Abstract
Prolonged neutropenia increases the risk for lethal invasive fungal infections (IFIs) such as those caused by Rhizopus species. Isavuconazonium sulfate is a new triazole that lacks pediatric dosing recommendations. Clinical courses of 4 pediatric patients with IFIs in the peri-allogeneic hematopoietic cell transplantation (alloHCT) period between 2015 and 2017 were reviewed. The reviews included previously unreported pharmacokinetic and safety data, and the IFIs included Rhizopus. Isavuconazonium sulfate was initiated with a loading dose followed by daily dosing, adjusted to a goal trough concentration of >3 mg/L based on adult literature. This target was achieved at a median of 7 days, demonstrating varying rates of metabolism. Renal insufficiency, electrolyte disturbances, and transaminitis were noted, although attribution was confounded by other alloHCT complications. One patient survived infection-free to hospital discharge and 1 of 3 deceased patients had evidence of an unresolved IFI (case 2). Case 2 was subtherapeutic for 39% of the duration of treatment, compared with others at an average of 29%, suggesting this target trough to be clinically relevant because case 2 demonstrated positive sinus and nasal cultures for Rhizopus on autopsy. We recommend initiation of isavuconazonium 10 mg/kg with a maximum dose of 372 mg. A loading dose of 10 mg/kg is used every 8 hours for 6 doses followed by 10 mg/kg dosing every 24 hours. Monitoring must continue beyond steady state. If early monitoring is not possible, we recommend a first drug level at week 3. If dose increases are required, a partial reload has been more successful instead of increasing daily doses. Further larger studies are needed to demonstrate optimum dosing in pediatric patients.
Keywords: clinical, mucormycosis, pharmacology, Rhizopus, stem cells, triazole
Introduction
Prolonged neutropenia, immunosuppression, and immune dysregulation increase the risk for opportunistic invasive fungal infection (IFI).1 Rhizopus, a Mucorales mold, can be focal or disseminated with mortality exceeding 70%.2–4 Best outcomes are achieved with early tissue diagnosis,5 surgical debridement,2,6 cellular immunity restoration, and antifungal pharmacotherapy. Isavuconazonium sulfate (a prodrug to isavuconazole) is a new broad spectrum triazole, targeting the CYP51A enzyme needed for fungal cell membrane formation, available in oral and IV formulations with less cardiotoxicity compared with other triazoles.7 Safety, pharmacokinetics, and dosing data are available for adults,8–10 but limited to case reports in pediatrics.4,11–14 We describe a case series of patients who received isavuconazonium sulfate for IFIs at our institution.
Cases
Case 1. A 2-year-old female with severe aplastic anemia developed Rhizopus sinusitis following 6 months of neutropenia, despite 5 months of voriconazole prophylaxis in addition to 1 month of micafungin therapy, added to treat a chest CT pulmonary nodule. Initial Rhizopus therapy included 4 weeks of IV liposomal amphotericin B (LAB) 5 mg/kg daily, oral posaconazole 6.25 mg/kg every 6 hours, and surgical debridements with amphotericin nasal irrigation 3 mg in 10 mL sterile solution. To restore cellular immunity, she underwent an 8/8 human leukocyte antigen (HLA)-matched unrelated donor allogeneic hematopoietic cell transplantation (alloHCT). To avoid drug interactions during reduced intensity conditioning (RIC) with cyclophosphamide, posaconazole was held. Posaconazole was restarted 48 hours after the last dose of cyclophosphamide. She received 7 granulocyte infusions starting day +0 until neutrophil engraftment on day +14 post-alloHCT. At week 9 of Rhizopus treatment, her isolate was found to be posaconazole resistant (MIC >32 mg/L) prompting transition to isavuconazonium sulfate (MIC 4) with a loading dose of 15 mg/kg IV every 12 hours for 4 doses, then maintenance dosing of 15 mg/kg daily. The dose was titrated to maintain the isavuconazole trough >3 mg/L. After 11 weeks of Rhizopus therapy and 3 weeks after neutrophil recovery, sinus endoscopy revealed resolution. Intranasal amphotericin B and debridements were discontinued (Image). She remained on LAB and isavuconazonium sulfate empirically until 6 months post-alloHCT. Isavuconazonium sulfate was well tolerated except for grade 1 transaminitis, which improved with 10% dose reduction and resolved upon discontinuation.
