ABSTRACT.
The American Zika virus (ZIKV) epidemic has highlighted the need to gain a better understanding of this emerging virus. The goal of this study was to describe the clinical symptoms, laboratory findings, and risk factors for symptomatic ZIKV infection in an area with ongoing transmission of other arboviral infections. We recruited patients at least 2 years of age seeking care at public health centers in León, Nicaragua, between January 2016 and August 2017, for fever, maculopapular rash, and/or nonsuppurative conjunctivitis with a duration of less than 1 week. A laboratory diagnosis of ZIKV was established using a combination of molecular and serological tests. Clinical and laboratory findings and potential risk factors were compared between participants with and without acute ZIKV infection. Fifty-eight (26%) of the 225 participants included in the analysis were found to have acute ZIKV infection. Pregnancy and reports of previous arboviral infection were associated with a higher risk of ZIKV infection. Rash, conjunctivitis, sore throat, and lower absolute neutrophil counts were associated with acute ZIKV infection. The clinical characteristics and risk factors identified were consistent with those identified by previous studies; however, we found sore throat to be a feature of ZIKV infection. We also found that neutrophil counts were lower in ZIKV-infected subjects. These clinical symptoms and laboratory data may help clinicians suspect ZIKV infection during future outbreaks.
INTRODUCTION
The emergence of Zika virus (ZIKV) has created new challenges for clinicians and public health officials, especially in regions with autochthonous transmission. During the outbreak on Yap Island in 2007, only 20% of infections were symptomatic; furthermore, among symptomatic individuals, the disease was generally mild, with rash, fever, arthralgia, and conjunctivitis as the most commonly reported symptoms.1 The American ZIKV epidemic of 2015 to 2017 highlighted the unusual ability of this mosquito-borne flavivirus to be transmitted congenitally and its potential to cause a constellation of anomalies in the developing fetus that may profoundly affect child development.2 Additionally, ZIKV can cause rare, but sometimes severe, neurological complications such as Guillain-Barre syndrome.3 The rare but severe clinical outcomes of ZIKV infection highlight the need to efficiently identify ZIKV infections at the individual level and population level to contain its spread.
An additional challenge is that ZIKV often circulates alongside other arboviruses, such as dengue virus (DENV), which is another flavivirus, and chikungunya virus. These viruses are transmitted by the same mosquito vector (Aedes aegypti) and have clinical symptoms that overlap those of ZIKV infection. Clinicians need to gain a better understanding of the unique clinical features of ZIKV infection in this setting. Few studies have examined the parameters of routine laboratory tests of acute ZIKV infection4–7; however, knowledge of these parameters could further aid clinicians in making appropriate diagnoses.
The identification of patient-level risk factors and behaviors affecting the risk for ZIKV infection can inform public health efforts to reduce disease burden. A seroprevalence study in Nicaragua found that women and older individuals were at increased risk for ZIKV infection.8 Because ZIKV is primarily transmitted by mosquitoes, it is assumed that individuals with increased exposure to the vector are at increased risk. For example, one household study found that those who store water in their households, thus providing breeding sites for mosquitoes, were at increased risk for ZIKV infection.9 However, documented sexual transmission and higher risk for women suggest that ZIKV may have some risk factors distinct from those of other arboviruses. There is limited knowledge of other risk factors for ZIKV disease, especially modifiable risk factors.
The objective of this study was to describe the clinical symptoms, laboratory findings, and risk factors for symptomatic ZIKV infection in an area with ongoing transmission of other arboviral infections. Better characterization of the clinical features and risk factors for ZIKV infection may assist clinicians in recognizing ZIKV infection, pursue appropriate diagnostic testing and clinical management, and guide public health efforts to prevent ZIKV burden.
METHODS
Study design and population.
We conducted an observational study in León, Nicaragua, between January 2016 and August 2017. Patients two years and older seeking care at any of the three Sistema Local de Atención Integral a la Salud-León (SILAIS-León) Health Centers for subjective fever, maculopapular rash, and/or nonsuppurative conjunctivitis with a duration less than 1 week were invited to participate in the study.
Clinical and demographic data collection.
At enrollment, all subjects were administered a structured questionnaire to collect data about demographics, medical history, clinical symptoms, vector avoidance behaviors, and other potential risk factors for ZIKV infection. Temperature data were obtained from the Health Center records.
