ABSTRACT.
To evaluate percutaneous transsplenic varices embolization (PTSVE) in the treatment of upper gastrointestinal bleeding (UGIB) in patients with chronic hepatic schistosomiasis japonicum (CHS), 29 CHS patients (20 males and 9 females) complicated with UGIB were selected as the investigation subjects. The patients were treated by PTSVE under the guidance of X-ray fluoroscopy. The success rate of PTSVE and the rate of complications were observed. In addition, the degrees of varices before and after PTSVE were evaluated by abdominal computed tomography (CT). Results showed that 26 CHS patients (89.6%) were successfully treated with PTSVE. Three cases (10.3%) failed, and two experienced intraperitoneal bleeding within 1 week after PTSVE. The abdominal CT showed a significant decrease of the varices stage in coronary (P < 0.001), esophageal (P = 0.006), and paraesophageal (P = 0.013) varices, but slightly increased perisplenic varices within 1 month of the intervention (P = 0.014). PTSVE may be a safe and effective procedure for the treatment of UGIB in CHS patients, particularly suitable for those with a widened hepatic fissure and exposed hepatic portal vein trunk and an enlarged spleen.
INTRODUCTION
Chronic hepatic schistosomiasis japonicum (CHS) can lead to severe portal hypertension, gastroesophageal varices, and upper gastrointestinal bleeding (UGIB).1 The massive bleeding from gastroesophageal varices is a life-threatening emergency.2 The common treatments of UGIB caused by gastroesophageal varices rupture include surgical, endoscopy, and interventional method. Endoscopy can only solve current bleeding. The result from long-term follow-up indicates that endoscopy cannot be used to prevent the occurrence of rebleeding, which will further increase surgical and mortality risk.3
For intervention, transjugular intrahepatic portosystemic shunt (TIPSS) and percutaneous transhepatic varices embolization (PTHVE) are the most commonly used methods.4 However, TIPSS may cause severe hepatic encephalopathy.5 The embolization of gastroesophageal varices by percutaneous transhepatic portal vein has good hemostatic effect. However, liver deformation and liver lobe disproportion are evident in CHS patients.6 Studies showed that approximately 85% to 90% of CHS patients have severe liver lobe imbalance, with the volume of the right lobe significantly reduced, volume of the left lobe and caudate lobe enlarged, the hepatic fissure widened, the main trunk of portal vein exposed, and the volume of spleen increased.6–8 These characteristic changes significantly increase the potential risk of percutaneous transhepatic portal vein puncture, and lead to surgical failure and intraperitoneal bleeding. Therefore, it is of great clinical significance to find another safe and effective approach.9,10
Percutaneous transsplenic varices embolization (PTSVE) has previously been used as a selective approach in cases with multiple intrahepatic lesions, large tumors affecting transhepatic portal vein puncture, or portal vein occlusion.9,10 To control variceal bleeding in CHS patients unsuitable for percutaneous transhepatic portal vein puncture, it is crucial to find another approach for catheterizing their portal vein. This study investigated the feasibility and efficacy of PTSVE for UGIB caused by gastroesophageal varices in CHS patients.
MATERIALS AND METHODS
Patients.
This retrospective study was approval by the institutional review board of our hospital, and informed consent was waived. From January 2013 to June 2020, the electronic medical record of CHS patients accompanied with UGIB treated by PTSVE were accessed and evaluated with the following inclusion criterion: 1) CHS diagnosed by typical liver calcification on computed tomography (CT) and clinical history, 2) CHS patients with UGIB treated by PTSVE, and 3) abdominal CT performed before and at 1 month follow-up after PTSVE. Exclusion criterionwere as follows: 1) no abdominal CT evaluation before PTSVE and 2) patients loss to follow-up after PTSVE. Twenty-nine CHS patients accompanied with UGIB treated by PTSVE were enrolled in this study.
Varicose veins stage.
