Abstract
Background
There is a limited understanding of the cognitive and psychiatric sequelae of COVID-19 during the post-acute phase, particularly among racially and ethnically diverse patients. Objective: We sought to prospectively characterize cognition, mental health symptoms, and functioning approximately four months after an initial diagnosis of COVID-19 in a racially and ethnically diverse group of patients.
Methods
Approximately four months after COVID-19 diagnosis, patients in the Johns Hopkins Post-Acute COVID-19 Team Pulmonary Clinic underwent a clinical telephone-based assessment of cognition, depression, anxiety, trauma, and function.
Results
Most Johns Hopkins Post-Acute COVID-19 Team patients assessed were women (59%) and members of racial/ethnic minority groups (65%). Of 82 patients, 67% demonstrated ≥1 abnormally low cognitive score. Patients requiring intensive care unit (ICU) stays displayed greater breadth and severity of impairment than those requiring less intensive treatment. Processing speed (35%), verbal fluency (26%–32%), learning (27%), and memory (27%) were most commonly impaired. Among all patients, 35% had moderate symptoms of depression (23%), anxiety (15%), or functional decline (15%); 25% of ICU patients reported trauma-related distress. Neuropsychiatric symptoms and functional decline did not differ by post-ICU versus non-ICU status and were unrelated to global cognitive composite scores.
Conclusions
At approximately 4 months after acute illness, cognitive dysfunction, emotional distress, and functional decline were common among a diverse clinical sample of COVID-19 survivors varying in acute illness severity. Patients requiring ICU stays demonstrated greater breadth and severity of cognitive impairment than those requiring less intensive treatment. Findings help extend our understanding of the nature, severity, and potential duration of neuropsychiatric morbidity after COVID-19 and point to the need for longitudinal assessment of cognitive and mental health outcomes among COVID-19 survivors of different demographic backgrounds and illness characteristics.
Key words: COVID-19, cognitive impairment, critical illness, depression, anxiety, functional decline
Introduction
The potential for neuropsychiatric complications of COVID-19 was appreciated early in the pandemic based on observations from prior coronavirus infections. Both the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics were characterized by delirium, cognitive impairment, mood disturbances, and anxiety that persisted beyond the acute phase of illness.1 Against this background, early reports of neurologic symptoms and delirium coupled with an ever-growing infection rate raised concerns that cognitive and mental health sequelae of COVID-19 could have significant individual and population health implications.2 , 3
While critical illness and hospitalization alone represent risk factors for impaired cognition and neuropsychiatric sequelae, combined hypoxemia, coagulopathy, need for greater sedation, social isolation, and related limits in access to rehabilitation services implies a potential for compounded neuropsychiatric risk in both short- and long-term outcomes.3, 4, 5, 6, 7, 8 At present, most available data characterize psychiatric and cognitive symptoms during the acute phase of COVID-19 illness, including delirium/altered mentation at presentation or during hospitalization.9 , 10 Existing knowledge of post-acute neuropsychiatric symptoms associated with COVID-19 infection is limited and primarily focused on psychiatric rather than cognitive outcomes. When assessed one month after hospital discharge, more than half of patients in one study produced scores in the clinically elevated range across measures of anxiety (42%), insomnia (40%), depression (31%), and post-traumatic stress (28%).11 Among COVID-19 patients who lack prior psychiatric histories, data suggest heightened risk of new-onset psychiatric diagnosis within 90 days of a positive COVID-19 test relative to that occurring after a range of other medical events.12
To date, most studies have relied on brief cognitive screening measures or small case series rather than detailed neuropsychological test batteries in samples of sufficient size to permit detection of patterns of impairment and associated disease and treatment characteristics.13, 14, 15, 16, 17 Much remains to be learned about the nature and severity of cognitive and psychiatric functioning across patients of varying disease severity and at various time points over the course of recovery from COVID-19.18 Furthermore, it is appreciated that racial and ethnic minority groups are disproportionately impacted by the pandemic and may be at higher risk of poor clinical outcomes, yet little data exist on long-term cognitive and psychiatric functioning in these groups.19, 20, 21
In order to characterize post-acute neuropsychiatric functioning, we prospectively evaluated a broad range of cognitive abilities, mental health symptoms, and functioning at approximately four months after an initial diagnosis of COVID-19 in a racially and ethnically diverse group of patients.
Materials and Methods
Study Design
A comprehensive battery of cognitive tests and measures of mental health and functional decline were administered as part of routine clinical care and analyzed retrospectively. The Johns Hopkins Medicine Institutional Review Board approved the study.
Study Population
Patients included adults receiving care in the Johns Hopkins Post-Acute COVID-19 Team (JH PACT) pulmonary clinic, requiring referral from a treating physician.22 Qualifying criteria for post-ICU clinic services included acute illness from COVID-19 requiring ≥48 hours of ICU care. Referrals to the JH PACT were a standard component of the ICU discharge procedures. Qualifying criteria for non-ICU clinic services included either (1) recovery from acute COVID-19 hospitalization (non-ICU) with ongoing pulmonary and/or rehabilitation needs at the time of hospital discharge or (2) persistent symptoms at 4–6 weeks after acute infection without hospitalization. Residual symptoms prompting JH PACT referral included persistent pulmonary issues, dyspnea, dysautonomia, fatigue, cognitive complaints, pain, and other nonresolving symptoms after COVID-19 infection.
