retrospective | Multicenter | Adults and children | 121 | 23.2 | 1.42:1 | ON: 29% TM: 12.4% NMO: 28.1% ADEM: 12.4% Others: 18.2% |
1.82 (0.74–3.4) b | 1.59 |
| Study (authors and year) | Other immunotherapies prior to MMF, AZA, and RTX | Concomitant CS | Concomitant IVIG/PE | Immunosuppressant (no. of cases) and dosage | Available index | Median follow-up duration, years | Quality score | ||
| Montcuquet et al. 16 | None | NA | None | MMF (5): 2000 mg daily | ARR, EDSS | 3.58 | 9 | ||
| Ramanathan et al. 17 | None | MMF: 16/16 RTX: 6/6 |
RTX: IVIG 1/6, PE 1/6 MMF: None |
MMF (16): 1000–2000mg daily; RTX (6): NA |
ARR | MMF 1.25; RTX 1.04 |
10 | ||
| Study (authors and year) | Other immunotherapies prior to MMF, AZA, and RTX | Concomitant CS | Concomitant IVIG/PE | Immunosuppressant (no. of cases) and dosage | Available index | Median follow-up duration, years | Quality score | ||
| Zhou et al. 20 | None | None | None | RTX (8): NA; MMF (3): NA; AZA (3): NA |
ARR | 0.92 | 9 | ||
| Pedapati et al. 25 | None | None | None | AZA (15): NA; MMF (3): NA |
ARR | AZA 1.0; MMF 3.25 |
8 | ||
| Tzartos et al. 24 | None | None | None | AZA (5): NA; MMF (2): NA |
ARR | 7.8 | 8 | ||
| Mao et al. 19 | None | None | IVIG: 5/5 PE: None |
RTX (5): 2 × 750mg/m2, Days 1 and 15 | ARR | 1.25 | 8 | ||
| Cobo-Calvo et al. 18 | Prior to AZA: 21.1% Prior to MMF: 50% Prior to RTX: 36.7% |
None | None | AZA (11): 150 mg daily; MMF (11): 2000 mg daily; RTX (26): 1000 mg infused every 6 months |
ARR, EDSS | AZA 2.1; MMF 1.7; RTX 1.7 |
8 | ||
| Albassam et al. 23 | None | None | None | RTX (12): Two doses of 500 mg/m2(max 1 g) given 10–14 days apart. Subsequently, one dose of 500 mg/m2 (max 1 g) was administered when CD19 cell counts >0 | EDSS | 2.0 | 10 | ||
| Durozard et al. 26 | Prior to RTX: 31.2% c | NA | None | RTX (16): Induction: 1000 mg infused twice at a 2-week interval. Maintenance: a single infusion of 1000 mg at 6 months or CD27-positive B-cells reached 0.05% | ARR, EDSS | 1.59 | 9 | ||
| Chen et al. 22 | Prior to MMF: 53% Prior to AZA: 23% Prior to RTX: 32% |
MMF: 2/19 AZA: 10/22 RTX: 5/37 |
None | MMF (19): NA; AZA (22): NA; RTX (37): NA |
ARR | MMF 1.1; AZA 1.7; RTX 1.2 |
9 | ||
| Whittam et al. 21 | Prior to RTX: 45.6% d | 32/121 | IVIG: 7/121 PE: None |
RTX (121): Initial 1000 mg on Day 0 and 15 or 375 mg/m2 weekly for 4 weeks. Interval between subsequent treatment was either fixed at 6-months or determined by periodic testing of circulating CD19 + B-cell or CD19 + CD27 + memory B-cell levels | ARR, EDSS | 1.0 | 8 | ||
ADEM, acute disseminated encephalomyelitis; ARR, annualized relapse rate; AZA, azathioprine; CS, corticosteroids; EDSS, Expanded Disability Status Scale; IVIG, intravenous immune globulins; MMF, mycophenolate mofetil; NA, not available; NMO, neuromyelitis optica; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; PE, plasma exchange; RTX, rituximab; TM, transverse myelitis.
Represents mean ± SD.
Represents median (interquartile range).
Previous treatments included mycophenolate mofetil for two and natalizumab, fingolimod, and teriflunomide for the remaining three patients.