Abdominal pre- and post-contrasted CT was performed to evaluate liver volume; spleen volume; the position of portal vein and splenic vein trunk and branches; varicose veins; and location, direction, and depth of the puncture point. The presence, anatomy, and stage of the varicose veins were evaluated separately using a 4-point scale8: stage 1 = no varices (score = 0), stage 2 = small and nontortuous (score = 1), stage 3 = mild tortuous and < 50% radius of veins (score = 2), and stage 4 = very large and tortuous (score = 3). Thirteen varices veins were identified and evaluated (i.e., coronary vein short gastric vein, perisplenic vein, gastroepiploic vein, splenorenal vein, paraoesophageal vein, esophageal vein, gastrorenal vein, mesenteric vein, retroperitoneal-paravertebral vein, omental vein, paraumbilical vein, and abdominal wall vein). The stage of each varicose vein was evaluated independently by two radiologists (with 10 years of experience in abdominal imaging and intervention). Any discrepancies were solved by consensus.
Clinical data acquisition.
Clinical data pre- and postintervention in each patient were collected, including gender, age, alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, total bilirubin (TB), prothrombin time (PT), albumin, and blood ammonia.
Intervention procedure.
All procedure was performed under the X-ray fluoroscopy. The skin puncture point was between the left midaxillary and posterior axillary lines at the splenic-portal level (mostly between the seventh and eighth intercostal space). After local anesthesia, a 21-G Chiba needle was used to penetrate the spleen (toward the splenic portal vein). When reaching approximately 2 to 3 cm from the splenic hilum, the core of the needle was removed. Then, the needle was connected with a syringe and pulled back slowly and gently. When the blood was continuously pumped out from the needle, an injection of 5-mL contrast agent (Ultravist 300, Bayer, Berlin, Germany) was performed for the direct photography of the splenic portal vein. A 0.457-mm guidewire was imported into the splenic vein trunk or even mesenteric vein, and a 5-F RH catheter sheath was imported with the guidance of the guidewire.
Twenty milliliters of contrast agent was injected at the rate of 6 mL/s to display the hepatic portal vein and the varicose veins. A 5F Cobra catheter was used to catheterize the gastroesophageal varices. According to the blood flow velocity, an appropriate amount of gelfoam was used to embolize the main trunk of varicose veins. After the blood flow velocity had slowed, steel coils were used to embolize the varicose veins completely, followed by the splenic puncture duct.
Postintervention follow-up.
The success rate and the incidence of complications such as intraperitoneal bleeding, infection or pneumothorax were recorded. The clinical laboratory tests and the degree of varicose veins were compared pre- and post-invention after one month.
Statistics analysis.
All data were analyzed using R software (version 4.0.2; http://www.r-project.org/). Student’s t-test was used to compare the clinical laboratory tests. The χ2 test was used to compare the degree of varicose veins of pre- and postinvention. Data are expressed as mean ± SD, and P < 0.05 indicated a statistically significant difference.
RESULTS
Patient characteristics.
Of the 29 patients, 20 were men (aged 76 ± 7.1 years), and nine were women (aged 74 ± 7.3 years). All patients had a history of CHS and of UGIB. Eleven had a history of esophageal vein ligation and/or sclerotherapy and experienced a rebleeding after endoscopic treatment. The PTSVE intervention methods was chosen according to the following CT findings: CHS patients with small liver right lobe or exposed portal vein trunk and branches, which is not suitable for performing TIPSS or PTHVE (Table 1).
Table 1.
Abdominal computed tomography evaluation for percutaneous transsplenic varices embolization and postprocedure evaluation
| Before evaluation | After evaluation (30 days) |
|---|---|
| Small right liver lobe but enlarged splenic parenchyma | Coronary vein |
| Exposed portal vein trunk and left/right branch | Esophageal vein |
| Intrahepatic portal vein occlusion | Paraesophageal vein |
| Multiple lesions/tumors affecting portal vein | Perisplenic vein |
Success rate of PTSVE.
PTSVE was successfully performed in 26 of the 29 (89.6%) patients, with one to five splenic vein punctures and two or three steel coils per patient. Effective hemostasis was confirmed in these patients. The procedure was unsuccessful in the remaining three cases (10.3%) due to failure of the splenic vein puncture.
Follow-up.
After PTSVE procedure, no continuous bleeding was observed within 1 week in the 26 CHS patients with UGIB, and vital signs were stable. Two patients (6.9%) had intraperitoneal bleeding within 1 week after intervention, which stopped after partial embolization of the splenic artery. The remaining 24 cases had no complications, including intraperitoneal bleeding, infection, or pneumothorax. Within 1 month of follow-up, the 26 patients did not experience rebleeding of the upper gastrointestinal tract.
