ABSTRACT.
There are a number of available and emerging malaria intervention tools that require innovative trial designs to find the optimal combinations at given epidemiologic settings. We simulated intervention strategies based on adaptive interventions, which included long-lasting insecticidal nets (LLINs), piperonyl butoxide–treated LLINs (PBO-LLINs), indoor residual spraying (IRS), and long-lasting microbial larviciding (LLML). The aims were to determine if PBO-LLINs or LLIN+IRS combination is more effective for initial interventions than LLINs and to identify the most effective intervention. We used a clustered, randomized adaptive trial design with malaria infection prevalence (MIP) as the outcome variable. The results indicate that during the initial stage of interventions, compared with regular LLINs, PBO-LLINs (relative reduction [RR]: 29.3%) and LLIN plus IRS with alternative-insecticide (RR: 26.8%) significantly reduced MIP. In the subsequent interventions, adding alternative insecticide IRS (RR: 23.8%) or LLML (RR: 31.2%) to existing PBO-LLIN was effective in further reducing MIP. During the next stage of interventions, adding LLML on top of PBO-LLIN+IRS (with alternative insecticides) had a significant impact on MIP (RR: 39.2%). However, adding IRS (with alternative insecticides) on top of PBO-LLIN+LLML did not significantly reduce MIP (11.6%). Overall, in clusters initiated with PBO-LLIN, adding LLML would be the most effective strategy in reducing MIP; in clusters initiated with LLIN+IRS, replacing LLIN+IRS with PBO-LLIN and LLML would be the most effective in reducing MIP. This study provides a new pathway for informing the optimal integrated malaria vector interventions, and the new strategy can be tested in field trials.
INTRODUCTION
Malaria remains a global public health challenge, with an estimated 228 million cases occurring annually worldwide in 2018.1 In addition, there is a growing realization that the enormous progress achieved in malaria control since 2000 appears to have slowed recently in part due to increased insecticide resistance and outdoor transmission.1–3 In fact, globally reported clinical malaria cases have increased since 2015 despite the high coverage of long-lasting insecticidal nets (LLINs) and other first-line interventions.1 The dramatic increase and widespread observations of insecticide resistance and outdoor malaria transmission have become an important challenge in malaria control.4–8 The malERA Refresh Consultative Panel on Tools for Malaria Elimination has called for “strategies for combining new and existing approaches are developed for different settings to maximize their longevity and effectiveness in areas with continuing transmission and receptivity.”9
There are many available and emerging interventions for malaria vector control,10,11 including LLINs, piperonyl butoxide-treated LLINs (PBO-LLINs),12 G2 nets,13 indoor residual spraying (IRS),14 topical repellent,15 space spraying/repellent,15 attractive toxic sugar bait (ATSB),16 larval habitat management and larviciding (chemical and microbial),17,18 genetically engineered (genetically modified or Wolbachia-transinfected) mosquitoes,19 mass trapping,20 and other emerging interventions under development.9,11 Integrated vector management is considered a promising tool for combating malaria in the era of insecticide resistance and outdoor transmission.10 In fact, the strategy of combining different interventions to control malaria has been tested in many field trials or implemented in the field.12,14,21 However, the challenge is to understand which interventions or combinations of interventions are appropriate in different settings and how to evaluate their effectiveness.9
Several issues need to be considered in determining the optimal mix of intervention strategies. First, transmission heterogeneity must be exploited (i.e., interventions must be tailored to the local ecological and epidemiological conditions, such as vector species composition and transmission intensity). Heterogeneity in transmission can occur at a local scale. For example, interventions or combination of interventions required in the lowland Ahero area east of Kisumu may be different from that in the highlands of Kakamega and Kisii areas of western Kenya because the composition of vector species differs among these areas.4,22–26 Heterogeneity and seasonality can also occur at the country or regional level.27,28 The heterogeneity in transmission results in the currently implemented control interventions being effective in some settings but not in other settings.1,3,29,30 For example, in western Kenya, currently implemented interventions can maintain malaria transmission at a moderate level (malaria infection prevalence [MIP] around 15%) in Kakamega county. However, with the same interventions, MIPs are around 50% in Kisumu county.22,29 In other words, different or additional interventions are required in Kisumu county to reduce the current malaria transmission.
Second, the suite of interventions may need to be adjusted over time if the disease burden changes and/or new interventions are made available and permitted for public health use. For example, the scale-up of LLINs and IRS in Africa has caused rapid and widespread development of vector resistance to multiple insecticides as well as increased outdoor transmission.4,8 Surveillance of vector pesticide resistance and behavioral changes may inform the choice of control strategies.8,23 In field trials, IRS with alternative insecticides has effectively reduced malaria transmission in areas with insecticide resistance.12,14 In addition, cost should be considered when planning a trial study; local community acceptability and field implementation feasibility are also of concern when implementing the interventions. Therefore, the integrated vector management strategy is an adaptive approach to altering and delivering an optimal mix of interventions in response to changing epidemiology—not merely an a priori optimal mix for a given transmission setting, which will become irrelevant once epidemiological and/or entomological conditions change. From a practical perspective, adaptive interventions have been implemented in malaria-endemic countries, for example, changes from ordinary insecticide-treated bed nets to LLIN since 2006,12,31,32 changes from pyrethroid IRS to the recently implemented Actellic® IRS,12,33–37 and implementing a combination of IRS with LLINs.12,34–37
Randomized controlled trials (RCTs) are considered the gold standard for assessing the relative efficacy of competing treatment options in evidence-based disease management.38 In an RCT, investigators randomly assign interventions or treatments to an experimental arm in comparison to a control arm. Randomized controlled trials are widely used in malaria intervention trials.12,14,15,18 Integrated vector intervention will potentially involve a combination of different types of interventions, such as LLINs plus IRS with alternative insecticide or LLINs plus LLML or ATSB for outdoor vector control.12,14,17,20 It is essential that different types of interventions be combined to combat insecticide-resistant and outdoor-biting/resting vectors. Due to the potentially large sample size requirement, the complete factorial design of a RCT or cluster-randomized trial (CRT) is not a practical way to find the optimal combination of interventions among the many available interventions. As the malERA Refresh Consultative Panel on Tools for Malaria Elimination pointed out, “Randomised controlled trials are expensive and time consuming, and new pathways should be explored for generating evidence for large-scale implementation of new interventions.”5 In addition, a combination of three or four interventions in one cluster in a trial or in the field can be costly and likely redundant from the cost-effectiveness point of view.12 Therefore, an innovative trial design is needed to fill this gap. Moreover, many of these interventions have been tested in the field, so a complete factorial design may not be necessary. Instead, multi-stage adaptive interventions may be an appropriate and cost-saving approach (i.e., utilizing individual variables such as ecology and epidemiology to adapt the intervention and then dynamically readapt interventions based on intervention outcomes).
