Abstract
The prospective randomized, placebo-controlled CALGB 10603/RATIFY trial (Alliance) demonstrated a statistically significant overall survival benefit from the addition of midostaurin to standard frontline chemotherapy in a genotypically-defined subgroup of 717 patients with FLT3-mutant acute myeloid leukemia (AML). The risk of death was reduced by 22% on the midostaurin-containing arm. In this post hoc analysis, we analyzed the cumulative incidence of relapse (CIR) on this study and also evaluated the impact of 12 4-week cycles of maintenance therapy. CIR analyses treated relapses and AML deaths as events, deaths from other causes as competing risks, and survivors in remission were censored. CIR was improved on the midostaurin arm (HR=0.71 (95% CI, 0.54–0.93); p=0.01), both overall and within European LeukemiaNet 2017 risk classification subsets when post-transplant events were considered in the analysis as events. However, when transplantation was considered as a competing risk, there was overall no significant difference between the risks of relapse on the two randomized arms. Patients still in remission after consolidation with high-dose cytarabine entered the maintenance phase, continuing with either midostaurin or placebo. Analyses were inconclusive in quantifying the impact of the maintenance phase on the overall outcome. In summary, midostaurin reduces the CIR.
Keywords: midostaurin, FLT3-mutant, acute myeloid leukemia
INTRODUCTION
Myeloblasts from 25 – 30% of adults with acute myeloid leukemia (AML) have an activating mutation in the gene encoding the trans-membrane tyrosine kinase FLT3.(1–3) Three-quarters are a FLT3 internal tandem duplication (ITD) mutation resulting in a duplication of between 1 and greater than 100 amino acids most commonly located in the juxtamembrane region. Such length mutations are associated with an adverse prognosis due to a high relapse rate, particularly in those with a high variant allele fraction relative to wild-type FLT3 alleles.(4,5) Tyrosine kinase domain (TKD) point mutations occur in about 8% of patients with de novo AML and have uncertain prognostic impact.(6,7) Both subtypes of FLT3 mutations yield proteins that spontaneously dimerize, thus bypassing ligand-mediated activation. Small molecule inhibitors of activated FLT3 specifically inhibit proliferation of leukemia cells in preclinical models (8–10) and demonstrate clinical benefit.(11,12)
Midostaurin is a multi-targeted kinase inhibitor that inhibits FLT3 signaling.(13–14) Clinical trials demonstrated that midostaurin could be given orally with an acceptable side-effect profile in combination with standard daunorubicin and cytarabine chemotherapy during courses of remission induction and post-remission chemotherapy.(15–16) A prospective, multi-national, randomized, double-blind, placebo-controlled trial (Cancer and Leukemia Group B (CALGB) 10603/RATIFY) demonstrated a statistically significant overall survival (OS) benefit from the addition of midostaurin to standard frontline chemotherapy in a genotypically-defined subgroup of 717 patients with FLT3-mutant AML.(17) The risk of death was reduced by 22% on the midostaurin-containing arm of the trial, and a benefit was observed in subsets with either high or low mutant allelic fractions or with the TKD mutation. These results contributed to the regulatory approval for frontline use of midostaurin with chemotherapy during induction and consolidation by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and in other countries. The EMA’s approval also specifically included maintenance.(18) Although allogeneic hematopoietic cell transplantation (allo-HCT) was not part of the treatment schedule in this study, a substantial proportion of patients received allo-HCT in first remission (25%) and overall (57%).(17) This was mainly motivated by favorable results of allo-HCT in high risk patients with activating FLT3 mutations.(19) In this report we describe the impact of midostaurin on the cumulative incidence of relapse (CIR), and we examine the outcomes of patients who received any amount of maintenance therapy on this trial.
METHODS
Trial Design
A detailed description of the CALGB 10603/RATIFY trial and its principal endpoints has been published.(17) Briefly, patients with newly diagnosed AML between ages 18 – 59 years provided written informed consent to allow obtaining a diagnostic marrow sample that was then submitted to one of nine academic laboratories for FLT3 mutation testing. If the patient’s leukemia cells were documented to have a FLT3-ITD or FLT3-TKD mutation and other standard eligibility criteria were met, they could be registered to the treatment trial. Cytogenetic analyses (545 patients, 76%) and NPM1 mutation analyses (475 patients, 66%) were performed on most patients. All available cytogenetic results plus newly obtained mutation data (NPM1, FLT3-ITD, FLT3-TKD, RUNX1, ASXL1, TP53) allowed subgroup classification according to the 2017 European LeukemiaNet (ELN) criteria for 441 patients (62%) as shown in Table 1.(20–22) Patients with the presence of any favorable characteristic were classified as “favorable” and otherwise as “intermediate” or “adverse” only when all components had been evaluated (cytogenetics and mutations).
Table 1.
Pretreatment characteristics for the 441 CRind patients and all 717 randomized patients
| All Induction CRs (CRind) (N=441) | All 717 randomized patients | ||
|---|---|---|---|
| Midostaurin (N=234) | Placebo (N=207) | ||
| Age in years, median (range) | 47.8 (20–60) | 49.9 (18–60) | 48 (18–61) |
| Female, N (%) | 114 (49%) | 120 (58%) | 398 (56%) |
| Randomization strata: FLT3 mutation, N (%) | |||
| TKD (No ITD) | 56 (24%) | 48 (23%) | 162 (23%) |
| ITD (ratio <0.7) | 116 (50%) | 93 (45%) | 341 (48%) |
| ITD (ratio ≥0.7) | 62 (27%) | 66 (32%) | 214 (30%) |
| ELN 2017 Groups (see text) | |||
| Favorable | 77 (50%) | 66 (51%) | 197 (45%) |
| Intermediate | 37 (24%) | 39 (30%) | 123 (28%) |
| Adverse | 40 (26%) | 23 (18%) | 121 (27%) |
| Pre-treatment WBC, median x103/ul (range) | 35 (0.6–421.8) | 31.3 (0.8–308.8) | 34.9 (0.6–421.8) |
ELN denotes European LeukemiaNet; WBC, white blood cell count.
CRind patients achieved a CR at any time during their induction period, prior to starting consolidation.
Hydroxyurea therapy was allowed for up to five days prior to the start of protocol therapy. During induction and four cycles of consolidation, midostaurin 50 mg or placebo were given twice daily on Days 8–21. Shortly after the protocol was opened in April 2008, the duration of study drug during 12 maintenance cycles was increased from 14 days every four weeks to 28 days continuously. The institutional review board at each participating center reviewed and approved the study. The trial was conducted in accordance with the Declaration of Helsinki.
