ABSTRACT.
Chikungunya virus (CHIKV) is an arbovirus endemic to South Asia with frequent outbreaks. A wide spectrum of neurological complications has been described in Chikungunya infections. Myeloneuropathy is a rare complication seen in Chikungunya and is proposed to have an underlying immune mediated pathogenesis. We report a case of a 45-year-old man presenting to the emergency services with acute onset of quadriparesis, breathlessness, urinary retention, profound pain, and sensory disturbances 6 weeks after the onset of high-grade fever and arthralgia. On examination, the patient had Medical Research Council grade 1 flaccid quadriparesis with prominent wasting and areflexia with distinct sensory level at T4. Immunoglobulin M CHIKV antibodies were positive, tested twice at a 1-week interval. He had notable magnetic resonance imaging (MRI) findings in the form of patchy T2 hyperintensities involving the entire length of the cervical and thoracic cord with normal brain imaging and extensive short tau inversion recovery hyperintense signal changes on muscle MRI. He was treated with five cycles of plasmapheresis and intravenous methylprednisolone followed by oral steroids for 8 weeks. At 20-week follow-up, the patient had improvement in upper limb weakness, but paraparesis persisted. The case highlights the presence of unusual MRI findings and also the importance of early recognition of after infective neurological complications, and prompt treatment with immunomodulation may be beneficial.
INTRODUCTION
Chikungunya virus (CHIKV) is an arbovirus transmitted by Aedes spp. mosquitoes.1 India is hyperendemic for CHIKV with frequent outbreaks.2 Neurological complications involving both the central and peripheral nervous systems (CNS and PNS) have been described in chikungunya infections, with encephalopathy and encephalitis being the most common neurological manifestations.3,4 Disease affecting multiple components of the nervous system—for example, encephalomyelitis or myeloneuropathy—are described, and the prognosis in these cases is poor.4 We describe a case of post-Chikungunya myeloneuropathy with unusual spinal cord magnetic resonance imaging (MRI) findings.
CASE REPORT
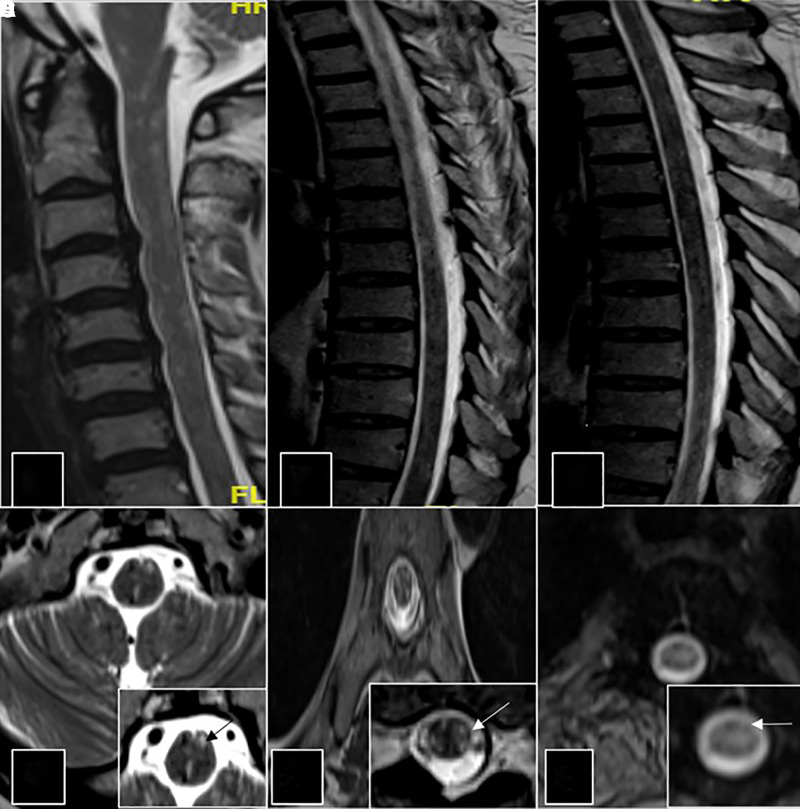
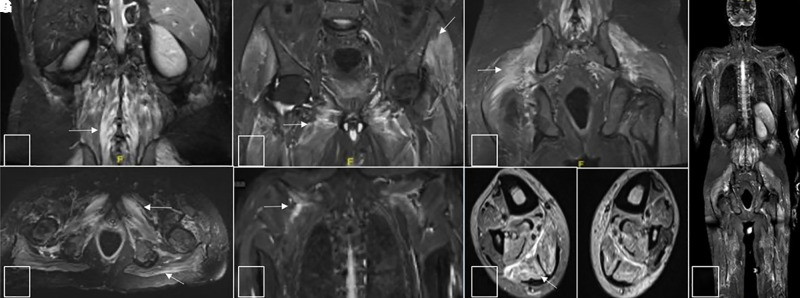
A 45-year-old man presented to the emergency services during February 2020 4 weeks after the onset of flaccid quadriparesis associated with urinary retention and sensory disturbances that evolved over 2 days. He had severe arthralgia involving large joints of the upper and lower limbs and excruciating neck pain. The patient had history of high-grade fever of 4 days duration with arthralgia, 6 weeks before the onset of quadriparesis but was not investigated for specific causes. The patient also had severe, persistent joint pain and muscle tenderness since the onset of fever and developed breathlessness