Previous treatments included azathioprine (27), mycophenolate mofetil (20), intravenous immune globulins (7), maintenance plasma exchange (2), cyclophosphamide (6), mitoxantrone (3), methotrexate (2), tacrolimus (1), ciclosporin (1), and multiple sclerosis—disease-modifying therapies (11).
The clinical characteristics of each study were also presented in Table 1, including pre-ARR, clinical phenotype, immunotherapies prior, concomitant maintenance corticosteroids and immunosuppressants. In 4 of 11 included studies,18,21,22,26 a varied proportion of patients had received other immunotherapies prior to RTX, MMF, or AZA, while patients received RTX, MMF, and AZA as first immunosuppressants to prevent relapses in the other studies.16,17,19,20,23,24,25 With regard to concomitant maintenance corticosteroids, some patients received concomitant corticosteroids in two studies,17,21 meanwhile, patients in seven studies18–20,22–25 did not, and data were not available in the remaining two studies.16,26 A certain proportion of patients received concomitant intravenous immune globulins or plasma exchange in three studies,17,19,21 while patients in other studies16,18,20,22–26 did not. Details of the RTX regimen were available for 180/231 (77.9%) patients18,19,21,23,26 and varied among studies: 137 (60.9%) patients21,26 received two fortnightly 1000-mg infusions, followed by 1000-mg infusion every 6 months or when memory B-cells re-emerged, 26 (11.6%) patients 18 received 1000-mg infusions every 6 months, 12 (5.3%) patients 23 were treated with 500 mg/m2 (maximum 1000 mg) infusion every 2 weeks for two times, followed by 500 mg/m2 (maximum 1000 mg) infusion when CD19 + memory B-cells re-emerged, and 5 (2.2%) patients 19 received two fortnightly 750 mg/m2 infusions. Details of the MMF regimen was available for 32/59 (54.2%) patients:16–18 16 (27.1%) patients16,18 were treated with 2000 mg daily and other 16 (27.1%) patients 17 were treated with 1000–2000 mg daily. Details of the AZA regimen was available for 11/56 (19.6%) patients: 18 all of them received 150 mg daily, as shown in Table 1.
Efficacy of RTX on the ARR and EDSS score
ARRs before and after RTX therapy were reported in seven studies,17–22,26 including 199 patients with MOGAD. A forest plot of the MD in the ARR was shown in Figure 2. This finding suggested that the MD reduction of ARR after RTX therapy was 1.35 (95% CI: 0.85–1.85; p_heterogeneity = 0.008, I2 = 65.6%) using the random-effect model. Subgroup analyses by age (adults vs children) and study design (retrospective vs prospective) were performed. Similar MDs were observed in the subgroup of adults (–1.67, 95% CI, –2.63 to –0.72; p_heterogeneity = 0.001, I2 = 81.9%) and children (–1.06, 95% CI, –1.99 to –0.13; p_heterogeneity = 0.071, I2 = 57.2%; Table 2). After excluding one prospective study, 27 the MD of ARR among the remaining six retrospective studies17–22 was –1.17 (95% CI: –1.60 to –0.75; p_heterogeneity = 0.057, I2 = 53.3%). Sensitivity analyses were performed successively by removing each study in turn and re-analyzed. No studies were found to significantly affect the MD and heterogeneity (MD ranged from –1.17, 95%CI: –1.60 to –0.75 to –1.50, 95% CI: –2.00 to –1.00). No obvious publication bias was suggested from the results of Begg’s test for ARR (p = 0.548), as shown in Supplemental eFigure 1.
Figure 2.
Forest plot of the mean difference in annualized relapse rate associated with the rituximab therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
Table 2.