Image.
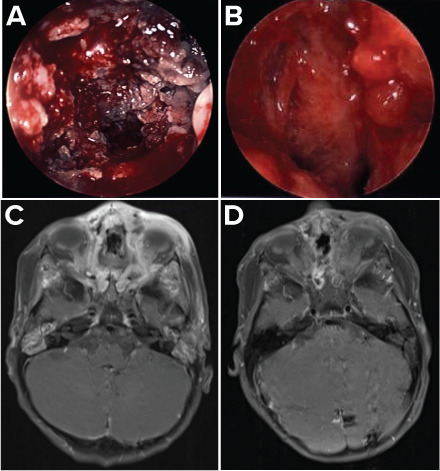
Comparison of Rhizopus infection imaging early (week 5) and late (week 16) in the treatment course. (A) Endoscopic photography, week 5; (B) endoscopic photography, week 16; (C) 3-tesla MRI of face with and without contrast (T1-weighted images with fat saturation in the axial plane post-contrast), week 5; (D) 3-tesla MRI of face with and without contrast (T1-weighted images with fat saturation in the axial plane post-contrast), week 16.
Case 2. An 11-year-old female with severe congenital neutropenia was admitted with angioinvasive Rhizopus sinusitis on day +270 following an 8/8 HLA-matched unrelated donor alloHCT. Her alloHCT course was complicated by graft failure, resulting in 17 days of neutropenia preceding Rhizopus infection. The MIC evaluations suggested resistance to voriconazole (>32), posaconazole (>32), and micafungin (>32) but susceptibility to amphotericin B (2) and isavuconazonium sulfate (1). Treatment began with amphotericin B sinus irrigation 3 mg in 10 mL sterile solution, IV LAB 5 mg/kg daily, isavuconazonium sulfate (initial loading dose of 10 mg/kg IV every 8 hours for 6 doses, then maintenance of 10 mg/kg daily), and surgical debridement. Target isavuconazole trough >3 mg/L of 6.98 mg/L was achieved on day 6. Sinus cultures remained negative after 7 and pathology after 18 days of therapy. On day +16 of Rhizopus treatment, the patient underwent a 4/8 HLA-haploidentical RIC HCT. The LAB dose was increased to 10 mg/kg daily and isavuconazonium sulfate held for 2 weeks to avoid drug interaction with cyclophosphamide. Isavuconazonium sulfate IV was reinitiated with a loading dose of 7.5 mg/kg every 8 hours for 6 doses then 7.5 mg/kg daily (75% of the initial dosing due to the high initial drug level). Weekly troughs were low, requiring dose escalation to 13 mg/kg to achieve isavuconazole trough >3 mg/L. Twelve granulocyte infusions were administered between day 0 and +41 of Rhizopus treatment, discontinued secondary to a serious transfusion reaction. By day +33 post-haploidentical HCT (haploHCT), she developed renal failure requiring dialysis and eventual respiratory failure requiring ventilation. She never recovered neutrophils, despite a CD34+-selected stem cell boost. On day +42 post-haploHCT, with declining status, surgical debridement was discontinued. With graft failure, systemic bacterial and viral infection, and multiorgan failure, she died on day +43. Despite absent findings of Rhizopus prior to her death, the postmortem evaluation revealed left sinus and nasal cultures positive for Rhizopus.
Case 3. An 8-year-old male with adrenoleukodys-trophy presented with palate ulceration on day +21 following a 5/8 HLA-matched umbilical cord blood transplant (UCBT) complicated by graft failure and 4 weeks of preceding neutropenia. A palate biopsy confirmed Zygomycetes by histopathologic silver and periodic acid-Schiff stains. The necrosis of affected tissue precluded aggressive debridement given the risk for severe disfigurement, and the parenchymal lung lesions on chest imaging suggested disseminated disease. Treatment was initiated with isavuconazonium sulfate (initial loading dose of 7 mg/kg IV every 8 hours for 6 doses, then maintenance of 7 mg/kg daily, reaching the individual maximum adult dose of 372 mg), and IV LAB 5 mg/kg daily. The initial isavuconazole level was therapeutic at 5.3 mg/L on day 6 of therapy. He additionally received 4 granulocyte transfusions in the first week of fungal therapy. One month after IFI diagnosis, the patient underwent a 5/8-HLA RIC haploHCT. For 2 weeks, isavuconazonium sulfate was held to avoid cyclophosphamide drug interaction, resuming on day +5 post-haploHCT at a loading dose of 7 mg/kg every 8 hours for 6 doses then 7 mg/kg daily. On day +19 post-haploHCT (day +49 of Zygomycetes treatment), multifactorial multiorgan failure from adenoviral and enterococcal sepsis prompted isavuconazonium sulfate discontinuation. Despite achieving neutrophil engraftment, he died on day +22 post-haploHCT. The family declined a postmortem evaluation.
