ABSTRACT.
Our aim was to identify the risk factors associated with unsuccessful outcomes of tuberculosis (TB) treatment in patients diagnosed between 2014 and 2016 in the 125 municipalities of Antioquia, Colombia. We studied a retrospective cohort of patients with TB diagnosed between 2014 and 2016, from national routine surveillance systems, in 125 municipalities of Antioquia. Factors associated with unsuccessful tuberculosis treatment outcomes (treatment failed, lost to follow up, or death) were identified utilizing a Poisson regression with robust variance. Over 3 years, of the 6,739 drug-susceptible tuberculosis patients, 73.4% had successful treatment and 26.6% unsuccessful outcomes (17% lost to follow up, 8.9% deaths, and 0.7% treatment failures). Patients with subsidized health insurance (Relative risk [RR]: 2.4; 95% CI: 2.1–2.8) and without health insurance (RR: 2.5; 95% CI: 2.1–3.0) had a higher risk for unsuccessful tuberculosis treatment compared to those with contributive health insurance. Other risk factors included age over 15 years, male sex, homelessness, people living with HIV, previous treatment, and primary diagnosis during hospitalization. Protective factors were living in a rural area and extrapulmonary disease. It is important to generate strategies that improves tuberculosis diagnosis in primary healthcare institutions. In addition, it is imperative to initiate new research about the barriers and obstacles related to patients, healthcare workers and services, and the health system, including the analysis of urban violence, to understand why the goal of TB treatment success has not been reached.
INTRODUCTION
In 2019, there were more than 10 million new tuberculosis (TB) patients and 1.2 million deaths in the world.1 In 2014, the WHO launched the End TB Strategy with targets to be met by 2035, including a 90% reduction in TB incidence compared with 2015. The achievement of this goal is compromised by the high percentage of unsuccessful TB treatment that perpetuates TB transmission at the public level.2
In previous studies, the following factors have been associated as risk factors with lost to follow up, failure or death in patients with drug-susceptible TB: male sex, being older than 75 years, weight < 35 kg, living in rural area, low education level, previously treated patients, abnormal X-ray, HIV coinfection, diabetes, other associated diseases, drug users, pulmonary TB with sputum smear-negative at the time of diagnosis, extrapulmonary TB, sputum smear-positive at second month of TB treatment.3–15
The state of Antioquia, with 125 municipalities, has historically had the highest number of TB cases in Colombia.16 Treatment success has been achieved in only 73% of patients,17 which is far from the national and global goal of 90%.2
In this context, and to generate strategies that positively impact the outcome of TB treatment, the aim of this study was to identify the risk factors associated with unsuccessful outcomes of TB treatment in patients diagnosed between 2014 and 2016 within the 125 municipalities of Antioquia.
MATERIALS AND METHODS
Settings.
Colombia is an upper middle-income country in South America.18 On June 2018, 6,651,890 people lived in a total of 125 municipalities of the state of Antioquia.19
The standard regimen for drug-susceptible TB in Colombia is the fixed-dose combination tablets paid by the government. For patients without comorbidities, treatment is prescribed for 6 months, the intensive phase (56 doses) includes isoniazid, rifampin, ethambutol, and pyrazinamide, and the continuation phase (56 doses), isoniazid and rifampin. For those with diabetes or HIV, treatment is 9 months, and 12 months for people with meningeal or osteoarticular TB. HIV testing is offered to all people with TB diagnosis, free of charge.
Study design and population.
We conducted a retrospective cohort-based study using surveillance data from the Tuberculosis Control Program in the state of Antioquia. The study population included all drug-susceptible TB patients notified during 2014, 2015, and 2016. We analyzed drug-susceptible TB patients only, because they represent 99.3% of all TB patients in the state and TB treatment is different from those with resistant TB (efficacy of first- and second-line drugs, adverse events and duration).20,21
Source of data.
We used two databases: 1) The Surveillance Health Public System (SIVIGILA, by the Spanish acronym) that records the mandatory reporting of diseases and conditions for national public health surveillance, including TB; and 2) the Tuberculosis Information System of Antioquia (SITB, by the Spanish acronym) that records all patients who are admitted to the TB program in Antioquia and registers follow-up information. Both databases were merged using the identification number of the patients and their full names. The data was then anonymized. In a total of 7,258 records, we excluded 472 duplicated patients (because two or more institutions notified the same patient) and 47 patients with drug resistance who were diagnosed during drug-susceptible TB treatment; therefore, the study population totaled 6,739 cases. The database is available in the Supplemental Material S1.