Sample collection.
At enrollment, blood, saliva, and urine were collected from each participant. For the first 101 subjects, samples were collected only at the time of enrollment, blood types were not tested, and participant temperatures were not recorded. Subsequently, the study protocol was changed to include the collection of convalescent serum 2 to 4 weeks after enrollment, blood type testing, and documentation of participant temperatures.
Definition of acute ZIKV infection.
The laboratory diagnosis of ZIKV was performed using a combination of molecular and serological tests. Participants were considered to have an acute ZIKV infection if ZIKV RNA was detected in the blood, urine, or saliva, or if their serological assay results were consistent with acute ZIKV infection (Supplemental Table S1). All others were considered noncases. Flaviviral IgM and IgG ELISAs were performed for acute and convalescent samples to distinguish acute primary and secondary infections, recent infections, past infections, and flavivirus-naïve status (Supplemental Table S1). If a participant did not provide a convalescent serum sample, then the ZIKV infection status was classified using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) results alone. Because of the potential for false-positive results for the molecular diagnosis of ZIKV,10 we followed the approach of a prior study and performed triplicate qRT-PCR testing to define acute ZIKV infections.11 All positive qRT-PCR samples at baseline were repeated in triplicate; if all three replicates were positive (cycle threshold < 38), then the sample was considered a true positive, and these subjects were considered acute ZIKV cases.
Molecular detection of ZIKV.
Viral RNA was extracted from whole blood, urine, or saliva using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). qRT-PCR was performed first for RNA extracted from blood using published primers and methods previously described by the United States CDC.12 If a participant’s blood sample was negative for ZIKV according to qRT-PCR, then their urine and saliva samples were tested (screening samples). If any screening sample was positive, then all three body fluids, when available, were tested in triplicate as described to eliminate false-positive results. A positive test result in triplicate for any of these three body fluids was considered ZIKV PCR-positive.
Measurement of IgG binding to ZIKV/DENV by ELISA.
Because of the known cross-reactivity in the humoral response between ZIKV and DENV,13 we simultaneously tested for antibody binding to both flaviviruses. ZIKV or DENV virions were captured by a fusion loop-specific anti-E mouse mAb (4G2) blocked with 3% nonfat dry milk (weight/volume) in Tris-buffered saline containing 0.05% (volume/volume) Tween 20 (3% milk blocking buffer). Human plasma or serum was diluted to 1:100 and 1:1,000 in 3% milk blocking buffer and incubated at 37°C for 1 hour. Bound ZIKV/DENV-specific serum antibodies were detected with an alkaline phosphatase-conjugated goat anti-human IgG Ab and p-nitrophenyl phosphate substrate. Absorbance at 405 nm for each of the duplicate samples was measured using Epoch plate reader systems (Biotek Instruments, Winooski, VT). The mean antibody binding signal of each serum to ZIKV or DENV was calculated by subtracting the mean background signal from wells incubated with pooled normal human serum. A mean absorbance value ≥ 0.2 was considered positive, and a value < 0.2 was considered negative.
Measurement of IgM binding to ZIKV or DENV by MAC ELISA.
The assay was performed as described in the CDC MAC ELISA instructions with modifications, including diluting samples at 1:40 using ZIKV or DENV1-4 mix from the C6/36 cell culture supernatant as an antigen, and washing the plates three times between each step. Briefly, a 96-well high-binding plate was coated with 75 μL of 1:50 diluted goat anti-human IgM in 0.1 M carbonate buffer at 4°C overnight. The plate was washed and then blocked with 200 μL of 5% nonfat dairy milk in 1× phosphate-buffered saline with 0.5% Tween (5% milk blocking buffer) for 30 minutes at room temperature. Serum or plasma at 1:40 diluted in 5% milk blocking buffer was incubated at 37°C for 1 hour. After washing, 50 μL of ZIKV or DENV antigen from C6/36 cell culture supernatant was added and incubated at 4°C overnight. Bound antigen was detected with horseradish peroxidase-conjugated 6B6C-1 mAb and enhanced K-Blue TMB substrate. Absorbance at 450 nm for each duplicate sample was measured using Epoch plate reader systems (Biotek Instruments, Winooski, VT). A positive/negative (P/N) ratio was calculated as follows: P/N = mean optical density (OD) of a sample reacted with antigen/mean OD of normal human serum reacted with antigen. A P/N value ≥ 3 was considered positive and a value < 3 was considered negative.
