Abstract
Motivation
Platform designs - master protocols that allow for new treatment arms to be added over time - have gained considerable attention in recent years. Between 2001 and 2019, 16 platform trials were initiated globally. The COVID-19 pandemic seems to have provided a new motivation for these designs. We conducted a rapid review to quantify and describe platform trials used in COVID-19.
Methods
We cross-referenced PubMed, ClinicalTrials.gov, and the Cytel COVID-19 Clinical Trials Tracker to identify platform trials, defined by their stated ability to add future arms.
Results
We identified 58 COVID-19 platform trials globally registered between January 2020 and May 2021. According to trial registries, 16 trials have added new therapies (median 3, IQR 4) and 11 have dropped arms (median 3, IQR 2.5). About 50% of trials publicly share their protocol, and 31 trials (53%) intend to share trial data. Forty-nine trials (84%) explicitly report adaptive features, and 21 trials (36%) state Bayesian methods.
Conclusions
During the pandemic, there has been a surge in the number of platform trials compared to historical use. While transparency in statistical methods and clarity of data sharing policies needs improvement, platform trials appear particularly well-suited for rapid evidence generation. Trials secured funding quickly and many succeeded in adding new therapies in a short time period, thus demonstrating the potential for these trial designs to be implemented beyond the pandemic. The evidence gathered here may provide ample insight to further inform operational, statistical, and regulatory aspects of future platform trial conduct.
Keywords: COVID-19, Master protocols, Platform trials, Drug development, Adaptive design
1. Introduction
The urgency posed by the COVID-19 pandemic was met with a large number of clinical trials worldwide. Within two months of the World Health Organization declaring a global pandemic, over 1000 interventional clinical trials had been registered, and this number has risen above 6000 as of June 2021 [1,2]. The number of these trials has highlighted many of the known efficiencies of clinical development, while also providing an opportunity to implement novel designs.
Platform clinical trials have gathered significant attention in recent years. They are considered a type of master protocol, which provides an overarching protocol designed to answer multiple questions; platform protocols adaptively allow for experimental therapies to enter or leave the trial over the course of the trial [3].
The simultaneous assessment of multiple experimental therapies under a single protocol offers many attractive and efficient features compared to traditional two-arm trials, including fewer required patients, shorter time to treatment evaluation, and greater power to identify effective therapies [4]. These advantages are similarly found in other adaptive designs, whereby trial features are amended during the trial based on prespecified criteria [5]. However, platform designs also introduce additional complexities in the design, analysis, and logistics of conduct [4,6,7].
In a recent study, Park et al. (2019) identified 16 platform trials initiated between 2001 and 2019, with numbers increasing over time [8]. While the majority of platform trials have been conducted in cancer, their use for viral illnesses and disease outbreaks is not a new concept. REMAP-CAP, a large-scale international platform trial, was initiated in 2016 for community-acquired pneumonia [9]. Berry et al. (2016) designed an adaptive platform trial to assess therapies for the 2014 West Africa Ebola outbreak, although the epidemic was contained before the trial started [10].
More recently, the COVID-19 pandemic has provided a new motivation for using these designs. Perhaps most well-known in the UK is the RECOVERY trial, which launched in March 2020 and has received considerable attention from the research community and public alike. Not only was RECOVERY extremely effective in its initiation, recruiting its first patients 9 days after protocol submission, but it was also able to release conclusive results in a timely manner. By April 2020, at least 97 registered clinical trials were testing the efficacy of hydroxychloroquine [11]. On June 3, 2020, RECOVERY trial investigators declared the treatment ineffective, and by June 17, 2020 the UK Medicines and Healthcare products Regulatory Agency (MHRA) instructed trials to discontinue recruitment to hydroxychloroquine arms [[12], [13], [14], [15]].
In addition to the RECOVERY trial, REMAP-CAP has not only been running since 2016, but was able to quickly expand from community-acquired pneumonia to also COVID-19[[80], [81]]. The Solidarity master protocol by the World Health Organization has also initiated multiple substudies on a global scale. The efficiency of such large trials makes it clear why platform trials have become a central point of discussion. However, the extent of their use in the broader COVID-19 clinical trial landscape has not yet been quantified. Details of their implementation, including design and conduct, may provide crucial information for our understanding of these innovative designs. We conducted a rapid review with an emphasis on trial operations (e.g. adding/dropping treatment arms), data sharing policies, transparency in methods and trial operations, and trial design (e.g. use of adaptive features).
2. Methods
We performed database searches at two time-points in October 2020 and June 2021. On October 21, 2020 (search 1) and June 22, 2021 (search 2), we searched ClinicalTrials.gov and PubMed for master protocols in any indication. The full Cytel COVID-19 Clinical Trials Tracker [16] dataset was retrieved on November 4th for COVID-19 trials of any design. Additional trials were identified through reference by trials retrieved from searches. Details on the search strategy are provided in Table S1 in the Supplementary material.
Platform definitions vary across the literature with regard to drug class, randomization, and other features, and the lack of consensus in these areas has been previously documented [[17], [18], [19], [20], [21]]. The most pervasive defining characteristic is the flexibility to add/drop arms from the platform. In looking at characteristics of published platform trials, it is unclear whether a trial must allow for both adding and dropping to be considered platform. For example, the plasmaMATCH trial in breast cancer consists of multiple non-comparative single-arm substudies, where it allows for the addition of experimental arms but each arm does not necessarily have early stopping criteria, therefore it is not clear whether an arm can be dropped. It is however referred to as a “phase 2a, platform trial” [22]. Therefore, in this study, we have labeled a trial as platform if it is stated on the trial registration webpage, in a publication, or in the full study protocol that the trial allows for the addition of future arms [3]. A trial was labeled as “potential” platform if the listed sources contained language that suggested but could not confirm the flexibility to add arms, or the trial self-identifies as platform but has no further information; for example, trial NCT04426695 defines itself as a master protocol without further detail, but the ClinicalTrials.gov history of registration changes shows that an arm was added in the two months following the trial start date in June 2020 [23].
Search results were split and screened by two independent reviewers (AMV and ZY); a sample of 28 trials was reviewed to verify consistency (Cohen's kappa 0.89, 95% CI: (0.77, 1.0)). Platform trial information was retrieved through manual search for the identified trials between December 1–3, 2020 for search 1 and June 30 – July 13, 2021 for search 2. Information was collected from trial registration pages, trial websites, study documents, and publications. Data was extracted by AMV and confirmed by ZY and CY. Disagreements were resolved through discussion and consensus.
In the results below, we descriptively examine trial operations and conduct, transparency and data sharing, and design features.
3. Results
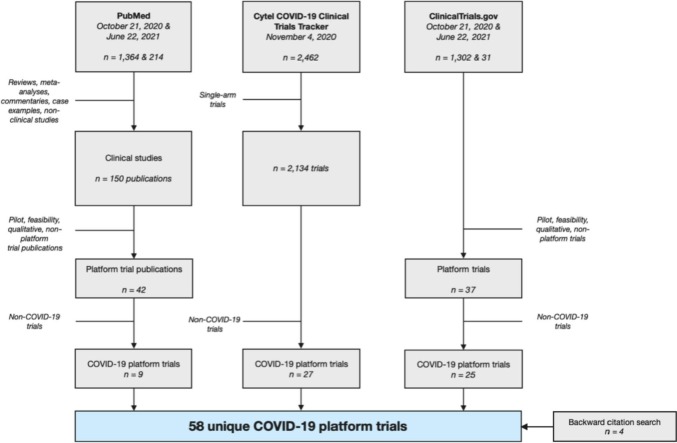
We identified a total of 58 registered platform trials (45 in search 1, 9 in search 2, and 4 by backward citation searching through the 9 trials from search 2) in COVID-19 treatments and prophylaxis (including 10 potential) registered between January 2020 and May 2021 (Figs. 1 , S1, S2) [9,13,[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]]. Of these, 19 (33%) are trials within a larger master protocol or research initiative, including Solidarity (CATCO [74], DisCoVeRy [75], and EU SolidAct [55]), ACTT (ACTT-1 [34], ACTT-2 [40], ACTT-3 [35], ACTT-4 [36]), TACTIC (TACTIC-R [70] and TACTIC-E [62]), and ACTIV (ACTIV-1 IM [28], ACTIV-2 [29], ACTIV-3 [25], ACTIV-4 [[30], [31], [32]], ACTIV-5 BET-A and ACTIV-5 BET-B [24], and ACTIV-6 [33]), and TOGETHER (TOGETHER 1, 2 [52], and 3 [73]). We found no details about TOGETHER 1, other than referencing in the TOGETHER 2 protocol [52].