Variables.
The TB treatment outcomes are reported in SITB according to the definitions of the Ministry of Health and Social Protection of Colombia22 and the WHO.23
Successful treatment was defined as the sum of cured and treatment completed. Unsuccessful outcome of TB treatment was defined as the sum of those whose treatment failed, died, or were lost to follow up. In addition, we analyzed the lost to follow up and death outcomes separately, comparing with those who had successful treatment outcomes (Supplemental Tables 1-S2 and 2-S3). Only 45 cases failed treatment, so they were not considered in this separate analysis.
We evaluated the following sociodemographic variables: age, sex (female and male), ethnicity (indigenous, Afro-Colombian and other), living area (urban [refer to towns, cities, and suburbs, with ≥ 25,000 persons], rural [≤ 25,000 inhabitants and population densities between 10 and 100 inhabitants/km2] ,and scattered rural [≤ 25,000 inhabitants and population densities less than 50 inhabitants/km2]);24 health insurance (contributive, subsidized, and poor without health insurance); healthcare worker; homeless; and prisoners. The clinical variables analyzed included HIV, diabetes, chronic obstructive pulmonary disease (COPD), drug disorder, chronic kidney disease, other immunodeficiency disorders, previously treated patients, year of notification, if the patient got the diagnosis in a hospital and disease form (pulmonary or extrapulmonary). The latter was classified according to the WHO definitions,23 which states that both pulmonary and extrapulmonary TB should be included as a pulmonary TB case. Regarding the health insurance, in Colombia, “contributive” means people who have work as independent or as employee and pay the health insurance through their companies or by themselves. “Subsidized” are those who cannot afford health insurance and are paid by the national, state, or local governments. For those classified as “poor without health insurance,” financial assistance was provided by the municipality where they lived, and the hospitalization or assistance in medium- or high-complexity institutions, by the state level of support. TB treatment, cultures, drug susceptibility testing, and hospitalization costs are paid by health insurance companies, and for “poor without health insurance” charges are paid by the state.
Although height and weight variables were available in the database, the data were inconsistent, so we decided not to include these in study.
Statistical analysis.
Tuberculosis cases were described by sociodemographic, clinical variables, and TB treatment outcomes. Relative risks (RR) and 95%CI were estimated using a Poisson regression with robust variance to identify factors associated with unsuccessful TB treatment outcomes in bivariate and multivariate models. For the multivariate model, we used a stepwise forward selection method using variables with a P value < 0.25 in the bivariate analysis and preserved the variables with a P < 0.05. To adjust for possible changes in TB control recommendations during the study period, we included the year of notification in all models as an adjustment variable.
Our primary analysis was based upon a completed case analysis. We ran a sensitivity analysis to address missing data by transforming all “not evaluated” patients to successful outcomes and then to unsuccessful outcomes (Supplemental Table 3-S5). We also used multiple imputation for all abovementioned predictor variables with missing data, including the TB treatment outcome. Variables with greater than 20% missing data were only imputed and not used as predictors.25 Body mass index (BMI) was not considered in the analysis because in addition of having more than 20% missing data, it had also more than 15% inconsistent and unreal values.
We estimated the fraction of unsuccessful TB treatment outcomes that was attributable to the health insurance system (PAF)26 using the formula:
where p′ refers to the prevalence of people who received subsidized health care insurance, people without health insurance and people with contributive health insurance in people with unsuccessful outcome of TB treatment, and θ is the RR estimated by a multivariate model.
Confidence intervals for the PAF were calculated using a nonlinear combination of parameters estimated based upon the delta method.27
Statistical analyses were performed with the Stata® statistical package version 12.0 (StataCorp., College Station, TX), and for multiple imputation R® version 3.3.1 Amelia library.25
Ethics statement.
The study was approved by the Ethics Committee of Universidad Pontificia Bolivariana and the Ethics Committee of the Pan American Health Organization. Researchers had written permission to use information from SIVIGILA and SITB by the Secretaria Seccional de Salud y Protección Social de Antioquia.