Case 4. A 19-month-old male with Hurler syndrome presented on day +52 post-5/6 HLA-matched UCBT, complicated by graft failure and 20 days of neutropenia; he also had an abdominal eschar concerning for fungal infection. When Rhizopus species was identified on the fungal wound culture, prophylactic micafungin was transitioned to LAB 5 mg/kg daily (MIC 1) and the lesion surgically debrided. Biopsy confirmed angioinvasive mucormycosis. Upon further examination 2 days later, an additional Alternaria species (unable to be further characterized) was isolated and resected from the left nasal turbinate, triggering empiric addition of isavuconazonium sulfate (initial dose of 10 mg/kg IV every 8 hours for 6 loading doses followed by 10 mg/kg IV daily) given the unavailability of susceptibilities. The patient underwent a second 5/6 HLA-matched RIC UCBT 8 days after IFI diagnosis. On day +19 post-second UCBT/day +9 of isavuconazonium sulfate, his initial isavuconazole trough returned therapeutic at 4.2 mg/L. However, the day 31 isavuconazole trough declined to 2.69 mg/L, prompting a 50% dose increase to 15 mg/kg IV daily. No subsequent levels were sent. At the last endoscopic sinus biopsy on day +38 post-second UCBT, fungal elements were absent. The patient died on day +67 post-second UCBT (day +57 of isavuconazonium sulfate treatment) of presumed non-infectious cryptogenic organizing pneumonia, with the postmortem evaluation showing diffuse alveolar hemorrhage. Autopsy of the abdominal skin by Gomori methenamine silver staining revealed a healing ulcer with no fungal organisms. Fungal cultures of the blood and lung were also negative.
Discussion
In this series, 1 patient was successfully discharged from the hospital without evidence of IFI, whereas others had negative cultures at death. This series demonstrates the aggressiveness of mucormycosis and the role of isavuconazonium sulfate in a multimodal antifungal approach.
At our institution, isavuconazonium sulfate is indicated for antifungal drug resistance and as an empiric or a dual coverage for progressive disease. Although posaconazole remains the standard of care, obtaining a therapeutic trough in pediatric patients after alloHCT is difficult.15 Nephrotoxicity can be seen with IV posaconazole, and oral posaconazole was not logistically possible for our patients.16
Pediatric dosing for isavuconazonium sulfate was extrapolated from adult literature although this approach was variably effective in achieving target troughs due to varying pharmacokinetics (Figure).17 In this series, various initiating doses were selected for the following reasons: concern for toxicity during azole overlap in transitioning between posaconazole and isavuconazonium sulfate, concern for higher metabolism of drug based on patient medication dosing history, and the stock availability of isavuconazonium sulfate. Timing of levels varied due to clinical concerns and unfamiliarity with the drug. Isavuconazonium sulfate was well tolerated, held for known drug interactions with chemotherapy, and dose reduced for side effects including nephrotoxicity (rising blood urea nitrogen and/or creatinine), electrolyte abnormalities (hyperkalemia, hypernatremia, and/or hypocalcemia), and hepatic dysfunction (transaminitis).
Figure.