An adaptive design is loosely defined as a trial design that allows for modifications to the trial procedure after its initiation without undermining its validity and integrity.39–42 An adaptive trial design is a sequence of decision rules that specify how the intervention should change to suit the current epidemiological conditions. It consists of three key elements: a set of available interventions, a sequence of decision rules to be followed when implementing the interventions, and a set of tailoring variables (evaluation criteria or outcomes). This design has frequently been used in clinical studies in areas such as psychology, mental health, and cancer treatment,40–42 but it has not been used in studies on vector and vector-borne infectious disease control. There are three major reasons for considering adaptive trial designs over traditional RCTs/CRTs for the control of vector-borne infectious diseases. First, different ecological and/or epidemiological settings may require different interventions or combinations of interventions. For example, a high-transmission setting may require a different strategy than a near-elimination setting.43 Second, given the many available and emerging new interventions, adding more interventions into a trial can potentially increase effectiveness. Practically speaking, however, adding interventions means adding cost,21,44,45 and logistically it can be very difficult if not impossible to implement the combined interventions in scale. Third, the effectiveness of an intervention may change over time due to changes in epidemiology (e.g., reduced transmission intensity), dynamically evolving risk (e.g., vector species shift or development of resistance), or ecology (environmental changes).5,22,28,43 Decisions must be made concerning if and when an intervention needs to be replaced or terminated and, accordingly, which intervention should follow. In this context, future subjects are randomized with bias toward the best-performing interventions, unlike in conventional RCT/CRT, which treats all interventions/subjects equally throughout a trial. In addition, decisions may take into account both effectiveness and cost.21,44,45 Clearly, adding interventions on top of existing interventions usually increases effectiveness; meanwhile, adding interventions may significantly increase cost and sometimes redundant, making the overall cost-effectiveness suboptimal.12,21,44,45
There are many types of adaptive trial designs,39,42,46,47 and the useful adaptive trial designs for malaria vector control may include adaptive individual randomization (e.g., LLIN, IRS), cluster randomization (e.g., larviciding), and drop-the-loser design.39 Conventional RCT/CRT has been used before in malaria control studies12,14,16; these designs do not drop any subject or cluster of subjects during trials. Drop-the-loser is a multistage design that allows three types of potential selections for the next stage: 1) dropping the inferior intervention, 2) modifying the intervention, and/or 3) adding an intervention. It is especially useful in selecting the optimal combination of interventions to form an effective integrated strategy when many interventions are available.
The aim of this study is to use malaria vector control as an example to provide a framework for developing the optimal integrated intervention using adaptive trial design with simulations based on local climatic-environmental and eco-epidemiological conditions. The simulated adaptive strategy here used four types of interventions: regular LLIN and IRS with pyrethroids or Actellic®, PBO-LLIN, and LLML. The strategy presented here can be tested in future field trials.
MATERIALS AND METHODS
Study area and simulation settings.
The biological and epidemiological data used in this study are from the Kombewa area of Kisumu County, western Kenya.22 Kombewa is located in the lowland Lake Victoria shore area (1,140–1,300 m elevation) and is 20 km west of Kisumu town. It is a semiarid area with poor drainage, semipermanent swampy streams, and some large swampy areas. It is a rural agricultural area with maize as the staple crop. The area is warm, with an average monthly maximum temperature of 29.1°C and minimum of 18.4°C. The warmest period is between January and March, and the coolest period is from June to August.22,48 The annual precipitation in the area is about 1,450 mm with two rainy seasons: the long rainy season from April to June and short rainy season from October to November.
In Kombewa, MIP was 50% in 2015, despite high LLIN coverage (> 90%), and the MIP was relatively stable (around 50%) since 2004, except for short periods of declines in MIPs immediately after the LLIN campaigns in 2006 and 2010.29,49 All three major African malaria vector species occurred in the area in 2015: Anopheles gambiae s.s. (28%), Anopheles funestus (42%), and Anopheles arabiensis (30%).29 Vector mortality against pyrethroids was around 50%, whereas vectors had no resistance against organophosphate.8,49 Outdoor transmission was found to be high in nearby areas.4 The malaria transmission intensity, measured by entomological inoculation rate (EIR), in the area in recent years was not available, but EIR was estimated to be around 50 infectious bites/person/year in 2017, based on the results of several previous studies conducted in the same or nearby areas.4,48,50 These field observed parameters, including vector species compositions, resistance levels (WHO tube/cone test mortality), and parasite infection prevalence in the past several years were used as baseline for the simulations (Table 1). The distribution of free universal artemisinin-based combination therapy has been implemented by the government of Kenya since 2006. In the study area, the government has also conducted four rounds of mass LLIN distributions, respectively, in 2006, 2010, 2014 and 2018. Long-lasting insecticidal nets are considered the standard intervention for the study area. No formal IRS or other vector interventions have been implemented in the area. The aim of this study is to find the optimal combination of interventions for vector control in this area.
Table 1.
Parameters used in simulations
| Parameter | Details | Value | References |
|---|---|---|---|
| Malaria infection prevalence | 50% | 29, 49 | |
| Vector composition | Anopheles. gambiae s.s. | 28% | 29, 49, 50 |
| Anopheles funestus | 42% | 29, 49, 50 | |
| Anopheles arabiensis | 30% | 29, 49, 50 | |
| Vector resistance level* | Pyrethroids | 50% | 8, 49, 55 |
| Organophosphate (Actellic®) | > 90% | 56 | |
| Intervention efficacy† | Regular LLIN | 50% | 55 |
| PBO-LLIN | 90% | 50 | |
| Regular insecticide IRS | 50% | 8, 49, 50 | |
| Alternative insecticide IRS | 80% | 56 | |
| Bti/Bs larvicide (LLML) | 80% | 17 | |
| Re-application interval (months) | IRS‡ | 6 | 50, 56 |
| Bti/Bs larvicide | 4 | 17 | |
Bti/Bs = Bacillus thuringiensis var israelensis/Bacillus sphaericus; IRS = indoor residual spraying; LLIN = long-lasting insecticidal net; PBO-LLIN = piperonyl butoxide-treated LLINs; PMI = President’s Malaria Initiative.
Mortality measured by WHO tube/cone test. Same morality for all species.
Measured by vector mortality.
Current Actellic® IRS is sprayed once a year by PMI.
We ran the simulations using the Epidemiological Modeling for Malaria Transmission (EMOD-MT), a comprehensive, individual-based stochastic model that takes into consideration human demography, entomology, climate, and other factors.51,52 The EMOD-MT is best suited for comparing the potential entomological and epidemiological impacts of various interventions. EMOD-MT was developed by the Institute for Disease Modeling (https://docs.idmod.org/projects/emod-malaria/en/latest/malaria-model-overview.html, Bill & Melinda Gates Foundation, Seattle, WA). The model consists of immature mosquito development, vector feeding and infection, vector-human interaction, human malaria infection, and within-host parasite dynamics. The model requires input of climatic data for vector and parasite development, habitat setting for immature vector simulation, and demographic inputs for infections simulations. The specifics of the model are described in detail elsewhere.51–54 The model includes both vector control and human-based interventions, such as bed nets, IRS, larviciding, and antimalarial drugs, among others. The software is freely available.
As reference climatic data for the simulations, we used the monthly average maximum, minimum, and mean temperatures and the monthly average cumulative precipitation from Kisumu, also on the Lake Victoria shore lowland area about 20 km east of Kombewa. Because LLIN is the routine government-implemented intervention, the effectiveness of LLIN was simulated as baseline and compared against our field monthly surveillance data.29 The most recent free LLIN mass distribution was conducted in June 2018 in the study area.