RESULTS
A total population of 6,739 TB cases diagnosed and admitted to the tuberculosis program were included in the study. Among them, 95.9% (6,465/6,739) were tested for HIV (in 182 patients the HIV test was not done, nine refused the testing, and 15 did not have results available). Seventy percent of patients were 15 to 54 years old, 62.8% were men, 8.7% were homeless, 82.2% had pulmonary TB, 14.1% were HIV coinfected, 13.4% previously treated for TB, and 43.9% received a primary diagnosis during hospitalization. The average percentage of successful TB treatment of the 3 years was 73.4% (Supplemental Figure 1-S4).
The highest percentage of lost to follow up were in patients between 25 and 44 years old, male, who did not have health insurance, and homeless. People ≥ 65 years old and male had higher mortality percentage (Table 1), as well as, people with HIV, COPD, chronic kidney disease, and for patients who had TB diagnosed during hospitalization (Table 2). Previously treated patients and those with drug use disorders had a higher percentage of lost to follow up (Table 2).
Table 1.
Sociodemographic variables of tuberculosis patients according to tuberculosis treatment outcome, Antioquia, Colombia, 2014 to 2016
| Sociodemographic variables | Cured, N = 1,987, n (%) |
Treatment completed, N = 2,536, n (%) |
Lost to follow up, N = 1,041, n (%) |
Treatment failed, N = 45, n (%) |
Died, N = 547, n (%) |
Not evaluated, N = 583, n (%) |
|---|---|---|---|---|---|---|
| Age in years | ||||||
| Less than 1 | 0 (0) | 10 (55.6) | 5 (27.8) | 0 (0) | 1 (5.6) | 2 (11.1) |
| 1–4 | 3 (2.9) | 89 (84.8) | 9 (8.6) | 0 (0) | 0 (0) | 4 (3.8) |
| 5–14 | 20 (17.2) | 70 (60.3) | 9 (7.8) | 0 (0) | 3 (2.6) | 14 (12.1) |
| 15–24 | 340 (32.4) | 416 (39.7) | 183 (17.4) | 5 (0.5) | 28 (2.7) | 77 (7.3) |
| 25–34 | 438 (29.1) | 542 (36) | 334 (22.2) | 8 (0.5) | 67 (4.4) | 118 (7.8) |
| 35–44 | 257 (26.4) | 370 (38) | 186 (19.1) | 10 (1) | 67 (6.9) | 84 (8.6) |
| 45–54 | 342 (30.2) | 405 (35.8) | 173 (15.3) | 11 (1) | 86 (7.6) | 114 (10.1) |
| 55–64 | 333 (34) | 346 (35.3) | 90 (9.2) | 5 (0.5) | 123 (12.6) | 82 (8.4) |
| ≥ 65 | 254 (29.5) | 288 (33.5) | 52 (6) | 6 (0.7) | 172 (20) | 88 (10.2) |
| Sex | ||||||
| Female | 809 (32.3) | 1,041 (41.5) | 283 (11.3) | 19 (0.8) | 150 (6) | 206 (8.2) |
| Male | 1,178 (27.8) | 1,495 (35.3) | 758 (17.9) | 26 (0.6) | 397 (9.4) | 377 (8.9) |
| Ethnicity | ||||||