during the course of admission. He had developed generalized patchy hyperpigmentation of the skin and particularly over the face. On examination, he had tachypnoea, flaccid areflexic quadriparesis with Medical Research Council (MRC) Grade 1 power with severe wasting and a definite sensory level at T4 with loss of all modalities of sensation below this level. He had severe muscle pains and muscle tenderness and generalized patchy hyperpigmentation. Cranial muscles were normal. His hemogram, liver and renal functions, serum creatine kinase level, and thyroid function tests were normal. Erythrocyte sedimentation rate was 92 mm at the first hour, and C-reactive protein was 12 mg/dL. Serum immunoglobulin M antibodies to Chikungunya antigen was positive, tested twice at 1-week intervals. MRI of the spine showed patchy, discrete, and confluent (short-segment) T2 hyperintense lesions resulting in a speckled pattern involving the cervical and thoracic cord with extension up to the cervicomedullary junction (Figure 1). No foci of blooming/diffusion restriction were seen. The lesions were indistinct on T1-weighted imaging. No postcontrast enhancement was observed. Brain imaging was normal. Whole body muscle MRI showed extensive short tau inversion recovery hyperintense signal changes in the posterior paraspinal group, gluteal muscles, muscles of pelvis (obturator internus and externus), the thigh adductors, gastrocnemius, bilateral soleus, trapezius, and supraspinatus muscles of the shoulder girdle suggestive of inflammation (Figure 2). Cerebrospinal fluid (CSF) analysis showed five cells (four lymphocytes and one polymorph) with glucose of 65 mg/dL and protein of 28 mg/dL. Test for CHIKV antibodies in CSF was not available. Nerve conduction studies showed evidence of sensorimotor axonopathy and left superficial radial nerve biopsy showed features of mild chronic axonopathy. Workup for other causes of long segment myelitis including autoimmune (antinuclear antibody [ANA], ANA profile, antineutrophil cytoplasmic antibodies, and angiotensin-converting enzyme levels), paraneoplastic profile, and neuromyelitis optica and myelin oligodendrocyte glycoprotein (NMO-MOG) antibodies were negative. His HIV testing, hepatitis virus screen and serum VDRL was negative. He was treated with intravenous methylprednisolone for 5 days and five cycles of plasmapheresis followed by maintenance on oral steroids for 8 weeks. He remained status quo for 2 weeks followed by gradual improvement in upper limb power to MRC Grade 4. At 20 weeks of follow-up, paraparesis and bladder symptoms persisted.
Figure 1.
Sagittal T2-weighted images of the spine (A, B, and C) show multiple foci of T2 hyperintensities in the cervical and thoracic cord. Axial T2-weighted images at cervical and thoracic spine levels with insets (D, E, and F) shows these punctate hyperintense foci.
Figure 2.