Four interventions were considered in the simulations: LLIN, PBO-LLIN, IRS with/without alternative insecticides, and Bacillus thuringiensis var israelensis/Bacillus sphaericus (Bti/Bs) LLML.17 Piperonyl butoxide–treated LLINs may help to restore the effectiveness of LLINs by increasing their killing power against insecticide resistant vectors.12 Similarly, alternative-insecticide IRS has proven effective against insecticide-resistant vectors.12,30 Long-lasting microbial larviciding kills larvae but requires repeated applications every 3–4 months.17 Simulation conditions are summarized in Table 1. Mosquito species composition were based on recently published field studies conducted in the study area or nearby areas.4,29 Efficacy of regular LLIN and pyrethroid IRS was also based on field observations (i.e., mosquito resistance levels as tested by WHO tube or cone bioassays).8,49,55 We have also assumed that Anopheles mosquitoes were susceptible to Actellic@ based on recent President’s Malaria Initiative entomological monitoring conducted in eastern Kisumu county.56
For simplicity, in the simulation model, we assumed that the efficacy of LLINs and PBO-LLINs was slowly waning after 2 years of use and lasted beyond 2 years with an exponential decaying killing power until the end of year 4.57,58 This assumption was based on the “catch-up” routine distribution of LLINs through in the government-run health facilities and Child Welfare Clinics, which provided LLINs to pregnant women and children under 5 years of age.59,60 Although the mass distribution of LLINs was made once every 4 years, this “catch-up” LLIN distribution may fill some of the gaps in LLIN coverage between two mass distributions, thus partially maintaining the LLIN effectiveness.59,60 The efficacy of IRS was assumed to be effective for 6 months, with exponential decaying effects after 2 months of application.8,56 Long-lasting microbial larviciding efficacy decays with a Gaussian distribution and lasted for 4 months.17
Trial design.
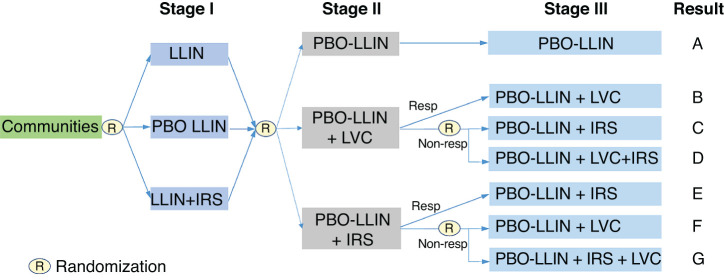
A cluster-randomized design was considered due to the use of LLML in the aquatic habitats rather than in individual houses.17,18 Three major factors were taken into consideration for the design. First, because LLIN is universally implemented in the study area,29 it was chosen as the reference intervention (i.e., the control arm). The same initial intervention (LLIN) was provided to all subjects in all clusters at stage I intervention. Second, given the exophagic and exophilic nature of some vector species, outdoor transmission may be important,4 and therefore LLML should be considered. Third, the study duration was 4 years (including 1 year of baseline prior to any intervention) because LLIN may lose most of its efficacy afterwards, with an evaluation period of 12 months after the initiation of each intervention. New interventions always started at the beginning of each year to minimize seasonal effects. A potential adaptive design flowchart is shown in Figure 1, and the potential timeline is shown in Supplemental Table 1.
Figure 1.
An example of adaptive intervention trial design. R means that randomization is required. Resp/Non-resp represents responded (i.e., showed effective reduction) and non-responded (i.e., did not show effective reduction) clusters. In Stage I intervention, we assumed that PBO-LLIN had a significant added effect on the reduction of malaria infection prevalence (MIP) compared to regular LLIN, therefore, LLINs are replaced by PBO-LLINs in Stage II. LVC is larval control. This figure appears in color at www.ajtmh.org.
The study’s primary aim was to determine if beginning with PBO-LLINs or LLIN+IRS is more effective in reducing MIP than that with regular LLINs. The second aim was to estimate the mean outcomes of the embedded adaptive interventions and identify the most effective intervention. Sample size calculation was based on reduction in MIP. The sample size (i.e., number of clusters required) was calculated assuming an intercluster correlation coefficient of 0.05,61 a significance level of 0.05, power of 80%, cluster size of 200 individuals, and detectable reduction of 10%. A sample size of five clusters is required for a non-adaptive design. Assuming 30% relative reduction is achieved at stage I intervention, then a maximum of 10 clusters are required at stage II interventions. Similarly, if 30% relative reduction is achieved at stage II intervention, then a maximum of 30 clusters are required at stage III interventions. This calculation assumes 1:1 response/nonresponse ratio. We started with 84 clusters for the simulations because this sample size is likely powered to detect smaller changes in MIP.
At stage I, each cluster was randomly assigned to one of the three arms: regular LLIN, PBO-LLIN, or regular LLIN+IRS (i.e., only indoor interventions were simulated at this stage). Selection of initial interventions may affect the subsequent selection of interventions and potentially the overall optimal combination of interventions. Therefore, the selection of initial interventions is important. In this study, the selection of initial interventions was based on the results of recently published field trial studies.12,14–18,30 An intervention was considered non-effective (or a cluster was considered a nonresponder) based on two criteria: 1) the stringent criterion for nonresponse, in which the reduction in MIP was < 20%, or 2) the lenient criterion for nonresponse, in which the reduction in MIP was statistically insignificant. Cost was not included in this study. The minimum 20% reduction was selected arbitrarily yet reasonably because a number of past trials have reached effectiveness of > 20% reduction in MIP or clinical malaria incidence.12,14,18,30,52
Field data for model validation.
To validate the model simulated trend of malaria prevalence, we used malaria prevalence data collected from randomly selected school children 6–13 years old in Kombewa from 2013 to 2018. The data collection process has been described previously, and some of the data have already been published.22,29,49 Briefly, each month, at least 100 volunteer school children from at least three schools were sampled to determine parasite prevalence. Blood samples were collected by the standard finger-prick method, and thin and thick blood smears were prepared for laboratory examination.22,29 Parasite species and gametocytes were identified microscopically.22,29 Parasite prevalence was calculated as number of positive samples divided by the total number of samples examined.
Outcome measures and data analysis.
The primary outcome measure was the percentage reduction in MIP rate. A primary aim was to find the combination of interventions that yielded the greatest reduction in MIP. Differences in MIP between treatments and control groups were compared with Poisson multivariate regression models with intervention, cluster, and calendar time as covariates, using a generalized estimating equations approach.62,63 Intervention was a time-varying covariate because the treatment in a cluster may be adapted and re-adapted pending the response to the previous treatment. For the second aim of analysis (estimation of outcomes for the embedded adaptive interventions), weighted and replicated generalized estimating equations were used to estimate the means in the infection prevalence among the embedded adaptive interventions and to compare the slopes at each stage for each adaptive intervention. Here, weighting is to account for the potential over- or under-presentation of some groups (e.g., nonresponders and responders could have different chances to be assigned to different treatment groups).64,65
Scientific and ethical clearance.
Scientific and ethical clearance was given by the institutional review boards of Kenya Medical Research Institute, Kenya, and the University of California at Irvine. Volunteers were enrolled from primary schools in the study sites through school administrators with the permission of the division office of the Ministry of Health, written consented by school principal on behalf of the parents, and oral assent from participants. Inclusion criteria included providing informed consent and having no reported chronic or acute illness except malaria. Exclusion criteria included unwillingness to participate in the study.
RESULTS
A potential adaptive intervention trial design.