Fig. 1.
PRISMA flowchart for combined search and selection at two time points. We searched ClinicalTrials.gov for master protocols in any indication (October 21, 2020) and COVID-19 (June 22, 2021). We searched PubMed for master protocols in any indication (October 21, 2020 and June 22, 2020). The full Cytel COVID-19 Clinical Trials Tracker dataset was retrieved on November 4th for COVID-19-specific trials of any design. A trial was labeled as platform if it is stated on the trial registration webpage, in a publication, or in the full study protocol that the trial allows for the addition of future arms [3]. A trial was labeled as “potential” platform if the listed sources contained language that suggested but could not confirm the flexibility to add arms, or the trial self-identifies as platform but has no further information.
Thirty-eight trials (65%) identify themselves as “platform”. Of these, 30 trials also give a statement about the flexibility to add arms, while the remaining 8 trials have insufficient further information on trial design. Overall, 48 trials (83%) reference an ability to add future interventions.
3.1. Operations and conduct
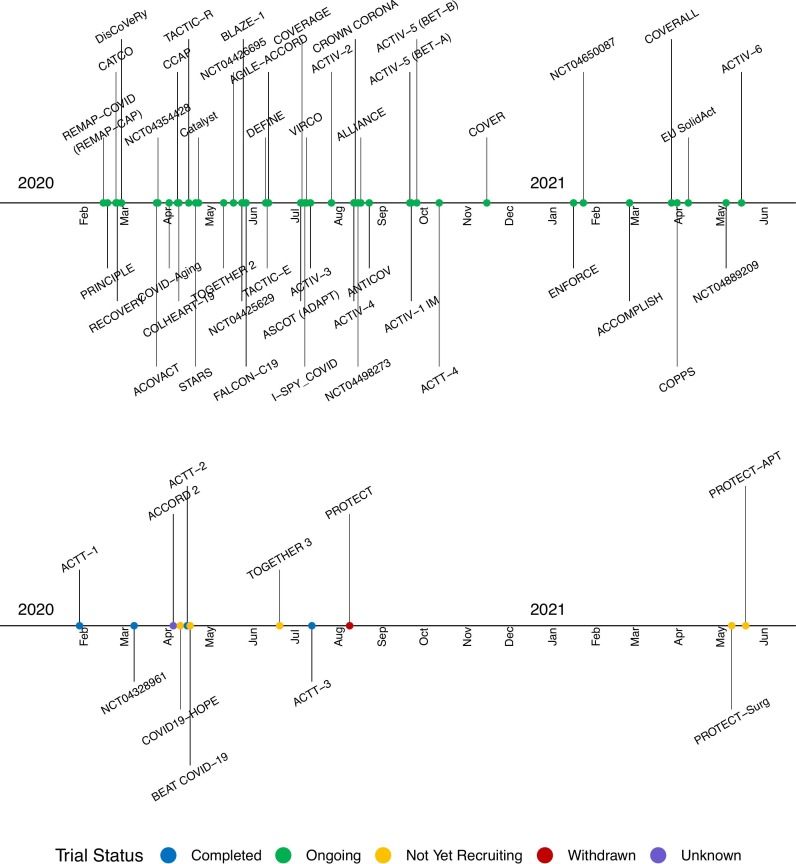
The earliest registered trial ACTT-1 began recruiting patients on February 21st in the US [34]. REMAP-CAP enrolled its first COVID-19 patient on March 9th in its sub-platform REMAP-COVID [80,81]. Nearly all trials are entirely publicly funded (48, 83%), and all but a few trials are ongoing and actively recruiting patients (Fig. 2 ). Four trials (CROWN CORONA [48], ENFORCE [63], COVERALL [60], and NCT04889209 [50]) assess potential vaccines. As of July 12, 2021, 46 trials (79%) are ongoing, 4 (7%) are completed, 5 (9%) are not yet recruiting, 1 (PROTECT) is withdrawn [66], and the status of 2 (ACCORD 2 [27] and TOGETHER 1[52]) are unknown, according to the latest information on trial registry websites.
Fig. 2.
COVID-19 platform trial initiation from January 2020 through May 2021. Trial status is shown as classified in ClinicalTrials.gov. A completed trial is one where the final data collection for the primary endpoint has been done for all enrolled participants. None of the four completed trials (ACTT-1, ACTT-2, ACTT-3, and NCT04328961) showed evidence of having added any treatment arms.
Using ClinicalTrials.gov's History of Changes, we found that several trials have added and/or closed arms over the course of conduct. Due to the variability in updates to registration pages, we may underestimate exact numbers, particularly for dropped or suspended arms. In total, 16 trials have recorded the addition of experimental therapies [9,25,26,32,37,48,57,59,68,72,74,75,77,79,82,78] and 12 have recorded arm discontinuations [25,30,48,53,59,68,72,74,75,77,78,82] (Table 1 ). For trials with available information, there have been a median of 3 added arms (IQR 4) and 3 dropped arms (IQR 2.5). Four trials stand out. As of May 5, 2021, RECOVERY has added 10 arms and closed 8. REMAP-COVID was initiated with 5 interventions and has since added 9 new therapies as of October 8, 2020, although the number of dropped arms was unavailable [83]. ACTIV-2 has added 9 arms and dropped 3 [82]. And, I-SPY COVID has added 6 arms and dropped 4 [59]. None of the four completed trials (ACTT-1 [34], ACTT-2 [84], ACTT-3 [35], and NCT04328961 [85]) showed evidence of having added any treatment arms.
Table 1.
Selected features of COVID-19 platform trials, as explicitly stated in registry pages, study documents, or trial websites as of July 13, 2020. If information was not available across these sources, it is designated as NA (“—”).⁎
| Trial registration ID | Trial phase | Platform designation | Adaptive features | Pre-specified decision rules | Bayesian methods | Available protocol | Available statistical analysis plan | Data sharing policy | Arms added | Arms dropped | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTT-1 | NCT04280705 | III | Yes | Sample size reassessment, graduation to control, futility stopping, efficacy stopping. | No | – | Yes | Yes | Public | – | – |
| REMAP-COVID (REMAP-CAP)⁎ | NCT02735707 | IV | Yes | Response-adaptive randomization, futility stopping, efficacy stopping, safety stopping, change in endpoint | Yes | Yes | Yes | Yes | By request | 9 | – |
| PRINCIPLE | – | III | Yes | Sample size reassessment, adaptive randomization, futility stopping, efficacy stopping, graduation to control | Yes | Yes | Yes | Yes | By request | – | – |
| CATCO | NCT04330690 | II | Yes | Change in primary endpoint, sample size reassessment | No | – | – | – | unclear | 3 | 2 |
| RECOVERY | NCT04381936 | II/III | Yes | Yes, unclear. | No | Yes, for pediatrics | Yes | Yes | By request | 10 | 8 |
| DisCoVeRy | NCT04315948 | III | Yes | Efficacy stopping, futility stopping, graduation to control | Yes | – | Yes | – | By request | 2 | 3 |
| NCT04328961 | NCT04328961 | II/III | Yes | Sample size reassessment | No | – | Yes | – | By request | – | – |
| ACOVACT | NCT04351724 | II/III | Potential | – | No | – | – | – | By request | 5 | 2 |
| NCT04354428 | NCT04354428 | II/III | Yes | Sample size reassessment, futility stopping, safety stopping, adaptive randomization | Yes | Yes | Yes | – | By request | 5 | 2 |
| COVID-Aging | NCT04359953 | III | Yes | – | No | – | – | – | – | – | – |
| CCAP | NCT04345289 | III | Yes | Futility stopping, graduation to control | No | – | – | – | – | – | 3 |
| COLHEART-19 | NCT04355143 | II | Potential | – | No | – | – | – | – | – | – |
| COVID19-HOPE | NCT04374903 | – | Yes | Yes, unclear | No | – | – | – | – | – | – |
| ACTT-2 | NCT04401579 | III | Yes | Futility stopping, efficacy stopping, safety stopping, sample size reassessment, graduation to control | No | – | Yes | – | Public | – | – |
| TACTIC-R | NCT04390464 | IV | Yes | Response adaptive randomization, futility stopping, efficacy stopping | Yes | Yes | Yes | – | – | – | – |
| BEAT COVID-19 | – | III | Yes | Response adaptive randomization | No | Yes | – | – | – | – | – |
| STARS | NCT04357730 | II | Yes | Efficacy stopping, safety stopping, futility stopping, adaptive enrichment, sample size reassessment | Yes | – | – | – | By request | – | – |
| Catalyst | – | II | Yes | Futility stopping, efficacy stopping | Yes | – | – | – | – | – | – |
| NCT04426695 | NCT04426695 | I/II/III | Potential | – | – | – | – | Public | 1 | – | |
| NCT04425629 | NCT04425629 | I/II/III | Yes | Futility stopping, efficacy stopping, population enrichment, sample size estimation, endpoint selection for III | No | Yes | Yes | – | Public/none | – | – |
| BLAZE-1 | NCT04427501 | II | Yes | Adaptive randomization, sample size reassessment, futility stopping | No | Yes | Yes | Yes | Public/none | 1 | – |
| FALCON-C19 | NCT04408170 | observational | Potential | Yes, unclear | No | – | – | – | – | – | – |
| DEFINE | NCT04473053 | II/III | Yes | Safety stopping | No | – | Yes | – | – | – | – |
| TACTIC-E | NCT04393246 | II/III | Yes | Futility stopping, safety stopping, efficacy stopping | Yes | Yes | Yes | – | – | – | – |
| TOGETHER 3 | – | – | Potential | Yes, unclear | No | – | – | – | Unclear | – | – |