Turbo inversion recovery magnitude (TIRM) images of muscle magnetic resonance images. (A–E) TIRM hyperintense signals in posterior paraspinal group of muscles, gluteal muscles as well as muscles of pelvis (obturator internus and externus), thigh adductors, trapezius, and supraspinatus muscles of the shoulder girdle. Axial images at calf levels (F) show TIRM hyperintense signals in gastrocnemius and soleus of bilateral calf. Fused TIRM whole body image (G) shows signal abnormalities involving multiple muscle groups of the shoulder, back, hip, pelvis, and thigh.
DISCUSSION
India is hyperendemic for Chikungunya infection. Fever and arthralgia are common presenting symptoms of Chikungunya fever.2 Our patient had high-grade fever with severe persistent arthralgia, highly suggestive of Chikungunya fever.
Neurological manifestations have been described in Chikungunya viral infections, with the most common being encephalopathy or encephalitis.4 However, disease affecting the peripheral nervous system or multiple components of the nervous system is also known to occur. Myeloneuropathy has been reported in Chikungunya viral infections, with a proposed immune-mediated basis.5 Our patient had multiaxial involvement of the nervous system in the form of myeloneuropathy occurring 6 weeks after an episode of high-grade fever, suggesting a postinfectious immune-mediated etiology. In a reported series of CHIKV myelopathy/myeloneuropathy comprising 14 cases, symptoms of myelopathy occurred within 20 days of CHIKV infection. In comparison, our case developed neurological symptoms 6 weeks after the infection. This temporal pattern is somewhat unusual because most of the cases of post-Chikungunya myelitis have been reported between 0 and 3 weeks of primary infection.
The pathogenesis of postviral myeloneuropathy is unclear; however, immune-mediated mechanisms have been proposed.6 These include molecular mimicry, polyclonal T/B-cell activation, and immune complex deposition.6
Imaging findings in postviral myelitis are varied and nonspecific. In some cases, the site and characteristic of the lesions and the presence of associated nerve root or leptomeningeal enhancement may give a clue to the etiology as in case of varicella zoster infection with dorsal root entry zone hyperintensity and enhancement and hyperintensity of anterior horn cells as in Coxsackie virus infections.6 Myelitis related to CHIKV infection is seldom reported.3,7–10 Myelopathy as an isolated neurological presentation occurs infrequently (∼20%).4,10 A few prior reports of diffuse, T2 hyperintense lesions involving the cord in the entirety with no significant postcontrast enhancement exist.11,12 A study of neurological complications of Chikungunya during the 2016 outbreak in Delhi showed that myelitis was a rare manifestation, seen only in one patient out of 42 cases, and MRI spine showed T2/fluid-attenuated inversion recovery hyperintensities suggestive of long-segment myelitis in the lumbar region.12 In a large series of 49 patients with CHIKV infections, myelopathy/myeloneuropathy occurred in 7 (14.6%) of the cases, and only three out of those had MRI changes suggestive of demyelination in the cord.3 Another case of myeloradiculopathy with positive CHIKV serology had left lower limb weakness with a corresponding enhancing lesion at T12 level in the spinal cord.5 Our case although similar in signal characteristics, was unique in the topographical distribution of the lesions, wherein they occurred as short segments with a tendency to appear confluent at certain locations. It is conceivable that such an imaging phenotype may represent an earlier time point in the natural course of the disease evolution.
A case of Chikungunya myelitis and myositis has been reported with MRI showing T2 hyperintensities extending from cervicomedullary junction to C6 level.13 The index case thus happens to be the second report of coexistent CHIKV-related myelopathy and muscle signal changes.
The tropism of CHIKV to fibroblasts, explaining its propensity to involve the joints, muscles, connective tissue has been documented earlier,11 which may in part offer a probable explanation to the imaging findings. Although the possibility of muscle changes secondary to radiculoneuropathy cannot be excluded, no distinctive muscle volume loss was seen. Thus, our case possibly had diffuse myositis, which is well correlated with the muscle pains and tenderness. Brain imaging was normal. This differs from previous reports of CNS involvement with brain MRI changes in CHIKV myeloradiculopathy described.