A potential adaptive intervention flowchart is shown in Figure 1, and the timeline is shown in Supplemental Table 1. The trial study lasted for 3 years (Supplemental Table 1). In this trial, we proposed to use regular LLIN, PBO-LLIN, IRS (vector-resistant insecticides or alternative insecticides), and larviciding with LLML. At stage I, each cluster was randomly assigned to one of the three arms with all indoor interventions: regular LLIN, PBO-LLIN, or regular LLIN+IRS. Assuming PBO-LLIN is more effective than regular LLIN at stage i, stage II will also consist of three arms all including PBO-LLIN (replace LLIN) as default intervention. Stage III interventions depend on the effectiveness of IRS and LLML at stage II (Figure 1). Regular LLIN is currently the routine intervention implemented by all malaria endemic South Saharan African countries. Therefore, LLIN served as the control arm at stage I intervention.
Performance of model simulation.
Figure 2 illustrated the observed MIP in the study area and model simulated MIP in the study area. The model-simulated dynamics of MIP matched the observed results well assuming 90% LLIN coverage, which is similar to the observed LLIN coverage in the study area (Figure 2).29
Figure 2.
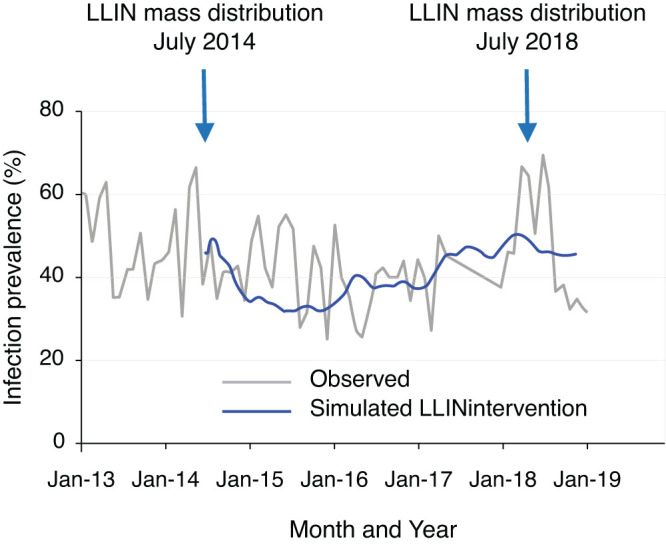
Dynamics of observed malaria infection prevalence (MIP) (gray curve) and model simulated MIP (blue curve). Model simulated MIP (blue curve) are based on 90% coverage of LLIN. This figure appears in color at www.ajtmh.org.
Stage I intervention.
Based on the current MIP of 50% (50.5% as simulated) and vector mortality rate of 50% against regular LLINs, switching from 90% regular LLIN to 90% PBO-LLIN yielded a MIP of 24.7%, a 51.1% reduction in MIP from the baseline, or 29.3% additional reduction against regular LLIN (MIP 35.0%), which was considered effective (Figure 3; Supplemental Figure 1). Therefore, all LLINs were replaced by PBO-LLINs for stage II. Compared with regular LLIN alone, adding IRS on top of LLIN reduced MIP by an additional 9.1% when using vector-resistant insecticides or by an additional 26.8% when using alternative low-resistance insecticides (Figure 3; Supplemental Figure 2).
Figure 3.
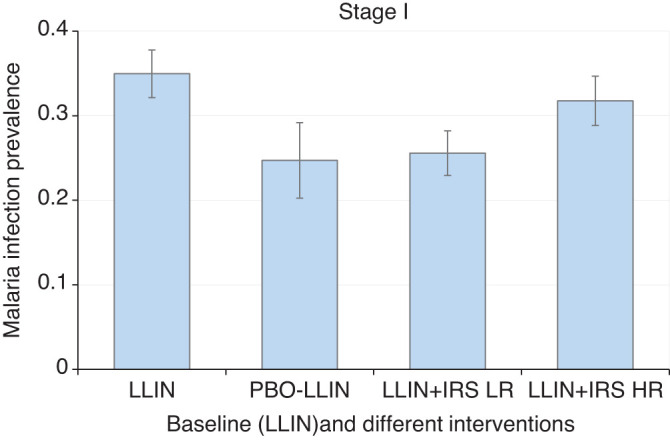
Simulated mean malaria infection prevalence rate under different intervention scenarios during stage I interventions. Error bar was standard deviation. LLIN, long-lasting insecticidal net; PBO-LLIN, piperonylbutoxide-treated LLIN; LLIN+IRS LR, LLIN plus indoor residual spraying using low resistance insecticides; LLIN+IRS HR, LLIN plus indoor residual spraying using high resistance insecticides. This figure appears in color at www.ajtmh.org.
In summary, PBO-LLIN and alternative-insecticide IRS were effective interventions and were considered in subsequent stages. Regular LLIN and IRS with vector-resistant insecticides were terminated after stage I.
Stage II intervention.
In stage II, all regular LLINs were replaced by PBO-LLINs (Figure 1). Adding alternative-insecticide IRS on top of PBO-LLIN had an overall 23.9% added effect on MIP (Figures 1 and 4; Supplemental Figure 3), which was considered effective. With relatively high rates of outdoor transmission and pyrethroid insecticide resistance, adding LLML (with reapplication every 4 months) on top of PBO-LLIN produced an overall 31.2% additional relative reduction in MIP (Figures 1 and 4; Supplemental Figure 3).
Figure 4.
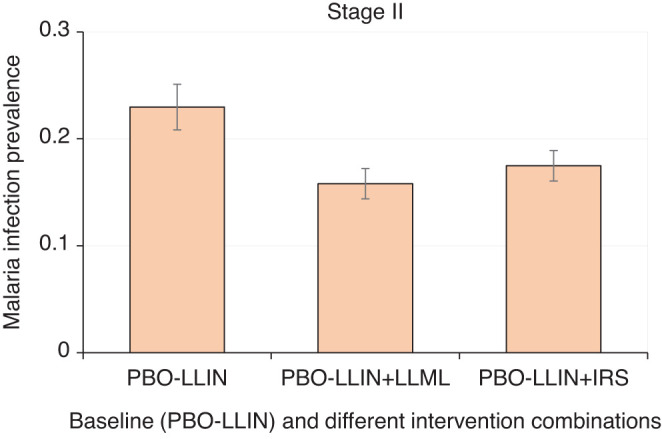
Simulated mean malaria infection prevalence rate under different intervention scenarios during stage II interventions. Error bar was standard deviation. PBO-LLIN, piperonylbutoxide-treated LLIN; PBO-LLIN+LLML, PBO-LLIN plus long-lasting microbial larviciding; PBO-LLIN+IRS, PBO-LLIN plus indoor residual spraying. This figure appears in color at www.ajtmh.org.
In summary, both alternative insecticides IRS and LLML were effective interventions when added to existing PBO-LLIN. If only two interventions could be considered due to cost and/or logistical concerns, PBO-LLIN+LLML would be the optimal combination for reducing MIP in the study area.
Stage III intervention.
As in stage II, PBO-LLIN was used as the baseline. For nonresponded clusters with LLML, interventions were switched to IRS, and vice versa. For responded clusters, both LLML and IRS were switched to LLML+IRS. Simulation results indicated that adding LLML on top of PBO-LLIN+IRS yielded an additional 39.2% relative reduction in MIP (Figures 1 and 5; Supplemental Figure 4), whereas adding IRS on top of PBO-LLIN+LLML yielded just an 11.6% additional reduction in MIP (Figures 1 and 5; Supplemental Figure 5), which fell below the 20% threshold.
Figure 5.