| ASCOT (ADAPT) | NCT04483960 | III | Yes | Efficacy stopping, safety stopping, adaptive randomization | Yes | Planned as frequentist, has now moved to Bayesian | Yes | Yes | By request | 4 | 2 |
| COVERAGE | NCT04356495 | III | Yes | Futility stopping, efficacy stopping, safety stopping, graduation to control | Yes | – | Yes | – | By request | 2 | – |
| I-SPY_COVID | NCT04488081 | II | Potential | Futility stopping, efficacy stopping, graduation to control | No | Yes | – | – | By request | 6 | 4 |
| VIRCO | NCT04445467 | II | Yes | Yes, unclear | No | – | Yes | – | By request | – | – |
| ACTIV-3 | NCT04501978 | III | Yes | Efficacy stopping, futility stopping, safety stopping, graduation to control, sample size reassessment | Yes | – | Yes | – | By request | 3 | 1 |
| ACTIV-2 | NCT04518410 | II/III | Yes | Efficacy stopping, safety stopping | Yes | Yes | Yes | – | – | 9 | 3 |
| ACTIV-4 | NCT04505774 | IV | Yes | Efficacy stopping, futility stopping, safety stopping, graduation to control | Yes | Yes | Yes | – | Unclear | 2 | 1 |
| CROWN CORONA | NCT04333732 | III | Yes | Response adaptive randomization, futility stopping | No | Yes | – | – | By request | 1 | 3 |
| NCT04498273 | NCT04498273 | III | Potential | Yes, unclear | No | – | – | – | – | – | – |
| ALLIANCE | NCT04395768 | II | Yes | Sample size reassessment, efficacy stopping, futility stopping safety stopping | No | – | – | – | – | – | – |
| ANTICOV | – | III | Yes | Futility stopping, efficacy stopping, response adaptive randomization | Yes | Yes | Yes | – | Unclear | – | – |
| ACTIV-5 (BET-A) | NCT04583956 | II | Yes | Futility stopping, efficacy stopping | Yes | – | Yes | – | By request | – | – |
| ACTIV-1 IM | NCT04593940 | III | Yes | Futility stopping, efficacy stopping, safety stopping, sample size reassessment, graduation to control | Yes | – | Yes | – | – | – | – |
| ACTIV-5 (BET-B) | NCT04583969 | II | Yes | Futility stopping, efficacy stopping | Yes | – | Yes | – | By request | – | – |
| PROTECT-Surg | NCT04386070 | III | Yes | Futility stopping, efficacy stopping | No | Yes | – | – | – | – | – |
| AGILE-ACCORD | NCT04746183 | I/II | Yes | Futility stopping, efficacy stopping, sample size reassessment, change in primary endpoint | Yes | Yes | Yes | – | By request | 4 | – |
| ACCORD 2 | – | II | Yes | Futility stopping, sample size reassessment | No | – | Yes | – | Public | – | – |
| COVER | NCT04561063 | II | Yes | Yes, unclear | No | – | – | – | – | – | – |
| PROTECT | NCT04389359 | II/III | Yes | Sample size reassessment | No | Yes | – | – | – | – | – |
| COPPS | NCT04662086 | II | Potential | Yes, unclear. | – | – | – | – | – | – | |
| EU SolidAct | NCT04891133 | II/III | Yes | Graduation to control | No | – | Yes | – | Public | – | – |
| PROTECT-APT | NCT04844541 | Observational | Yes | – | No | – | – | – | – | – | – |
| NCT04650087 | NCT04650087 | III | Potential | – | No | – | – | – | – | – | – |
| ENFORCE | NCT04760132 | IV | Yes | – | No | – | – | – | – | – | – |
| COVERALL | NCT04805125 | III | Yes | – | No | – | – | – | – | – | – |
| NCT04889209 | NCT04889209 | I/II | Yes | Yes, unclear | No | – | – | – | – | – | – |
| ACCOMPLISH | NCT04829188 | Not Applicable | Potential | Adaptive randomization | No | – | – | – | – | – | – |
| OPTIMISE-C19 | NCT04790786 | III | Yes | Sample size reassessment | – | Yes | Yes | – | By request | – | – |
| ACTT-3 | NCT04492475 | III | Yes | Efficacy stopping, futility stopping, safety stopping, graduation to control, sample size reassessment | No | – | – | – | – | – | – |
| ACTIV-6 | NCT04885530 | III | Yes | Sample size reassessment, futility stopping, efficacy stopping | Yes | Yes | Yes | – | By request | – | – |
| TOGETHER 2 | NCT04403100 | III | Yes | Adaptive randomization, efficacy stopping, sample size re-assessment | No | Yes | Yes | – | By request | – | – |
| TOGETHER 1 | – | Unknown | Yes | – | – | – | – | – | – | – | – |
| ACTT-4 | NCT04640168 | III | Yes | Efficacy stopping, futility stopping, safety stopping, graduation to control, sample size reassessment | No | – | – | – | – | – | – |
REMAP-CAP consists of many treatment domains.
3.2. Transparency and intention to share data
Transparency in research encompasses easy and free access to trial documents, clear description of design and statistical methods, and timely dissemination of results. At the fast rate of COVID-19 platform trial implementation, protocol release is likely the most direct means of accessing design and analysis information. We found that 29/58 (50%) platform trials made their full protocol publicly accessible. In December 2020, we found 23/45 trials (51%) had publicly accessible protocols; since then, 12/45 (27%) trials have released new or updated versions (Table S2). The proportion of industry-funded and non-industry-funded trials that share protocols are similar; 55% (5/9) and 50% (24/48), respectively. Of the 14 trials that fall under a larger master protocol, only CATCO does not have publicly accessible documents [74].
Accessing IPD is important for replicating final analyses, generating new hypotheses, and applying new statistical methods to real data [86,87]. We located trial data sharing statements through ClinicalTrials.gov, publications, and the official trial protocol where available.
Thirty-one trials (53%) have stated an intention to share IPD; 20 by private request, 7 on a public data sharing platform, and 4 are unclear about how data will be shared. Two trials have conflicting data sharing statements across sources. NCT04425629 and BLAZE-1 committed on ClinicalTrials.gov to provide IPD on the Vivli platform [88], but both later published statements in the New England Journal of Medicine saying that they would not be sharing IPD [26,[69], [89], [90]].
3.3. Design features and statistical methods
Design and analysis methods for platform trials are less established and potentially more complex than in standard trial designs, generating a need for clear presentation of methods. A trial design is adaptive when it has pre-planned and pre-specified opportunities to modify design aspects based on accumulating trial data while preserving the trial's validity and integrity [5,91]. We found that 49 trials (84%) clearly present adaptive features in addition to adding arms. These include futility stopping (29, 50%), early efficacy stopping (25, 44%), sample size reassessment (20, 34%), and adaptive randomization (11, 19%). Twelve trials (21%) included the ability for the control arm to be replaced by an experimental arm that has demonstrated sufficiently superior efficacy (“graduation” to control). Thirteen trials (23%) use a seamless design (2 phase I/II, 9 phase II/III and 2 phase I/II/III). Nine trials (16%) state themselves to be adaptive, but further details were unavailable (Table 1). Detailed statistical methods for these features are not listed on trial registry websites, but of the 29 trials with publicly available trial documents, 16 provide specific decision algorithms for at least one of their adaptive components. Six (10%) trials have made their full statistical analysis plans (SAPs) public – up from 3 (7%) in December 2020 – and others have given plans to release their SAPs before final analysis (Table S2).