Complete remission (CR) was conventionally assessed by blood counts and bone marrow examination. If CR was not achieved by day 60 (a protocol CR), it was considered an event for event-free survival (EFS), but later responders were permitted to continue on study treatment. Transplantation was not mandated in the protocol but was conducted at the discretion of the investigator. Approximately 28% of patients (n=101) on the midostaurin arm and 23% on placebo (n=81) underwent allo-HCT in first CR (p=0.10). Study drug was not given for maintenance after transplantation. Ten patients had started maintenance therapy (7 on the midostaurin arm and 3 on the placebo arm) prior to receiving allo-HCT while still in first CR. .Patients discontinued the study drug when they received any antileukemia therapy not in the protocol, including allo-HCT.
FLT3 Testing
The details of assay validation and mutation testing have been previously reported.(17,23) A minimum allelic ratio of 0.05 for FLT3-ITD to wild-type was necessary to assign AML as FLT3 mutated. Results including TKD point mutation status and high or low FLT3-ITD ratio were reported to investigators within 48 hours from receipt of sample in the laboratory.
Study Conduct
This study was conducted at 225 sites in 17 countries. Participating cooperative groups included: Alliance/CALGB, AMLSG, CETLAM, ECOG, EORTC/HOVON, GIMEMA, NCIC, OSHO, PETHEMA, LATAM, SAL, SWOG, ALLG, and individual sites. Alliance/CALGB was the lead group and held the clinical trial data. The study was sponsored in North America by the Cancer Therapy and Evaluation Program (CTEP) of the National Cancer Institute (NCI) and in non-North American sites by Novartis Pharmaceutical Company. Patients enrolled on the study were randomized with equal probability to the two treatments. These randomizations were double-blinded and were stratified by the FLT3 mutation subgroup: TKD, ITD with mutant allelic ratio <0.7 (low), and ITD with allelic ratio ≥0.7 (high).
Primary study results
From May 2008 through October 2011, 3277 newly diagnosed AML patients 18–59 years old were screened for FLT3 mutations. Seven-hundred seventeen patients (214 FLT3-ITD-high, 341 FLT3-ITD-low; 162 FLT3-TKD) were randomized to midostaurin (n=360) or placebo (n=357). As previously reported (17), arms were balanced for age, race, FLT3 subtype, cytogenetic risk group, and blood counts except for sex (midostaurin, 52% female; placebo, 59% female; p=0.04). Both OS (HR=0.78; one-sided p=0.009) and event-free survival (EFS; HR=0.78; one-sided p=0.002) were significantly better on the midostaurin arm. The benefit of midostaurin, in analyses both uncensored and censored for transplantation, was consistent across all three FLT3 subgroups.(17) The rate of grade 3–5 adverse events on the two arms was similar except for rash which was more common on the midostaurin arm.
Statistical considerations
The primary endpoint of the study was OS, defined as the time interval from randomization to death from any cause. EFS was defined as the time from randomization until the earliest qualifying event, including: failure to obtain a CR on or before 60 days after initiation of protocol therapy (protocol-specified CR), relapse, or death from any cause. Patients alive and event-free at the time of analysis were censored for this endpoint on the date of last clinical assessment.
Treating death from causes other than AML as a competing risk and stratifying on FLT3 subgroups, CIR analyses were performed using two definitions of complete remission: CR per protocol (CR60; by day 60) and CR during induction (CRind; any time while on induction therapy) to understand the ability of midostaurin compared with placebo to decrease the incidence of relapse. Only the results for the larger number of CRind patients are reported here. The differences between the ELN 2017 risk categories were also explored to evaluate whether any subgroup had a higher incidence of relapse. CIR analyses treated relapses and AML deaths as events, deaths from other causes as competing risks, and survivors in remission as censored. In some analyses, transplantation was neither censored nor treated as a competing risk. Gray’s test was used to test for significant differences between CIR curves and was stratified on FLT3 subgroup. A sensitivity analysis treated transplants and non-AML deaths as competing risks to understand if midostaurin or an ELN classification group affected relapse early in the course of the disease.
Landmark analyses (from the start of maintenance) were performed to understand the impact of midostaurin versus placebo on the subset of 205 CR patients (CR at any time on study) who received any amount of maintenance therapy. Here, disease-free survival (DFS) was defined as time from start of maintenance to the first of death or relapse, and censoring patients at the time of their most recent clinical assessment deemed to be disease-free prior to documented relapse. Landmark DFS analyses (from the end of maintenance therapy) were also performed to understand the long-term impact of midostaurin versus placebo on the subset of patients who completed all protocol treatment (i.e., we measured time from end of all planned maintenance to the first of death or relapse).
All p-values for these analyses are two-sided. All analyses are post hoc and exploratory.
RESULTS
All patients are now off active treatment. The median follow-up was 59 months from enrollment for surviving patients.
A CR was achieved within the protocol-specified 60 days by 403 patients (CR60; 56%) with no significant difference between arms: 212/360 (59%) on the midostaurin arm and 191/357 (54%) on placebo (p=0.15). A total of 441 patients (midostaurin, 234 (65%); placebo, 207 (58%)) achieved CR following 1 or 2 cycle of induction chemotherapy before starting protocol consolidation (CRind) [Table 2]. These 441 patients make up the sole analysis group for the evaluation of CIR.
Table 2.
Complete remission and relapse rates by treatment arm.
| Midostaurin (N=360) | Placebo (N=357) | p * | |
|---|---|---|---|
| CRind, N (%) | 234 (65%) | 207 (58%) | 0.05 |
| Time to CR, median (range) | 36.5 days (20–99) | 36 days (20–108) | |
| Relapses, N (%) | 98 (42%) | 101 (49%) | 0.15 |
2-sided Fisher’s exact p-value
CRind patients achieved a CR at any time during their induction period, prior to starting consolidation.