Statistical analysis.
Clinical and laboratory findings and risk factors for ZIKV infection were compared between participants with and without evidence of acute ZIKV infection using the χ2 test or Fisher’s exact test (for cells with < 5 observations) for categorical data, and using Student’s t test, the Kruskal-Wallis test, or the rank-sum test for continuous data. Multivariable logistic regression models were used to examine associations between clinical findings and risk factors. We fit one model using only clinical symptoms as covariates and one with both symptoms and laboratory variables as covariates. Variables were included in each model if they had a univariate P < 0.1; then, they were removed in a backwards stepwise fashion until all remaining variables had an adjusted P < 0.1. Models were validated, and the area under the receiver-operating characteristic curve was estimated using 10-fold repeated cross-validation. Missing data were handled using multiple imputation with chained equations and 20 imputations; this was nested within the cross-validation to prevent overestimation of the area under the receiver-operating characteristic curve.14 Statistical analyses were performed using SAS version 9.3 (SAS Corporation, Cary, NC), Stata version 12 (StataCorp LP, College Station, TX), and R (www.r-project.org).
RESULTS
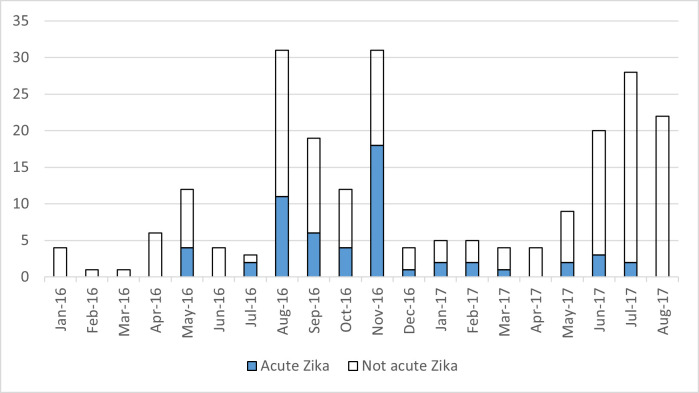
Between January 2016 and August 2017, 225 participants presenting with a history of fever, rash, and/or nonsuppurative conjunctivitis for less than 1 week were enrolled in the study. Six subjects who did not undergo qRT-PCR testing were categorized based on serological data. The first 101 participants did not have paired acute and convalescent samples for serological testing according to the initial protocol, and 26 of the subsequent participants did not return for convalescent sample collection. Participants without paired acute and convalescent samples were categorized by qRT-PCR test results alone (Supplemental Tables S1, S2, and S3). An additional 23 participants were enrolled but excluded from analyses because of missing or ambiguous serological and qRT-PCR test results. Most cases occurred during the second half of 2016 (Figure 1), corresponding to the reported peak of the ZIKV epidemic in Nicaragua.8,15 A second wave of enrollment occurred in 2017, which was the usual seasonal timing of arbovirus transmission. No acute ZIKV cases were detected after July 2017.
Figure 1.
Enrollment and proportion of Zika virus infections during the study period. This figure appears in color at www.ajtmh.org.
Among the 225 subjects included in the analysis, the mean age was 26.5 years (range, 1–80 years; SD, 17.4), and 146 (65%) were female. Fifty-eight subjects (26%) had acute ZIKV infection according to qRT-PCR and/or serology results. Thirty-eight subjects had ZIKV confirmed by positive qRT-PCR results, 44 had ZIKV confirmed by serology, and 24 had ZIKV confirmed by both types of testing. ZIKV-infected subjects were younger by a mean of 3 years, but this difference was not statistically significant (Table 1). The nine pregnant women enrolled in the study had an 11-times higher risk of ZIKV infection than nonpregnant women of childbearing age (Table 1).
Table 1.