Finally, 21 trials (36%) clearly state use of Bayesian methods. ASCOT stated in its protocol dated May 18, 2020 [72] that it may change the analysis from a frequentist to Bayesian framework; in a subsequent protocol dated October 30, 2020, it is now using Bayesian methods [92].
4. Discussion
We conducted a database search on October 21, 2020 and identified 45 COVID-19 platform trials. A follow-up search seven months later returned 13 more platform trials initiated in that span. We collected information on operations (e.g. adding arms), data sharing and transparency, and trial design and methods (e.g. statistics and adaptive features).
4.1. Definition of platform trial and relationship with adaptive feature reporting
Amidst the excitement surrounding platform trials, some facets of their design and conduct remain ambiguous. Many authors have cited inconsistencies in platform definitions found in the literature. For example, in a systematic review by Siden et al. (2019), they identified 25 unique definitions of “platform trial” [18]. Additionally, the most recent FDA draft guidance on master protocols (2018) lacks a concrete definition of “platform trial” [96].
In this review, we opted for an inclusive definition of “platform”, requiring self-identification and/or evidence suggesting that arms may be added. We relax other characteristic requirements sometimes included in the literature, such as dropping arms and randomization [18,93]. As explained previously, this was motivated by the design of ongoing trials [22]. While the flexibility to drop arms is likely implied or intended in the trials we identified, nearly half (26 trials, 44%) lack any documentation of this design feature. Thus, this broad definition allowed us to capture the magnitude of trials that may be considered platform, which may additionally facilitate future consensus on definition. It also highlights the lack of consensus regarding platform trial classification, as we saw 20 trials (35%) do not self-identify as platform.
Additionally, we restricted our data collection only to publicly available information. Trial methods and results are notoriously underreported, but readily and timely availability in a pandemic is arguably necessary. While non-publicly available trial documents may be obtained directly from trial investigators, this extra barrier can slow evidence generation in a setting where treatments have been urgently needed.
4.2. Design features and statistical methods
The flexibility to add arms throughout the trial is a central feature of platform design that offers substantial efficiencies over standard design – in theory. A review from 2015 showed that new treatment arms were not commonly added in practice [94]. This may be due to many factors, including statistical considerations, logistics, and short-term costs [94]. However, we found that at least 16 COVID-19 platform trials have successfully added arms in a short period of time, characterized by rapid amendments, uninterrupted accrual, quick release of information, and speedy results.
The CONSORT extension guidelines for adaptive trials can be applied to the reporting of platform trials, since the addition and dropping of treatment arms is itself adaptive [5]. The Adaptive Platform Trials Coalition (2017) also requires a that a platform trial pre-specify decision algorithms [93]. As the addition of arms is dependent on future emerging science, this feature may be exempt. Still, not all included trials satisfy these reporting requirements for other design features. However, there may be valid reasons for this, including the urgency these trials faced and the accelerated timelines from conception to recruitment. We encourage retrospective release of statistical analysis plans for greater insight into trial design.
The use of Bayesian methods distinguishes itself from the standard approaches to trial design [95]. However, it is difficult to compare Bayesian and Frequentist design reporting. The nature of these frameworks is different, and as Bayesian methods become increasingly implemented, investigators should be careful to adhere to appropriate reporting requirements [96]. Still, once more outcome data is available, it would be interesting to compare trials that use Bayesian vs. Frequentist methods with regard to number of enrolled patients, complexity of implementation, reporting quality, and replicability.
4.3. Statements on data sharing
About half of platform trials have given a statement with an intention to shared IPD. Dillman et al. (2020) found that 77% of randomized controlled trials in COVID-19 (completed as of June 2020) planned to share IPD [97]. One possible explanation for the slightly lower rates in our dataset is that 79% of COVID-19 platform trials are ongoing and their data sharing plans might be updated during conduct, whereas Dillman et al. examined only completed trials [72,96,95,97]. In fact, AGILE-ACCORD did update their policy; their protocol dated May 7, 2020 stated that access to IPD may be approved upon request, and in a publication submitted on May 27, 2020 and published on June 19, 2020, it stated that IPD will be uploaded to sharing platform Clinical Study Data Request [27,37].
Still, there are outstanding issues. ClinicalTrials.gov includes a field where it may be specified whether a trial intends to share IPD (“Yes”) or not (“No”), or if they're undecided (“Undecided”). For trials that say “Yes”, there are multiple degrees of sharing ranging from uploading the IPD to a data sharing platform to releasing IPD to investigators by approval and only for specific purposes [98]. “Data sharing” itself can mean many things, and details are not always provided.
An increasing number of journals are requiring data sharing policy statements as a condition of publication [[99], [100], [101]]. Still, the specificity of these statements varies greatly, and may not be sufficient to measure commitment to share data; even studies that have committed to sharing IPD on an open platform often don't comply [102]. When they do, the path to access is lengthy and tedious [103].
Data sharing rates in the non-COVID-19 trial sphere are dismal – one review found that 68% of published studies included a statement of intent to share data, but only 0.6% made them publicly available, and 19% made them available through data repositories [102]. Comparable rates of data sharing statements between the COVID-19 and non-COVID-19 domains is slightly discouraging. Research was rapidly initiated at the start of the pandemic, but there has not been an equally rapid and widespread global sharing of discoveries about the nature of the virus, symptoms, treatments, and risk factors – ideally through IPD [97,104]. This may be crucial for this and future outbreaks; however, structural changes are required and may be more efficiently enacted if enforced by funders.
Platform trials may face additional unique challenges, however; for example, relating to securing agreements with different partners or ongoing statistical comparisons with a shared control arm. The seemingly living nature of these data sharing policies may allow researchers to gain further insight into such operations. Future work may be done to explore this topic.
4.4. Platform trials for pandemics
Our findings show quick initiation of new platform trials in the first 10 months after declaring COVID-19 a global pandemic, which was followed by slower initiation since December 2020 (Fig. 2). This trend reflects the general COVID-19 trials landscape, according to ClinicalTrials.gov as of July 19, 2021 [2]. But given the success of many trials in adding new arms, this may have replaced the need for new trials, given already well-established platforms. It would be interesting to quantify the extent to which the conduct of these platform trials reduced the need for new RCTs, if possible and if at all.
It may be that COVID-19 has provided a unique research environment for which the speed to find effective treatments amongst many candidate therapies is vital, and platform designs are particularly well-suited to answer that call. Unprecedented levels of coordinated collaborative efforts (including from the government, regulators, funders, industry partners) and additional resources to help to overcome many challenges that have deterred their implementation in the past, have enabled many platform trials to be set up and recruit in record time.
Funding has historically been a challenge to secure and is a major hurdle to both starting new platform trials and adding arms to existing platforms [94]. In COVID-19 research, funding applications were fast-tracked which facilitated rapid trial initiations [105]. And we saw in our review that new arms were added in many trials. The pandemic has shown that perpetual trial conduct is not only possible, but effective. The use of platform design beyond the pandemic and the agility of the system to facilitate this uptake will depend on regulatory willingness and efforts. Moving forward, governments and funding bodies can learn from the success of these processes and establish infrastructure to better facilitate and secure funding for platform trials in the wider clinical trial landscape [106].
The public release of trial information during the pandemic reached record levels, both in volume and quickness. Press releases to communicate findings can facilitate adjustments in ongoing global research as was seen with RECOVERY [12,107]. However, press releases lack information needed to assess methods and translate findings into practice [107,108]. Science journalism can also lead to misinterpretation, and it is suggested that early release of vaccine trial data has contributed to vaccine hesitancy [109].
5. Conclusions
The scientific research community acted quickly in the implementation of clinical trials worldwide. We found that the number of platform trials far exceeds past numbers, and may offer ample insight into the design and conduct of platform trials, including barriers and shortcomings. Synthesizing such information can potentially accelerate our understanding of these designs, and may even transform evidence generation in drug development.