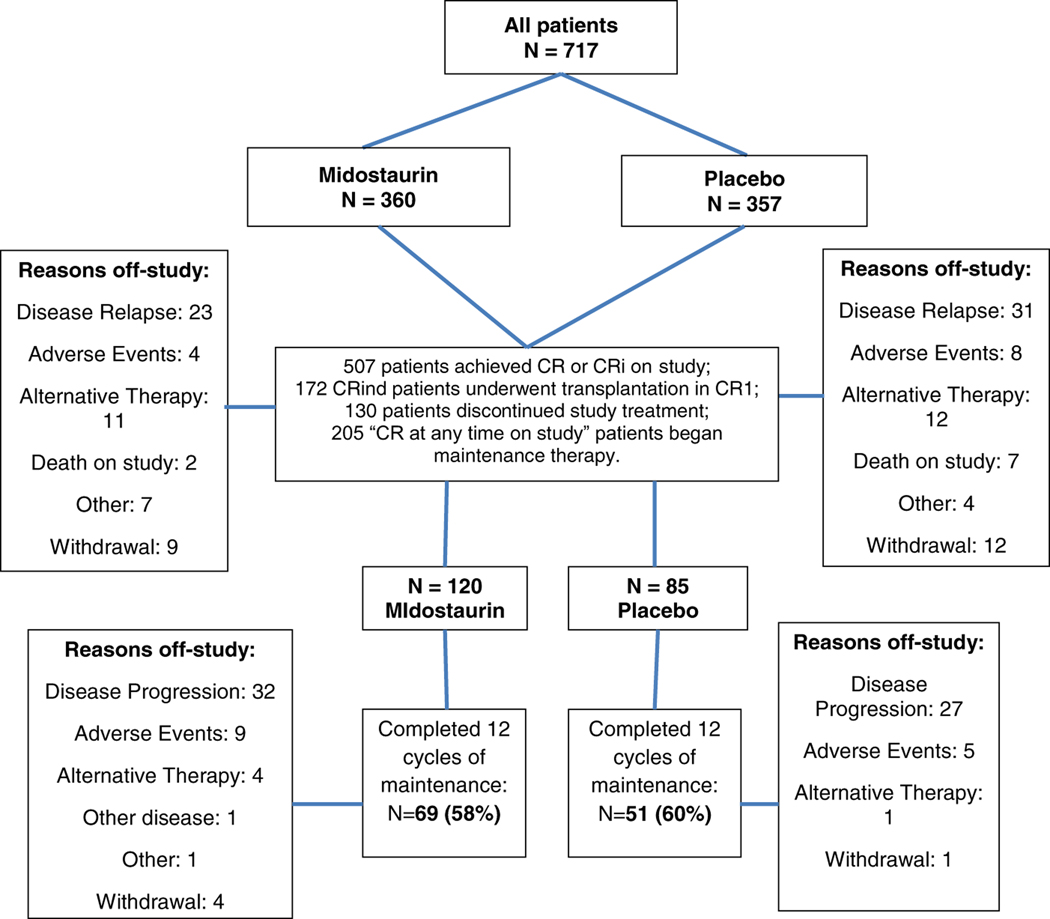
An additional 63 patients did not meet the definition of CR due to inadequate recovery of peripheral blood counts at the end of induction but stayed on study, received one or more courses of consolidation chemotherapy, and subsequently met all of the requirements for CR. Thus, 504 patients (70%) achieved CR at any time on study; 3 others had CRi. Of these, 172 underwent allo-HCT in first CR and 130 went off-study prior to starting maintenance, mostly for disease relapse, alternative therapy, or adverse events (Figure 1). After consolidation, maintenance patients remained on their originally assigned double-blind treatment arm, and were not re-randomized. Thus, a total of 205 patients who attained CR/CRi at any time and were not transplanted entered the maintenance phase of treatment (120 on the midostaurin arm and 85 on placebo). There was no significant difference in the time to start maintenance therapy between the two study arms (median, 6.9 months for midostaurin, and 7.5 months for placebo; p=0.17). See CONSORT diagram for details (Figure 1).
Figure 1.
CONSORT diagram
Analysis of the cumulative incidence of relapse
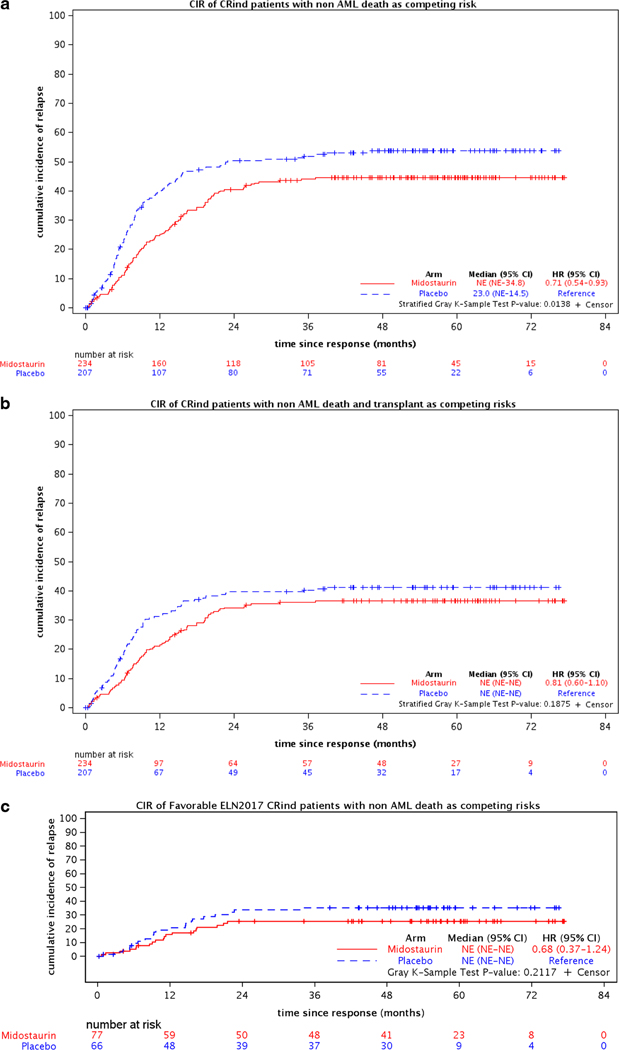
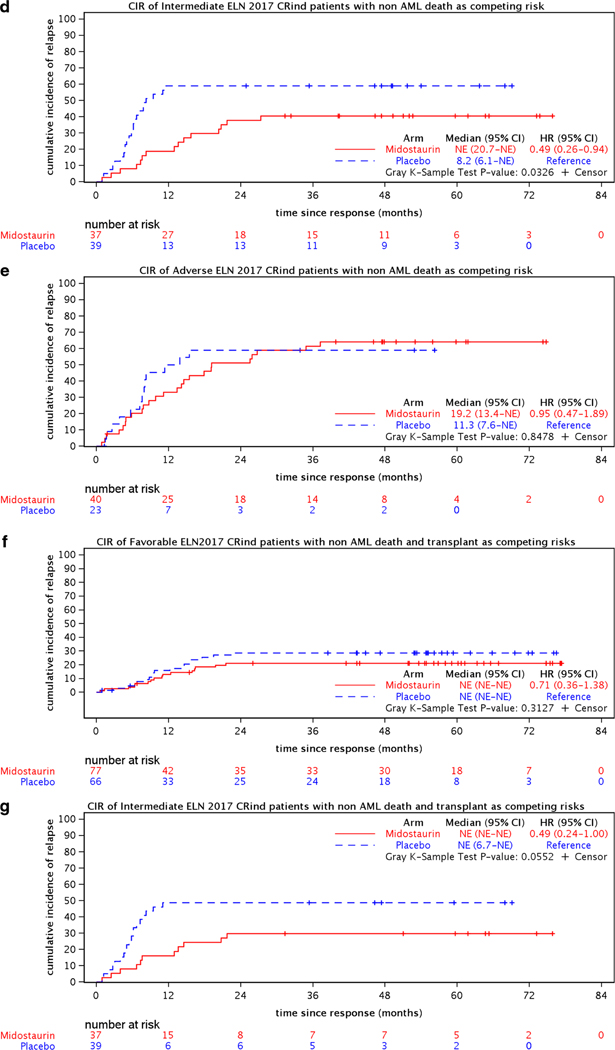
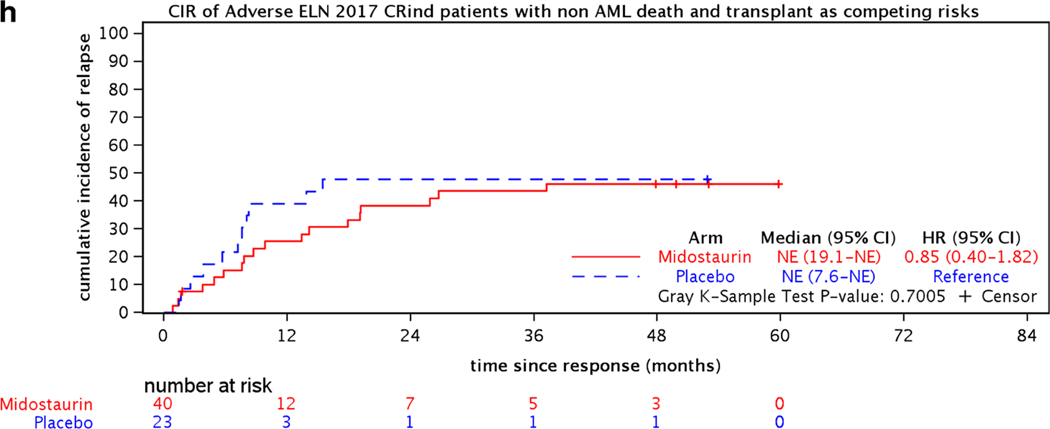
