ABSTRACT.
Canine visceral leishmaniasis (CVL) is a serious zoonotic disease in Brazil and Southern Europe. CVL is primarily caused by Leishmania infantum and its diagnosis relies largely on detection of parasites in bone marrow or lymph node aspirates by microscopic observation of the parasites in stained smears, parasite culture, or polymerase chain reaction (PCR). Serological tests exist but they do not distinguish active disease from simple exposure to parasite antigens. Here, we have assessed the utility of a new monoclonal antibody––based antigen (protein) detection test for the diagnosis of CVL. The test was positive in 70% of beagle dogs experimentally infected with L. infantum. In contrast, culture of the parasites from bone marrow aspirates was positive in only 40% of the infected animals. These preliminary results suggest that this antigen detection test, which we have recently described for the diagnosis of human VL, has the potential to be a useful diagnostic tool for CVL.
Canine visceral leishmaniasis (CVL) is a serious zoonotic disease, with high prevalence in several countries in Southern Europe and South America.1 CVL is primarily caused by Leishmania infantum. In humans, the disease is known as kala-azar and is caused by either L. infantum or Leishmania donovani. Dogs infected with L. infantum constitute an important reservoir of the parasites.
Dogs treated with the available human drugs can present a rapid reduction in parasite burden during the therapy, which unfortunately and frequently recrudesces after interruption of treatment.2
Definitive diagnosis of active CVL in most endemic areas of the disease relies primarily on direct microscopic observation of leishmania parasites in smears or their culture from aspirate biopsies from either bone marrow (BM) or peripheral lymph nodes, which are invasive and risky procedures. In addition, the sensitivity of these tests is in general modest and varies enormously.3 Nucleic acid amplification tests are also existing tools for the diagnosis of CVL.4 Although these tests are in general more sensitive than microscopy/culture they are relatively complicated and expensive, and are restricted to advanced hospitals/research centers. The currently available serological diagnostic tests measure antibodies against parasitic antigens and have moderate accuracy for the diagnosis of CVL.5 Most of the existing antibody detecting tests target the same antigens for the diagnosis of human visceral leishmaniasis (VL) and CVL.
Interesting alternatives to these diagnostic procedures are platforms that detect pathogen antigens in bodily fluids, which by definition are excellent assays to diagnose active disease. These antigen detection tests have been successfully used for many years for the diagnosis of several human infectious diseases including hepatitis B,6 sore throat caused by Streptococcus pyogenes,7 pneumonia caused by either Streptococcus pneumoniae,8 or Legionella pneumophilla,9 tuberculosis,10 malaria,11 amoebiasis,12 and COVID-19.13 Unfortunately, to our knowledge there is not any described and commercially available antigen detection assays for the diagnosis of CVL.
Over the past years, we used mass spectrometry to identify six L. infantum/L. donovani proteins present in urine of human VL patients (Table 1). These defined markers were used for the development of an accurate, sensitive, and specific protein-based antigen detection assay for human VL.14–16 Our latest version of the antigen detection test is formatted as a multiplexed capture enzyme-linked immunosorbent assay (ELISA) that is assembled to simultaneously detect all six L. infantum/L. donovani biomarkers. The test was assembled using as capture reagent a pool of six different purified monoclonal antibodies (mAbs) specific for the six individual biomarkers. Similarly, the developing reagent consisted of a pool of six biotinylated purified mAbs specific for different epitopes than those that were recognized by the six mAbs included in the capture pool. The initial clinical validation of this new mAb-based multiplexed capture ELISA was carried out using 69 well-characterized urine samples from patients with VL and showed a sensitivity of ≥ 93%. The test was negative with all 65 control samples (35 from healthy control subjects and 30 from patients with confirmed non-VL tropical diseases).14
Table 1.
Leishmania infantum/donovani proteins found in the urine of human patients with visceral leishmaniasis
| Protein | Abbreviature | MW (kDa) | NCBI Accession |
|---|---|---|---|
| Iron superoxide dismutase | Li-isd1 | 21.53 | XP_001467866.1 |
| Tryparedoxin | Li-txn1 | 16.7 | XP_001466642.1 |
| Nuclear transport factor 2 | Li-ntf2 | 13.89 | XP_001463738.1 |
| MaoC family dehydratase | Ld-mao1 | 16.97 | XP_003858460.1 |
| Peptidyl-prolyl cis-trans isomerase | Ld-ppi1 | 12.62 | XP_003858557.1 |
| Malate dehydrogenase | Ld-mad1 | 33.28 | XP_003864180.1 |
MW = molecular weight; NCBI = National Center for Biotechnology Information.