Limitations
We are limited by the amount and quality of data available, as registration pages are often sparse and lack detail. Thus, various aspects we report here, especially protocol amendments, adding and dropping treatment arms, and design features may be underestimated.
Declarations
Role of the funding source: ZY is funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The findings, interpretations and conclusions expressed in this paper are entirely those of the authors. The funding source did not have a role in the writing or decision to submit for publication. All authors have full access to the full data in the study and accept responsibility to submit for publication.
Potential conflicts of interest: CY serves as a consultant/independent contractor with Faron Pharmaceuticals, and as an honorarium recipient with Celgene. JMB reports grants and non-financial support from AstraZeneca, Merck Sharp & Dohme, Puma Biotechnology, Clovis Oncology, Pfizer, Janssen-Cilag, Novartis, Roche, and Eli Lilly.
Declaration of Competing Interest
CY serves as a consultant/independent contractor with Faron Pharmaceuticals, and as an honorarium recipient with Celgene. JMB reports grants and non-financial support from AstraZeneca, Merck Sharp & Dohme, Puma Biotechnology, Clovis Oncology, Pfizer, Janssen-Cilag, Novartis, Roche, and Eli Lilly.
Acknowledgements
We would like to thank Claire Snowdon, the editors, and the anonymous reviewers for their useful feedback.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2021.106625.
Appendix A. Supplementary data
PRISMA checklist
Supplementary materials
References
- 1.Nasrallah A.A., Farran S.H., Nasrallah Z.A., Chahrour M.A., Salhab H.A., Fares M.Y., Khachfe H.H., Akl E.A. A large number of COVID-19 interventional clinical trials were registered soon after the pandemic onset: a descriptive analysis. J. Clin. Epidemiol. 2020;125:170–178. doi: 10.1016/j.jclinepi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Library of Medicine ClinicalTrials.gov COVID-19 Studies. 2021. https://clinicaltrials.gov/ct2/results?cond=COVID-19&age_v=&gndr=&type=Intr&rslt=&Search=Apply (n.d.) (accessed July 19, 2021)
- 3.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 4.Saville B.R., Berry S.M. Efficiencies of platform clinical trials: a vision of the future. Clin. Trials. 2016;13:358–366. doi: 10.1177/1740774515626362. [DOI] [PubMed] [Google Scholar]
- 5.Dimairo M., Pallmann P., Wason J., Todd S., Jaki T., Julious S.A., Mander A.P., Weir C.J., Koenig F., Walton M.K., Nicholl J.P., Coates E., Biggs K., Hamasaki T., Proschan M.A., Scott J.A., Ando Y., Hind D., Altman D.G., ACE Consensus Group The Adaptive designs CONSORT extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design. BMJ. 2020;369:m115. doi: 10.1136/bmj.m115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiavone F., Bathia R., Letchemanan K., Masters L., Amos C., Bara A., Brown L., Gilson C., Pugh C., Atako N., Hudson F., Parmar M., Langley R., Kaplan R.S., Parker C., Attard G., Clarke N.W., Gillessen S., James N.D., Maughan T., Sydes M.R. This is a platform alteration: a trial management perspective on the operational aspects of adaptive and platform and umbrella protocols. Trials. 2019;20:264. doi: 10.1186/s13063-019-3216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hague D., Townsend S., Masters L., Rauchenberger M., Van Looy N., Diaz-Montana C., Gannon M., James N., Maughan T., Parmar M.K.B., Brown L., Sydes M.R., for the STAMPEDE and FOCUS4 investigators Changing platforms without stopping the train: experiences of data management and data management systems when adapting platform protocols by adding and closing comparisons. Trials. 2019;20:294. doi: 10.1186/s13063-019-3322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J.J.H., Siden E., Zoratti M.J., Dron L., Harari O., Singer J., Lester R.T., Thorlund K., Mills E.J. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angus D.C., Berry S., Lewis R.J., Al-Beidh F., Arabi Y., van Bentum-Puijk W., Bhimani Z., Bonten M., Broglio K., Brunkhorst F., Cheng A.C., Chiche J.-D., De Jong M., Detry M., Goossens H., Gordon A., Green C., Higgins A.M., Hullegie S.J., Kruger P., Lamontagne F., Litton E., Marshall J., McGlothlin A., McGuinness S., Mouncey P., Murthy S., Nichol A., O’Neill G.K., Parke R., Parker J., Rohde G., Rowan K., Turner A., Young P., Derde L., McArthur C., Webb S.A. The REMAP-CAP (randomized embedded multifactorial adaptive platform for community-acquired pneumonia) study. Rationale and design. Ann. Am. Thorac. Soc. 2020;17:879–891. doi: 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry S.M., Petzold E.A., Dull P., Thielman N.M., Cunningham C.K., Corey G.R., McClain M.T., Hoover D.L., Russell J., Griffiss J.M., Woods C.W. A response adaptive randomization platform trial for efficient evaluation of Ebola virus treatments: a model for pandemic response. Clin. Trials Lond. Engl. 2016;13:22–30. doi: 10.1177/1740774515621721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmeira V.A., Costa L.B., Perez L.G., Ribeiro V.T., Lanza K., Silva A.C.S.E. Do we have enough evidence to use chloroquine/hydroxychloroquine as a public health panacea for COVID-19? Clin. Sao Paulo Braz. 2020;75:e1928. doi: 10.6061/clinics/2020/e1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise J., Coombes R. Covid-19: the inside story of the RECOVERY trial. BMJ. 2020;370 doi: 10.1136/bmj.m2670. [DOI] [PubMed] [Google Scholar]
- 13.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized Trial of Hydroxychloroquine as Postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVERY Chief Investigator No Clinical Benefit from Use of Lopinavir-Ritonavir in Hospitalised COVID-19 Patients Studied in RECOVERY. 2020. https://www.recoverytrial.net/news/no-clinical-benefit-from-use-of-lopinavir-ritonavir-in-hospitalised-covid-19-patients-studied-in-recovery
- 15.Robinson J. MHRA instructs all UK hydroxychloroquine COVID-19 clinical trials to suspend recruitment. Pharm. J. 2020;305 doi: 10.1211/PJ.2020.20208075. [DOI] [Google Scholar]
- 16.Thorlund K., Dron L., Park J., Hsu G., Forrest J.I., Mills E.J. A real-time dashboard of clinical trials for COVID-19, lancet digit. Health. 2020;2:e286–e287. doi: 10.1016/S2589-7500(20)30086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janiaud P., Serghiou S., Ioannidis J.P.A. New clinical trial designs in the era of precision medicine: an overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat. Rev. 2019;73:20–30. doi: 10.1016/j.ctrv.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Siden E.G., Park J.J.H., Zoratti M.J., Dron L., Harari O., Thorlund K., Mills E.J. Reporting of master protocols towards a standardized approach: a systematic review. Contemp. Clin. Trials Commun. 2019;15 doi: 10.1016/j.conctc.2019.100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer E.L., Mesenbrink P., Dunger-Baldauf C., Fülle H.-J., Glimm E., Li Y., Posch M., König F. The evolution of master protocol clinical trial designs: a systematic literature review. Clin. Ther. 2020;42:1330–1360. doi: 10.1016/j.clinthera.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Berry D.A. The brave New World of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Clin. Trials Dev. Pers. Cancer Med. 2015;9:951–959. doi: 10.1016/j.molonc.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirakawa A., Asano J., Sato H., Teramukai S. Master protocol trials in oncology: review and new trial designs. Contemp. Clin. Trials Commun. 2018;12:1–8. doi: 10.1016/j.conctc.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner N.C., Kingston B., Kilburn L.S., Kernaghan S., Wardley A.M., Macpherson I.R., Baird R.D., Roylance R., Stephens P., Oikonomidou O., Braybrooke J.P., Tuthill M., Abraham J., Winter M.C., Bye H., Hubank M., Gevensleben H., Cutts R., Snowdon C., Rea D., Cameron D., Shaaban A., Randle K., Martin S., Wilkinson K., Moretti L., Bliss J.M., Ring A. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296–1308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regeneron Pharmaceuticals, Safety, Tolerability, and Efficacy of Anti-Spike (S) ClinicalTrials.Gov; 2020. SARS-CoV-2 Monoclonal Antibodies for Hospitalized Adult Patients With COVID-19.https://clinicaltrials.gov/ct2/show/NCT04426695 (n.d.) (accessed December 3. [Google Scholar]