The demographics and key pretreatment characteristics for the 441 CRind patients are shown in Table 1. The CR rates, median times and ranges to CR, and relapse rates for the two treatment arms are shown in Table 2. Figure 2(a–h) shows the CIR for CRind patients, counting non-AML death as a competing risk and either considering events post-transplantation (c,d,e) or counting transplantation (f,g,h) as a competing risk for both treatment arms (midostaurin vs placebo) and by ELN classification (Favorable, Intermediate, and Adverse) subgroups.
Figure 2.
Cumulative incidence of relapse (CIR) for 441 CRind patients with either non-AML death or non-AML death and transplantation as competing risks, (a,b) overall and (c-h) for the 282 CRind patients with available cytogenetic and molecular data by ELN 2017 risk classification.
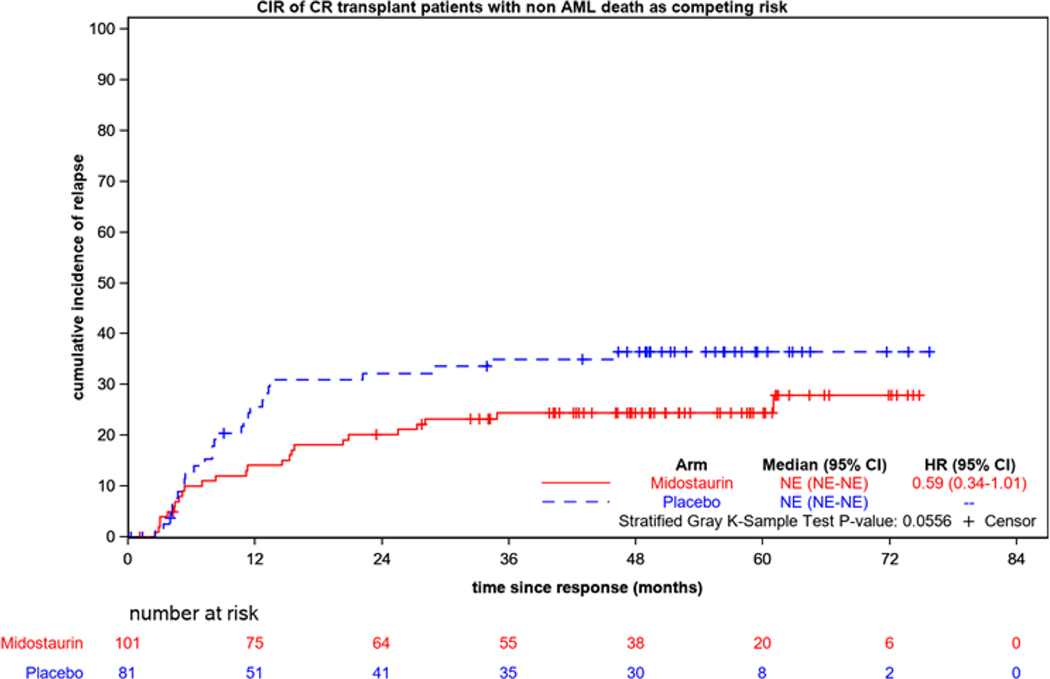
CIR was significantly improved on the midostaurin arm if transplantation was not taken into account (for the 441 CRind patients, HR=0.71 (95% CI, 0.54–0.93); p=0.01). If transplantation was considered a competing risk, there was no significant difference between the relapse risks on the midostaurin arm compared to the placebo arm (HR=0.81 (95% CI, 0.60–1.10); p=0.19). However, the CIR was lower for both treatment arms when transplantation was considered as a competing risk (Figures 2a and 2b). Figure 3 shows the CIR for the 182 patients who underwent allogeneic transplantation in first CR; this includes the CRind patients plus a few who achieved CR after starting consolidation. Thus, transplantation was important in preventing relapse.