Here, we report the preliminary experiments that aimed to assess the potential utility of this assay for the diagnosis of CVL. The experiments were performed using archived dog serum samples stored at −70°C. The sera were from a former study that used 20 beagle dogs experimentally infected with L. infantum.17 The option to test the utility of the multiplexed ELISA using canine sera instead of urine (as reported for humans) was based on a pragmatic reason, that is, in contrast to humans, collection of random urine specimens is not a routine procedure for dogs. However, the results of this pilot study show that the new antigen detection test that we described for the diagnosis of human VL using urine specimen, may also be an interesting new tool for the diagnosis of CVL if performed with the animal’s sera.
The 20 beagle dogs that were used in this study were previously housed at the animal facility from Cummings School of Veterinary Medicine at Tufts University, Grafton, MA. This former study aimed at the evaluation of a vaccine candidate for VL.17 The study followed all NIH guidelines for animal experimentation and was approved by the Institutional Animal Care and Use Committee at Tufts University (Protocol: #G2012-82, May 7, 2012). For these experiments, one group of 10 dogs was immunized three times with 50 µg of the leishmanial antigen nuclear transport factor 2 (Li-ntf2) emulsified in the adjuvant BpMPLA-SE (Institute Butantan, São Paulo, Brazil), which is an oil-in water emulsion containing monophosphoryl lipid A (MPLA) derived from Bordetella pertussis. A second group of 10 dogs was used as control. The latter animals were inoculated with phosphate-buffered saline only. At the end of the inoculations, all the animals in both vaccinated and control groups were inoculated intravenously with 107 live, virulent metacyclic promastigotes of L. infantum (MHOM/BR/00/1669), kindly supplied by Dr. Mary E. Wilson (University of Iowa, Iowa City, IA). Confirmation of infection was done by parasite culture in biphasic NNN medium using BM aspirate from anesthetized animals. Cultures were performed at 11 months after the challenge of the animals with L. infantum. BM samples were obtained by puncturing the trochanteric fossa of proximal femur and dispensed into citrated containing tubes. The NNN culture tubes were inoculated with 100 µl of the aspirates followed by incubation at 25–27°C for 2 weeks. A sample was considered as positive when parasites were observed by microscopic examination of liquid phase the NNN culture medium. In addition, all 20 dogs were bled before immunizations, before challenge, and at 11 months after the challenge with L. infantum. Sera were then prepared and stored frozen (at −70°C) till use.
We used these archived sera to assess the diagnostic utility for CVL of the antigen detection test that we have recently described for the diagnosis of human VL. To begin to assess the utility of this new test for the diagnosis of CVL, in addition to the frozen stored serum samples from the infected dogs we used as control frozen pools containing sera obtained from dogs bled previously to the immunization with Li-ntf2. Unfortunately, we no longer had stored individual serum samples that were collected before immunization of the animals. However, we had three frozen pools of serum from these animals. To compensate for this limitation, we used additional freshly collected sera from apparently healthy dogs and from animals with diseases different from CVL. These samples were obtained from the Foster Hospital for Small Animals, Cummings School of Veterinary Medicine at Tufts University, Grafton, MA. Specifically, these samples were categorized in the following groups: normal healthy animals (N = 11); dogs suspected of having infections other than CVL (N = 6) such as pneumonia caused by either mycoplasma or of undetermined etiology, Lyme disease, and gastroenteritis; dogs with neoplastic diseases (N = 16) including B cell lymphoma, multicentric T cell lymphoma, carcinomas, osteosarcoma, multiple myeloma and others; and finally, animals with metabolic diseases (N = 13), including immune-mediated hemolytic anemia, Cushing’s disease, diabetes mellitus, possible Addison’s disease, and others.
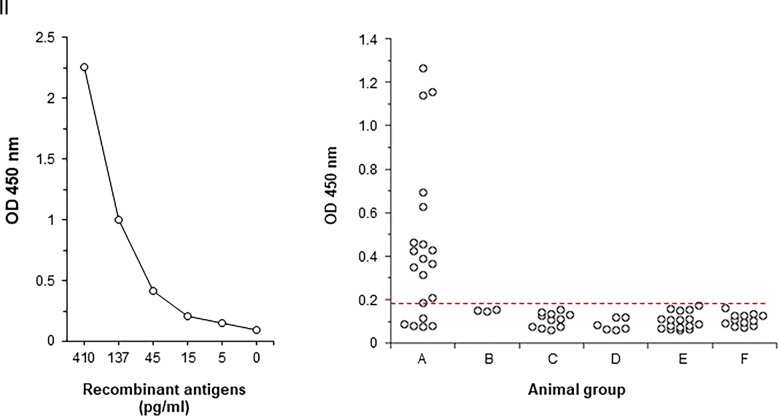
The results of this initial pilot study show that the multiplexed assay had a high biochemical sensitivity (∼15 pg/mL) and was positive for the detection of CVL in 14 out of the 20 infected animals (Figure 1A and B, respectively). These results are very promising in view of the fact that the multiplexed antigen detection test had a higher sensitivity (70%) than the expected 40% obtained with the gold standard diagnostic test (parasite culture) of CVL (Table 2). It is important to emphasize that these sensitivities were calculated under the theoretical assumption that all 20 infected animals developed CVL. Nonetheless, it is worth mentioning that out the eight animals positive for parasite culture seven were positive for the antigen detection test and one was borderline for the test (Table 2). Moreover, the antigen detection test was also positive in seven animals that were negative in the parasite culture assay. Because of its higher sensitivity, the antigen detection test (in contrast to parasite culture assay), has the potential to also diagnose CVL without clinical signs. Finally, the test was negative when performed with serum samples from dogs with diseases different from CVL (Figure 1). Therefore, these preliminary results also point to a high specificity of the test.