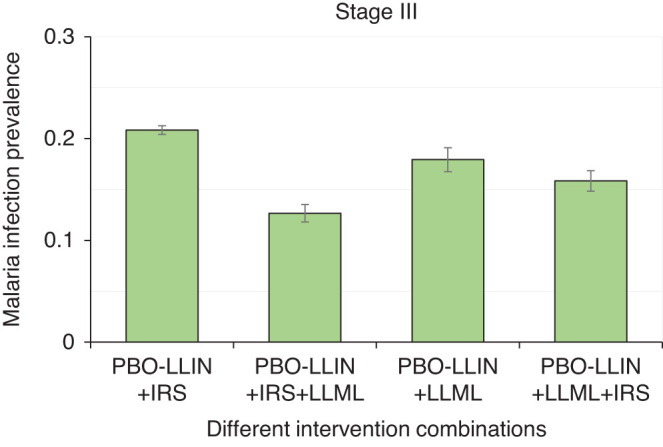
Simulated mean malaria infection prevalence rate under different intervention scenarios during stage III interventions. Error bar was standard deviation. PBO-LLIN, piperonylbutoxide-treated LLIN; PBO-LLIN+LLML, PBO-LLIN plus long-lasting microbial larviciding; PBO-LLIN+LLML+IRS, PBO-LLIN plus LLML and then indoor residual spraying; PBO-LLIN+IRS, PBO-LLIN plus indoor residual spraying; PBO-LLIN+IRS+LLML, PBO-LLIN plus IRS and then LLML. This figure appears in color at www.ajtmh.org.
In summary, adding LLML on top of PBO-LLIN+IRS (with alternative insecticides) had a significant impact on MIP. However, if PBO-LLIN+LLML has already been implemented, adding IRS may not be recommended. In other words, PBO-LLIN+LLML might be the optimal effective combination of interventions when only LLIN, PBO-LLIN, IRS, and LLML are considered.
Optimized intervention strategy.
Overall, in clusters initiated with PBO-LLIN, PBO-LLIN+LLML would be the most effective in reducing MIP; in clusters initiated with LLIN+IRS, then PBO-LLIN+IRS+LLML would be the most effective in reducing MIP. Note that IRS was simulated using alternative insecticides, and only LLIN, PBO-LLIN, IRS, and LLML interventions were considered. The cost of interventions was not included in this analysis.
DISCUSSION
Malaria is still a major public health threat, despite that significant progress has been made in the past two decades. Insecticide resistance and outdoor malaria transmission are widespread, though their public health impact is not fully understood. Integrated vector management is considered a promising tool for combating insecticide resistance and outdoor transmission.10 Here, using simulations, we show that an adaptive trial design can be used as an alternative strategy to find the optimal integrated intervention that is suitable in given settings. A field trial of malaria vector intervention using adaptive trial design is ongoing in eastern Kisumu, Kenya, and it will serve as a test case for future studies.66
Adaptive trial design and field implications.
This study provides an alternative approach to a multi-intervention trial design, and the simulation shows how field trials might be conducted (e.g., how to sequentially implement different interventions and what potential outcomes to expect). The RCT, which is considered the gold standard of conventional trial designs, is balanced across all interventions (i.e., it treats all intervention arms equally), and, once randomized, the interventions remain unchanged throughout the trial. By contrast, the adaptive trial approach uses multistage optimization to find a robust mix of interventions by eliminating or replacing inferior interventions. Given the large number of available interventions, it can be difficult to determine the optimal integrated strategy using an RCT design, which would require a large sample size to include all potential interventions.9 So far, no field trials have tested three or more interventions using a full factorial design,12,14,20,30,52 which is considered one of the main challenges for generating evidence to support large-scale implementation of integrated interventions.9 The only three-intervention trial that has been conducted involved parallel studies of two competing interventions, meaning no additive effect was assessed.12 The adaptive strategy could greatly reduce the potentially large sample size required by the RCT design and thus provides a new pathway to developing the optimal integrated vector management strategy. For example, in a trial done by Protopopoff et al.12 to test LLIN, PBO-LLIN, and IRS for their effectiveness in malaria control, PBO-LLIN was in competition with LLIN; therefore, four intervention arms were needed. If adaptive intervention were used in this case, two arms would be sufficient (although two stages would be required). Furthermore, if more interventions are considered, adaptive design may reduce the sample size required. This simulation demonstrates the effectiveness of this strategy and thus the potential for using this strategy in a field trial.
Several field trials have tested interventions similar to those in this study and yielded levels of effectiveness similar to those shown by our simulations.12,14,20,30,62 In the trials by Protopopoff et al.,12 PBO-LLIN outperformed regular LLIN by a 25% reduction in MIP, and adding alternative-insecticide IRS on top of LLIN/PBO-LLIN resulted in a 36% additional reduction in MIP. Similarly, in a trial by Tiono et al.,62 LLINs treated with permethrin plus pyriproxyfen showed a 25% reduction in clinical malaria in children compared with regular LLINs. A small-size cluster-randomized trial conducted by Afrane et al.17 found that LLML reduced indoor-biting malaria vector density by 76–82% and outdoor-biting density by 67–75% and was effective up to 3–4 months. In the Fillinger et al.18 conventional Bti/Bs trial, intervention reduced 48% of new infections after 6 months of continuous applications. The results of our simulation are consistent with these field trials (i.e., adding alternative-insecticide IRS or LLML or switching from regular LLIN to PBO-LLIN reduces MIP by 20–40%). In addition, the simulated dynamics of MIP reflected the field observations. Therefore, that the results from these field trials reflects our simulation findings provides support that our simulations are reliable.
Our new trial design and simulation went beyond combining two interventions, and no field trial has tested a combination of three interventions with complete factorial designs. As shown by field trials and in our simulations, alternative-insecticide IRS and LLML may be equally effective in reducing malaria transmission (measured by MIP or clinical malaria). Because IRS and LLML work on different stages of vector development, and LLML reduces overall vector density,17 so adding LLML on top of IRS may further reduce indoor vector density. Assuming moderate outdoor transmission, our simulation results indicate that adding IRS on top of LLML yielded less reduction in MIP compared with its counterpart (i.e., adding LLML on top of IRS), presumably because LLML also reduces outdoor transmission. These simulation results can be tested in field trials. In this context, our study provides a guideline or new pathways that are desperately needed for future multi-intervention trial designs.9
The major drawback of adaptive intervention trial design is the potentially longer test duration needed. There is a trade-off between large sample size and long test duration when more interventions are included. However, it is not feasible to do a RCT when there are many available interventions.
Selection of initial interventions and starting time of each intervention.
Selection of initial interventions is important in adaptive interventions because the eventual optimal combination of interventions depends on the initial interventions. Expert opinions are key for selecting the initial interventions and are based on previously evaluated interventions. For example, for malaria control, alternative insecticide IRS and PBO-LLIN have been shown to be effective in further reducing malaria transmission.12,30 In fact, pyrimiphos-methyl or bendiocarb IRS has been implemented in Kenya, Uganda, and Ethiopia, and PBO-LLIN will be rolled out in 2020 in Kenya according to the PMI plan.35–37 However, other factors must also be considered, such as the cost of implementation, feasibility for field implementation, and community acceptability. In this study, we have also considered mode of delivery. For example, larviciding has been shown to be effective in reducing malaria transmission. However, finding all breeding habitats is challenging. Because LLINs, IRS, and PBO-LLINs are only required for each household, presumably implementing these interventions is logistically easier and possibly less costly. In addition, IRS, LLINs, and PBO-LLINs are indoor interventions. Thus, larviciding, an outdoor intervention, complements IRS and PBO-LLIN because it targets a different stage of the mosquito life cycle. Therefore, larviciding was implemented separately after IRS, LLINs, and PBO-LLIN. In other words, we first assessed the performance of each adult intervention tool, then added larval intervention tools. Last, and the most importantly, the purpose of using an adaptive design strategy is to reduce the intervention sample size without undermining the integrity of the intervention. Therefore, the number of initial interventions should be kept minimal; otherwise, the sample size will increase, and the advantage of doing an adaptive trial will be lost.