The results of meta-analysis and subgroup analysis in myelin oligodendrocyte glycoprotein antibody–associated disease.
| Subgroup | Studies no. | Patients no. | MD (95% CI) fixed model | MD (95% CI) random model | Heterogeneity test | |
|---|---|---|---|---|---|---|
| I2 (95%) | P | |||||
| RTX on ARR | 7 | 199 | –1.25 (–1.50, –0.99) | –1.35 (–1.85, –0.85) | 65.6% | 0.008 |
| Adults | 4 | 143 | –1.30 (–1.67, –0.93) | –1.67 (–2.63, –0.72) | 81.9% | 0.001 |
| Children | 4 | 50 | –1.09 (–1.60, –0.58) | –1.06 (–1.99, –0.13) | 57.2% | 0.071 |
| RTX on EDSS | 4 | 175 | –0.80 (–1.08, –0.53) | –0.80 (–1.08, –0.53) | 0.0% | 0.955 |
| MMF on ARR | 7 | 59 | –1.00 (–1.00, –1.00) | –0.83 (–1.35, –0.31) | 67.8% | 0.005 |
| Adults | 3 | 28 | –1.12 (–1.77, –0.47) | –1.12 (–1.77, –0.47) | 0.0% | 0.697 |
| Children | 2 | 7 | –1.40 (–2.40, –0.40) | –1.40 (–2.40, –0.40) | 0.0% | 0.843 |
| MMF on EDSS | 2 | 16 | –0.23 (–1.50, 1.04) | –0.23 (–1.50, 1.04) | 0.0% | 0.666 |
| AZA on ARR | 5 | 56 | –1.49 (–2.04, –0.95) | –1.71 (–2.58, –0.83) | 55.7% | 0.061 |
| Adults | 3 | 30 | –0.97 (–1.60, –0.34) | –0.97 (–1.60, –0.34) | 0.0% | 0.482 |
| Children | 2 | 11 | –2.01 (–3.42, –0.61) | –2.01 (–3.42, –0.61) | 0.0% | 0.753 |
ARR, annualized relapse rate; AZA, azathioprine; CI, confidence interval; EDSS, Expanded Disability Status Scale; MD, mean difference; MMF, mycophenolate mofetil; RTX, rituximab.
EDSS scores were described in four studies with RTX therapy,18,21,23,26 including 175 patients. A forest plot of the MD in the EDSS before and after RTX therapy was shown in Figure 3. The result revealed that the MD of EDSS after RTX therapy was –0.80 (95% CI: –1.08 to –0.53; p_heterogeneity = 0.955, I2 = 0.0%) on the fixed-effect model. No obvious publication bias was found from the results of Begg’s test for EDSS (p = 0.308), as shown in Supplemental eFigure 2.
Figure 3.
Forest plot of the mean difference in Expanded Disability Status Scale score associated with the rituximab therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
Efficacy of MMF on the ARR and EDSS score
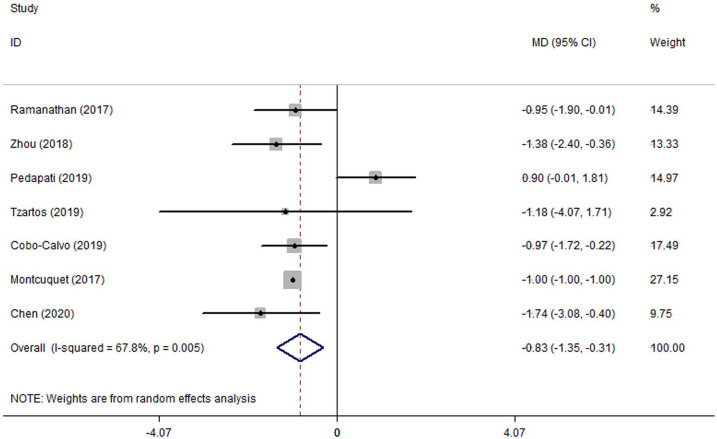
ARRs before and after MMF therapy were reported in seven studies16–18,20,22,24,25 including 59 patients for the meta-analysis. As seen in Figure 4, a forest plot indicated the MD of ARR after MMF therapy on random-effect model was –0.83 (95% CI: –1.35 to –0.31; p_heterogeneity = 0.005, I2 = 67.8%). When conducting the subgroup analysis by age, the MD of ARR after MMF therapy was –1.12 (95% CI: –1.77 to –0.47; p_heterogeneity = 0.697, I2 = 0.0%) in adults and –1.40 (95% CI: –2.40 to –0.40; p_heterogeneity = 0.843, I2 = 0.0%) in children, showing the heterogeneity was well eliminated (Table 2). No obvious publication bias was suggested from the results of Begg’s test for ARR (p = 0.368), as shown in Supplemental eFigure 3.