Figure 3.
Cumulative incidence of relapse (CIR) for the 182 patients who underwent allogeneic transplantation in first CR according to randomized treatment arm.
Analyzing the CIR within each ELN risk group (282 evaluable CRind patients), the hazard ratio was significantly better for the midostaurin arm compared to the placebo arm (reference) in the Intermediate risk group (HR=0.49 (95% CI, 0.26–0.94); p=0.03) if transplantation was ignored, but not for the Favorable or Adverse ELN groups [Figure 2(c,d,e)]. If transplantation was considered a competing risk, there were no significant differences between the relapse risks on the midostaurin arm compared to placebo in any of the 3 ELN risk groups [Figure 2(f,g,h)]. However, relatively few patients were available for analysis within the several subgroups.
Analyzing the CIR between the ELN risk groups, when non-AML death and transplantation were considered competing risks, the CIR was better in the Favorable ELN risk group patients overall compared to the Intermediate (HR=1.79 (95% CI, 1.02–3.16) or Adverse risk groups (HR=2.15 (95% CI, 1.17–3.96), p=0.04; Favorable is the reference). (Supplemental Figure S1.)
Analysis of maintenance treatment
Pretreatment characteristics differed between those who entered maintenance treatment and those who did not (Table 3). Patients who entered the maintenance phase of treatment were slightly older as a group, less often female, and had more favorable FLT3 mutation status (i.e., fewer patients had allelic ratio >0.7), cytogenetics, and ELN risk status than those who did not enter the maintenance phase. However, among the patients who actually commenced maintenance, there were no significant differences between the pretreatment characteristics on the two maintenance arms (Table 4).
Table 3:
Comparison of pretreatment characteristics of patients who began maintenance therapy and those who did not
| 205 patients who began maintenance therapy | 512 patients who did not receive maintenance therapy | p-value* | |
|---|---|---|---|
| Age in years, median (range) | 49 (19–60) | 47 (18–61) | 0.081 |
| Female, N (%) | 103 (50%) | 295 (58%) | 0.072 |
| FLT3 mutation, N (%) | 0.00162 | ||
| TKD (No ITD) | 59 (29%) | 103 (20%) | |
| ITD (ratio <0.7 | 103 (50%) | 238 (47%) | |
| ITD (ratio ≥0.7) | 43 (21%) | 171 (33%) | |
| ELN 2017, N (%) | <0.012 | ||
| Favorable | 80 (57%) | 117 (39%) | |
| Intermediate | 33 (24%) | 90 (30%) | |
| Adverse | 25 (18%) | 96 (32%) | |
| Pre-treatment WBC, | 32.0 (0.6–421.8) | 35.8 (0.8–329.8) | 0.441 |
| x103/ul, median (range) | |||
p-values
Kruskal Wallis
Chi square
Table 4.
Pretreatment characteristics for the 205 maintenance patients by treatment arm
| Midostaurin (N=120) | Placebo (N=85) | p-value* | |
|---|---|---|---|
| Age, median years (range) | 48 (20–60) | 51 (19–60) | 0.061 |
| Female, N (%) | 56 (47%) | 47 (55%) | 0.222 |
| FLT3 mutation, N (%) | 0.672 | ||
| TKD (No ITD) | 32 (27%) | 27 (32%) | |
| ITD (ratio <0.7) | 61 (51%) | 42 (49%) | |
| ITD (ratio ≥0.7) | 27 (23%) | 16 (19%) | |
| ELN 2017, N (%) | 0.242 | ||
| Favorable | 43 (54%) | 37 (63%) | |
| Intermediate | 19 (24%) | 14 (24%) | |
| Adverse | 17 (22%) | 8 (14%) | |
| Pre-treatment WBC, x103/ul, median (range) | 30.4 (0.6–421.8) | 38.3 (1.2–231.0) | 0.701 |
p-values
Kruskal Wallis
Chi Square
Maintenance was well tolerated, and the median duration of exposure was the same on both arms (48 weeks, which was the planned treatment period). Discontinuation due to adverse events was infrequent (8% for midostaurin; 6% for placebo). Relapses during maintenance were reported in 32 patients on midostaurin (27%) and 30 patients on placebo (35%). One patient on each arm died during the maintenance period without relapse.
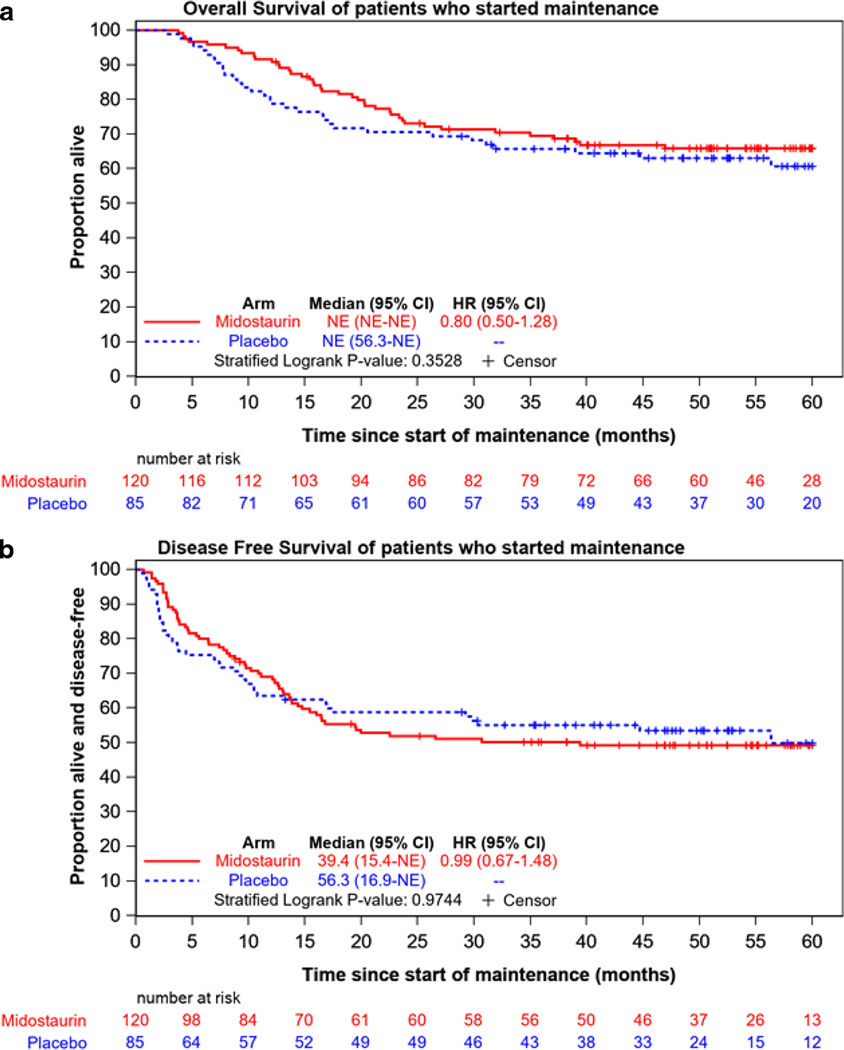
Landmark analyses were performed for all patients who started maintenance. There were no significant differences in OS or DFS between the two treatment arms for patients who started maintenance (Figure 4). There was no significant difference in CIR between the two arms for the entire period after starting maintenance (HR=0.98 for midostaurin [95% CI, 0.65–1.49]; p=0.93) [Supplemental Figure S9]. DFS was not significantly different between the two arms during the 12 cycles of maintenance (HR=0.74 for midostaurin versus placebo [95% CI, 0.45–1.19]; stratified logrank p=0.21) [Supplemental Figure S4], or by the ELN 2017 classification overall (stratified logrank p=0.19) [Supplemental Figure S5]. DFS was not significantly different between the two arms from the end of treatment for the 120 patients who completed all planned maintenance (Supplemental Figure S6).