Abdominal CT showed a significant decrease of the varices stage in coronary (P < 0.001), esophageal (P = 0.006), and paraesophageal (P = 0.013) varices, but a slightly increased stage of perisplenic varices within 1 month after intervention (P = 0.014) (Figures 1 and 2). The clinical characteristics and stage of the varices pre- and postintervention are summarized in Table 2.
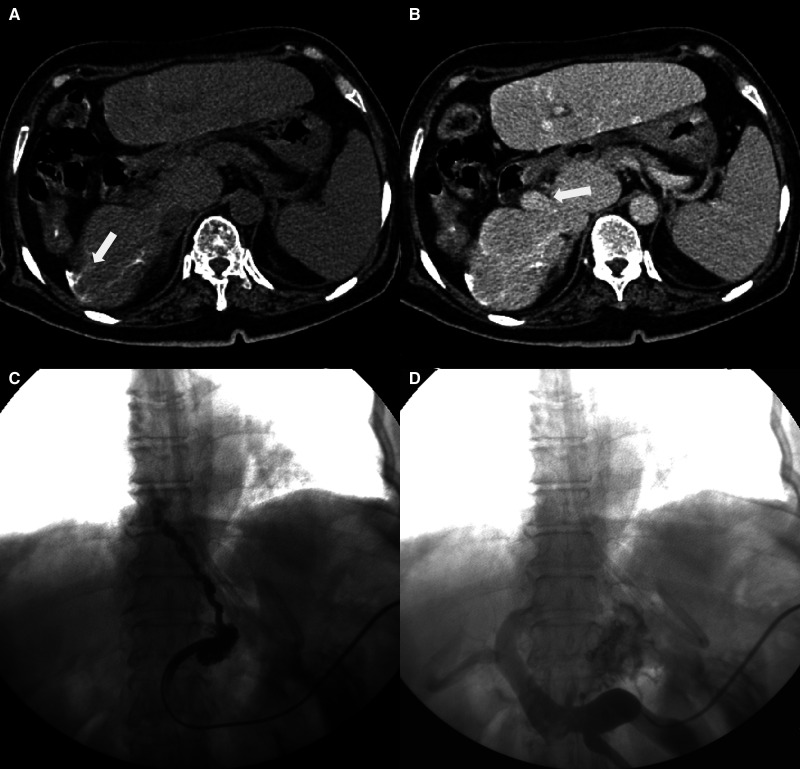
Figure 1.
A 75-year-old chronic hepatic schistosomiasis japonicum (CHS) patient with upper gastrointestinal bleeding (UGIB). Preintervention computed tomography (A and B) shows typical multilinear calcifications, a significantly reduced right liver and increased left liver lobe volume, an obviously widened liver fissure and exposed hepatic portal vein trunk, and a significantly increased spleen volume. A 5F Cobra catheter was transsplenicly inserted into the coronary vein, and portography showed tortuous gastroesophageal varices (C). After gastroesophageal varices embolization, the varicose veins disappeared. Patency of the hepatic portal vein with tortuous branches is seen (D).
Figure 2.
The radar chart shows significantly decreased varices stage in coronary, esophageal, and paraesophageal varices, but slightly increased perisplenic varices within 1 month postintervention. RPV = retroperitoneal paravertebral vein. *P < 0.05, **P < 0.01, ***P < 0.0001. This figure appears in color at www.ajtmh.org.
Table 2.