Characterization
COVID-19 diagnosis was made via real-time reverse transcription PCR test. Demographic characteristics including age, sex, race, and ethnicity as well as years of educational attainment and occupational status were obtained through chart review and patient self-report. The Barona Index combined demographic variables (age, sex, race, education, occupational status, and geographic region) with correction for the tendency for intelligence test scores to increase over time, known as the Flynn effect, to predict Wechsler Adult Intelligence Scale 4th edition Full Scale IQ scores.23 See Supplementary Table 1.
Cognitive Assessment
Patients underwent a telephone-based neuropsychological assessment battery; telephone assessment has been shown to be feasible and provides valid assessment of cognitive function relative to in-person examinations.24, 25, 26 This battery has been successfully implemented in diverse patient populations.27 , 28 Assessments occurred between July 6, 2020, and January 22, 2021.
The cognitive battery consisted of eight scores. The Rey Auditory Verbal Learning Test (RAVLT29 , 30) assessed acquisition of a 15-item word list that was presented over multiple exposure trials as well as memory as indexed by delayed recall for word list items. Oral Trail Making Test parts A and B assessed processing speed by having patients count aloud to 25 as quickly as possible (part A) and executive functioning (part B) by having them do so while switching between numbers and letters.31 Attention and working memory were assessed with a number span task during which patients repeated increasingly lengthy digit strings in forward and backward sequences.28 Letter-cued verbal fluency assessed speeded word retrieval in response to phonetic cues by asking patients to name as many words as possible beginning with a certain letter of the alphabet over two 60-second trials (cues F and L28). Category-cued verbal fluency assessed rapid access to semantic information by asking patients to name as many items of a given semantic category as possible over two 60-second trials (cues animals and vegetables28). Standardized scores were derived from age-adjusted published normative data.28 , 30 , 31 A global cognitive composite was computed as the mean of age-adjusted standardized scores across up to 8 available cognitive scores.
Mental Health and Functional Assessment
Patients completed the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7) on which scores ≥5 and ≥10 reflect the presence of clinically elevated mild and moderate symptoms of depression and anxiety, respectively.32 , 33 Trauma-related distress was assessed in post-ICU patients via the Impact of Events Scale-6, on which scores of ≥1.75 reflect symptomatic levels of post-traumatic distress.34 , 35 The Quick Dementia Rating Scale, patient version, (QDRS) assessed subjective change across 10 domains of cognition, mood, and daily functioning. Scores ≥1.5 and ≥6 reflect clinically meaningful mild and moderate functional decline, respectively.36 , 37
Missing Data
One patient declined to report race, and two declined to report ethnicity. Four patients could not complete Oral Trail Making Test part B, and one declined to attempt the task. An additional patient declined to complete the letter and category fluency tasks as well as the GAD-7. Those with missing cognitive data were similar to those with complete data with respect to age, sex, racial/ethnic minority status, education, estimated IQ, cognitive composite scores, and psychiatric and functional assessment scores (P > 0.05).
Statistical Analyses
Cognitive outcomes
Independent samples t-tests compared PACT clinics (non-ICU/post-ICU) by age, educational attainment, estimated IQ, and time since diagnosis. Chi-square analyses compared PACT clinics by sex, race, and ethnicity. These demographic and clinical characteristics were also explored in relation to global cognitive composite scores via independent samples t-tests, analysis of variance, and chi-square analyses. Analysis of covariance compared global cognitive composite scores by PACT clinic (non-ICU/post-ICU) adjusting for variables that were either imbalanced across clinics or associated with global cognitive composite scores. Frequencies were calculated for the proportion of individuals overall and within each PACT clinic producing ≥1 cognitive score in the impaired range. Mild/moderate and severe cognitive impairment were defined as performances ≥1.5 and ≥2 standard deviations below published age-adjusted normative means for each cognitive test and the global cognitive composite score. Because neurologically healthy individuals may produce one or more low test scores on a cognitive battery, reliance on a single low score may increase the likelihood of false-positive findings of impairment.38, 39, 40 The likelihood of obtaining one or more low scores increases along with the number of tests in the battery.39 , 40 Based on the binomial probability distribution, in a cognitive battery such as this yielding 8 primary scores, there is a 10% probability that 2 scores will exceed the ≥1.5 standard deviation cutoff for mild/moderate impairment and a 1% probability that 2 scores will exceed the ≥2 standard deviation cutoff for severe impairment.41 Tests for the significance of proportions determined whether the observed proportion of individuals within each clinic producing at least 2 scores ≥1.5 and ≥2 standard deviations below demographic means exceeded expectation (i.e., 10% and 1% probabilities, respectively).
Mental health and functioning
Independent samples t-tests compared scores on measures of the PHQ-9, GAD-7, and QDRS by PACT clinic. Frequencies were calculated for the proportion of individuals scoring above clinical cutoffs on mental health and functional outcome measures. Pearson correlations assessed the association between cognitive test performances and PHQ-9, GAD-7, Impact of Events Scale-6, and QDRS.
Statistical analyses were performed with IBM SPSS statistics version 25.