Demographics of patients with and without acute Zika virus infection
| Acute ZIKV infection N = 58 |
No acute ZIKV infection N = 167 |
OR (95% CI) or P value |
|
|---|---|---|---|
| Age, years, mean (SD) | 24.5 (15.6) | 27.3 (18.0) | 0.26 |
| Younger than 18 years, n (%) | 21/58 (36%) | 56/161 (35%) | 1.07 (0.56–1.99) |
| Female, n (%) | 39/58 (67%) | 107/167 (64%) | 1.15 (0.61–2.20) |
| Pregnant, n (% of females 12–49 years) | 7/27 (26%) | 2/69 (3%) | 11.35 (1.96–1.21) |
| Education, n (%) | 0.72 | ||
| Elementary school or less | 10/32 (31%) | 36/98 (37%) | |
| Middle or high school | 7/32 (22%) | 24/98 (24%) | |
| Technical school/college | 15/32 (47%) | 38/98 (39%) | |
| Unknown/missing | 26 | 69 | |
| Occupation, n (%) | 0.79 | ||
| Student | 17/32 (53%) | 47/101 (47%) | |
| Teacher/healthcare worker | 2/32 (6%) | 9/101 (9%) | |
| Housewife | 6/32 (19%) | 13/101 (13%) | |
| Street vendor | 0 | 1/101 (1%) | |
| Factory worker | 0 | 3/101 (3%) | |
| Farm worker | 0 | 1/101 (1%) | |
| Business/sales | 2/32 (6%) | 3/101 (3%) | |
| Other | 2/32 (6%) | 16/101 (16%) | |
| Unemployed | 0 | 0 | |
| Not applicable/young child | 3/32 (9%) | 8/101 (8%) | |
| Unknown/missing | 26 | 66 |
OR = odds ratio; ZIKV = Zika virus.
Clinical characteristics are described in Table 2. Only 20% of participants with acute ZIKV infection had a measured temperature > 38°C at enrollment, but 86% reported fever during the previous 7 days. Reported fever was less common for participants with acute ZIKV infection than for noncases. Self-reported rash, red eyes, and sore throat were more commonly associated with acute ZIKV infection. ZIKV-infected participants had significantly lower neutrophil counts; the mean absolute neutrophil count was 3,480 for ZIKV-infected participants compared with 4,800 for noninfected participants. However, this difference is probably not clinically important because the majority of all participants had neutrophil counts in the normal range. Although medical comorbidities were associated with a higher risk of acute ZIKV infection, they were uncommon; this relationship did not reach statistical significance. Blood type was not associated with acute ZIKV infection.
Table 2.
Clinical characteristics and laboratory data of patients with and without acute Zika virus infection
| Acute ZIKV infection |
No acute ZIKV infection |
aOR (95% CI) Model 1 |
aOR (95% CI) Model 2 |
||
|---|---|---|---|---|---|
| N = 58 | N = 167 | OR (95% CI) or P value |
N = 225 | N = 225 | |
| Temperature > 38°C | 3/15 (20%) | 25/73 (34%) | 0.48 (0.08–2.03) | ||
| Missing* | 43 | 94 | |||
| White blood cell count/μL, mean (SD) | 6120 (2,680) | 7,600 (3,840) | 0.002 | ||
| ALC, mean (SD) | 2320 (1,040) | 2,470 (1,290) | 0.41 | ||
| ANC,† mean (SD) | 3480 (2,350) | 4,800 (3,370) | 0.001 | 0.87 (0.75–1.01) | |
| ANC < 1,500, mean (SD) | 5/57 (9%) | 7/162 (4%) | 2.14 (0.59–7.15) | ||
| Mean hemoglobin, g/mL (SD) | 13.5 (2.3) | 13.2 (1.5) | 0.42 | ||
| Mean platelet count/μL (SD) | 257,000 (80,100) | 283,000 (87,400) | 0.06 | ||