Figure 1.
Initial clinical validation of a multiplexed antigen detection assay for the diagnosis of canine visceral leishmaniasis (CVL) in dogs experimentally infected with Leishmania infantum. Validation was carried out by capture enzyme-linked immunosorbent assay (ELISA) assembled with a pool of affinity purified monoclonal antibodies (mAbs) specific for the biomarkers Li-isd1, Li-txn1, Li-ntf2, Ld-mao1, Ld-ppi1, and Ld-mad1. (I) Determination of the biochemical sensitivity of the assay using different concentrations of a pool of the six recombinant biomarkers. (II) Clinical sensitivity and specificity of the assay, which was performed using the following dog serum samples: (A), experimentally infected intravenously with 107 promastigote cells of L. infantum (N = 20); (B), three pools of serum samples from six different animals obtained before challenge; (C), randomly and freshly obtained normal serum samples (N = 11); (D), suspected of having infections other than CVL (N = 6); (E), neoplastic diseases (N = 16); and (F), metabolic diseases (N = 13). Sera from dogs of groups C, D, E, and F were from the Foster Hospital for Small Animals, Cummings School of Veterinary Medicine at Tufts University, Grafton, MA (see text for detailed description of these samples). Plates were washed and wells were incubated with a second pool containing purified biotinylated mAbs specific for the six leishmanial antigens. Wells were then incubated with streptavidin labeled peroxidase, the substrate H2O2, and the chromophore 3,3′,5,5′-tetramethylbenzidine. optical density (OD) was then read at 450 nm. The dashed red line represents the cut-off value (0.1573), which was calculated using the average of the OD obtained from the average of the OD obtained with the three serum pools from pre-immunized/challenged animals + 3 SD. All individual serum samples were tested in duplicates. This figure appears in color at www.ajtmh.org.
Table 2.
Sensitivity of parasite culture compared with a multiplexed leishmanial antigen detection test for the diagnosis of CVL
| Dog number * | Parasite culture (BM) | Antigen detection test |
|---|---|---|
| 1 | − | − |
| 2 | − | + |
| 3 | − | − |
| 4 | − | − |
| 5 | − | − |
| 6 | + | + |
| 7 | − | + |
| 8 | − | + |
| 9 | + | + |
| 10 | + | + |
| 11 | − | + |
| 12 | + | + |
| 13 | + | + |
| 14 | + | + |
| 15 | − | − |
| 16 | − | + |
| 17 | + | +/− |
| 18 | − | + |
| 19 | + | + |
| 20 | − | + |
| Assay sensitivity | 40% | 70% |
CVL = canine visceral leishmaniasis.
Dogs 1–10 were from the group of animals previously vaccinated with Li-ntf2 and dogs 11–20 were from the group of control, nonvaccinated animals.
We are aware of the difficulties to clearly diagnose active CVL versus CVL without clinical signs particularly in kenneled dogs experimentally infected with L. infantum.18 Nonetheless, it is worth mentioning that after the last blood collection, the animals were killed and necropsied. The post mortem examination of all 20 dogs experimentally infected with L. infantum, showed, to different degrees, histopathological lesions in lymph nodes, liver, and spleen consistent with L. infantum infection.17 The overall lesions were granulomas with variable lymphoplasmacytic components and rare intracytoplasmic structures resembling parasitic forms. These results suggest different degrees of disease severity in the infected animals. However, we could not precisely quantify the intensity of these lesions to be able to use this metric to judiciously rank the animals in groups of CVL without clinical signs versus active CVL.19 Therefore, the suggestion that the test might be useful for diagnosing both disease conditions although attractive, needs further investigation, ideally performed in animals naturally infected with L. infantum.