Results
Characteristics
Of 82 PACT patients for whom English is the primary language, N = 48 (59%) required ≥48 hours of ICU care (i.e., post-ICU clinic). The majority were women (59%) and members of racial/ethnic minority groups. The mean (SD) age was 54.5 (14.6) years. The majority (95%) had at least a high school (12 years) education with mean (SD) 14.7 (3.1) years. The mean (SD) estimated IQ score was 98.2 (8.6). Post-ICU patients were older and had fewer years of education as well as lower estimated IQ scores than non-ICU patients but did not differ with respect to sex, race, ethnicity, or time since initial COVID-19 diagnosis. See Table 1 .
Table 1.
Baseline Demographic, Cognitive, and Mental Health Characteristics Overall and Differences in ICU Status
| Variables | Overall (N = 82) | Non-ICU (N = 34) | Post-ICU (N = 48) | T/Chi-square statistic (df) | P value |
|---|---|---|---|---|---|
| Age, mean (sd); range, years | 54.5 (14.6); 26–85 | 49.5 (13.0); 26–82 | 58.0 (14.8); 29–85 | −2.7 (80) | <0.01 |
| Male, n (%) | 34 (41.5) | 11 (32.4) | 23 (47.9) | 2.0 (1, 82) | 0.16 |
| Race, n (%) | 4.2 (4, 82) | 0.38 | |||
| White or Caucasian | 29 (35.4) | 16 (47.1) | 13 (27.1) | ||
| Black or African American | 44 (53.7) | 15 (44.1) | 29 (60.4) | ||
| Asian | 2 (2.4) | 1 (2.9) | 1 (2.1) | ||
| Other | 6 (7.3) | 2 (5.9) | 4 (8.3) | ||
| Unspecified | 1 (1.0) | — | 1 (1.2) | ||
| Ethnicity, n (%) | 1.5 (2, 82) | 0.47 | |||
| Not Hispanic or Latino | 78 (95.1) | 33 (97.1) | 45 (93.8) | ||
| Hispanic or Latino | 2 (2.4) | 1 (2.9) | 1 (2.9) | ||
| Unspecified | 2 (2.4) | — | 2 (2.4) | ||
| Education, mean (sd); range, years | 14.7 (3.1); 2–22 | 15.7 (3.1); 12–22 | 14.0 (3.0); 2–21 | 2.5 (80) | 0.02 |
| Estimated IQ, mean (sd); range, years | 98.2 (8.6); 70–114† | 100.7 (8.5); 84–114 | 96.4 (8.4); 70–112‖ | 2.3 (79) | 0.02 |
| Time from diagnosis, mean (sd); range, days | 126.5 (70.1); 19–282 | 122.7 (75.7); 27–294 | 129.0 (66.9); 19–301 | −0.4 (78) | 0.69 |
| RAVLT acquisition, mean (sd); range# | 42.6 (11.2); 17–63 | 46.7 (10.8); 20–63 | 39.7 (10.7); 17–63 | 2.9 (80) | <0.01 |
| RAVLT delayed recall, mean (sd); range# | 7.5 (3.5); 0–14 | 8.4 (3.5) 0–13 | 6.9 (3.3); 1–14 | 1.9 (80) | 0.06 |
| Oral Trail Making Test Part A, mean (sd); range# | 10.1 (6.7); 4–56 | 10.3 (9.0); 5–56 | 10.0 (4.6); 4–26 | 0.2 (80) | 0.85 |
| Oral Trail Making Test Part B, mean (sd); range# | 42.5 (34.4); 13–231 | 37.2 (38.6); 13–231‡ | 46.5 (30.7); 17–145§ | −1.2 (75) | 0.24 |
| Number span forward, mean (sd); range# | 9.9 (2.7); 3–16∗ | 10.4 (2.4); 7–16 | 9.6 (2.9) 3–16 | 1.5 (80) | 0.15 |
| Number span backward, mean (sd); range# | 7.2 (2.8); 1–15 | 7.6 (2.4); 3–13 | 6.9 (3.1); 1–15 | 1.1 (80) | 0.28 |
| Letter-cued verbal fluency, mean (sd); range# | 23.2 (8.7); 5–48† | 27.0 (8.1); 11–48‡ | 20.6 (8.2); 5–40 | 3.5 (79) | <0.01 |
| Category-cued verbal fluency, mean (sd); range# | 30.8 (9.3); 13–50† | 32.6 (9.2); 14–50‡ | 29.5 (9.2); 13–48 | 1.5 (79) | 0.15 |
| PHQ-9, mean (sd); range | 7.0 (5.0); 0–20 | 7.5 (5.5); 0–20 | 6.7 (4.6); 0–20 | 0.71 (80) | 0.48 |
| GAD-7, mean (sd); range | 5.3 (4.7); 0–19 | 5.5 (4.6); 0–19 | 5.1 (4.9); 0–19 | 0.35 (79) | 0.72 |
| IES-6, mean (sd); range | — | — | 1.2 (1.4); 0–7 | — | — |
| QRDS, mean (sd); range | 3.1 (2.9); 0–12 | 3.2 (3.3); 0–11 | 3.0 (2.7); 0–12 | 0.27 (80) | 0.79 |
Abbreviations: GAD-7 = Generalized Anxiety Disorder-7; IES-6 = Impact of Events Scale-6; PHQ-9 = Patient Health Questionnaire-9; QDRS = Quick Dementia Rating Scale; RAVLT = Rey Auditory Verbal Learning Test.
n = 77.
n = 81.
n = 33.
n = 44.
n = 47.