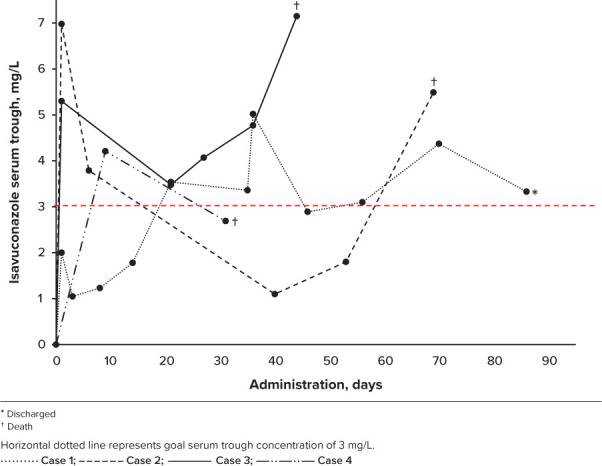
Isavuconazole serum trough goal achieved in majority of patients by day 21 of administration.
The goal trough concentration for isavuconazonium sulfate remains unclear with studies unable to identify a clinically efficacious concentration level. A trial using isavuconazonium sulfate for invasive aspergillosis and in which the average trough level was 3.9 mg/L did not show a significant association between concentration and efficacy.9 A risk-versus-benefit analysis suggested a higher dose was preferable. As such, to avoid toxicity, the standard goal at our institution is isavuconazole trough >3 mg/L.18,19 Additionally, in our report, case 2 was subtherapeutic for 39% of the duration of treatment, compared with others at an average of 29%, suggesting this target trough to be clinically relevant. Accordingly, in our cohort, no toxicities were noted with supra-therapeutic troughs, and death was not attributed to high troughs.
Our recommendation to achieve target troughs is to initiate isavuconazonium sulfate at a loading dose of 10 mg/kg every 8 hours for 6 doses followed by 10 mg/kg dosing every 24 hours, using an individual maximum dose of 372 mg (per adult recommendations). Given a significantly long drug half-life, early and frequent monitoring presents some challenges. Variable time to therapeutic trough was noted in our cohort of pediatric patients and knowledge about pharmacokinetics unfortunately remains scarce. Steady state was not achieved until approximately 3 weeks after initiation. Although we acquired levels as early as 6 days to ensure sufficient levels, we would not recommend dose changes be made until ~3 weeks of therapy. Early therapeutic levels reflect the loading dose, so monitoring must continue beyond steady state. If frequent monitoring is not possible, we recommend a first drug level at week 3 because week 2 levels have been misleading, in our experience. If dose increases are required, a partial reload in addition to dose increase has been more successful given long drug half-life. The recommendation to consider a half load before increasing the dose of isavuconazonium sulfate is based purely on our institutional experiences. Generally speaking, the use of a loading dose should be used to assist in reaching the steady-state concentration sooner while a therapeutic drug level is in process.
Currently, there is no consensus on what would be considered the upper limit of normal for levels. Furfaro et al20 examined serial levels and suggested a trough of ~5 mg/L as a threshold for gastrointestinal toxicity. In our cohort, we did not see any levels >5 mg/L at steady state. Once out of the acute phase, serum drug concentrations are monitored monthly, then every 2 months per provider comfort for infection control.
In summary, isavuconazonium sulfate is a well-tolerated triazole, but goal levels are not clearly established, and interpatient pharmacokinetic variability and long half-life make it challenging to achieve desired levels. Future studies of pharmacokinetic association with IFI response will better inform trough goals.
ABBREVIATIONS
- alloHCT
allogeneic hematopoietic cell transplantation
- CT
computed tomography
- haploHCT
haploidentical hematopoietic stem cell transplant
- HLA
human leukocyte antigen
- IFI
invasive fungal infection
- IV
intravenous
- LAB
liposomal amphotericin B
- MIC
minimum inhibitory concentration
- MRI
magnetic resonance imaging
- RIC
reduced intensity conditioning
- UCBT
umbilical cord blood transplant
Footnotes
Disclosures. This research was supported by the National Institutes of Health's National Center for Advancing Translational Sciences, grants KL2TR002492 and UL1TR002494. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health's National Center for Advancing Translational Sciences. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Ethical Approval and Informed Consent. Given the nature of this work, the institution/board of ethics committee did not require review or informed consent.