Figure 4.
Forest plot of the mean difference in annualized relapse rate associated with the mycophenolate mofetil therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
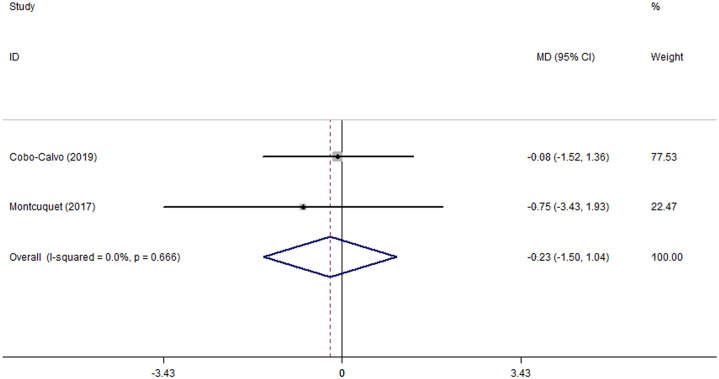
EDSS scores were reported in two studies with MMF therapy,16,18 including 16 patients for the analysis. A forest plot suggested that the MD of EDSS after MMF therapy was –0.23 (95% CI: –1.50 to 1.04; p_heterogeneity = 0.666, I2 = 0.0%, Figure 5). No obvious publication bias was observed by the Begg’s test for EDSS (p = 1.000), as shown in Supplemental eFigure 4.
Figure 5.
Forest plot of the mean difference in Expanded Disability Status Scale score associated with the mycophenolate mofetil therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
Efficacy of AZA on the ARR and EDSS score
ARRs before and after AZA therapy were reported in five studies,18,20,22,24,25 including 56 patients for the meta-analysis. Our study demonstrated that the MD of ARR after AZA therapy on random-effect model was –1.71 (95% CI: –2.58 to –0.83; p_heterogeneity = 0.061, I2 = 55.7%, Figure 6). Given the underlying heterogeneity, subgroup analysis by age was performed. The MD of ARR after AZA therapy was –0.97 (95% CI: –1.60 to –0.34; p_heterogeneity = 0.482, I2 = 0.0%) in adults and –2.01 (95% CI: –3.42 to –0.61; p_heterogeneity = 0.753, I2 = 0.0%) in children on the fixed-effect model, revealing that the heterogeneity was well subsided. No publication bias was indicated from the result of Begg’s test for ARR (p = 0.462) and the Begg’s funnel plot was symmetrical (Supplemental eFigure 5). EDSS scores before and after AZA therapy were only reported in one of the included studies 18 which was not sufficient to conduct a meta-analysis.
Figure 6.
Forest plot of the mean difference in the annualized relapse rate associated with the azathioprine therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
Safety and discontinuation of treatment
As seen in Table 3, drug discontinuation was recorded in nine studies.16–21,23,25,26 In six studies18–21,23,26 reporting the RTX discontinuation, 27/197 (13.71%) patients discontinued RTX therapy due to adverse effects (3/197, 1.52%), the others discontinued RTX because of physician or patient decision (6/197, 3.05%) and treatment failure (18/197, 9.14%). In five studies16–18,20,25 with the details of MMF discontinuation, 13/39 (33.33%) patients discontinued MMF therapy due to adverse effects (3/39, 7.69%). In three studies18,20,25 reporting AZA discontinuation, 9/37 (24.32%) patients discontinued AZA therapy due to adverse effects (4/37, 10.81%).
Table 3.