| Platelets < 150,000 | 0/50 (0%) | 3/123 (2%) | 0 (0–5.98) | ||
| Self-reported symptoms during the past 7 days | |||||
| Fever | 44/56 (79%) | 149/162 (92%) | 0.32 (0.14–0.77) | ||
| Rash | 50/57 (88%) | 106/162 (65%) | 3.69 (1.65–9.50) | 2.88 (1.20–6.91) | 2.55 (1.04–6.23) |
| Red eyes | 42/56 (75%) | 79/162 (49%) | 3.12 (1.61–6.35) | 2.25 (1.11–4.57) | 2.10 (1.01–4.34) |
| Headache | 40/57 (70%) | 113/161 (70%) | 1.00 (0.52–1.97) | ||
| Retro-orbital pain | 29/57 (51%) | 85/163 (52%) | 0.95 (0.52–1.75) | ||
| Nausea/vomiting | 25/57 (44%) | 59/163 (36%) | 1.38 (0.74–2.55) | ||
| Abdominal pain | 19/57 (33%) | 47/163 (29%) | 1.24 (0.64–2.35) | ||
| Sore throat | 32/57 (56%) | 48/162 (30%) | 3.02 (1.62–5.69) | 2.38 (1.24–4.58) | 2.37 (1.22–4.62) |
| Lethargy | 28/57 (49%) | 78/161 (48%) | 1.03 (0.56–1.89) | ||
| Myalgias | 28/57 (49%) | 81/163 (50%) | 0.98 (0.53–1.79) | ||
| Arthralgias | 37/57 (65%) | 93/163 (57%) | 1.39 (0.75–2.64) | ||
| Bruising or petechiae | 20/57 (35%) | 46/160 (29%) | 1.34 (0.69–2.54) | ||
| Missing | 1–2 | 4–7 | |||
| Blood type | 0.34 | ||||
| A | 5/31 (16%) | 24/96 (25%) | |||
| B | 4/31 (13%) | 6/96 (6%) | |||
| AB | 0 | 0 | |||
| O | 22/31 (71%) | 66/96 (69%) | |||
| Missing* | 27 | 71 | |||
| Self-reported history of arbovirus diagnosis, n (%) | |||||
| Any arbovirus | 23/51 (45%) | 32/144 (22%) | 2.86 (1.44–5.67) | ||
| Dengue | 16/49 (33%) | 21/141 (15%) | 2.76 (1.28–5.90) | ||
| Missing | 1–3 | 23–26 | |||
| Comorbid chronic illness | |||||
| Diabetes | 4/50 (8%) | 4/143 (3%) | 3.00 (0.54–16.8) | ||
| Cancer | 0 | 4/144 (3%) | 0 (0–4.38) | ||
| Kidney disease | 4/49 (8%) | 2/143 (1%) | 6.19 (0.86–70.7) | ||
| Neurological disease | 2/50 (4%) | 2/144 (1%) | 2.94 (0.21–41.5) | ||
| Missing | 1–3 | 23–24 | |||
ALC = absolute leukocyte count; ANC = absolute neutrophil count; aOR = adjusted odds ratio; OR = odds ratio; ZIKV = Zika virus.
As mentioned in the Methods section, the initial study protocol did not include documentation of temperature.
Included in Model 2 in units of 1,000 cells per μL.
We constructed multivariable predictive models to distinguish patients acutely infected with ZIKV from those not infected with ZIKV. Model 1 incorporated clinical signs and symptoms that would be readily available to a clinician; it showed that rash, red eyes, and sore throat were independently associated with an increased risk of ZIKV (Table 2). Model 2 incorporated both clinical symptoms and laboratory data; it showed that each 1,000-cell/μL decrease in the neutrophil count was associated with a 15% higher risk of ZIKV infection (Table 2).
No vector control activities were associated with the risk of ZIKV infection (Table 3). Participants with acute ZIKV infection had a 2.9-times greater chance of reporting a previous arboviral infection (Table 2). Among adults, recent sexual contact was not a risk factor for ZIKV infection. No men who had sex with men (N = 4) had acute ZIKV infection.
Table 3.