Age-adjusted standard scores, M = 100, SD = 15; allowable range, 61–139.
Cognitive Outcomes
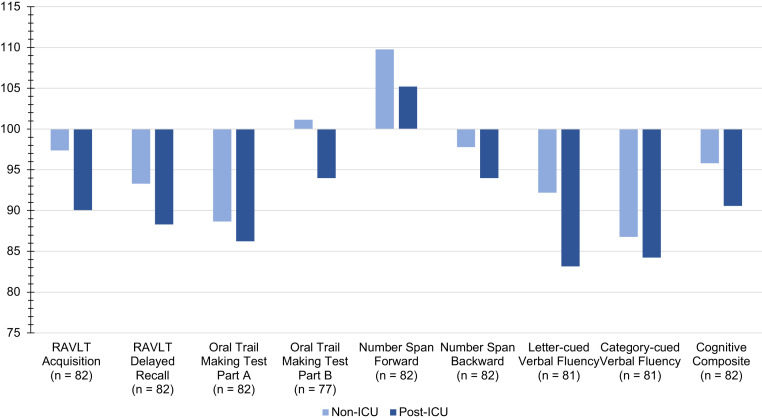
Cognitive testing occurred approximately 4 months after the first positive test for COVID-19 (mean [SD], 126.5 [70.1] days). Mean age-adjusted standard scores are presented in Figure 1 . Overall, 67% of PACT patients produced ≥1 score in the mild/moderate or severe range of impairment (65% non-ICU, 69% post-ICU).
Figure 1.
Age-Adjusted Standard Scores by ICU Status. Age-adjusted standard scores have a mean of 100 and standard deviation 15, with higher scores reflecting better performance. The cognitive composite was computed as the mean of age-adjusted standard scores across the cognitive scores.
Global cognitive composite scores differed by ICU status (post-ICU versus non-ICU, t(80) = 2.18, P = 0.03), racial/ethnic minority status (t(79) = 3.32, P = 0.001), and estimated IQ (F(1,79) = 14.47, P < 0.001) but did not differ by age, sex, education, or time since initial COVID-19 diagnosis (all P > 0.05). After accounting for age, education, estimated IQ, and racial/ethnic minority status, post-ICU clinic patients produced lower cognitive composite scores than non-ICU patients (mean post-ICU = 90.6, SD = 11.0, mean non-ICU = 95.8, SD = 10.3, F(1, 75) = 4.6, P = 0.04). Among non-ICU patients, cognitive composite scores did not differ between those who were hospitalized (n = 21, 62%) and those who were not hospitalized (n = 13, 38%, t(32) = −0.19, P = 0.85). Racial/ethnic minorities showed a insignificant trend toward disproportional receipt of care in the ICU (minority post-ICU = 65.4%, minority non-ICU = 34.6%, white post-ICU = 44.8%, white non-ICU = 55.2%, chi-square (1, n = 81) = 3.2, P = 0.07). Within racial/ethnic minority patients, those treated in the post-ICU clinic demonstrated a trend toward poorer global cognitive functioning relative to racial/ethnic minority patients treated in the non-ICU clinic (mean post-ICU = 87.9, SD = 10.1, mean non-ICU = 93.3, SD = 10.9, t(50) = 1.77, P = 0.083) while not differing with respect to age, education, or estimated IQ (all P > 0.42).
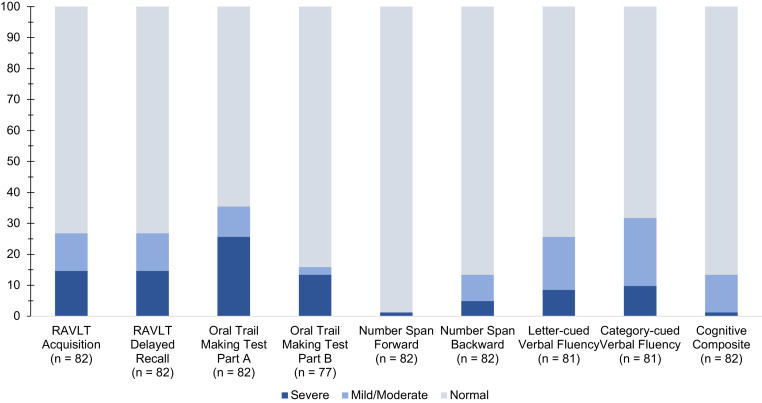
On a global cognitive composite measure, the average score among non-ICU patients was 0.28 standard deviations below demographic expectation, with 6% producing global cognitive functioning scores below the cutoff for mild/moderate impairment. The observed proportion of non-ICU patients producing ≥2 cognitive scores beyond the cutoff for mild/moderate impairment (32%) exceeded the expected 10% proportion (P < 0.001).41 Mild/moderate impairment was particularly common on Oral Trail Making Test part A, category-cued verbal fluency, RAVLT acquisition, and RAVLT delayed recall. The observed proportion of non-ICU patients producing ≥2 cognitive scores beyond the cutoff for severe impairment (15%) exceeded the expected 1% proportion (P < 0.001). Severe impairment was most common on the Oral Trail Making Test part A (21%). See Figure 2 , Table 2 , and Supplementary Table 2.