| Indigenous | 12 (18.8) | 35 (54.7) | 10 (15.6) | 0 (0) | 3 (4.7) | 4 (6.3) |
| Afro-Colombian | 39 (27.9) | 63 (45) | 24 (17.1) | 2 (1.4) | 3 (2.1) | 9 (6.4) |
| Other | 1,936 (29.6) | 2,438 (37.3) | 1,007 (15.4) | 43 (0.7) | 541 (8.3) | 570 (8.7) |
| Living area | ||||||
| Urban | 1,460 (28.3) | 1,919 (37.2) | 851 (16.5) | 18 (0.3) | 402 (7.8) | 508 (9.8) |
| Rural | 101 (38.1) | 91 (34.3) | 27 (10.2) | 3 (1.1) | 21 (7.9) | 22 (8.3) |
| Scattered rural | 76 (30.5) | 94 (37.8) | 21 (8.4) | 0 (0) | 25 (10) | 33 (13.3) |
| Health insurance | ||||||
| Contributive | 921 (30.4) | 1,490 (49.1) | 156 (5.1) | 17 (0.6) | 181 (6) | 267 (8.8) |
| Subsidized | 944 (29.4) | 933 (29) | 698 (21.7) | 23 (0.7) | 328 (10.2) | 289 (9) |
| Poor people without health insurance | 122 (24.8) | 113 (23) | 187 (38) | 5 (1) | 38 (7.7) | 27 (5.5) |
| Healthcare worker | ||||||
| No | 1,574 (28.7) | 2,023 (36.9) | 869 (15.9) | 20 (0.4) | 441 (8) | 553 (10.1) |
| Yes | 31 (30.7) | 52 (51.5) | 6 (5.9) | 1 (1) | 2 (2) | 9 (8.9) |
| Homeless | ||||||
| No | 1,880 (30.6) | 2,447 (39.8) | 721 (11.7) | 41 (0.7) | 519 (8.4) | 542 (8.8) |
| Yes | 107 (18.2) | 89 (15.1) | 320 (54.3) | 4 (0.7) | 28 (4.8) | 41 (7) |
| Prisoners | ||||||
| No | 1,950 (29.3) | 2,505 (37.7) | 1,027 (15.5) | 45 (0.7) | 546 (8.2) | 574 (8.6) |
| Yes | 37 (40.2) | 31 (33.7) | 14 (15.2) | 0 (0) | 1 (1.1) | 9 (9.8) |
Table 2.
Clinical variables of tuberculosis patients according to tuberculosis treatment outcome. Antioquia, Colombia, 2014 to 2016
| Clinical variables | Cured, N = 1,987, n (%) |
Treatment completed, N = 2,536, n (%) |
Lost to follow up, N = 1,041, n (%) |
Treatment failed, N = 45, n (%) |
Death, N = 547, n (%) |
Not evaluated, N = 583, n (%) |
|---|---|---|---|---|---|---|
| Disease form | ||||||
| Pulmonary | 1,984 (35.8) | 1,673 (30.2) | 940 (17) | 40 (0.7) | 443 (8) | 461 (8.3) |
| Extrapulmonary | 3 (0.3) | 863 (72) | 101 (8.4) | 5 (0.4) | 104 (8.7) | 122 (10.2) |
| HIV | ||||||
| No | 1,831 (33.2) | 2,096 (38) | 762 (13.8) | 34 (0.6) | 357 (6.5) | 438 (7.9) |
| Yes | 121 (12.8) | 365 (38.5) | 209 (22.1) | 10 (1.1) | 125 (13.2) | 117 (12.4) |
| Diabetes | ||||||
| No | 1,927 (29.1) | 2,495 (37.7) | 1,031 , (15.6) | 42 (0.6) | 540 (8.2) | 583 (8.8) |
| Yes | 60 (49.6) | 41 (33.9) | 10 (8.3) | 3 (2.5) | 7 (5.8) | 0 (0) |
| Chronic obstructive pulmonary disease | ||||||
| No | 1,968 (29.4) | 2,520 (37.6) | 1,039 (15.5) | 45 (0.7) | 541 (8.1) | 583 (8.7) |