- 24.National Institute of Allergy and Infectious Diseases A Multicenter Platform Trial of Putative Therapeutics for the Treatment of COVID-19 in Hospitalized Adults. 2020. https://fnih.org/sites/default/files/2021-04/activ-5.pdf
- 25.Lundgren J.D., Grund B., Barkauskas C.E., Holland T.L., Gottlieb R.L., Sandkovsky U., Brown S.M., Knowlton K.U., Self W.H., Files D.C., Jain M.K., Benfield T., Bowdish M.E., Leshnower B.G., Baker J.V., Jensen J.-U., Gardner E.M., Ginde A.A., Harris E.S., Johansen I.S., Markowitz N., Matthay M.A., Østergaard L., Chang C.C., Davey V.J., Goodman A., Higgs E.S., Murray D.D., Murray T.A., Paredes R., Parmar M.K.B., Phillips A.N., Reilly C., Sharma S., Dewar R.L., Teitelbaum M., Wentworth D., Cao H., Klekotka P., Babiker A.G., Gelijns A.C., Kan V.L., Polizzotto M.N., Thompson B.T., Lane H.C., Neaton J.D. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lilly Eli, Company A. ClinicalTrials.Gov; 2021. Study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in Participants With Mild to Moderate COVID-19 Illness (BLAZE-1)https://www.clinicaltrials.gov/ct2/show/NCT04427501 (n.d.) (accessed June 20. [Google Scholar]
- 27.Wilkinson T., Dixon R., Page C., Carroll M., Griffiths G., Ho L.-P., De Soyza A., Felton T., Lewis K.E., Phekoo K., Chalmers J.D., Gordon A., McGarvey L., Doherty J., Read R.C., Shankar-Hari M., Martinez-Alier N., O’Kelly M., Duncan G., Walles R., Sykes J., Summers C., Singh D., on behalf of the ACCORD Collaborators ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:691. doi: 10.1186/s13063-020-04584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duke Clinical Research Institute ACTIV-1 IM: Randomized Master Protocol for Immune Modulators for Treating COVID-19. 2020. https://www.fnih.org/sites/default/files/2021-04/activ-1.pdf
- 29.National Institute of Allergy, and Infectious Diseases ACTIV-2/A5401 Adaptive Platform Treatment Trial for Outpatients with COVID-19 (Adapt Out COVID) 2021. https://fnih.org/sites/default/files/2021-04/Protocol%20ACTIV-2-A5401%20Version%204%20dated%2031March2021_0.pdf
- 30.NYU School of Medicine ACTIV-4 A Multicenter, Adaptive, Randomized Controlled Platform Trial of the Safety and Efficacy of Antithrombotic Strategies in Hospitalized Adults with COVID-19. 2021. https://fnih.org/sites/default/files/2021-04/ACTIV4A_Protocol_v.1.1_Feb.1.2021.pdf
- 31.University of Pittsburgh ACTIV-4 COVID-19 Outpatient Thrombosis Prevention Trial. 2020. https://fnih.org/sites/default/files/2021-03/activ-4b.pdf
- 32.Duke University ACTIV-4 COVID-19 Thrombosis Prevention Trials: Post-Hospital Thromboprophylaxis. 2020. https://fnih.org/sites/default/files/2021-03/activ-4c.pdf
- 33.National Center for Advancing Translational Sciences ACTIV-6: COVID-19 Outpatient Randomized Trial to Evaluate Efficacy of Repurposed Medications. 2021. https://www.fnih.org/sites/default/files/2021-07/ACTIV-6%20Protocol%20V1.0_01APR2021.pdf
- 34.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2020. Adaptive COVID-19 Treatment Trial (ACTT)https://clinicaltrials.gov/ct2/show/NCT04280705 (n.d.) (accessed December 3. [Google Scholar]
- 35.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2021. Adaptive COVID-19 Treatment Trial 3 (ACTT-3)https://clinicaltrials.gov/ct2/show/NCT04492475 (n.d.) [Google Scholar]
- 36.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2021. Adaptive COVID-19 Treatment Trial 4 (ACTT-4)https://clinicaltrials.gov/ct2/show/NCT04640168 (n.d.) [Google Scholar]
- 37.Griffiths G., Fitzgerald R., Jaki T., Corkhill A., Marwood E., Reynolds H., Stanton L., Ewings S., Condie S., Wrixon E., Norton A., Radford M., Yeats S., Robertson J., Darby-Dowman R., Walker L., Khoo S. AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, Safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial. Trials. 2020;21:544. doi: 10.1186/s13063-020-04473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon J.H., Lau J.S.Y., Roney J., Rogers B.A., Trubiano J., Sasadeusz J., Molton J.S., Gardiner B., Lee S.J., Hoy J.F., Cheng A., Peleg A.Y. An adaptive randomised placebo controlled phase II trial of antivirals for COVID-19 infection (VIRCO): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:847. doi: 10.1186/s13063-020-04766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An open-label, multicentre, randomised, adaptive platform trial of the safety and efficacy of several therapies, including antiviral therapies, versus control in mild / moderate cases of COVID-19 2020. https://covid19crc.org/wp-content/uploads/2020/09/ANTICOV_01_COV_MasterProtocol_V5_09July2020.pdf
- 40.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M.-D., Kim E.-S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.-C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenith T., Fisher B.A., Slade D., Rowe A., Sharpe R., Thickett D.R., Whitehouse T., Rowland M., Scriven J., Parekh D., Bowden S.J., Savage J.S., Richards D., Bion J., Kearns P., Gates S., on behalf of CATALYST investigators CATALYST trial protocol: a multicentre, open-label, phase II, multi-arm trial for an early and accelerated evaluation of the potential treatments for COVID-19 in hospitalised adults. MedRxiv. 2021 doi: 10.1101/2021.02.10.21251478. 2021.02.10.21251478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.University of California . ClinicalTrials.Gov; 2021. Colchicine to Reduce Cardiac Injury in COVID-19 (COLHEART-19)https://clinicaltrials.gov/ct2/show/NCT04355143 (n.d.) [Google Scholar]
- 43.University of Pittsburgh . ClinicalTrials.Gov; 2021. Comparative Effectiveness of Readmission Reduction Interventions for Individuals With Sepsis or Pneumonia (ACCOMPLISH)https://clinicaltrials.gov/ct2/show/NCT04829188 (n.d.) [Google Scholar]
- 44.Stanford University . ClinicalTrials.Gov; 2021. COVID-19 Outpatient Pragmatic Platform Study (COPPS) - Master Protocol.https://clinicaltrials.gov/ct2/show/NCT04662086 (n.d.) [Google Scholar]
- 45.University of Pittsburgh . ClinicalTrials.Gov; 2021. COVID-19 Positive Outpatient Thrombosis Prevention in Adults Aged 40–80.https://clinicaltrials.gov/ct2/show/NCT04498273 (n.d.) [Google Scholar]
- 46.University of Witwatersrand, South Africa . ClinicalTrials.Gov; 2021. COVID-19 Prophylaxis South Africa (COVER HCW)https://clinicaltrials.gov/ct2/show/NCT04561063 (n.d.) [Google Scholar]
- 47.Duke University . ClinicalTrials.Gov; 2021. COVID-19 Thrombosis Prevention Trials: Post-hospital Thromboprophylaxis.https://clinicaltrials.gov/ct2/show/NCT04650087 (n.d.) [Google Scholar]
- 48.Washington University School of Medicine . ClinicalTrials.Gov; 2021. CROWN CORONATION: COVID-19 Research Outcomes Worldwide Network for CORONAvirus prevenTION.https://clinicaltrials.gov/ct2/show/NCT04333732 (n.d.) [Google Scholar]
- 49.Gaughan E., Quinn T., Bruce A., Antonelli J., Young V., Mair J., Akram A., Hirani N., Koch O., Mackintosh C., Norrie J., Dear J., Dhaliwal K. DEFINE: a phase IIa randomised controlled trial to evaluate repurposed treatments for COVID-19. MedRxiv. 2021 doi: 10.1101/2021.05.20.21257513. 2021.05.20.21257513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2021. Delayed Heterologous SARS-CoV-2 Vaccine Dosing (Boost) After Receipt of EUA Vaccines.https://clinicaltrials.gov/ct2/show/NCT04889209 (n.d.) [Google Scholar]
- 51.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Klekotka P., Shen L., Skovronsky D.M. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical Trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reis G., Moreira Silva E.A.D.S., Medeiros Silva D.C., Thabane L., Singh G., Park J.J.H., Forrest J.I., Harari O., Quirino Dos Santos C.V., Guimarães de Almeida A.P.F., de Figueiredo Neto A.D., Savassi L.C.M., Milagres A.C., Teixeira M.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Mills E.J. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical Trial. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hvidovre University Hospital Efficacy and Safety of Novel Treatment Options for Adults With COVID-19 Pneumonia (CCAP) https://clinicaltrials.gov/ct2/show/NCT04345289 (n.d.)