Figure 2.
Proportion of PACT Patients Producing Scores at or Below Cutoffs for Mild/Moderate and Severe Cognitive Impairment Across Cognitive Tests. Mild/moderate impairment is defined as sores ≥1.5 standard deviation below published age-adjusted normative means. Severe impairment is defined as scores ≥2 standard deviations below published age-adjusted normative means.
Table 2.
Proportion of PACT Patients Performing at or Below the Mild/Moderate and Severe Ranges of Impairment Across Cognitive Tests by ICU Status
| Domain | Overall (N = 82), % impaired∗ |
Non-ICU (N = 34), % Impaired |
Post-ICU (N = 48), % Impaired |
|||
|---|---|---|---|---|---|---|
| Mild/Moderate | Severe | Mild/Moderate | Severe | Mild/Moderate | Severe | |
| RAVLT acquisition | 26.8 | 14.6 | 14.7 | 8.8 | 35.4 | 18.8 |
| RAVLT delayed recall | 26.8 | 14.6 | 14.7 | 8.8 | 35.4 | 18.8 |
| Oral Trail Making Test Part A | 35.4 | 25.6 | 32.4 | 20.6 | 37.5 | 29.2 |
| Oral Trail Making Test Part B | 15.9† | 13.4† | 11.8§ | 8.8§ | 18.8‖ | 16.7‖ |
| Number span forward | 1.2 | 1.2 | 0 | 0 | 2.1 | 2.1 |
| Number span backward | 13.4 | 4.9 | 8.8 | 2.9 | 16.7 | 6.3 |
| Letter-cued verbal fluency | 25.6‡ | 8.5‡ | 11.8§ | 2.9§ | 35.4 | 12.5 |
| Category-cued verbal fluency | 31.7‡ | 9.8‡ | 26.5§ | 5.9§ | 35.4 | 12.5 |
| Cognitive composite score | 13.4 | 1.2 | 5.9 | 2.9 | 18.8 | 0 |
Abbreviations: ICU = intensive care unit; PACT = Post-Acute COVID-19 Team; RAVLT = Rey Auditory Verbal Learning Test.
Mild/moderate and severe cognitive impairment were defined as performances ≥1.5 and ≥2 standard deviations below published age-adjusted normative means. Hence, 7% of normative control group participants would be expected to produce a score in the mild/moderate range of impairment on any single cognitive test, and 2% would be expected to produce a score in the severe range of impairment on any single cognitive test.
n = 77.
n = 81.
n = 33.
n = 44.
Post-ICU patients performed, on average, 0.63 standard deviations below expectation on a global cognitive composite measure, with 19% producing global cognitive functioning scores below the cutoff for mild/moderate impairment. The observed proportion of post-ICU patients producing ≥2 scores below the cutoff for mild/moderate impairment (58%) exceeded the expected 10% proportion (P < 0.001). Elevated rates of impairment were observed across all domains with the exception of number span forward. More than one-third of post-ICU patients performed in the mild/moderately impaired range on the Oral Trail Making Test part A, letter- and category-cued verbal fluency, RAVLT acquisition, and RAVLT delayed recall. The observed proportion of post-ICU patients producing ≥2 cognitive scores beyond the cutoff for severe impairment (31%) exceeded the expected 1% proportion (P < 0.001). Severe impairment was most common on a test of processing speed (29%).
Mental Health and Functioning
Scores on the PHQ-9, GAD-7, and QDRS did not differ based on ICU status (all P > 0.05). Overall, 78% of PACT patients produced ≥1 mildly elevated score across measures of psychiatric distress and functional decline (74% non-ICU, 81% post-ICU), with 35% producing ≥1 moderately elevated score across these measures (35% non-ICU, 35% post-ICU). Mildly elevated PHQ-9 or GAD-7 were reported by 70% of PACT patients, with 27% reporting moderate to severe elevations. In addition, 62% and 69% of patients reported mild functional declines on the QDRS across non-ICU and post-ICU clinics, with 18% and 13% reporting moderate functional declines, respectively. One quarter of post-ICU patients reported symptomatic levels of trauma-related distress on the Impact of Events Scale-6. See Table 3 .
Table 3.
Proportion of PACT Patients Scoring in the Symptomatic Range Across Mental Health and Functional Outcomes by ICU Status
| Overall (N = 82), % symptomatic |
Non-ICU (N = 34), % symptomatic |
Post-ICU (N = 48), % symptomatic |
||||
|---|---|---|---|---|---|---|
| IES-6 |
-- |
-- |
-- |
-- |
25.0 |
|
| Mild | Moderate | Mild | Moderate | Mild | Moderate | |
| PHQ-9 | 67.1 | 23.2 | 67.6 | 29.4 | 66.7 | 18.8 |
| GAD-7 | 50.0∗ | 14.8 | 52.9† | 15.2 | 47.9 | 14.6 |
| QRDS | 65.9 | 14.6 | 61.8 | 17.6 | 68.8 | 12.5 |
Abbreviations: GAD-7 = Generalized Anxiety Disorder-7; IES-6 = Impact of Events Scale-6; PHQ-9 = Patient Health Questionnaire-9; QDRS = Quick Dementia Rating Scale.
n = 81.
n = 33.
Global cognitive composite scores were not associated with scores on the PHQ-9, GAD-7, Impact of Events Scale-6, or QDRS (all P > 0.08).