Another issue is the impact of the intervention starting time. Because the study area experiences two rainy and two dry seasons each year, mosquito larval breeding habitats and mosquito population dynamics show strong seasonality.22 Therefore, intervention starting season may have short-term impacts on reducing malaria transmission and the cost-effectiveness of the intervention. For example, during the dry season, larval habitats are less common and likely concentrated in certain areas where water is available.67–69 Larviciding intervention during this period may have increased effectiveness and reduced costs. Because larviciding must be repeated several times a year, the effects that one application has on long-term malaria transmission are not clear. In general, the effects of the starting time of an intervention on the overall effectiveness of an adaptive trial is a subject of further investigations.
A further question is the impact of carryover effect. Different interventions and different intervention starting times will potentially yield different patterns of transmission in the intervention area by the end of each stage of interventions, and this result at the end of each year is used to determine the selection of specific intervention in a given cluster in the next step.63–65 This strategy is the nature of the adaptive trial design and the key difference between adaptive and conventional cluster randomized controlled trials. In adaptive trials, effective interventions will be continued in the next stage, whereas clusters with ineffective interventions will be re-randomized and assigned to effective or new interventions. The re-randomization process acts to minimize the carryover effect, whereas re-randomization is not allowed in conventional cluster randomized controlled trials. The key is the inter-stage evaluation; RCT design does not allow such evaluations.
Sequential multiple assignment randomized trial.
A sequential multiple assignment randomized trial (SMART) is one way to inform the development of adaptive trials.40,42 As mentioned earlier, it is logistically difficult, if not impossible—and perhaps not even necessary—to include all of the potentially effective interventions or combinations of interventions in one trial all at once. However, it is possible to sequentially add selected interventions into the trial based on outcomes at each step and existing evidence, such as previous laboratory experiments, field trials, or mathematical model simulations. A SMART usually includes multiple intervention stages, with each stage corresponding to one of the critical decisions in the adaptive trial process.40 Data from a SMART design can be used to address many questions regarding the construction of optimal adaptive intervention (i.e., the comparison of different intervention options at different stages of the intervention). For example, assume we have LLIN, IRS, and larviciding. We can start with two arms: LLIN and LLIN+IRS (i.e., begin with indoor interventions; we add larviciding at stage II). What is the main effect at different stages? What is the difference in the final outcome between two intervention strategies that begin with different stage I interventions (e.g., LLIN versus LLIN+IRS)? And, of course, in terms of the overall optimal strategy, which intervention strategy produces the most cost-effective outcome? Remember, both IRS and larviciding may be eliminated at the middle or by the end of the trial if they are deemed ineffective.
The sequence of the interventions may have impacts on the overall outcomes (i.e., the optimal combination of interventions at a given ecological setting). This sequential assignment and outdoor transmission likely explain the difference in overall reduction in MIP between sequential intervention combinations PBO-LLIN+LLML+IRS and PBO-LLIN+IRS+LLML. Long-lasting microbial larviciding and alternative insecticide IRS may be equally effective in reducing overall MIP. However, LLML reduces both indoor and outdoor vector density, thereby reducing potential future overall transmission, whereas IRS only reduces indoor vector density but has limited impact on outdoor vectors, especially outdoor resting vectors. Therefore, IRS application results in a higher potential of future transmission because the outdoor resting/biting female insects will maintain the overall vector density or with a compromised reduction in overall vector density. In other words, the sequential applications of PBO-LLIN → LLML and PBO-LLIN → IRS will result in different entomological conditions, which in turn, affects the effectiveness of the subsequent interventions.
Optimal integrated strategy in different ecological-epidemiological settings.
As mentioned earlier, ecological and epidemiological heterogeneity may affect intervention outcomes, and thus different intervention strategies may be required in different settings. For example, in the Kakamega highlands of western Kenya, A. gambiae s.s. is the predominant malaria vector, accounting for > 90% of all vectors, which is very different from Kombewa.4,22 Although outdoor transmission exists, A.. gambiae s.s. is still considered as primarily indoor-resting and human-feeding. Here, simulation results indicated that adding LLML on top of LLIN may add just a 29.4% relative reduction in entomological inoculation rate (EIR), and adding LLML on top of LLIN+IRS added a 22.8% relative reduction in EIR (Zhou, unpublished data). In Kakamega, MIP was around 40% in 2002 and 13% in 2016.22,29 Assuming a high killing rate (80%) for LLIN, simulation results indicated that LLIN alone (Note: universal artemisinin-based combination therapies and LLIN have been implemented since 2006) had reduced MIP by 76.8% since 2006,22,49 which is similar to field observations made when vector insecticide resistance was relatively low.22,29 Further reductions in MIP may require a different intervention strategy in these areas, for example, IRS with alternative insecticides.30 Although these results must be assessed further through field trials, they demonstrate the potential power of an adaptive trial design.
Future emerging interventions for outdoor transmission control.
Outdoor transmission has become increasingly important due to the selection pressure posed by the indoor interventions such as LLINs and IRS.4,9,23 Larviciding reduces both indoor and outdoor transmission in high-transmission areas, as demonstrated by field trials and model simulations.17,18 However, larval control is highly labor intensive and requires continual enhancement due to the potential loss of larvicides (e.g., rain wash), the creation of new breeding habitats after the rain, and the loss of habitats during dry season. Other interventions, such as ATSB and spatial repellent, may be alternative choices against outdoor transmission.9,11 In addition, new interventions may become available for public health application (e.g., G2 LLINs, a new LLIN based on chlorfenapy).9 Adding new interventions into the adaptive design simply adds additional arms or stages. Modeling simulations can be done in the same way, although field evaluations may become more intensive because they require more field work.
Selection of optimal strategy: Multiple criteria.
In malaria epidemiology and control, EIR is considered a good entomological indicator for measuring the intensity of transmission70; EIR is relatively easy to observe in the field. However, MIP or clinical incidence rate (CIR) gives a more direct measurement of transmission. If multiple criteria are considered, intervention evaluation can be done using Q-learning with a weighted reward combining EIR, CIR, and MIP.71–74 In this case, a reduction in MIP, CIR, or EIR can only be obtained after the intervention—a time-delayed reward. We can define a weighted Q-function of EIR, MIP, and CIR to measure the reduction in transmission. The goal is to find the best possible Q-score at the end of each intervention stage after performing different interventions.71,72,74
Selection of optimal strategy: Cost-effectiveness.
Cost-effectiveness is key to the selection of optimal intervention strategies. There is substantial literature on the cost and cost-effectiveness of malaria interventions.21,45,75,76 Previous cost-effectiveness analysis has focused primarily on individual interventions; the cost-effectiveness of an integrated vector management program has not been thoroughly evaluated. Adding more interventions on top of existing interventions may further reduce disease transmission, but it may also increase material and operational costs. Cost-effectiveness can be measured as a discounted reduction in transmission in the Q-functions73,74; that is, if an additional intervention will increase the cost, the reduction in transmission is discounted by the cost (e.g., weighted inversely against the increased cost) at each intervention stage. Further discounts may be applied at subsequent stages of the intervention.