Health behaviors of patients with and without acute Zika virus infection
| Acute ZIKV infection | No acute ZIKV infection | OR (95% CI) | |
|---|---|---|---|
| N = 32 | N = 102 | ||
| Currently has mosquito bites | 15/28 (54%) | 53/102 (52%) | 1.06 (0.46–2.51) |
| Stored water during the past month | 23/32 (72%) | 62/102 (61%) | 1.63 (0.70–4.09) |
| Uses air conditioner | 3/32 (9%) | 4/101 (4%) | 2.49 (0.34–15.6) |
| Uses fan | 30/32 (94%) | 91/101 (90%) | 1.64 (0.32–16.3) |
| Screens on all windows | 1/32 (3%) | 5/99 (5%) | 0.61 (0.01–5.75) |
| Used bed net during the past month | 3/32 (9%) | 10/101 (10%) | 0.94 (0.16–4.00) |
| Used insect repellent during the past month | 7/32 (22%) | 14/101 (14%) | 1.75 (0.60–4.75) |
| Used insect repellent more than once per week | 7/32 (22%) | 11/98 (11%) | 2.21 (0.73–6.32) |
| MOH sprayed outside of home | 26/32 (81%) | 86/101 (85%) | 0.75 (0.27–2.32) |
| MOH sprayed inside of home | 29/31 (94%) | 86/101 (85%) | 2.51 (0.53–24.0) |
| MOH applied larvicide around home | 28/30 (93%) | 98/102 (96%) | 0.57 (0.08–6.66) |
| Resident applied insecticide or repellent inside home during the past month | 14/32 (44%) | 33/102 (32%) | 1.62 (0.71–3.68) |
| Keeps animals | 22/31 (71%) | 74/102 (73%) | 0.92 (0.38–2.35) |
| Had sex with a man during the past 3 months (females older than 11 years) | 9/16 (56%) | 16/43 (37%) | 2.13 (0.66–7.21) |
| Had sex with a woman during the past 3 months (males older than 11 years) | 3/5 (60%) | 11/17 (65%) | 0.83 (0.07–12.5) |
MOH = Ministry of Health; OR = odds ratio; ZIKV = Zika virus. Data represent a subset of the study because the first 101 participants were not administered this questionnaire.
DISCUSSION
We describe clinical and epidemiological features of acute ZIKV infections in a Nicaraguan population living where dengue is hyperendemic. The clinical features of acute ZIKV infection identified were similar to those reported for other cohorts,1,10,16,17,24–28 except we found that sore throat was associated with ZIKV infection. We also found that neutrophil counts were lower for ZIKV cases compared with noncases.
Acute ZIKV infection was generally associated with a mild disease course. Rash was the cardinal feature and was present in 88% of ZIKV-infected patients; it was highly predictive of ZIKV infection in the context of this epidemic. As in Singapore, conjunctivitis and lack of fever were associated with ZIKV rather than other etiologies. In contrast to the majority of studies conducted in the Americas, the Singapore study did not find a strong association between rash and ZIKV infection, demonstrating the need for context-specific data.4 We found that sore throat was predictive of ZIKV infection, as has been reported in some series6,7 but not in others.5,16 This finding may represent an under-recognized feature of the virus. We did not observe any neurological complications such as Guillain-Barre Syndrome in our cohort.
During our evaluation of laboratory results, the total white blood cell counts were slightly lower in ZIKV-infected subjects, although the majority of participants in both groups had white blood cell counts in the normal range. Leukopenia has been observed with both DENV and chikungunya virus infections.17 Interestingly, absolute neutrophil counts were significantly lower in ZIKV-infected subjects than in noncases (mean, 3,480 versus 4,800, respectively), suggesting that absolute neutrophil counts may be a more specific marker of ZIKV infection than total white blood cell counts. One study of dengue found that monocytes were elevated early during infection but that lymphocytes increased and neutrophils decreased during the course of infection.18 Platelet counts were also slightly lower in ZIKV-infected individuals than in noncases (mean, 257,000 versus 283,000, respectively); however, the platelet count did not remain an important predictor of acute ZIKV infection in multivariable models, and clinically significant thrombocytopenia occurred only in subjects without acute ZIKV infection. Thrombocytopenia has been well-characterized in dengue infections.19 Previous studies that compared laboratory features of ZIKV to those of other arbovirus infections found that normal platelet counts were predictive of ZIKV infection rather than DENV infection.4
During our evaluation of risk factors, pregnant women in our study were at high risk for ZIKV infection (odds ratio, 11.4). Lozier et al. showed that women were more likely to have a symptomatic ZIKV infection than men, but that their risk of asymptomatic infection was the same, suggesting possible sex-specific differences in viral pathogenesis.16 These findings likely reflect increased care-seeking behavior by pregnant women; however, other factors like pregnancy-associated immunosuppression and the transmissibility of ZIKV via sex may increase the risk in this population.20–22 Additionally, pregnant women can remain viremic for prolonged periods of time,23 which could increase the sensitivity of qRT-PCR for the diagnosis of acute infection.
We found no association among age, occupation, or educational level and risk of ZIKV infection, implying that exposure was uniform across the socioeconomic spectrum; this was supported by multiple studies that have shown that in the ZIKV-naïve populations of the Americas, large proportions of the population were infected during the epidemic. Another study performed in Nicaragua found an increasing risk of ZIKV infection with age8; this is in contrast to the high rate of DENV for children in endemic areas and highlights the epidemic nature of ZIKV transmission in this naïve population.