Discussion
At approximately 4 months after acute illness, two-third of COVID-19 patients presenting to a post-COVID-19 clinic showed impairment in one or more domains of cognition. Cognitive deficits were widespread in those with and without ICU stays and occurred most commonly on measures of oral processing speed and verbal fluency as well as learning and memory. Patients requiring at least 48 hours of ICU care demonstrated poorer global cognition and also demonstrated more frequent impairment in executive functioning and working memory relative to those requiring less intensive treatment. Psychiatric distress and functional decline were also common, with 35% of COVID-19 survivors producing at least one moderately elevated score across measures of anxiety, depression, trauma, and functional decline. One in four patients requiring treatment in the ICU reported trauma-related distress. Severity of psychiatric distress and functional decline were similar among patients requiring more and less intense COVID-19 treatment and were unrelated to cognitive functioning.
Our understanding of post-acute cognitive and mental health functioning remains in the early stages and is limited to a handful of studies that vary with respect to patient characteristics, COVID-19 severity, the time in which testing occurred relative to infection, and the breadth and depth of cognitive assessment. In an Italian study, 12 previously neurologically healthy adults (age 48–80 years) hospitalized for neurologic complications of COVID-19 performed more poorly than matched controls on an executive functioning composite and measures of reaction time and vigilance when tested near the end of their course of inpatient rehabilitation.42 More recently, 81% of patients (n = 57, mean age 65 years) recovering from COVID-19 infection on an inpatient rehabilitation unit showed mild to severe cognitive impairment on a battery assessing memory and executive functioning, with deficits occurring most frequently in working memory, set shifting, divided, attention, and processing speed.43
Two studies have examined cognition in relatively younger and seemingly less severely ill patients early in the post-acute phase. Among 35 previously cognitively healthy COVID-19 survivors in Spain (age 24–60 years) who were tested 10 to 35 days after hospital discharge, severe impairments (T score ≤ 30) were most frequent on tests of letter-cued verbal fluency (11%), mental flexibility (9%), and working memory (9%), with less frequent deficits (6%) observed on tests of processing speed, category-cued verbal fluency, attention, learning, and memory.44 Subjective cognitive complaints were associated with the severity of anxiety and depressive symptoms, but not objective cognitive performance. A group of 29 hospitalized adults in China (age 30–64 years) underwent tablet-based cognitive testing of select cognitive domains 2 to 3 weeks after COVID-19 infection. Patients performed more poorly than age- and sex-matched peers on aspects of sustained attention but did not differ from controls with respect to processing speed, executive functioning, attention, or working memory nor did they report elevated symptoms of anxiety or depression.45
Data on longer term cognitive outcomes after COVID-19 are just starting to take shape and suggest that outcomes may vary based on markers of disease severity. Of 179 previously cognitively and psychiatrically healthy patients in Spain (age 22–81 years) assessed roughly two months after hospital discharge, 58% demonstrated moderate deficits (T score ≤ 40) on at least one of four cognitive tests, most frequently learning and category-cued verbal fluency. Clinically elevated symptoms of depression, anxiety, and/or trauma were reported by 39% of patients.46 In contrast, in an Australian sample of 78 patients tested two to three months after diagnosis and of whom only 12% required hospitalization, objective cognitive impairment was demonstrated by only 10% of patients, most frequently on a test of psychomotor speed, with most deficits (63%) being of mild severity.47 Symptoms of depression were reported by 21%. More recently, a prospective US cohort of 100 nonhospitalized patients found high rates of relatively young individuals (mean age 43 ± 11), females (70%), and those with pre-existing anxiety/depression (42%) and autoimmune disease (16%) presented to a post-COVID clinic. When assessed five to six months after the illness, there was no difference in subjective rates of “brain fog” or cognition-related quality of life between those who had tested positive versus negative for SARS-CoV-2. Among a smaller subset undergoing objective assessment (n = 34), the groups did not differ in their performance on a brief cognitive battery although the SARS-CoV-2 positive group did underperform relative to demographic controls on measures of attention and working memory.48
To date, predictions as to the potential for long-term neuropsychiatric sequelae of COVID-19 have been based largely on observations of neurologic symptoms and delirium in the acute stage of illness coupled with literature from prior coronavirus epidemics and observations made in the study of critical illness survivors. More than 15% of patients affected by the SARS and MERS epidemics demonstrated cognitive impairment and psychiatric disturbance 6–39 months after recovery.1 Similarly, reports that at one year after hospitalization for critical illness, cognitive deficits persist in 24%–34%, symptoms of post-traumatic distress persist in 34%, and symptoms of depression persist in 29% suggest that long-term follow-up of COVID-19 patients is warranted.6, 7, 8 While depression has been found to independently predict long-term cognitive impairment in survivors of critical illness,49 the present study did not find psychiatric symptoms to track with the intensity of treatment or severity of cognitive dysfunction. More work is needed to understand the mechanisms driving cognitive and psychiatric morbidity among individuals treated for COVID-19.