Discontinuation and adverse effects of immunosuppressive therapy in patients with myelin oligodendrocyte glycoprotein antibody–associated disease.
| Immunosuppressant | Discontinuation of treatment | Manifestations of adverse effects |
|---|---|---|
| RTX18–21,23,26 | 27/197 (13.71%) discontinued 3/197 (1.52%) adverse effects 6/197 (3.05%) physician or patient decision 18/197 (9.14%) treatment failure |
7/149 (4.70%) experienced infusion related reactions 8/149 (5.37%) developed leucopenia 6/149 (4.02%) developed hypogammaglobulinemia 2/149 (1.34%) developed infection |
| MMF16–18,20,25 | 13/39 (33.33%) discontinued 3/39 (7.69%) adverse effects 2/39 (5.13%) physician or patient decision 8/39 (20.51%) treatment failure |
1/3 (33.3%) developed diarrhea |
| AZA18,20,25 | 9/37 (24.32%) discontinued 4/37 (10.81%) adverse effects 3/37 (8.11%) physician or patient decision 2/37 (5.41%) treatment failure |
0/15 (0%) experienced adverse effects |
AZA, azathioprine; MMF, mycophenolate mofetil; RTX, rituximab.
The detailed adverse effects were recorded in four of included studies.21,23,25,26 Among the patients receiving RTX therapy with the records of adverse effects,21,23,26 7/149 (4.70%) patients experienced infusion related reactions, 8/149 (5.37%) patients developed leucopenia, 6/149 (4.02%) patients developed hypogammaglobulinemia, 2/149 (1.34%) patients developed infection. The information about adverse effects of MMF and AZA were recorded only in one of the studies, 25 in which 1/3 (33.3%) patient on MMF therapy developed diarrhea while 0/15 (0%) patient on AZA therapy experienced any adverse effects.
Discussion
Frequent attacks contributed to disability in patients with MOGAD. As previous studies suggested, 62.2% of patients remitted completely or almost completely after the initial attack, while the proportion became lower for subsequent attacks (40.6%) and dropped to 26.4% after the fifth relapse. 6 Some studies have suggested that long-term immunosuppressive therapy was potentially related to a reduced relapse rate, similar to that in patients with MS and NMOSD.7,8,27,28 To the best of our knowledge, this is the first meta-analysis on the efficacy and safety of RTX, MMF, and AZA in disease prevention of MOGAD.
RTX is a human and mouse chimeric IgG1 monoclonal antibody that targets the B-cell CD20 antigen, which is involved in cell cycle progression. RTX was originally approved for the treatment of B-cell lymphoma, but it has been increasingly used in B-cell-related autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, autoimmune encephalitis, MS, and NMOSD.29–34 In MOGAD, the pathogenic role of MOG antibody remains uncertain. Some studies suggested that MOG antibody might lead to MOGAD via two possible mechanisms: (1) MOG antibody–induced demyelination mediated by complements and (2) MOG-reactive T-cell-induced inflammation through antigen-presenting cells. 35
In this study, we found that RTX treatment was robustly associated with reduced ARR and disability levels in patients with MOGAD, and it was still effective in adults and children when stratified by age. The MD reduction of ARR after RTX therapy in patients with MOGAD was 1.35 (95% CI: 0.85–1.85), which was close to that in those with NMOSD (1.56, 95% CI: 1.29–1.82) and MS (1.00, 95% CI: 0.83–1.17).33,34 A multicenter observational study indicated that the relapse-free rate during RTX treatment was 92.9% (13/14), consistent with our result. 36 Concurrently, another study showed that the relapse-free rate during RTX therapy dropped to 22.2% (2/9), which might be affected by the prolonged follow-up duration (6.25 years), the small sample and other potential confounders. 6 A recent systematic review conducted by Lu et al. 37 also revealed that RTX could reduce the relapse rate in the patients with MOGAD, using a qualitative analysis based on the Oxford Center for Evidence-Based Medicine 2011 Levels. As for safety concerns, we identified that adverse events occurred infrequently and only a few patients discontinued RTX therapy due to adverse effects. Thus, it can be concluded that RTX is an effective and safe treatment in patients with MOGAD.