Figure 4.
Landmark analysis of overall survival (a) and disease-free survival (b) for the 205 patients who began maintenance therapy by treatment arm.
At the end of the maintenance portion of the trial (twelve 4-week cycles), 69 patients (58%) had completed all maintenance treatment on the midostaurin arm and 51 (60%) had completed treatment on the placebo arm for a total of 120 patients. Subsequently during the follow-up period, there were 26 post-maintenance DFS events: 17 relapses on the midostaurin arm, and 7 relapses and 2 deaths on the placebo arm. These events occurred in 7/40 (20%) patients with FLT3-TKD, 15/59 (29%) with ITD-low, and 4/21 (22%) with ITD-high (X2, p=0.61).
A landmark analysis of DFS by treatment arm for the 120 patients who completed all planned maintenance, starting from the last dose of study drug is shown in Figure S6. There was no significant difference in DFS between the two arms (HR=1.55 for midostaurin [95% CI, 0.69–3.49]; p=0.28). However, DFS at 1-year from the end of maintenance was 77% [95% CI, 65–85%] for midostaurin and 92% [95% CI, 80–97%] for placebo. This was due to a greater number of early relapses seen on the midostaurin arm within 6 months after ending study drug, and the shape of the curves suggests that midostaurin may have delayed but not prevented relapse in some of these patients (Supplemental Table 1).
DISCUSSION
We analyzed the CIR and evaluated the impact of maintenance therapy observed within the large prospective CALGB 10603/RATIFY trial for adults with untreated FLT3-mutated AML.(17) Our objective was to understand if the benefit of midostaurin was due to a reduction in the risk of relapse. If relapses after allo-HCT were considered an event, CIR was significantly improved on the midostaurin arm. However, when transplantation was considered as a competing risk, there was no significant difference between the risks of relapse on the two randomized arms. Of note, when transplantation was treated as a competing risk, the CIR was lower for both treatment arms (Figures 2a and 2b). Thus, transplantation in CR1 appeared important for preventing relapse (Figure 3). We conclude that midostaurin decreases the risk of relapse, perhaps by leading to a lower residual tumor burden. Unfortunately, this trial did not include serial assessments of measurable residual disease (MRD) following induction therapy or prior to transplantation or beginning maintenance therapy though banked samples are being retrospectively analyzed.(24)
There are several important limitations of these unplanned post hoc subset analyses and their generalizability. Any significant findings should be handled with caution. In some cases, only small numbers of patients were available in each subgroup. For this reason, stratified analyses were not performed for the CIR analyses within ELN 2017 risk groups. Only newly diagnosed patients 18 – 59 years old were enrolled on the study. Thus, this randomized trial provides no information about the benefit of midostaurin for older AML patients. However, a subsequent phase 2 trial in which midostaurin was added to intensive chemotherapy followed by allo-HCT for patients with FLT3-ITD AML included 86 older (61–70 years) patients.(25) There were no unanticipated toxicities reported in that study from the combination with midostaurin, and compared with historical controls, midostaurin significantly improved EFS in both older and younger patients. Twelve cycles of midostaurin maintenance therapy were also planned following conventional consolidation and after allo-HCT on that study; 97 patients (34%) started maintenance, but 62% discontinued early mainly due to nonrelapse causes (gastrointestinal toxicity and infections).
In our study, patients were not re-randomized at the start of the maintenance treatment. A second randomization at this point was considered and had been suggested by the US FDA when designing the study, but investigators felt that a 2 X 2 randomization scheme was not feasible due to the much larger number of patients required. While it was not possible to determine definitively the additional benefit of maintenance therapy without a second randomization, midostaurin maintenance was specifically approved by the EMA,(18) but was not commented upon in the FDA approval. There were more relapses after stopping the drug on the midostaurin arm (17/69 = 25% versus 7/51 = 14% on the placebo arm), and more of these relapses occurred within the first six months (14 (20%) versus 2 (4%); Supplemental Table 1). These numbers are too small for any clinically meaningful comparisons.
The benefit of post-remission maintenance therapy in AML is under active investigation. QUAZAR AML-001 (NCT01757535) is a phase 3, randomized, placebo-controlled trial investigating the use of CC-486 (an oral formulation of azacitidine) as maintenance therapy for patients with AML, aged 55 or over, who were in CR1 after intensive induction chemotherapy.(26) At a median follow-up of 41.2 months, OS was significantly improved with CC-486 vs placebo; median OS was 24.7 months vs 14.8 months from time of randomization, respectively (P<0.001). Relapse free survival (RFS) was also significantly prolonged; median RFS was 10.2 months in the CC-486 arm, compared with 4.8 months in the placebo arm (P<0.001).