References
- 1.Marty FM, Ostrosky-Zeichner L, Cornely OA et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis . 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 2.Roden MM, Zaoutis TE, Buchanan WL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis . 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 3.Marr KA, Carter RA, Crippa F et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis . 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 4.Muggeo P, Calore E, Decembrino N et al. Invasive mucormycosis in children with cancer: a retrospective study from the Infection Working Group of Italian Pediatric Hematology Oncology Association. Mycoses . 2019;62(2):165–170. doi: 10.1111/myc.12862. [DOI] [PubMed] [Google Scholar]
- 5.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis . 2008;47(4):503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 6.Ardeshirpour F, Bohm LA, Belani KK et al. Surgery for pediatric invasive fungal sinonasal disease. Laryngoscope . 2014;124(4):1008–1012. doi: 10.1002/lary.24369. [DOI] [PubMed] [Google Scholar]
- 7.Pomorska A, Malecka A, Jaworski R et al. Isavuconazole in a successful combination treatment of disseminated mucormycosis in a child with acute lymphoblastic leukaemia and generalized haemochromatosis: a case report and review of the literature. Mycopathologia . 2019;184(1):81–88. doi: 10.1007/s11046-018-0287-0. [DOI] [PubMed] [Google Scholar]
- 8.Viljoen J, Azie N, Schmitt-Hoffmann AH, Ghannoum M. A phase 2, randomized, double-blind, multicenter trial to evaluate the safety and efficacy of three dosing regimens of isavuconazole compared with fluconazole in patients with uncomplicated esophageal candidiasis. Antimicrob Agents Chemother . 2015;59(3):1671–1679. doi: 10.1128/AAC.04586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maertens JA, Raad II, Marr KA et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet . 2016;387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Gachot B, Akan H et al. Epidemiology and outcome of fungemia in a cancer cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031) Clin Infect Dis . 2015;61(3):324–331. doi: 10.1093/cid/civ293. [DOI] [PubMed] [Google Scholar]
- 11.Barg AA, Malkiel S, Bartuv M et al. Successful treatment of invasive mucormycosis with isavuconazole in pediatric patients. Pediatr Blood Cancer . 2018;65(10):e27281. doi: 10.1002/pbc.27281. [DOI] [PubMed] [Google Scholar]
- 12.Cornu M, Bruno B, Loridant S et al. Successful outcome of disseminated mucormycosis in a 3-year-old child suffering from acute leukaemia: the role of isavuconazole? A case report. BMC Pharmacol Toxicol . 2018;19(1):81. doi: 10.1186/s40360-018-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Leonardis F, Novielli C, Giannico B et al. Isavuconazole treatment of cerebral and pulmonary aspergillosis in a pediatric patient with acute lymphoblastic leukemia: case report and review of literature. J Pediatr Hematol Oncol . 2020;42(6):e469–e471. doi: 10.1097/MPH.0000000000001508. [DOI] [PubMed] [Google Scholar]
- 14.Brivio E, Verna M, Migliorino GM et al. Isavuconazole-induced acute liver failure in a pediatric patient with invasive aspergillosis. Pediatr Infect Dis J . 2019;38(10):1035–1037. doi: 10.1097/INF.0000000000002418. [DOI] [PubMed] [Google Scholar]
- 15.Bernardo VA, Cross SJ, Crews KR et al. Posaconazole therapeutic drug monitoring in pediatric patients and young adults with cancer. Ann Pharmacother . 2013;47(7–8):976–983. doi: 10.1345/aph.1R775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otu A, Bongomin F, Kosmidis C, Denning DW. Acute kidney injury: an unusual complication of posaconazole use. J Chemother . 2018;30(6–8):380–383. doi: 10.1080/1120009X.2018.1522472. [DOI] [PubMed] [Google Scholar]
- 17.Andes D, Kovanda L, Desai A et al. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother . 2018;62(7):e00585–18. doi: 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decembrino N, Perruccio K, Zecca M et al. A case series and literature review of isavuconazole use in pediatric patients with hemato-oncologic diseases and hematopoietic stem cell transplantation. Antimicrob Agents Chemother . 2020;64(3):e01783–19. doi: 10.1128/AAC.01783-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaindl T, Andes D, Engelhardt M et al. Variability and exposure-response relationships of isavuconazole plasma concentrations in the phase 3 SECURE trial of patients with invasive mould diseases. J Antimicrob Chemother . 2019;74(3):761–767. doi: 10.1093/jac/dky463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furfaro E, Signori A, Di Grazia C et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother . 2019;74(8):2341–2346. doi: 10.1093/jac/dkz188. [DOI] [PubMed] [Google Scholar]