MMF is a prodrug of mycophenolic acid (MPA), which inhibits de novo guanosine nucleotide synthesis and selectively targets proliferation of T and B lymphocytes.38,39 In the past few years, MMF has been used for the prevention of allograft rejection in organ transplantation and is increasingly used in the treatment of autoimmune diseases, such as NMOSD because of its potential efficacy and good tolerance. 40
Our study demonstrated that MMF treatment is effective in reducing the ARR in patients with MOGAD. Li et al.’s 41 study conducted a prospective observational cohort study with 79 MOGAD patients, suggesting the relapse-free rate in the MMF group was 92.6% (50/54) with the median follow-up of 400 days, consistent with our result. However, with an extension of follow-up, the relapse-free rate probably drops. In a retrospective multicenter study, the relapse-free rate after MMF treatment was 46.7% (7/15) during a median follow-up of 5.5 years. 42 When subgroup analysis was performed by age, the heterogeneity subsided, suggesting that age may be a potential source of heterogeneity. A particular concern was the different clinical phenotypes in patients with MOGAD, which changed from acute disseminated encephalomyelitis (ADEM) like (ADEM, ADEM-optic neuritis, and multiphasic disseminated encephalomyelitis) in children to opticospinal (optic neuritis and myelitis) in adults. 43 Our study was not qualified enough to conclude on the association between MMF treatment and EDSS scores, mainly due to the limited number of studies. Therefore, further studies on disability levels after MMF treatment are required. Notably, reported MMF discontinuation rate due to adverse effects was 7.69%, which was not rare. Our result supported the notion that MMF was effective for the maintenance treatment in patients with MOGAD.
Similar to MMF, AZA is an immune-modulating drug, originally developed for the prevention of graft rejection in transplant surgery. 44 Considering widespread application, popular price, and reliable efficacy, AZA has gradually become one of the most commonly prescribed preventive treatment in immune-mediated neurological diseases, including myasthenia gravis, autoimmune encephalitis and NMOSD.45–47 As a consequence, an international questionnaire investigation for neurologists found that AZA was the most popular first-choice maintenance therapy in MOGAD. 11
Unsurprisingly, our study also identified AZA treatment effective in reducing the ARR in patients with MOGAD, which was in agreement with Lu et al.’s 37 qualitative review. In a retrospective multicenter study, the relapse-free rate after AZA treatment was 50.0% (10/20) and ARR was also lowered with a median follow-up of 5.5 years. 42 Subgroup analysis exhibited that AZA treatment is effective in both adults and children. In addition, there seemed to be a moderate tendency that AZA was more effective in children than in adults, probably due to different clinical phenotypes between them. This finding suggested that it would be better for future studies to be aware of the age stratification and different phenotypes. Reported discontinuation of AZA treatment due to adverse effects was 10.81%. To conclude, AZA is an effective therapy for preventing disease relapses in patients with MOGAD.
This study has several limitations. First, all the studies included in our meta-analysis were retrospective or prospective observational studies with heterogeneous designs, which may not control sufficiently all the related confounders. Second, potential heterogeneities were indeed observed among the pooled estimates of ARRs after RTX, MMF, or AZA therapy. We speculated that disease course, number of attacks before therapy, treatment protocols, delayed efficacy of drugs and combination of corticosteroids might contribute to the heterogeneities across the studies. Of note, a certain proportion of patients were on concomitant corticosteroids or combination therapy which might inflate the therapeutic effect of RTX, MMF, and AZA. Third, we converted non-normally distributed statistics (median with range or interquartile range, individual data) to normally distributed statistics (mean with SD), which may lead to bias. Fourth, the efficacy and safety of MMF and AZA needed to be explained with caution, due to the small sample, incomplete information on dosing regimens and adverse event data.
Conclusion
In summary, this systematic review and meta-analysis provided further evidence that RTX is associated with reduced relapse rates and disability levels in patients with MOGAD. MMF and AZA were also identified to be effective in preventing disease relapses. Our findings highlighted the necessity of large randomized clinical trials to thoroughly evaluate the efficacy and safety of RTX, MMF, and AZA as maintenance therapy in patients with MOGAD.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-3-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-4-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-5-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-6-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-7-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: QLL and YXZ conceptualized and designed the study, searched relevant articles, collected and evaluated the data, drafted and revised the initial manuscript. MTC conceptualized and designed the study, collected and analyzed the data, revised the initial manuscript. YZ collected and analyzed the data, revised the initial manuscript. SQ and GLF collected and analyzed the data. CHS conceptualized and designed the study, coordinated and supervised data collection and analyzation, critically reviewed and revised the manuscript. All authors approved the final version of the manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: All analyses were based on previously published data, thus no ethical approval and patient consents are required.