There is a rationale for continuing treatment with agents that inhibit FLT3 signaling. Mathew and colleagues showed that sorafenib, another multi-targeted tyrosine kinase inhibitor, increased IL-15 production by FLT3-ITD leukemia cells.(27) This synergized with the allogeneic CD8+ T-cell response post-transplant, leading to long-term survival in six mouse models of FLT3-ITD AML. Human FLT3-ITD AML cells obtained from sorafenib responders following sorafenib therapy showed increased levels of IL-15, suggesting the potential for an immune-mediated anti-leukemia effect. When sorafenib was used as monotherapy in 29 patients with FLT3-ITD AML who relapsed after alloHCT, five (17%) achieved sustained CR, and four were in treatment-free remission for a median of 4.4 years when reported.(28) Maintenance therapy with sorafenib after alloHCT was evaluated in the SORMAIN trial, a multicenter, randomized, double-blind, placebo-controlled trial of single agent sorafenib, starting 60–100 days after transplantation for FLT3-ITD AML.(29) Among 83 patients enrolled, after a median follow up of 41.8 months, the 2-year relapse-free survival (RFS) was 53.3% (95% CI, 36.5%−67.5%) with the placebo versus 85.0% (69.5%−93.0%) for the sorafenib group (HR 0.39; 95% CI, 0.18 −0.85; p=0.0135).
In a small open-label, randomized, phase II RADIUS trial with 60 patients, investigators evaluated whether adding midostaurin to standard of care (SOC) extended RFS, compared with SOC alone, for patients with FLT3-ITD AML after allo-HCT.(30) SOC included anti-infective and graft-versus-host disease prophylaxis and treatment; midostaurin 50 mg was administered twice daily in 28-day treatment cycles. The estimated 18-month RFS was 89% in the midostaurin arm and 76% in the SOC arm (HR = 0.46; 95% CI 0.12–1.86; p=0.27).
In a phase III trial that enrolled 371 patients with relapsed or refractory FLT3-mutant AML, the 247 randomly assigned (2:1) to monotherapy with gilteritinib had significantly longer OS than the 124 assigned to salvage chemotherapy (median OS 9.3 months vs 5.6 months; HR 0.64; 95% CI 0.49 to 0.83; P<0.001).(31) CR with full or partial hematologic recovery was observed in 34.0% of the gilteritinib patients and 15.3% in the chemotherapy group. Gilteritinib is now being evaluated in the phase III, randomized, double-blind, placebo-controlled GOSSAMER trial as maintenance therapy following induction/consolidation therapy for FLT3-ITD AML in first CR (NCT02927262). The MORPHO trial is a randomized, double-blind, placebo-controlled, multi-center trial that compares gilteritinib to placebo as maintenance therapy over a period of two years following hematopoietic stem cell transplantation in patients with FLT3-ITD AML in first CR. The primary endpoint is RFS (NCT02997202). Other trials are now comparing gilteritinib and midostaurin (NCT04027309).
When quizartinib was used as a single agent in a large randomized controlled trial for 367 patients with relapsed or refractory FLT3-ITD AML, the composite CR rate was 48% (95% CI, 42%−55%) with quizartinib and 27% (95% CI, 19%−36%) for SOC.(32) The duration of CR was 12.1 (95% CI, 10.4–27.1) weeks vs 5.0 (95% CI, 3.3–12.6) weeks, respectively. The transplant rate was 32% in the quizartinib arm, and 49 of 79 (62%) of these patients resumed single-agent quizartinib post-transplant. The median OS was 6.2 (95% CI, 5.3–7.2) months for the quizartinib-treated patients, with an estimated 12-month OS probability of 27%. A front-line phase III, randomized, placebo-controlled trial of quizartinib (QuANTUM First) is now evaluating this FLT3 inhibitor during induction and consolidation chemotherapy in AML patients 18–75 years old, followed by 36 months of maintenance therapy (NCT02668653).
The results from our unplanned subset analysis of maintenance treatment on the CALGB 10603/RATIFY trial do not allow firm conclusions on the clinical benefit from maintenance therapy with midostaurin. It is difficult from this trial’s data set to isolate the clinical benefit gained from any single component of the trial or phase of therapy as the survival benefit was observed by intention-to-treat for the whole treatment plan, including the use of post-remission consolidation therapy, maintenance, and allo-HCT. The decision to use 12 cycles of maintenance was arbitrary. The shape of the DFS curve which shows a high number of relapses occurring during the first six months after completing midostaurin maintenance supports the hypothesis that midostaurin may suppress but not eradicate MRD. We conclude that 12 cycles of midostaurin maintenance was well-tolerated, but the definitive impact of maintenance strategies using midostaurin or any other targeted agent would need to be addressed by randomization.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients who participated and their families, the clinical and laboratory research staff members of multiple international cooperative groups, CTEP of the NCI, and Novartis Pharmaceuticals. We acknowledge Dr. Francesco Lo-Coco’s and Dr. Clara D. Bloomfield’s key roles in the design and completion of this study.
CALGB is now part of the Alliance for Clinical Trials in Oncology. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821, U10CA180882, U24CA198171 (to the Alliance for Clinical Trials in Oncology), U10CA032291, U10CA041287, U10CA077651, U10CA077658, U10CA180791, U10CA180820 (ECOG-ACRIN), UG1CA233290, U10CA180836, U10CA180850, U10CA180863 (CCTG), U10CA180867, U10CA180888 (SWOG), and UG1CA233338. [https://acknowledgments.alliancefound.org] This study was also supported in part by funds from Novartis.