Clearly, when cost-effectiveness is evaluated, the optimal intervention strategies may change. For example, adding IRS on existing LLIN is more effective in reducing MIP than that by PBO-LLIN alone. However, IRS must be reapplied periodically, whereas PBO-LLIN may be effective continuously for up to 2–3 years. Thus, the differences in implementation and material costs must be considered with regard to the gains in reduction in malaria cases.
CONCLUSIONS.
In summary, integrated vector management is considered the optimal solution for preventing vector-borne infectious diseases. The scale-up for universal coverage of LLINs and IRS for malaria control has caused widespread mosquito resistance and increased outdoor transmission. Thus, new viable integrated intervention strategies are urgently needed to adapt to the changing ecology and epidemiology. With so many available and emerging interventions, conventional RCT/CRT or similar trial designs will not be a cost-effective way to find the optimal combination of interventions. An adaptive trial design may provide the high-quality data needed to determine the optimal cost-effective combination of interventions that can adapt to transmission heterogeneity and changing ecology and epidemiology. Adaptive trial design is both conceptually and scientifically appealing. Based on an adaptive trial design, we described an example in malaria control using the drop-the-loser design to address the question of finding the optimal integrated intervention. Modeling simulation conditions can be modified so that results can be obtained in different transmission settings, and results can be evaluated in field trials. Most importantly, our example provides a framework or new pathways for informing the optimal integrated intervention when field assessment supports the simulation results. Even if field tests only partially support the simulation results, the model can be modified and the parameters recalibrated so that the results better suit the field conditions. Malaria remains one of the most serious vector-borne infectious diseases, and adaptive trial designs provide an alternative pathway to improve the efficacy and effectiveness of interventions in vector-borne disease control. These designs and simulation results deserve continued research attention.
Supplemental Material
Note: Supplemental table and figures appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization , 2019. World Malaria Report 2019. Geneva, Switzerland: WHO. [Google Scholar]
- 2. World Health Organization , 2012. World Malaria Report 2012. Geneva, Switzerland: WHO. [Google Scholar]
- 3. Bhatt S. et al. , 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, Yan G, 2017. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J 16: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The malERA Refresh Consultative Panel on Insecticide and Drug Resistance, 2017. malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS Med 14: e1002450.
- 6. Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, Djègbé I, Guis H, Corbel V, 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 206: 1622–1629. [DOI] [PubMed] [Google Scholar]
- 7. Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, Birungi J, Wondji CS, 2014. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS One 9: e110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, Zhou G, Githeko AK, Yan G, 2015. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerg Infect Dis 21: 2178–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The malERA Refresh Consultative Panel on Tools for Malaria Elimination, 2017. An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication. PLoS Med 14: e1002455.
- 10. Benelli G, Beier JC, 2017. Current vector control challenges in the fight against malaria. Acta Trop 174: 91–96. [DOI] [PubMed] [Google Scholar]
- 11. Tizifa TA, Kabaghe AN, McCann RS, van den Berg H, Van Vugt M, Phiri KS, 2018. Prevention efforts for malaria. Curr Trop Med Rep 5: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Protopopoff N. et al. , 2018. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391: 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Achee NL. et al. , 2019. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis 13: e0006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinder M. et al. , 2015. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet 385: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 15. Sluydts V. et al. , 2016. Efficacy of topical mosquito repellent (picaridin) plus long-lasting insecticidal nets versus long-lasting insecticidal nets alone for control of malaria: a cluster randomised controlled trial. Lancet Infect Dis 16: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 16. Qualls WA, Müller GC, Traore SF, Traore MM, Arheart KL, Doumbia S, Schlein Y, Kravchenko VD, Xue RD, Beier JC, 2015. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar J 14: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Afrane YA, Mweresa NG, Wanjala CL, Gilbreath TM, III, Zhou G, Lee MC, Githeko AK, Yan G, 2016. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar J 15: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fillinger U, Ndenga B, Githeko A, Lindsay SW, 2009. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Organ 87: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw WR, Marcenac P, Childs LM, Buckee CO, Baldini F, Sawadogo SP, Dabiré RK, Diabaté A, Catteruccia F, 2016. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun 7: 11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Homan T. et al. , 2016. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster-randomised trial. Lancet 388: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 21. Dambach P, Schleicher M, Stahl H-C, Traoré I, Becker N, Kaiser A, Sié A, Sauerborn R, 2016. Routine implementation costs of larviciding with Bacillus thuringiensis israelensis against malaria vectors in a district in rural Burkina Faso. Malar J 15: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, Himeidan YE, Minakawa N, Githeko AK, Yan G, 2011. Changing patterns of malaria epidemiology between 2002 and 2010 in western Kenya: the fall and rise of malaria. PLoS One 6: e20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Killeen GF. et al. , 2017. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob Health 2: e000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ndenga BA, Mulaya NL, Musaki SK, Shiroko JN, Dongus S, Fillinger U, 2016. Malaria vectors and their blood-meal sources in an area of high bed net ownership in the western Kenya highlands. Malar J 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obala AA, Kutima HL, Nyamogoba HD, Mwangi AW, Simiyu CJ, Magak GN, Khwa-Otsyula BO, Ouma JH, 2012. Anopheles gambiae and Anopheles arabiensis population densities and infectivity in Kopere village, western Kenya. J Infect Devel Countr 6: 637–643. [DOI] [PubMed] [Google Scholar]
- 26. Githeko AK, Service MW, Mbogo CM, Atieli FK, Jurna FO, 1994. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop 58: 307–316. [DOI] [PubMed] [Google Scholar]
- 27. Awine T, Malm K, Bart-Plange C, Silal SP, 2017. Towards malaria control and elimination in Ghana: challenges and decision making tools to guide planning. Glob Health Action 10: 1381471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, Yan G, Zhou G, 2018. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou G, Lee M-C, Githeko AK, Atieli HE, Yan G, 2016. Insecticide-treated net campaign and malaria transmission in western Kenya: 2003–2015. Front Public Health 4: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katureebe A. et al. , 2016. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med 13: e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curtis CF, Maxwell CA, Magesa SM, Rwegoshora RT, Wilkes TJ, 2006. Insecticide-treated bed-nets for malaria mosquito control. J Am Mosq Control Assoc 22: 501–506. [DOI] [PubMed] [Google Scholar]
- 32. Carnevale P, Gay F, 2019. Insecticide-treated mosquito nets. Methods Mol Biol 2013: 221–232. [DOI] [PubMed] [Google Scholar]
- 33. Tangena JA. et al. , 2020. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: an adjusted retrospective analysis. Malar J 19: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi L, Pryce J, Garner P, 2019. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Database Syst Rev 5: CD012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.USAID/PMI, 2018. Kenya Malaria Operational Plan FY 2019. Available at: https://www.pmi.gov/where-we-work/kenya/. Accessed March 5, 2020.
- 36.USAID/PMI, 2017. Uganda Malaria Operational Plan FY 2018. Available at: https://www.pmi.gov/where-we-work/kenya/. Accessed March 5, 2020.
- 37.USAID/PMI, 2018. Ethiopia Malaria Operational Plan FY 2019. Available at: https://www.pmi.gov/where-we-work/kenya/. Accessed March 5, 2020.