It is important to mention that one of the leishmanial antigens detected by the multiplexed assay is Li-ntf2, which is the antigen that was initially used in the former study that provided the dog sera used in the present investigation. Because this antigen was inoculated in 10 of the 20 animals used in the current study, this pre-exposure to Li-ntf2 could theoretically constitute a complication in the assessment of the multiplexed antigen detection assay. However, it is unlikely that the antigen would remain in the animal’s system, particularly the serum, for over 12 months after its inoculation (the serum was obtained 11 months after challenge, which was 1 month after the last inoculation with the antigen). Moreover, it is important to emphasize that eight out of the 14 positive sera illustrated in Figure 1 and Table 2 are from dogs that were never exposed to Li-ntf2. Therefore, it is unlikely that preimmunization with the antigen Li-ntf2 impacted the interpretation of the results described earlier.
We recognize that the sensitivity/specificity of the multiplexed assay thus far described in this pilot study is based on a limited number of dogs experimentally infected with L. infantum. Nonetheless, although these results are preliminary, they encourage further validation involving a larger sample of dogs, which likely should result in a higher level of confidence. We are in the process of translating this study to a large clinical validation, which will include sera from animals naturally infected with L. infantum in endemic areas for CVL in Brazil as well as sera from healthy dogs and from animals having several other non-CVL infectious diseases. In addition, we are evaluating the suitability of these mAbs for the assembly of a reliable immunochromatographic rapid test for point-of-care diagnosis of CVL.
Finally, the multiplexed assay has the potential to be of great utility not only for the diagnosis of CVL but also as an important tool to monitor the treatment efficacy of this disease, a complicated issue that veterinary doctors frequently struggle with. We have preliminary proof-of-concept evidence of this possible utility from our former studies with human VL.20
REFERENCES
- 1. Belo VS. et al. , 2013. Factors associated with visceral leishmaniasis in the americas: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvar J. et al. , 1994. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol 88: 371–378. [DOI] [PubMed] [Google Scholar]
- 3. Gomes YM, Paiva Cavalcanti M, Lira RA, Abath FG, Alves LC, 2008. Diagnosis of canine visceral leishmaniasis: biotechnological advances. Vet J 175: 45–52. [DOI] [PubMed] [Google Scholar]
- 4. Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miró G, 2018. Canine visceral leishmaniasis: diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis 12: e0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peixoto HM, de Oliveira MR, Romero GA, 2015. Serological diagnosis of canine visceral leishmaniasis in Brazil: systematic review and meta-analysis. Trop Med Int Health 20: 334–352. [DOI] [PubMed] [Google Scholar]
- 6. Liu YP, Yao CY, 2015. Rapid and quantitative detection of hepatitis B virus. World J Gastroenterol 21: 11954–11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen JF. et al. 2013. Rapid-antigen detection tests for group a streptococcal pharyngitis: revisiting false-positive results using polymerase chain reaction testing. J Pediatr 162: 1282–1284. [DOI] [PubMed] [Google Scholar]
- 8. Mandell LA. et al. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 ( Suppl 2 ): S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dionne M, Hatchette T, Forward K, 2003. Clinical utility of a Legionella pneumophila urinary antigen test in a large university teaching hospital. Can J Infect Dis 14: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollock NR. et al. , 2013. Validation of Mycobacterium tuberculosis Rv1681 protein as a diagnostic marker of active pulmonary tuberculosis. J Clin Microbiol 51: 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beadle C. et al. , 1994. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 343: 564–568. [DOI] [PubMed] [Google Scholar]
- 12. Tanyuksel M, Petri WA, Jr, 2003. Laboratory diagnosis of amebiasis. Clin Microbiol Rev 16: 713–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mak GC. et al. , 2020. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol 129: 104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abeijon C. et al. , 2020. Urine-based antigen detection assay for diagnosis of visceral leishmaniasis using monoclonal antibodies specific for six protein biomarkers of Leishmania infantum/Leishmania donovani . PLoS Negl Trop Dis 14: e0008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abeijon C. et al. , 2019. Development of a multiplexed assay for detection of Leishmania donovani and Leishmania infantum protein biomarkers in urine samples of patients with visceral leishmaniasis. J Clin Microbiol 57: e02076–e02018. doi: 02010.01128/JCM.02076-02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abeijon C, Campos-Neto A, 2013. Potential non-invasive urine-based antigen (protein) detection assay to diagnose active visceral leishmaniasis. PLoS Negl Trop Dis 7: e2161–e2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abeijon C. et al. , 2016. Immunogenicity in dogs and protection against visceral leishmaniasis induced by a 14kDa Leishmania infantum recombinant polypeptide. Trials Vaccinol 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otranto D. et al. , 2009. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin Vaccine Immunol 16: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solano-Gallego L. et al. , 2011. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 4: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abeijon C, Singh OP, Chakravarty J, Sundar S, Campos-Neto A, 2016. Novel antigen detection assay to monitor therapeutic efficacy of visceral leishmaniasis. Am J Trop Med Hyg 95: 800–802. [DOI] [PMC free article] [PubMed] [Google Scholar]