Conflict-of-interest disclosure: RAL has acted as a consultant or advisor to Novartis, Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, and MorphoSys, and has received clinical research support from Novartis, Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven, Rafael Pharmaceuticals, and royalties from UpToDate. SJM has acted as a consultant or advisor for Pfizer and Pique Therapeutics, and has another relationship with BeiGene. CT is the Chief Executive Officer and a co-owner of AgenDix, a company performing molecular diagnostics, and has acted as a consultant or advisor for Novartis and Astellas, and has received clinical research support from Bayer. KD has acted as a consultant or advisor for Astellas, Celgene, Daiichi Sankyo, Janssen, Novartis, and Roche and has received clinical research support from Astex, Celgene, and Novartis. GM, RBK, DN, and HS have acted as consultants or advisors for Novartis. AHW has acted as a consultant or advisor for Novartis, Astellas, Pfizer, MacroGenics, AbbVie, Genentech, Servier, Celgene, Amgen, Astra Zeneca, and Janssen, and is a member of the speakers bureau for AbbVie/Genentech and Novartis, and has received research funding from Novartis, Celgene, AbbVie, Servier, Astra Zeneca, and Amgen, and is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax. JS has acted as a consultant or advisor for Pfizer, Daiichi Sankyo, AbbVie, Novartis, Astellas, and Roche and is a member of the speakers bureau for Novartis, Pfizer, Daiichi Sankyo, and AbbVie. MAS has acted as a consultant or advisor for Teva Pharmaceutical Industries, Daiichi-Sankyo, Orsenix, AbbVie, Novartis, and Pfizer. TdW has acted as a consultant or advisor for Novartis, Celgene, Johnson & Johnson, and Incyte and has received clinical research support from Novartis, Celgene, and Johnson & Johnson. BCM has acted as a consultant or advisor for Celgene, Novartis, and Astellas and is currently employed by Roche/Genentech. MST has acted as a consultant or advisor for AbbVie, BioLineRx, Daiichi-Sankyo, Orsenix, KAHR Medical, Rigel Pharmaceuticals, Nohla, Delta Fly Pharma, Tetraphase, Oncolyze, and Jazz Pharmaceuticals, and has received clinical research funding from AbbVie, Cellerant Therapeutics, Orsenix, ADC Therapeutics, and BioSight, and has received royalties from UpToDate. JK has acted as a consultant or advisor for Amgen, Astellas, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. AG has received clinical research support from Novartis. RFS has acted as a consultant or advisor for Pfizer and Daiichi Sankyo, is a member of the speakers bureau for Novartis, Pfizer, and Daiichi Sankyo, and has received clinical research funding from Pfizer, Daiichi Sankyo, PharmaMar, Astra Zeneca, and Roche. SA has acted as a consultant or advisor for Novartis and Daiichi-Sankyo. IGathmann is an employee of Novartis. HD has acted as a consultant or advisor for AbbVie, Agios, Amgen, Astellas, Astex Pharmaceuticals, Celgene, Helsinn, Janssen, Jazz Pharmaceuticals, Novartis, Oxford Biomedicals, and Roche, and has received institutional research support from Amgen, AROG Pharmaceuticals, BristolMyers Squibb, Celgene, Jazz Pharmaceuticals, Novartis, Pfizer, and Sunesis. RMS has acted as a consultant or advisor for AbbVie, Actinium, and Agios, has received personal fees from Amgen, Argenx, AROG, Astellas, AstraZeneca, BioLineRx, Celgene, Cornerstone, Daiichi-Sankyo, Fujifilm, Jazz Pharmaceuticals, MacroGenics, Novartis, Ono/Theradex Oncology, Orsenix, Otsuka/Astex, Pfizer, Roche, Stemline Therapeutics, Takeda, and Trovagene, and has received institutional research support from AbbVie, Agios, AROG, and Novartis.
Footnotes
Clinicaltrials.gov Identifier number: NCT00651261
Competing Interests:
The remaining authors declare no competing financial interests.
REFERENCES
- 1.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10(12): 1911–1918. [PubMed] [Google Scholar]
- 2.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759. [DOI] [PubMed] [Google Scholar]
- 3.Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol 2017; 96: 1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335. [DOI] [PubMed] [Google Scholar]
- 5.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res 2001; 61: 7233–7239. [PubMed] [Google Scholar]
- 6.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood 2007; 110: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 7.Whitman SP, Ruppert AS, Radmacher MD, Mrózek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood 2008; 111: 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002; 1: 433–443. [DOI] [PubMed] [Google Scholar]
- 9.Pratz KW, Levis MJ. Bench to bedside targeting of FLT3 in acute leukemia. Curr Drug Targets 2010; 11(7): 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 2004; 104(4): 1145–1150. [DOI] [PubMed] [Google Scholar]
- 11.Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 2017. August; 18(8): 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes J, Perl AE, Döhner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2018. July; 19(7): 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone RM, Manley PW, Larson RA, and Capdeville R. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Advances 27 February 2018; 2(4): 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 15.Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al.Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia 2012; 26(9): 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer T, Stone RM, DeAngelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 2010; 28(28): 4339–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017; 377(5): 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzogani K, Yu Y, Meulendijks D, Herberts C, Hennik P, Verheijen R, et al. European Medicines Agency review of midostaurin (Rydapt) for the treatment of adult patients with acute myeloid leukaemia and systemic mastocytosis. ESMO Open 2019; 4 :e000606. doi: 10.1136/esmoopen-2019-000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014. November 27; 124(23): 3441–3449. [DOI] [PubMed] [Google Scholar]
- 20.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Döhner K, Thiede C, Jahn N, Panina E, Gambietz A, Larson RA, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood 2020. January 30; 135(5): 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voso MT, Larson RA, Jones D, Marcucci G, Prior T, Krauter J, et al. Midostaurin in patients with acute myeloid leukemia and FLT3-TKD mutations: a sub-analysis from the RATIFY trial. Blood Advances 2020. October 13; 4(19): 4945–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiede C, Prior TW, Lo-Coco F, Krauter J, Barragán E, Nomdedeu J, et al. FLT3 Mutation Assay Laboratory Cross Validation: Results from the CALGB 10603/RATIFY (Alliance) trial in patients with newly diagnosed FLT3-mutated acute myeloid leukemia. Blood 2018; 132 (Supplement 1): abstract 2800. [Google Scholar]
- 24.Levis M, Shi W, Chang K, Laing C, Pollner R, Gocke C, et al. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood 2020; 135(1): 75–78. [DOI] [PubMed] [Google Scholar]
- 25.Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood 2019; 133(8): 840–851. [DOI] [PubMed] [Google Scholar]
- 26.Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med 2020; 383(26): 2526–2537. [DOI] [PubMed] [Google Scholar]
- 27.Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med 2018. March; 24(3): 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzelder SK, Schroeder T, Lübbert M, Ditschkowski M, Götze K, Scholl S, et al. Long-term survival of sorafenib-treated FLT3-ITD-positive acute myeloid leukaemia patients relapsing after allogeneic stem cell transplantation. Eur J Cancer 2017. November; 86: 233–239. [DOI] [PubMed] [Google Scholar]
- 29.Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol 2020. September 10; 38(26): 2993–3002. [DOI] [PubMed] [Google Scholar]
- 30.Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant 2020. December 7; 10.1038/s41409-020-01153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 2019; 381(18): 1728–1740. [DOI] [PubMed] [Google Scholar]
- 32.Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2019; 20(7): 984–997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.