- 54.University Hospital Strasbourg, France, Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on the Survival of Hospitalized Elderly Patients with COVID-19 (COVID-Aging) https://clinicaltrials.gov/ct2/show/NCT04359953 (n.d.)
- 55.Oslo University Hospital European DisCoVeRy for Solidarity: An Adaptive Pandemic and Emerging Infection Platform Trial. 2021. https://eu-response.eu/wp-content/uploads/2021/07/2021-04-07-Protocol-EU-SolidAct-v1.1-1.pdf
- 56.Manchester University NHS Foundation Trust . ClinicalTrials.Gov; 2021. Facilitating AcceLerated Clinical Validation Of Novel Diagnostics for COVID-19 (FALCON-C19)https://clinicaltrials.gov/ct2/show/NCT04408170 (n.d.) [Google Scholar]
- 57.Duvignaud A., Lhomme E., Pistone T., Onaisi R., Sitta R., Journot V., Nguyen D., Peiffer-Smadja N., Crémer A., Bouchet S., Darnaud T., Poitrenaud D., Piroth L., Binquet C., Michel J.-F., Lefèvre B., Lebeaux D., Lebel J., Dupouy J., Roussillon C., Gimbert A., Wittkop L., Thiébaut R., Orne-Gliemann J., Joseph J.-P., Richert L., Anglaret X., Malvy D. Home treatment of older people with symptomatic SARS-CoV-2 infection (COVID-19): a structured summary of a study protocol for a multi-arm multi-stage (MAMS) randomized Trial to evaluate the efficacy and tolerability of several experimental treatments to reduce the risk of hospitalisation or death in outpatients aged 65 years or older (COVERAGE trial) Trials. 2020;21:846. doi: 10.1186/s13063-020-04619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King Hussein Cancer Center . ClinicalTrials.Gov; 2021. Hydroxychloroquine in Combination With Azithromycin or Sirolimus for Treating COVID-19 Patients (COVID19-HOPE)https://clinicaltrials.gov/ct2/show/NCT04374903 (n.d.) [Google Scholar]
- 59.QuantumLeap Healthcare Collaborative . ClinicalTrials.Gov; 2021. I-SPY COVID-19 TRIAL: An Adaptive Platform Trial for Critically Ill Patients.https://clinicaltrials.gov/ct2/show/NCT04488081 (n.d.) [Google Scholar]
- 60.University Hospital, Basel, Switzerland . ClinicalTrials.Gov; 2021. Immunocompromised Swiss Cohorts Based Trial Platform (COVERALL)https://clinicaltrials.gov/ct2/show/NCT04805125 (n.d.) [Google Scholar]
- 61.National Institute of Integrative Medicine, Australia . ClinicalTrials.Gov; 2021. International ALLIANCE Study of Therapies to Prevent Progression of COVID-19.https://clinicaltrials.gov/ct2/show/NCT04395768 (n.d.) [Google Scholar]
- 62.Lu I.N., Kulkarni S., Fisk M., Kostapanos M., Banham-Hall E., Kadyan S., Bond S., Norton S., Cope A., Galloway J., Hall F., Jayne D., Wilkinson I.B., J. Cheriyan, muLTi-arm therapeutic study in pre-ICu patients admitted with Covid-19-experimental drugs and mechanisms (TACTIC-E): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:690. doi: 10.1186/s13063-020-04618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rigshospitalet, Denmark . ClinicalTrials.Gov; 2021. National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 Vaccines (ENFORCE)https://clinicaltrials.gov/ct2/show/NCT04760132 (n.d.) [Google Scholar]
- 64.University of Birmingham . ClinicalTrials.Gov; 2021. Preventing Pulmonary Complications in Surgical Patients at Risk of COVID-19 (PROTECT-Surg)https://clinicaltrials.gov/ct2/show/NCT04386070 (n.d.) [Google Scholar]
- 65.Henry M. ClinicalTrials.Gov; 2021. Jackson Foundation for the Advancement of Military Medicine, Prophylaxis and Treatment of COVID-19 (PROTECT-APT)https://clinicaltrials.gov/ct2/show/NCT04844541 (n.d.) [Google Scholar]
- 66.Cambridge University Hospitals NHS Foundation Trust . ClinicalTrials.Gov; 2021. PROphylaxis for paTiEnts at Risk of COVID-19 infecTion (PROTECT)https://clinicaltrials.gov/ct2/show/NCT04389359 (n.d.) [Google Scholar]
- 67.Woolcock Institute of Medical Research . Aust. N. Z. Clin. Trials Regsitry; 2021. Randomised Clinical Trial of Interventions for the Treatment of COVID-19 in the Community Setting for High Risk Older People.https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379801&isReview=true (n.d.) [Google Scholar]
- 68.RECOVERY Trial Randomised Evaluation of COVID-19 Therapy (RECOVERY) 2021. https://www.recoverytrial.net/files/recovery-protocol-v14-0-2021-02-15.pdf n.d.
- 69.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulkarni S., Fisk M., Kostapanos M., Banham-Hall E., Bond S., Hernan-Sancho E., Norton S., Cheriyan J., Cope A., Galloway J., Hall F., Jayne D., Wilkinson I.B. Repurposed immunomodulatory drugs for Covid-19 in pre-ICu patients - mulTi-arm therapeutic study in pre-ICu patients admitted with Covid-19 - repurposed drugs (TACTIC-R): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:626. doi: 10.1186/s13063-020-04535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore H.B., Barrett C.D., Moore E.E., Jhunjhnuwala R., McIntyre R.C., Moore P.K., Wang J., Hajizadeh N., Talmor D.S., Sauaia A., Yaffe M.B. STudy of Alteplase for respiratory failure in SARS-Cov2/COVID-19: study design of the phase IIa STARS Trial. Res. Pract. Thromb. Haemost. 2020;4:984–996. doi: 10.1002/rth2.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denholm J.T., Davis J., Paterson D., Roberts J., Morpeth S., Snelling T., Zentner D., Rees M., O’Sullivan M., Price D., Bowen A., Tong S.Y.C. The Australasian COVID-19 Trial (ASCOT) to assess clinical outcomes in hospitalised patients with SARS-CoV-2 infection (COVID-19) treated with lopinavir/ritonavir and/or hydroxychloroquine compared to standard of care: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:646. doi: 10.1186/s13063-020-04576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.TOGETHER 3 Trial, Cochrane COVID-19 Study Regist. 2021. https://covid-19.cochrane.org/studies/crs-15334278 (n.d.)