| Yes | 19 (44.2) | 16 (37.2) | 2 (4.7) | 0 (0) | 6 (14) | 0 (0) |
| Drug disorder | ||||||
| No | 1,916 (29.4) | 2,485 (38.1) | 950 (14.6) | 40 (0.6) | 544 (8.3) | 583 (8.9) |
| Yes | 71 (32.1) | 51 (23.1) | 91 (41.2) | 5 (2.3) | 3 (1.4) | 0 (0) |
| Chronic kidney disease | ||||||
| No | 1,982 (29.6) | 2,518 (37.6) | 1,038 (15.5) | 43 (0.6) | 541 (8.1) | 583 (8.7) |
| Yes | 5 (14.7) | 18 (52.9) | 3 (8.8) | 2 (5.9) | 6 (17.6) | 0 (0) |
| Other immunodeficiency disorders | ||||||
| No | 1,970 (29.4) | 2,523 (37.7) | 1,036 (15.5) | 45 (0.7) | 541 (8.1) | 580 (8.7) |
| Yes | 17 (38.6) | 13 (29.5) | 5 (11.4) | 0 (0) | 6 (13.6) | 3 (6.8) |
| Previously treated patient | 191 (21.1) | 193 (21.3) | 367 (40.5) | 8 (0.9) | 79 (8.7) | 68 (7.5) |
| No | 1,785 (31) | 2,314 (40.2) | 665 (11.5) | 36 (0.6) | 460 (8) | 500 (8.7) |
| Yes | 191 (21.1) | 193 (21.3) | 367 (40.5) | 8 (0.9) | 79 (8.7) | 68 (7.5) |
| TB treatment center different to diagnostic center | ||||||
| No | 230 (30.1) | 237 (31) | 120 (15.7) | 2 (0.3) | 87 (11.4) | 89 (11.6) |
| Yes | 508 (23.9) | 874 (41.1) | 330 (15.5) | 4 (0.2) | 163 (7.7) | 248 (11.7) |
| Hospital diagnosis | ||||||
| No | 1,075 (39.7) | 895 (33) | 395 (14.6) | 14 (0.5) | 87 (3.2) | 244 (9) |
| Yes | 562 (19) | 1,209 (40.8) | 504 (17) | 7 (0.2) | 361 (12.2) | 319 (10.8) |
| Year of notification | ||||||
| 2014 | 793 (33.4) | 972 (40.9) | 371 (15.6) | 34 (1.4) | 186 (7.8) | 20 (0.8) |
| 2015 | 605 (29.7) | 740 (36.3) | 341 (16.7) | 4 (0.2) | 157 (7.7) | 189 (9.3) |
| 2016 | 589 (25.3) | 824 (35.4) | 329 (14.1) | 7 (0.3) | 204 (8.8) | 374 (16.1) |
TB = tuberculosis.
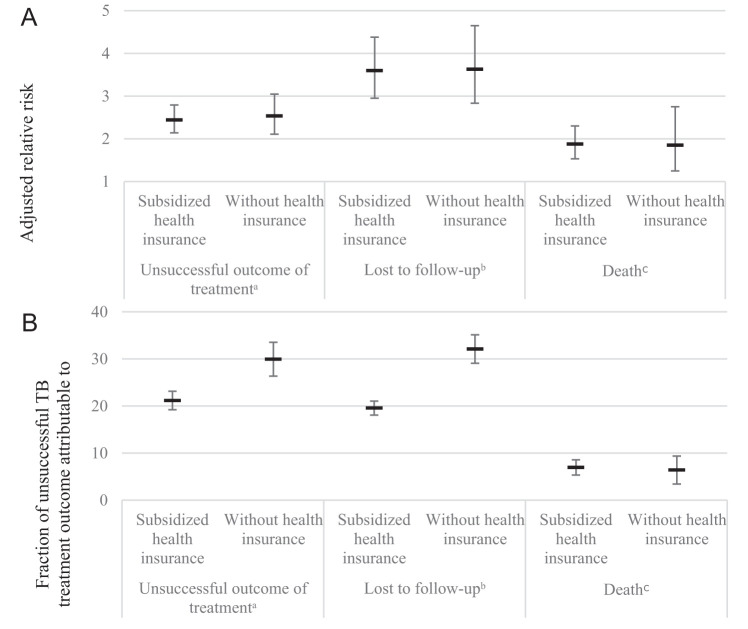
Patients with subsidized health insurance (RR: 2.4; 95% CI: 2.1–2.8) and without health insurance (RR: 2.5; 95% CI: 2.1–3.0) presented a higher risk for unsuccessful TB treatment compared with those with contributive health insurance (Table 3). This was also observed in the analysis with lost to follow up (RR for subsidized: 3.6, 95% CI: 3–4.4; RR for without: 3.6, 95% CI: 2.8–4.7, compared with contributive) (Supplemental Table 1-S2) and death (RR for subsidized: 1.9, 95% CI: 1.5–2.3; RR for without: 1.9, 95% CI: 1.2–2.8, compared with contributive) (Supplemental Table 2-S3). In addition, the three models that we ran for sensitivity analysis presented similar results for the association of health insurance and unsuccessful TB treatment (Supplemental Table 3-S5).
Table 3.