The causes of long-segment myelitis are diverse and include inflammatory, postinfectious, autoimmune, and paraneoplastic etiologies.14 Neuromyelitis optica and MOG-associated myelitis are important differentials in longitudinally extensive transverse myelitis. Lesions involving the entire spinal cord extent as in this case are known to occur in both NMO and MOG.15 In our case, workup for other causes such as autoimmune and paraneoplastic profile, myositis profile, serum and CSF NMO-MOG antibody, Brucella polymerase chain reaction, and HIV testing were negative. The mild chronic axonopathy seen on nerve biopsy may be due to post Chikungunya peripheral neuropathy. In a review of 856 cases chikungunya-related neurological illness, combined involvement of the CNS and PNS as in our case was seen in 9% of the cases.4 Almost half the patients identified with myelopathy had evidence of radiculopathy and neuropathy in a large series of 90 patients.4
Corticosteroids are the mainstay of treatment in postinfectious myelitis due to the underlying immune-mediated pathogenesis. We treated the patient with intravenous methylprednisolone followed by five cycles of plasmapheresis and oral steroids. Incomplete recovery of our patient may have been due to the extensive nature of spinal cord and muscle involvement evident on neuroimaging.
In conclusion, myeloneuropathy and myositis are rare complications of Chikungunya infection that warrant prompt identification and treatment with immunomodulation. The extensive but patchy spinal cord MRI findings observed in our case has not been reported earlier and are noteworthy. Clinical improvement may be delayed despite early institution of therapy for postinfective complications, and response is often incomplete.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. Furuya-Kanamori L, Liang S, Milinovich G. et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abhishek K, Chakravarti A, 2019. Simultaneous detection of IgM antibodies against dengue and chikungunya: coinfection or cross-reactivity? J Family Med Prim Care 8: 2420–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, Purohit HJ, Taori GM, Daginawala HF, 2009. Neurological complications of chikungunya virus infection. Neurol India 57: 177–180. [DOI] [PubMed] [Google Scholar]
- 4. Mehta R, Gerardin P, de Brito CAA, Soares CN, Ferreira MLB, Solomon T, 2018. The neurological complications of chikungunya virus: a systematic review. Rev Med Virol 28: e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bank AM, Batra A, Colorado RA, Lyons JL, 2016. Myeloradiculopathy associated with chikungunya virus infection. J Neurovirol 22: 125–128. [DOI] [PubMed] [Google Scholar]
- 6. Goh C, Desmond PM, Phal PM, 2014. MRI in transverse myelitis. J Magn Reson Imaging 40: 1267–1279. [DOI] [PubMed] [Google Scholar]
- 7. Hameed S, Memon M, Imtiaz H, Kanwar D, 2019. Longitudinally extensive transverse myelitis with seropositive chikungunya. BMJ Case Rep 12: e231745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khatri H, Shah H, Roy D, Tripathi KM, 2018. A case report on chikungunya virus-associated encephalomyelitis. Case Rep Infect Dis 2018: 8904753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinheiro TJ, Guimarães LF, Silva MT, Soares CN, 2016. Neurological manifestations of Chikungunya and Zika infections. Arq Neuropsiquiatr 74: 937–943. [DOI] [PubMed] [Google Scholar]
- 10. Jain RS, Khan I, Saini PK, 2017. Longitudinally extensive transverse myelitis caused by chikungunya virus. Indian J Med Specialities 8: 48–50. [Google Scholar]
- 11. Murthy JMK, 2009. Chikungunya virus: the neurology. Neurol India 57: 113–115. [DOI] [PubMed] [Google Scholar]
- 12. Anand KS, Agrawal AK, Garg J, Dhamija RK, Mahajan RK, 2019. Spectrum of neurological complications in chikungunya fever: experience at a tertiary care centre and review of literature. Trop Doct 49: 79–84. [DOI] [PubMed] [Google Scholar]
- 13. Choudhary N, Makhija P, Puri V, Khwaja GA, Duggal A, 2016. An unusual case of myelitis with myositis. J Clin Diagn Res 10: OD19–OD20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frohman EM, Wingerchuk DM, 2010. Transverse myelitis. N Engl J Med 363: 564–572. [DOI] [PubMed] [Google Scholar]
- 15. Denève M. et al. , 2019. MRI features of demyelinating disease associated with anti-MOG antibodies in adults. J Neuroradiol 46: 312–318. [DOI] [PubMed] [Google Scholar]