The present study reflects a detailed assessment of cognitive functioning, psychiatric distress, and functional decline in a sample of clinically referred COVID-19 patients during the post-acute phase. Patients spanned a wide age range and were of diverse racial and ethnic backgrounds. They varied in disease severity and level of care required during the acute phase of illness. Patients were not excluded based on pre-existing medical or neurological conditions; rather, findings reflect the neuropsychiatric functioning of patients who are likely to receive treatment in a post-acute COVID-19 pulmonary clinic. We used a comprehensive battery and methodologically rigorous definitions of cognitive impairment. Assessments also occurred later in the post-acute phase than has previously been explored and provide novel data on the potential duration of neuropsychiatric sequelae among COVID-19 survivors.
The study findings are limited by the selective nature of patients seen in the JH PACT clinic and are not generalizable to those who do not seek outpatient post-COVID-19 care. As these are clinically referred patients, we also lack baseline data that could help determine the extent to which observed cognitive and psychiatric dysfunction reflects sequelae of COVID-19 illness. Furthermore, ours is one of the first dedicated COVID-19 survivorship clinics in the United States, with data collection beginning relatively early in the pandemic.22 As such, we were unable to recruit healthy controls and instead relied on published normative data to establish rates of poor cognitive performance. The normative data for some tests were derived from samples that were relatively older and less racially and ethnically diverse than our own.28 The use of regression-based norms allowed for standardization of obtained cognitive scores for younger individuals who were not well represented in the normative data set. The finding that a substantial proportion of patients showed cognitive impairment even when applying norms for older adults suggests that the extent of cognitive dysfunction in younger COVID-19 survivors may be higher than appreciated in the present study. In addition, our finding that within racial/ethnic minority patients, those treated in ICU for ≥48 hours tended to perform more poorly than those requiring less intensive treatment, while not differing in age, education, or intelligence suggests that disease severity rather than systematic biases in clinic makeup underly the observation of poorer cognition outcomes in ICU survivors. Further work is needed to elucidate and combat systemic inequalities that are contributing to the disproportional impact of the COVID-19 pandemic among racial minority groups. We may have underestimated rates of executive functioning by excluding those who could not complete the tasks rather than classifying these individuals as impaired. Finally, study data were collected over seven months in which rapid advances were made in the treatment of COVID-19. It remains to be seen whether neuropsychiatric outcomes will improve in parallel with treatment advances.
Conclusions
The present study's findings of persistent cognitive deficits, psychiatric symptoms, and functional decline at approximately 4 months after the diagnosis help extend our understanding of the breadth, severity, and potential duration of neuropsychiatric morbidity among patients presenting to a post-COVID-19 clinic. Consistent with reports that racial and ethnic minority groups are disproportionately impacted by the pandemic, minority patients composed most individuals receiving care in the JH PACT clinic.20 , 21 This underscores the need for greater representation of minority groups in COVID-19 research to further understand the full impact of COVID-19 in vulnerable and traditionally underrepresented groups. Findings highlight the need for multidisciplinary integrated care teams aimed at providing comprehensive survivorship care for COVID-19 survivors throughout the recovery process. They also serve as a foundation for the longitudinal assessment of cognitive and mental health outcomes among COVID-19 survivors of different demographic backgrounds and illness characteristics.
Acknowledgments
The authors wish to thank Anna Agranovich, PhD; Adam Kaplan, MD, PhD; and Elizabeth Ryznar, MD, for their work in providing clinical care to patients seen in the JH PACT clinic.
Footnotes
Conflict of Interest: E.B. reports receiving speaker fees/honoraria from the University of Rochester, Johns Hopkins University, and Montana Area Health Education Center. A.M.P. reports receiving legal consulting fees; speaker fees/honoraria from Vizient, Johns Hopkins, ASHA, HCL Healthcare India, and Global Tracheostomy Collaborative; and Data Safety Monitoring/Advisory Board membership sponsored by Universidad de Chile. All other authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Funding: This work was supported by the National Institutes of Health (K23ES029105, K23HL138206, K12HL143957, R01AG057725); the Johns Hopkins Alzheimer's Disease Research Center (P30AG066507); and the Richman Precision Medicine Center of Excellence in Alzheimer's Disease.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaclp.2021.10.006.