Factors associated with unsuccessful tuberculosis treatment outcome. Antioquia, Colombia, 2014 to 2016
| Variables | Successful treatment, N = 4,523, n (%) |
Unsuccessful treatment, N = 1,633, n (%) |
RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|---|---|
| Age in years | ||||
| < 15 | 900 (80.8) | 214 (19.2) | 1 | 1 |
| 15–54 | 2,930 (73.2) | 1,073 (26.8) | 1.4 (1.2–1.6) | 1.2 (1–1.3) |
| ≥ 55 | 520 (70.8) | 214 (29.2) | 1.5 (1.3–1.8) | 1.7 (1.4–2) |
| Male sex | 2,673 (69.4) | 1,181 (30.6) | 1.6 (1.4–1.7) | 1.3 (1.2–1.4) |
| Ethnicity | ||||
| Indigenous | 47 (78.3) | 13 (21.7) | 1 | |
| Afro-Colombian | 102 (77.9) | 29 (22.1) | 1 (0.6–1.8) | |
| Other | 4,374 (73.3) | 1,591 (26.7) | 1.2 (0.8–2) | |
| Living area | ||||
| Urban | 3,379 (72.7) | 1,271 (27.3) | 1 | 1 |
| Rural | 192 (79) | 51 (21) | 0.8 (0.6–1) | 0.8 (0.6–1.1) |
| Scattered rural | 170 (78.7) | 46 (21.3) | 0.8 (0.6–1) | 0.7 (0.5–0.9) |
| Health insurance | ||||
| Contributive | 2,411 (87.2) | 354 (12.8) | 1 | 1 |
| Subsidized | 1,877 (64.1) | 1,049 (35.9) | 2.8 (2.5–3.1) | 2.4 (2.1–2.8) |
| Poor without health insurance | 235 (50.5) | 230 (49.5) | 3.9 (3.4–4.4) | 2.5 (2.1–3) |
| Healthcare worker | 83 (90.2) | 9 (9.8) | 0.4 (0.2–0.7) | |
| Homeless individuals | 196 (35.8) | 352 (64.2) | 2.8 (2.6–3) | 1.5 (1.3–1.7) |
| Prisoners | 68 (81.9) | 15 (18.1) | 0.7 (0.4–1.1) | |
| Disease form | ||||
| Pulmonary | 3,657 (72) | 1,423 (28) | 1 | 1 |
| Extrapulmonary | 866 (80.5) | 210 (19.5) | 0.5 (0.4–0.6) | 0.8 (0.7–1) |
| HIV | 486 (58.6) | 344 (41.4) | 1.8 (1.7–2) | 1.5 (1.4–1.7) |
| Diabetes | 101 (83.5) | 20 (16.5) | 0.6 (0.4–0.9) | |
| Chronic obstructive pulmonary disease | 35 (81.4) | 8 (18.6) | 0.7 (0.4–1.3) | |
| Drug disorder | 122 (55.2) | 99 (44.8) | 1.7 (1.9–2) | |
| Chronic kidney disease | 23 (67.6) | 11 (32.4) | 1.2 (0.7–2) | |
| Other immunodeficiency disorders | 30 (73.2) | 11 (26.8) | 1 (0.3–1.7) | |
| Previously treated patient | 384 (45.8) | 454 (54.2) | 2.5 (2.3–2.7) | 1.7 (1.5–1.9) |
| Hospital diagnosis | 1,771 (67) | 872 (33) | 1.6 (1.5–1.8) | 1.4 (1.3–1.6) |
| Year of notification | ||||
| 2014 | 1,765 (74.9) | 591 (25.1) | 1 | 1 |
| 2015 | 1,345 (72.8) | 502 (27.2) | 1.1 (0.9–1.2) | 1.01 (0.89–1.14) |
| 2016 | 1,413 (72.4) | 540 (27.6) | 1.1 (0.9–1.2) | 1.06 (0.94–1.2) |
RR = relative risk.
Other risk factors associated with unsuccessful TB treatment included age between 15 and 54 years, and ≥ 55 years, male sex, homeless individuals, HIV coinfection, previously treated patients, and primary diagnosis during hospitalization. Protector factors were living in scattered rural areas, compared with urban, and extrapulmonary disease form (Table 3).