Percutaneous transsplenic varices embolization before and after interventional treatment
| Preintervention (n = 29) | Postintervention (n = 26) |
P value | |
|---|---|---|---|
| Age | 75 ± 8.2 | 74 ± 7.1 | 0.842 |
| Gender (female/male) | 9/20 | 9/17 | 0.777 |
| Child-Pugh (A/B/C) | 9/14/6 | 6/14/6 | 0.803 |
| ALT (IU/L) | 20.0 ± 11.4 | 21.3 ± 11.3 | 0.672 |
| AST (IU/L) | 24.0 ± 8.9 | 22.6 ± 8.7 | 0.575 |
| Platelet count | 178 ± 61.6 | 162 ± 45 | 0.277 |
| Total bilirubin (μmol/L) | 23.0 ± 13.1 | 28.1 ± 14.0 | 0.186 |
| Prothrombin time (s) | 13.6 ± 1.7 | 13.2 ± 2.5 | 0.595 |
| Albumin (g/L) | 35.6 ± 3.3 | 34.5 ± 3.9 | 0.261 |
| Coronary vein (stage 1/2/3/4) | 0/7/19/3 | 4/22/0/0 | < 0.001 |
| Esophageal vein (stage 1/2/3/4) | 6/12/7/4 | 9/17/0/0 | 0.006 |
| Paraesophageal vein (stage 1/2/3/4) | 10/10/5/4 | 17/9/0/0 | 0.013 |
| Gastroepiploic vein (stage 1/2/3/4) | 10/13/4/2 | 8/13/5/0 | 0.537 |
| Short gastric vein (stage 1/2/3/4) | 15/14/0/0 | 11/11/4/0 | 0.089 |
| Perisplenic vein (stage 1/2/3/4) | 20/9/0/0 | 11/9/6/0 | 0.014 |
| Gastrorenal vein (stage 1/2/3/4) | 10/17/2/0 | 8/13/5/0 | 0.390 |
| Splenorenal vein (stage 1/2/3/4) | 15/13/1/0 | 9/16/1/0 | 0.437 |
| RPV (stage 1/2/3/4) | 19/10/0/0 | 15/11/0/0 | 0.550 |
| Mesenteric vein (stage 1/2/3/4) | 22/6/1/0 | 16/9/1/0 | 0.500 |
| Omental vein (stage 1/2/3/4) | 17/12/0/0 | 12/13/1/0 | 0.412 |
| Paraumbilical vein (stage 1/2/3/4) | 19/6/3/1 | 14/9/2/1 | 0.706 |
| Abdominal-wall vein (stage 1/2/3/4) | 17/10/1/1 | 17/8/1/0 | 0.786 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; RPV = retroperitoneal paravertebral vein.
DISCUSSION
Schistosomiasis japonicum was endemic in 12 provinces of China in 1950s. An estimated 11 million people were infected, and 0.7 million people are still at the risk of infection.11,12 Schistosomiasis japonicum eggs can lead to irreversible liver fibrosis through host immune response, which leads to severe portal hypertension, gastroesophageal varices, and UGIB.13 The main causes of death in CHS patients are severe UGIB secondary to portal hypertension, hepatic encephalopathy, and ascites with refractory infection.14
Interventional treatment for UGIB include shunts, such as TIPSS, and varices embolization. However, the portosystemic shunt after TIPSS may cause hepatic encephalopathy in CHS patients.5 PTHVE may be useful to control UGIB. However, the success of PTHVE depends on the condition of hepatic portal vein. An impeded puncture route or exposed portal vein trunk have limited the utility of PTHVE.9,15
PTSVE provides a good choice for the treatment of gastroesophageal varices rupture in CHS patients unsuitable for TIPSS or PTHVE. A previous study showed that PTSVE could be used primarily in the following cases: 1) patients with multiple intrahepatic lesions or large tumors affecting transhepatic portal vein puncture; 2) intrahepatic portal vein occlusion by extensive thrombus; and 3) exposed portal vein trunk and left/right branch, which is prone to causing intraperitoneal bleeding.10 In this study, CHS patients selected for PTSVE were evaluated before intervention by CT. All the cases showed significantly reduced right liver lobe volume, increased left and tail liver lobe volume, an obviously widened liver fissure, an exposed hepatic portal vein trunk, as well as a significantly increased spleen volume. TIPSS or percutaneous transhepatic embolization injured the exposed hepatic portal vein trunk and lead to intraperitoneal bleeding in 10.6% patients.16 The success rate of PTSVE in UGIB patients with CHS is 89.6% (26/29), which is similar to previous studies.9–11
According to the results of this study, the key to the success of PTSVE depends on the puncture of the splenic portal vein. The following points should be considered: 1) spleen ultrasound or contrast-enhanced CT should be performed before the intervention to evaluate the size of the spleen and the condition of the splenic artery and splenic portal vein and 2) to confirm whether the puncture needle is entering the splenic portal vein by injecting a small amount of contrast agent. However, multiple injections of contrast agent could lead to the residual contrast agent in the splenic parenchyma and affect the procedure. It was imperative to pull back the puncture needle slowly and pump the syringe gently and manually to confirm the position of the puncture needle. This method could reduce residual contrast agent in splenic parenchyma and provide clear visualization for the surgery. 3) A sponge and steel coils should be used in sequence to form a permanent embolism. 4) To avoid bleeding from the fragile and vascularly rich spleen, it is necessary to operate gently, reduce puncture time, and embolize the splenic puncture duct.