- 38. Bothwell LE, Greene JA, Podolsky SH, Jones DS, 2016. Assessing the gold standard – lessons from the history of RCTs. N Engl J Med 374: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 39. Chow S-C, 2014. Adaptive clinical trial design. Annu Rev Med 65: 405–415. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Mehta C, 2016. Adaptive designs for clinical trials. N Engl J Med 375: 65–74 Rev. [DOI] [PubMed] [Google Scholar]
- 41. Huskins WC, Fowler VG, Jr, Evans S, 2018. Adaptive designs for clinical trials: application to healthcare epidemiology research. Clin Infect Dis 66: 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA, 2012. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol 8: 21–48 Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rabinovich RN. et al. , 2017. malERA: an updated research agenda for malaria elimination and eradication. PLoS Med 14: e1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mulligan J-A, Yukich J, Hanson K, 2008. Costs and effects of the Tanzanian national voucher scheme for insecticide-treated nets. Malar J 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stelmach R, Colaço R, Lalji S, McFarland D, Reithinger R, 2018. Cost-effectiveness of indoor residual spraying of households with insecticide for malaria prevention and control in Tanzania. Am J Trop Med Hyg 99: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Biswas A, Bhattacharya R, 2016. Response-adaptive designs for continuous treatment responses in phase III clinical trials: a review. Stat Methods Med Res 25: 81–100 Rev. [DOI] [PubMed] [Google Scholar]
- 47. Grieve AP, 2017. Response-adaptive clinical trials: case studies in the medical literature. Pharm Stat 16: 64–86 Rev. [DOI] [PubMed] [Google Scholar]
- 48. Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, Mbogo C, Minakawa N, Zhou G, Yan G, 2006. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol 43: 200–206. [DOI] [PubMed] [Google Scholar]
- 49. Kapesa A, Kweka EJ, Atieli H, Kamugisha E, Zhou G, Githeko AK, Yan G, 2017. Why some sites are responding better to anti-malarial interventions? A case study from western Kenya. Malar J 16: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Machani MG, Ochomo E, Zhong D, Zhou G, Wang X, Githeko AK, Yan G, Afrane YA, 2020. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci Rep 10: 19063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eckhoff PA, 2011. A malaria transmission-directed model of mosquito life cycle and ecology. Malar J 10: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eckhoff PA, 2013. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg 88: 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eckhoff PA, 2012. Malaria parasite diversity and transmission intensity affect development of parasitological immunity in a mathematical model. Malar J 11: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eckhoff PA, 2012. P. falciparum infection durations and infectiousness are shaped by antigenic variation and innate and adaptive host immunity in a mathematical model. PLoS One 7: e44950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wanjala CL, Zhou G, Mbugi J, Simbauni J, Afrane YA, Ototo E, Gesuge M, Atieli H, Githeko AK, Yan G, 2015. Insecticidal decay effects of long-lasting insecticide nets and indoor residual spraying on Anopheles gambiae and Anopheles arabiensis in western Kenya. Parasit Vectors 8: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. PMI VectorLink Kenya , 2019. Annual Entomological Monitoring Report, October 2017–September 2018. Rockville, MD: The PMI VectorLink Project, Abt Associates Inc. [Google Scholar]
- 57. Minta AA, Landman KZ, Mwandama DA, Shah MP, Vanden Eng JL, Sutcliffe JF, Chisaka J, Lindblade KA, Mathanga DP, Steinhardt LC, 2016. The effect of holes in long-lasting insecticidal nets on malaria in Malawi: results from a case–control study. Malar J 16: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. WHO , 2013. Recommendations for Achieving Universal Coverage with Long-Lasting Insecticidal Nets in Malaria Control. Geneva, Switzerland: World Health Organization.
- 59. National Malaria Control Programme , 2018. Kenya Malaria Strategy 2019–2023. Nairobi, Kenya: Ministry of Health. [Google Scholar]
- 60. Mutuku FM, Khambira M, Bisanzio D, Mungai P, Mwanzo I, Muchiri EM, King CH, Kitron U, 2013. Physical condition and maintenance of mosquito bed nets in Kwale County, coastal Kenya. Malar J 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rotondi MA, Donner A, 2012. Sample size estimation in cluster randomized trials: an evidence-based perspective. Comput Stat Data Anal 56: 1174–1187. [Google Scholar]
- 62. Tiono AB. et al. , 2018. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. Lancet 392: 569–580. [DOI] [PubMed] [Google Scholar]
- 63. NeCamp T , Kilbourne A, Almirall D, 2017. Comparing cluster-level dynamic treatment regimens using sequential, multiple assignment, randomized trials: regression estimation and sample size considerations. Stat Methods Med Res 26: 1572–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano G, Waxmonsky JG, Yu J, Murphy SA, 2012. Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods 17: 457–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lu X, Nahum-Shani I, Kasari C, Lynch KG, Oslin DW, Pelham WE, Fabiano G, Almirall D, 2016. Comparing dynamic treatment regimes using repeated-measures outcomes: modeling considerations in SMART studies. Stat Med 35: 1595–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou G, Lee MC, Atieli HE, Githure JI, Githeko AK, Kazura JW, Yan G, 2020. Adaptive interventions for optimizing malaria control: an implementation study protocol for a block-cluster randomized, sequential multiple assignment trial. Trials 21: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, Yan G, 2005. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. Am J Trop Med Hyg 73: 157–165. [PubMed] [Google Scholar]
- 68. Himeidan YE, Zhou G, Yakob L, Afrane Y, Munga S, Atieli H, El-Rayah E, Githeko AK, Yan G, 2009. Habitat stability and occurrences of malaria vector larvae in western Kenya highlands. Malar J 8: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kweka EJ, Zhou G, Munga S, Lee M-C, Atieli HE, Nyindo M, Githeko AK, Yan G, 2012. Anopheline larval habitats seasonality and species distribution: a prerequisite for effective targeted larval habitats control programmes. PLoS One 7: e52084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Penny MA, Maire N, Bever CA, Pemberton-Ross P, Briët OJT, Smith DL, Gething PW, Smith TA, 2016. Distribution of malaria exposure in endemic countries in Africa considering country levels of effective treatment. Malar J 14: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, Waxmonsky JG, Yu J, Murphy SA, 2012. Q-learning: a data analysis method for constructing adaptive interventions. Psychol Methods 17: 478–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Murphy SA, Oslin DW, Rush AJ, Zhu J, MCATS, 2007. Methodological challenges in constructing effective treatment sequences for chronic psychiatric disorders. Neuropsychopharmacology 32: 257–262. [DOI] [PubMed] [Google Scholar]
- 73. Bergmeir P, 2018. Enhanced Machine Learning and Data Mining Methods for Analysing Large Hybrid Electric Vehicle Fleets based on Load Spectrum Data. Wiesbaden, Germany: Springer Verlag. [Google Scholar]
- 74. Liu N, Liu Y, Logan B, Xu Z, Tang J, Yang Y, 2019. Learning the dynamic treatment regimes from medical registry data through deep Q-network. Sci Rep 9: 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. White MT, Conteh L, Cibulskis R, Ghani AC, 2011. Costs and cost-effectiveness of malaria control interventions – a systematic review. Malar J 10: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dudley HJ, Goenka A, Orellana CJ, Martonosi SE, 2016. Multi-year optimization of malaria intervention: a mathematical model. Malar J 15: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.