ORCID iDs: Yin-Xi Zhang  https://orcid.org/0000-0002-4462-171X
https://orcid.org/0000-0002-4462-171X
Chun-Hong Shen  https://orcid.org/0000-0002-0832-0848
https://orcid.org/0000-0002-0832-0848
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Qi-Lun Lai, Department of Neurology, Zhejiang Hospital, Hangzhou, China.
Yin-Xi Zhang, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Meng-Ting Cai, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Yang Zheng, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Song Qiao, Department of Neurology, Zhejiang Hospital, Hangzhou, China.
Gao-Li Fang, Department of Neurology, Zhejiang Chinese Medicine and Western Medicine Integrated Hospital, Hangzhou, China.
Chun-Hong Shen, Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, 88 Jiefang Road, Hangzhou 310009, China.
References
- 1. Iglesias A, Bauer J, Litzenburger T, et al. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 2001; 36: 220–234. [DOI] [PubMed] [Google Scholar]
- 2. Lebar R, Baudrimont M, Vincent C. Chronic experimental autoimmune encephalomyelitis in the guinea pig. Presence of anti-M2 antibodies in central nervous system tissue and the possible role of M2 autoantigen in the induction of the disease. J Autoimmun 1989; 2: 115–132. [DOI] [PubMed] [Google Scholar]
- 3. Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016; 15: 307–324. [DOI] [PubMed] [Google Scholar]
- 4. Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol 2018; 75: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–3138. [DOI] [PubMed] [Google Scholar]
- 8. Cobo-Calvo A, Ruiz A, Maillart E, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 2018; 90: e1858–e1869. [DOI] [PubMed] [Google Scholar]
- 9. Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Chiriboga S, Sechi E, Buciuc M, et al. Long-term outcomes in patients with myelin oligodendrocyte glycoprotein immunoglobulin G-associated disorder. JAMA Neurol 2020; 8: e203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whittam DH, Karthikeayan V, Gibbons E, et al. Treatment of MOG antibody associated disorders: results of an international survey. J Neurol 2020; 267: 3565–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 14. Rostom A, Dubé C, Cranney A, et al. Celiac disease. Rockville, MD: Agency for Healthcare Research and Quality (US), 2004. (Evidence reports/technology assessments, no. 104. Appendix D. Quality assessment forms), https://www.ncbi.nlm.nih.gov/books/NBK35156/ [Google Scholar]
- 15. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montcuquet A, Collongues N, Papeix C, et al. Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler 2017; 23: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 17. Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018; 89: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cobo-Calvo A, Sepulveda M, Rollot F, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflammation 2019; 16: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao L, Yang L, Kessi M, et al. Myelin oligodendrocyte glycoprotein (MOG) antibody diseases in children in central south China: clinical features, treatments, influencing factors, and outcomes. Front Neurol 2019; 10: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou J, Lu X, Zhang Y, et al. Follow-up study on Chinese children with relapsing MOG-IgG-associated central nervous system demyelination. Mult Scler Relat Disord 2019; 28: 4–10. [DOI] [PubMed] [Google Scholar]
- 21. Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS, et al. Treatment of MOG-IgG-associated disorder with rituximab: an international study of 121 patients. Mult Scler Relat Disord 2020; 44: 102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen JJ, Flanagan EP, Bhatti MT, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology 2020; 95: e111–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albassam F, Longoni G, Yea C, et al. Rituximab in children with myelin oligodendrocyte glycoprotein antibody and relapsing neuroinflammatory disease. Dev Med Child Neurol 2020; 62: 390–395. [DOI] [PubMed] [Google Scholar]
- 24. Tzartos JS, Karagiorgou K, Tzanetakos D, et al. Deciphering anti-MOG IgG antibodies: clinical and radiological spectrum, and comparison of antibody detection assays. J Neurol Sci 2020; 410: 116673. [DOI] [PubMed] [Google Scholar]