The main causes for the failure of PTSVE include9–11: 1) lack of an enlarged splenic parenchyma, 2) slender splenic portal and intrasplenic veins, and 3) obvious perisplenic varices impeding the guidewire to reach the splenic portal vein trunk.
CONCLUSION
PTSVE can be considered a safe and effective procedure for the treatment of UGIB in CHS patients and is particularly suitable for the patients with a widened hepatic fissure, an exposed hepatic portal vein trunk, and an enlarged spleen. A study with a larger sample is needed to confirm these findings.
References
- 1. Song L. et al. , 2016. Assessment of the effect of treatment and assistance program on advanced patients with schistosomiasis japonica in China from 2009 to 2014. Parasitol Res 115: 4267–4273. [DOI] [PubMed] [Google Scholar]
- 2. Toews I, George AT, Peter JV, Kirubakaran R, Fontes LES, Ezekiel JPB, Meerpohl JJ, 2018. Interventions for preventing upper gastrointestinal bleeding in people admitted to intensive care units. Cochrane Database Syst Rev 6: CD8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho SH, Lee YS, Kim YJ, Sohn CH, Ahn S, Seo DW, Kim WY, Lee JH, Lim KS, 2018. Outcomes and role of urgent endoscopy in high-risk patients with acute nonvariceal gastrointestinal bleeding. Clin Gastroenterol Hepatol 16: 370–377. [DOI] [PubMed] [Google Scholar]
- 4. Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, Riggio O, 2019. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int 39: 871–877. [DOI] [PubMed] [Google Scholar]
- 5. Zhang K, Sun X, Wang G, Zhang M, Wu Z, Tian X, Zhang C, 2019. Treatment outcomes of percutaneous transhepatic variceal embolization versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding. Medicine (Baltimore) 98: e15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monzawa S, Uchiyama G, Ohtomo K, Araki T, 1993. Schistosomiasis japonica of the liver: contrast-enhanced CT findings in 113 patients. AJR Am J Roentgenol 161: 323–327. [DOI] [PubMed] [Google Scholar]
- 7. Wu S. et al. , 2018. Evaluation of transient elastography in assessing liver fibrosis in patients with advanced schistosomiasis japonica. Parasitol Int 67: 302–308. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Yang G, Qiang J, Cai S, Zhou H, 2016. Incidence of insulin resistance and diabetes in patients with portosystemic shunts without liver dysfunction. J Int Med Res 44: 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu K, Meng X, Zhou B, Qian J, Huang W, Deng M, Shan H, 2013. Percutaneous transsplenic portal vein catheterization: technical procedures, safety, and clinical applications. J Vasc Interv Radiol 24: 518–527. [DOI] [PubMed] [Google Scholar]
- 10. Gong GQ, Wang XL, Wang JH, Yan ZP, Cheng JM, Qian S, Chen Y, 2001. Percutaneous transsplenic embolization of esophageal and gastrio-fundal varices in 18 patients. World J Gastroenterol 7: 880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian C, Zhang Y, Zhang X, Yuan C, Gao Z, Yuan H, Zhong J, 2018. Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis. Parasite 25: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang LD. et al. , 2009. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360: 121–128. [DOI] [PubMed] [Google Scholar]
- 13. Chofle AA. et al. , 2014. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect Dis 14: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masi B, Perles-Barbacaru TA, Bernard M, Viola A, 2020. Clinical and preclinical imaging of hepatosplenic schistosomiasis. Trends Parasitol 36: 206–226. [DOI] [PubMed] [Google Scholar]
- 15. Chu HH, Kim HC, Jae HJ, Yi NJ, Lee KW, Suh KS, Chung JW, Park JH, 2012. Percutaneous transsplenic access to the portal vein for management of vascular complication in patients with chronic liver disease. Cardiovasc Intervent Radiol 35: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 16. Ohta M, Hashizume M, Kawanaka H, Akazawa K, Ueno K, Tomikawa M, Kishihara F, Tanoue K, Sugimachi K, 1996. Complications of percutaneous transhepatic catheterization of the portal venous system in patients with portal hypertension. J Gastroenterol Hepatol 11: 630–634. [DOI] [PubMed] [Google Scholar]