In general, vector avoidance and control measures were not strongly associated with ZIKV infection. Nicaraguan Ministry of Health vector control activities were reported by 80% to 90% of households in the study; therefore, homogenous coverage of the study area likely limited our ability to evaluate the effects of these activities at the individual level. It is possible that individuals living in areas with a high density of mosquitoes may be more likely to attempt vector control, which may obfuscate the relationship between vector control and Zika risk. Household water storage was not associated with an increased ZIKV risk, unlike in other studies.9,24 The ubiquity of Aedes vectors in tropical areas and their aggressive diurnal biting behavior are known to limit the effectiveness of vector control activities in many contexts,25 as was shown by our data.
Our conclusions are, to some extent, limited to the context of the American epidemic, but they are likely relevant to arbovirus-endemic areas and future ZIKV outbreaks. We acknowledge that some misclassification may have occurred for a few reasons. Serological diagnosis of ZIKV infection in the context of pre-existing DENV immunity or concurrent circulation of DENV can be complicated, but we used a rigorous approach to identify acute ZIKV infections (Supplemental Tables S1 and S3). We did not perform qRT-PCR testing for DENV in our cohort; however, the Nicaraguan Ministry of Health reported low rates of DENV during our study period, and this was corroborated by our serological studies (Supplemental Table S3). We did not have the complete diagnostic information of other pathogens, particularly bacterial causes of febrile syndromes. Other studies performed in Nicaragua have shown that a wide variety of pathogens can cause a nonspecific febrile syndrome that is easily confused with ZIKV, including leptospirosis and rickettsial infections.26–28 Additionally, qRT-PCR assays to confirm ZIKV were performed using samples that had undergone several freeze–thaw cycles, which may have compromised the sensitivity of the test. To avoid the potential for false-negative results caused by RNA instability or the presence of PCR inhibitors,10 we also considered participants with negative qRT-PCR results but positive serological conversion to have ZIKV infections, thus strengthening our laboratory diagnostic approach.
It remains unclear whether ZIKV will establish endemic human or zoonotic cycles in the tropical Americas.42,43 However, recent outbreaks in India29 and Singapore30 and epidemiological evidence of endemic ZIKV circulation in Africa,31–33 Southeast Asia,34 and the Pacific Islands35–37 indicate that diagnostic suspicion for ZIKV infection remains relevant. Future studies in a variety of epidemiological settings, particularly endemic areas where ZIKV circulates at lower levels, need to identify features predictive of ZIKV in these contexts.
Supplemental Tables
ACKNOWLEDGMENTS
We thank the study nurses who contributed to this study, including Xiomara Obando and Josseling Delgado. We thank the Health Center Staff, including Dr. Candidad Chavez, Carlos Adan Mairena, Jessenia Caballero, and Rosa Virginia. Most importantly, we thank the people who participated in this study.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Duffy MR. et al. , 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360: 2536–2543. [DOI] [PubMed] [Google Scholar]
- 2. Rice ME, 2018. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection—U.S. Territories and Freely Associated States, 2018. MMWR Morb Mortal Wkly Rep 67: 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. da Silva IRF, Frontera JA, Bispo de Filippis AM, Nascimento do OJM, RIO-GBS-ZIKV Research Group , 2017. Neurologic complications associated with the Zika virus in Brazilian adults. JAMA Neurol 74: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan G. et al. , 2018. Distinguishing Zika and dengue viruses through simple clinical assessment, Singapore. Emerg Infect Dis 24: 1565–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombo TE. et al. , 2017. Clinical, laboratory and virological data from suspected ZIKV patients in an endemic arbovirus area. J Clin Virol Off Publ Pan Am Soc Clin Virol. 96: 20–25. [DOI] [PubMed] [Google Scholar]