- 25. Pedapati R, Bhatia R, Singh N, et al. Anti-myelin oligodendrocyte glycoprotein antibody associated disease spectrum – a north Indian tertiary care centre experience and review of literature. J Neuroimmunol 2020; 340: 577143. [DOI] [PubMed] [Google Scholar]
- 26. Durozard P, Rico A, Boutiere C, et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann Neurol 2020; 87: 256–266. [DOI] [PubMed] [Google Scholar]
- 27. Holmoy T, Hoglund RA, Illes Z, et al. Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder. J Neurol. Epub ahead of print 3 October 2020. DOI: 10.1007/s00415-020-10235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hauser SL, Cree B. Treatment of multiple sclerosis: a review. Am J Med 2020; 133: 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rastetter W, Molina A, White CA. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med 2004; 55: 477–503. [DOI] [PubMed] [Google Scholar]
- 30. Iorio R, Damato V, Alboini PE, et al. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J Neurol 2015; 262: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 31. Nepal G, Shing YK, Yadav JK, et al. Efficacy and safety of rituximab in autoimmune encephalitis: a meta-analysis. Acta Neurol Scand 2020; 142: 449–459. [DOI] [PubMed] [Google Scholar]
- 32. Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol 2016; 73: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 33. Tian X, Chen C, Ma L, et al. Efficacy and safety of rituximab in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. J Neuroimmunol 2020; 347: 577317. [DOI] [PubMed] [Google Scholar]
- 34. Gao F, Chai B, Gu C, et al. Effectiveness of rituximab in neuromyelitis optica: a meta-analysis. BMC Neurol 2019; 19: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 2019; 15: 89–102. [DOI] [PubMed] [Google Scholar]
- 36. Armangue T, Olive-Cirera G, Martinez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol 2020; 19: 234–246. [DOI] [PubMed] [Google Scholar]
- 37. Lu Q, Luo J, Hao H, et al. Efficacy and safety of long-term immunotherapy in adult patients with MOG antibody disease: a systematic analysis. J Neurol. Epub ahead of print 30 September 2020. DOI: 10.1007/s00415-020-10236-4. [DOI] [PubMed] [Google Scholar]
- 38. Villarroel MC, Hidalgo M, Jimeno A. Mycophenolate mofetil: an update. Drugs Today 2009; 45: 521–532. [DOI] [PubMed] [Google Scholar]
- 39. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000; 47: 85–118. [DOI] [PubMed] [Google Scholar]
- 40. Huang W, Wang L, Zhang B, et al. Effectiveness and tolerability of immunosuppressants and monoclonal antibodies in preventive treatment of neuromyelitis optica spectrum disorders: a systematic review and network meta-analysis. Mult Scler Relat Disord 2019; 35: 246–252. [DOI] [PubMed] [Google Scholar]
- 41. Li S, Ren H, Xu Y, et al. Long-term efficacy of mycophenolate mofetil in myelin oligodendrocyte glycoprotein antibody-associated disorders: a prospective study. Neurol Neuroimmunol Neuroinflamm 2020; 7: e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol 2018; 75: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hegen H, Reindl M. Recent developments in MOG-IgG associated neurological disorders. Ther Adv Neurol Disord 2020; 13: 1756286420945135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol 2008; 64: 753–767. [DOI] [PubMed] [Google Scholar]
- 45. Espiritu AI, Pasco P. Efficacy and tolerability of azathioprine for neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord 2019; 33: 22–32. [DOI] [PubMed] [Google Scholar]
- 46. Matney SE, Huff DR. Diagnosis and treatment of myasthenia gravis. Consult Pharm 2007; 22: 239–248. [DOI] [PubMed] [Google Scholar]
- 47. Hermetter C, Fazekas F, Hochmeister S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol 2018; 9: 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-3-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-4-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-5-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-6-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-tif-7-tan-10.1177_17562864211055694 for Efficacy and safety of immunosuppressive therapy in myelin oligodendrocyte glycoprotein antibody–associated disease: a systematic review and meta-analysis by Qi-Lun Lai, Yin-Xi Zhang, Meng-Ting Cai, Yang Zheng, Song Qiao, Gao-Li Fang and Chun-Hong Shen in Therapeutic Advances in Neurological Disorders
Data Availability Statement
Data were available upon request. Interested researchers may contact the corresponding author.