Supplementary Data
Tables 1 and 2
References
- 1.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira A. Long-term neurological threats of COVID-19: a call to update the thinking about the outcomes of the coronavirus pandemic. Front Neurol. 2020;11:308. doi: 10.3389/fneur.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miners S., Kehoe P.G., Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12:1–16. doi: 10.1186/s13195-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon T. Neurological infection with SARS-CoV-2—the story so far. Nat Rev Neurol. 2021:1–2. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:1–2. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandharipande P.P., Girard T.D., Jackson J.C., et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43:1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 8.Rabiee A., Nikayin S., Hashem M.D., et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:1744. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varatharaj A., Thomas N., Ellul M.A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. The Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62354COVID -19cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negrini F., Ferrario I., Mazziotti D., et al. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Arch Phys Med Rehabil. 2021;102:155. doi: 10.1016/j.apmr.2020.09.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcloughlin B.C., Miles A., Webb T.E., et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11:857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo M.S., Malsy J., Pöttgen J., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groiss S.J., Balloff C., Elben S., et al. Prolonged neuropsychological deficits, central nervous system involvement, and brain stem affection after COVID-19—a case series. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.574004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vannorsdall T., Oh E. Post-acute cognitive and mental health outcomes among COVID-19 survivors: early findings and a call for further investigation. J Intern Med. 2021 doi: 10.1111/joim.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sze S., Pan D., Nevill C.R., et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100630. doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu T., Mack J.A., Salvatore M., et al. Characteristics associated with Racial/Ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw open. 2020;3 doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez D.A., Hinson J.S., Klein E.Y., et al. SARS-CoV-2 positivity rate for Latinos in the Baltimore–Washington, DC region. JAMA. 2020;324:392–395. doi: 10.1001/jama.2020.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brigham E., O'Toole J., Kim S.Y., et al. The Johns Hopkins post-acute COVID-19 team (PACT): a multidisciplinary, collaborative, ambulatory framework supporting COVID-19 survivors. Am J Med. 2021;134:462–467.e1. doi: 10.1016/j.amjmed.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirton J.W., Soble J.R., Marceaux J.C., et al. Comparison of models of premorbid IQ estimation using the TOPF, OPIE-3, and Barona equation, with corrections for the Flynn effect. Neuropsychology. 2020;34:43. doi: 10.1037/neu0000569. [DOI] [PubMed] [Google Scholar]
- 24.Lacritz L.H., Carlew A.R., Livingstone J., Bailey K.C., Parker A., Diaz A. Patient satisfaction with telephone neuropsychological assessment. Arch Clin Neuropsychol. 2020;35:1240–1248. doi: 10.1093/arclin/acaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanho T.C., Amorim L., Zihl J., Palha J.A., Sousa N., Santos N.C. Telephone-based screening tools for mild cognitive impairment and dementia in aging studies: a review of validated instruments. Front Aging Neurosci. 2014;6:16. doi: 10.3389/fnagi.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlew A.R., Fatima H., Livingstone J.R., et al. Cognitive assessment via telephone: a scoping review of instruments. Arch Clin Neuropsychol. 2020;35:1215–1233. doi: 10.1093/arclin/acaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matchanova A., Babicz M.A., Medina L.D., et al. Latent structure of a brief clinical battery of neuropsychological tests administered in-home via telephone. Arch Clin Neuropsychol. 2021;36:874–886. doi: 10.1093/arclin/acaa111. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub S., Besser L., Dodge H.H., et al. Version 3 of the Alzheimer Disease centers' neuropsychological test battery in the Uniform data set (UDS) Alzheimer Dis Assoc Disord. 2018;32:10–17. doi: 10.1097/WAD.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New York, NY: 2006. A compendium of neuropsychological tests: Administration, norms, and commentary. [Google Scholar]
- 30.Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey auditory verbal learning test: RAVLT: a handbook. [Google Scholar]
- 31.Mrazik M., Millis S., Drane D.L. The oral Trail making test: effects of age and concurrent validity. Arch Clin Neuropsychol. 2010;25:236–243. doi: 10.1093/arclin/acq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 34.Thoresen S., Tambs K., Hussain A., Heir T., Johansen V.A., Bisson J.I. Brief measure of posttraumatic stress reactions: impact of Event Scale-6. Soc Psychiatry Psychiatr Epidemiol. 2010;45:405–412. doi: 10.1007/s00127-009-0073-x. [DOI] [PubMed] [Google Scholar]
- 35.Hosey M.M., Leoutsakos J.S., Li X., et al. Screening for posttraumatic stress disorder in ARDS survivors: validation of the Impact of Event Scale-6 (IES-6) Crit Care. 2019;23:1–7. doi: 10.1186/s13054-019-2553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman S.E., Koscik R.L., Clark L.R., et al. Use of the Quick Dementia Rating System (QDRS) as an initial screening measure in a longitudinal cohort at risk for Alzheimer's disease. J Alzheimers Dis Rep. 2017;1:9–13. doi: 10.3233/ADR-170004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galvin J.E. The Quick Dementia Rating System (QDRS): a rapid dementia staging tool. Alzheimers Dement (Amst) 2015;1:249–259. doi: 10.1016/j.dadm.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binder L.M., Iverson G.L., Brooks B.L. To err is human:“Abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- 39.Schretlen D.J., Testa S.M., Winicki J.M., Pearlson G.D., Gordon B. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. J Int Neuropsychological Soc. 2008;14:436–445. doi: 10.1017/S1355617708080387. [DOI] [PubMed] [Google Scholar]
- 40.Oltra-Cucarella J., Sánchez-SanSegundo M., Rubio-Aparicio M., Arango-Lasprilla J.C., Ferrer-Cascales R. The association between the number of neuropsychological measures and the base rate of low scores. Assessment. 2021;28:955–963. doi: 10.1177/1073191119864646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingraham L.J., Aiken C.B. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10:120. [Google Scholar]
- 42.Ortelli P., Ferrazzoli D., Sebastianelli L., et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021:1–6. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H., Lu S., Chen J., et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendez R., Balanza-Martinez V., Luperdi S.C., et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;290:621–631. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darley D.R., Dore G.J., Cysique L., et al. Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med J Aust. 2021;214:279–280. doi: 10.5694/mja2.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham E.L., Clark J.R., Orban Z.S., et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 "long haulers". Ann Clin Transl Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordness M.F., Patel M.B., Erickson C.R., et al. Depression predicts long-term cognitive impairment in survivors of critical illness. J Trauma Acute Care Surg. 2021;90:79–86. doi: 10.1097/TA.0000000000002955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables 1 and 2