- 74.Sunnybrook Health Sciences Centre . ClinicalTrials.Gov; 2021. Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO)https://clinicaltrials.gov/ct2/show/NCT04330690 (n.d.) [Google Scholar]
- 75.Ader F. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang D.T., McCreary E.K., Bariola J.R., Wadas R.J., Kip K.E., Marroquin O.C., Koscumb S., Collins K., Shovel J.A., Schmidhofer M., Wisniewski M.K., Sullivan C., Yealy D.M., Axe M., Nace D.A., Haidar G., Khadem T., Linstrum K., Snyder G.M., Seymour C.W., Montgomery S.K., McVerry B.J., Berry L., Berry S., Meyers R., Weissman A., Peck-Palmer O.M., Wells A., Bart R., Albin D.L., Minnier T., Angus D.C. The UPMC OPTIMISE-C19 (OPtimizing treatment and impact of monoclonal antIbodieS through evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization. Trials. 2021;22:363. doi: 10.1186/s13063-021-05316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medical University of Vienna . ClinicalTrials.Gov; 2021. Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) (ACOVACT)https://clinicaltrials.gov/ct2/show/NCT04351724 (n.d.) [Google Scholar]
- 78.University of Washington Efficacy of Novel Agents for Treatment of SARS-CoV- 2 Infection Among High-Risk Outpatient Adults: An Adaptive Randomized Platform Trial. 2020. https://clinicaltrials.gov/ProvidedDocs/28/NCT04354428/Prot_003.pdf
- 79.Regeneron Pharmaceuticals . ClinicalTrials.Gov; 2021. Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for Hospitalized Adult Patients With COVID-19.https://clinicaltrials.gov/ct2/show/NCT04426695 (n.d.) [Google Scholar]
- 80.The Writing Committee for the REMAP-CAP Investigators Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang D.T., McVerry B.J., Horvat C., Adams P.W., Berry S., Buxton M., Clermont G., Garrard W., Girard T.D., Haidar G., King A.J., Linstrum K., Malakouti S., Mayr F.B., McCreary E.K., Montgomery S.K., Seymour C.W., Weissman A., Angus D.C., on behalf of the R.-C.I. The UPMC REMAP-COVID Group Implementation of the randomized embedded multifactorial adaptive platform for COVID-19 (REMAP-COVID) trial in a US health system—lessons learned and recommendations. Trials. 2021;22:100. doi: 10.1186/s13063-020-04997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2021. ACTIV-2: A Study for Outpatients With COVID-19.https://clinicaltrials.gov/ct2/show/NCT04518410 (n.d.) [Google Scholar]
- 83.Bonten M.J.M. ClinicalTrials.Gov; 2020. Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia (REMAP-CAP)https://www.clinicaltrials.gov/ct2/show/NCT02735707 (n.d.) (accessed December 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.Gov; 2021. Adaptive COVID-19 Treatment Trial 2 (ACTT-2)https://clinicaltrials.gov/ct2/show/NCT04401579 (n.d.) [Google Scholar]
- 85.University of Washington . ClinicalTrials.Gov; 2021. Hydroxychloroquine for COVID-19 Post-Exposure Prophylaxis (PEP)https://clinicaltrials.gov/ct2/show/NCT04328961 (n.d.) [Google Scholar]
- 86.Statham E.E., White S.A., Sonwane B., Bierer B.E. Primed to comply: individual participant data sharing statements on ClinicalTrials.gov. PLoS One. 2020;15 doi: 10.1371/journal.pone.0226143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.I. of M. Board on Health Sciences Policy . National Academic Press (US); 2015. Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk.https://www.ncbi.nlm.nih.gov/books/NBK285999/ [PubMed] [Google Scholar]
- 88.Multi-Regional Clinical Trials Center of Brigham and Women'’s Hospital and Harvard, Vivli https://vivli.org (n.d.)
- 89.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Adams A.C., Van Naarden J., Custer K.L., Shen L., Durante M., Oakley G., Schade A.E., Sabo J., Patel D.R., Klekotka P., Skovronsky D.M. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Regeneron Pharmaceuticals, Safety, Tolerability, and Efficacy of Anti-Spike (S) ClinicalTrials.Gov; 2021. SARS-CoV-2 Monoclonal Antibodies for the Treatment of Ambulatory Adult and Pediatric Patients With COVID-19.https://clinicaltrials.gov/ct2/show/NCT04425629 (n.d.) (accessed June 30. [Google Scholar]
- 91.Pallmann P., Bedding A.W., Choodari-Oskooei B., Dimairo M., Flight L., Hampson L.V., Holmes J., Mander A.P., Odondi L., Sydes M.R., Villar S.S., Wason J.M.S., Weir C.J., Wheeler G.M., Yap C., Jaki T. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018;16:29. doi: 10.1186/s12916-018-1017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.University of Melbourne . 2020. Australasian COVID-19 Trial (ASCOT) ADAptive Platform Trial. [Google Scholar]
- 96.Food and Drug Administration, Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics. Draft Guidance. 2018. https://www.fda.gov/media/120721/download
- 93.Angus D.C., Alexander B.M., Berry S., Buxton M., Lewis R., Paoloni M., Webb S.A.R., Arnold S., Barker A., Berry D.A., Bonten M.J.M., Brophy M., Butler C., Cloughesy T.F., Derde L.P.G., Esserman L.J., Ferguson R., Fiore L., Gaffey S.C., Gaziano J.M., Giusti K., Goossens H., Heritier S., Hyman B., Krams M., Larholt K., LaVange L.M., Lavori P., Lo A.W., London A.J., Manax V., McArthur C., O’Neill G., Parmigiani G., Perlmutter J., Petzold E.A., Ritchie C., Rowan K.M., Seymour C.W., Shapiro N.I., Simeone D.M., Smith B., Spellberg B., Stern A.D., Trippa L., Trusheim M., Viele K., Wen P.Y., Woodcock J. The adaptive platform trials coalition, adaptive platform trials: definition, design, conduct and reporting considerations. Nat. Rev. Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 94.Cohen D.R., Todd S., Gregory W.M., Brown J.M. Adding a treatment arm to an ongoing clinical trial: a review of methodology and practice. Trials. 2015;16:179. doi: 10.1186/s13063-015-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fors M., González P. Current status of Bayesian clinical trials for oncology, 2020. Contemp. Clin. Trials Commun. 2020;20 doi: 10.1016/j.conctc.2020.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kruschke J.K. Bayesian analysis reporting guidelines. Nat. Hum. Behav. 2021 doi: 10.1038/s41562-021-01177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dillman A., Park J.J.H., Zoratti M.J., Zannat N.-E., Lee Z., Dron L., Hsu G., Smith G., Khakabimamaghani S., Harari O., Thorlund K., Mills E.J. Reporting and design of randomized controlled trials for COVID-19: a systematic review. Contemp. Clin. Trials. 2021;101 doi: 10.1016/j.cct.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taichman D.B., Sahni P., Pinborg A., Peiperl L., Laine C., James A., Hong S.-T., Haileamlak A., Gollogly L., Godlee F., Frizelle F.A., Florenzano F., Drazen J.M., Bauchner H., Baethge C., Backus J. Data sharing statements for clinical trials — a requirement of the international committee of medical journal editors. N. Engl. J. Med. 2017;376:2277–2279. doi: 10.1056/NEJMe1705439. [DOI] [PubMed] [Google Scholar]
- 99.Resnik D.B., Morales M., Landrum R., Shi M., Minnier J., Vasilevsky N.A., Champieux R.E. Effect of impact factor and discipline on journal data sharing policies. Account. Res. 2019;26:139–156. doi: 10.1080/08989621.2019.1591277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCAIN K.W. Mandating sharing: journal policies in the natural sciences. Sci. Commun. 1995;16:403–431. doi: 10.1177/1075547095016004003. [DOI] [Google Scholar]
- 101.Vasilevsky N.A., Minnier J., Haendel M.A., Champieux R.E. Reproducible and reusable research: are journal data sharing policies meeting the mark? PeerJ. 2017;5 doi: 10.7717/peerj.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danchev V., Min Y., Borghi J., Baiocchi M., Ioannidis J.P.A. Evaluation of data sharing after implementation of the international committee of medical journal editors data sharing statement requirement. JAMA Netw. Open. 2021;4:e2033972. doi: 10.1001/jamanetworkopen.2020.33972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geifman N., Bollyky J., Bhattacharya S., Butte A.J. Opening clinical trial data: are the voluntary data-sharing portals enough? BMC Med. 2015;13:280. doi: 10.1186/s12916-015-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dron L., Dillman A., Zoratti M.J., Haggstrom J., Mills E.J., Park J.J.H. Clinical Trial data sharing for COVID-19-related research. J. Med. Internet Res. 2021;23(3):e26718. doi: 10.2196/26718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Department of Health and Social Care, UK Groundbreaking COVID-19 Treatments to be Fast-Tracked Through Clinical Trials. 2021. https://www.gov.uk/government/news/groundbreaking-covid-19-treatments-to-be-fast-tracked-through-clinical-trials
- 106.Kiszewski A.E., Cleary E.G., Jackson M.J., Ledley F.D. NIH funding for vaccine readiness before the COVID-19 pandemic. Vaccine. 2021;39:2458–2466. doi: 10.1016/j.vaccine.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siedner M.J., Gandhi R.T. Proposing minimum requirements for announcing clinical trial results during the COVID-19 pandemic. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McMahon J.H., Lydeamore M.J., Stewardson A.J. Bringing evidence from press release to the clinic in the era of COVID-19. J. Antimicrob. Chemother. 2021;76:547–549. doi: 10.1093/jac/dkaa506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Catalan-Matamoros D., Elías C. Vaccine hesitancy in the age of coronavirus and fake news: analysis of journalistic sources in the Spanish quality press. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17218136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist
Supplementary materials