- 6. Brasil P. et al. , 2016. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis 10: e0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braga JU. et al. , 2017. Accuracy of Zika virus disease case definition during simultaneous dengue and Chikungunya epidemics. PLoS One 12: e0179725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zambrana JV. et al. , 2018. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc Natl Acad Sci USA 115: 9294–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burger-Calderon R. et al. , 2018. Zika virus infection in Nicaraguan households. PLoS Negl Trop Dis 12: e0006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Passos SRL. et al. , 2017. Detection of Zika Virus in April 2013 patient samples, Rio de Janeiro, Brazil. Emerg Infect Dis 23: 2120–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paz-Bailey G. et al. , 2018. Persistence of Zika virus in body fluids - final report. N Engl J Med 379: 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanciotti RS. et al. , 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrade P. et al. 2019. Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat Commun 10: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wahl S, Boulesteix A-L, Zierer A, Thorand B, van de Wiel MA, 2016. Assessment of predictive performance in incomplete data by combining internal validation and multiple imputation. BMC Med Res Methodol 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon A. et al. , 2019. Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. PLoS Med 16: e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lozier MJ. et al. , 2016. Differences in prevalence of symptomatic Zika virus infection, by age and sex-Puerto Rico. J Infect Dis 217: 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomashek KM. et al. , 2017. Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012–2015. PLoS Negl Trop Dis 11: e0005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaloemwong J. et al. , 2018. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: a retrospective study. BMC Hematol 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogt MB, Lahon A, Arya RP, Spencer Clinton JL, Rico-Hesse R, 2019. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl Trop Dis 13: e0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hills SL. et al. , 2016. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 65: 215–216. [DOI] [PubMed] [Google Scholar]
- 21. Turmel JM. et al. , 2016. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 387: 2501. [DOI] [PubMed] [Google Scholar]
- 22.D’Ortenzio E. et al. , 2016. Evidence of sexual transmission of Zika virus. Available at: https://www.nejm.org/doi/10.1056/NEJMc1604449?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov. Accessed November 1, 2019.
- 23. Lozier MJ. et al. , 2018. Prolonged detection of Zika virus nucleic acid among symptomatic pregnant women: a cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am 67: 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Netto EM. et al. , 2017. High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. MBio 8: e01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell JR, Tabachnick WJ, 2013. History of domestication and spread of Aedes aegypti–a review. Mem Inst Oswaldo Cruz 108 ( Suppl 1 ): 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reller ME. et al. , 2016. First identification and description of rickettsioses and Q fever as causes of acute febrile illness in Nicaragua. PLoS Negl Trop Dis 10: e0005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chikeka I, Matute AJ, Dumler JS, Woods CW, Mayorga O, Reller ME, 2016. Use of peptide-based enzyme-linked immunosorbent assay followed by immunofluorescence assay to document Ehrlichia chaffeensis as a cause of febrile illness in Nicaragua. J Clin Microbiol 54: 1581–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reller ME. et al. , 2014. Unsuspected leptospirosis is a cause of acute febrile illness in Nicaragua. PLoS Negl Trop Dis 8: e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yadav PD. et al. , 2019. Zika virus outbreak in Rajasthan, India in 2018 was caused by a virus endemic to Asia. Infect Genet Evol 69: 199–202. [DOI] [PubMed] [Google Scholar]
- 30. Sadarangani SP, Hsu LY, 2016. The 2016 outbreak of Zika in Singapore. Ann Acad Med Singap 45: 381–382. [PubMed] [Google Scholar]
- 31. Ankrah GA, Bonney JHK, Agbosu EE, Pratt D, Adiku TK, 2019. Serological evidence of Zika virus infection in febrile patients at Greater Accra Regional Hospital, Accra Ghana. BMC Res Notes 12: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera BB. et al. , 2017. Continued transmission of Zika virus in humans in West Africa, 1992–2016. J Infect Dis 215: 1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willcox AC. et al. , 2018. Seroepidemiology of dengue, Zika, and yellow fever viruses among children in the Democratic Republic of the Congo. Am J Trop Med Hyg 99: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruchusatsawat K. et al. , 2019. Long-term circulation of Zika virus in Thailand: an observational study . Lancet Infect Dis 19: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kama M. et al. , 2019. Sustained low-level transmission of Zika and Chikungunya viruses after emergence in the Fiji Islands. Emerg Infect Dis 25: 1535–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woon YL. et al. , 2019. Zika virus infection in Malaysia: an epidemiological, clinical and virological analysis. BMC Infect Dis 19: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasmono RT. et al. , 2018. Zika virus seropositivity in 1–4-year-old children, Indonesia, 2014. Emerg Infect Dis 24: 1740–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.