The proportion of TB patients with subsidized health insurance who experienced an unsuccessful TB treatment outcome was 35.9%, and without health insurance was 49.5% (Supplemental Table 4-S6). PAF for unsuccessful TB treatment outcome was 21.2% (95% CI 19.2–23.1) and 29.8% (95% CI 26.2–33.5) for subsidized and without health insurance, respectively. Consequently, the joint PAF was 51% (Supplemental Table 4-S6). PAF was similar in patients without health insurance for the unsuccessful outcome of TB treatment and lost to follow up (Figure 1).
Figure 1.
(A) Adjusted relative risk and (B) fraction of unsuccessful outcome in a population of tuberculosis (TB) cases that was attributable to the health insurance (PAF). Antioquia, 2014 to 2016. *Baseline category “contributive.” aUnsuccessful outcome of treatment (death, lost to follow up, and failure) compared with successful treatment (cured and treatment completed). Relative risk adjusted by age, sex, living area, homeless, disease form, HIV, previously treated patient, hospital diagnosis, and year of notification. bLost to follow up compared with successful treatment (cured and treatment completed). Relative risk adjusted by age, sex, living area, homeless, HIV, previously treated patient, hospital diagnosis, and year of notification. cDeath compared with successful treatment (cured and treatment completed). Relative risk adjusted by age, sex, disease form, HIV, previously treated patient, hospital diagnosis, and year of notification.
DISCUSSION
Our study identified higher rates of unsuccessful TB treatment outcomes among Colombians who were subsidized or were without any health insurance. A separate analysis of lost to follow up and mortality outcomes also demonstrated that subsidized or absent health insurance were associated factors, providing corroborative results. The proportion of unsuccessful TB treatment outcomes in this population that was attributable to being subsidized or having the absence of health insurance was estimated to be 51%.
Although in Colombia the diagnosis and treatment of TB are paid for by the government, there are other costs that individuals must incur. Nieto et al. reported that patients and their families in Medellin assumed half of the total cost of TB treatment due to loss of productivity, and those costs constituted 15% of the biannual income of patients.28 In addition, Martínez-Sánchez et al. found in three cities within Colombia in 2017, that the main direct costs incurred during TB treatment were transportation and additional diagnostic testing, and that their monthly income decreased by 14.6% due to temporary work leaves as a result of TB treatment.29 Studies conducted in low- and middle-income countries, many of whom had provided free-of-charge diagnosis and treatment, has shown that during the process of seeking and receiving care for TB, approximately 20% (range 0–62%) of the total cost was due to direct medical costs, 20% (0–84%) to direct nonmedical costs, and 60% (16–94%) to income loss.30 Therefore, even in countries that provide free access to diagnosis and treatment, patients with low income may be highly affected by TB costs, creating a significant barrier to accessing appropriate TB treatment and follow up.
In the End TB Strategy, Universal Health Coverage (UHC) is considered as a bold and necessary policy to move toward to the end of TB.2 UHC means that all people receive quality, essential health services, without being exposed to financial hardship.31 Assuming a hypothetical causal association between lower treatment success with subsidized and absent health insurance, more than a half of the unsuccessful TB treatment outcomes in our population could be averted if we could eliminate these expenditures (subsidized and absence of health insurance). This suggests that integrated public policies to guarantee the UHC for the Colombia population could positively impact TB treatment outcomes and, therefore, should be prioritized. However, a critical health system challenge is to assure the financial sustainability of the system.
In addition to health insurance, we found other previously documented factors to be associated with poor TB treatment outcomes including young adults32 and elderly population,33 male sex,34 homeless population,34,35 HIV coinfected individuals33,34,36,37 and those who had previously been treated.33,36 However, the association with receiving a TB diagnosis during hospitalization, living in scatter rural area and extrapulmonary disease form needs further clarification.
The association of a TB diagnosis being established during hospitalization and unsuccessful TB treatment outcomes, may be related to diagnostic delays and limited access to healthcare. Possible explanations for the delay in TB diagnosis can be divided to patient characteristics, healthcare personnel, or healthcare system–related factors. Patient-related characteristics may include lack of awareness about the disease, stigma, discrimination, lack of health insurance, low-income conditions, nonaccessibility to healthcare facilities due to geographic and economic constraints, HIV coinfection and being homeless.34,35,38–43 Factors linked to healthcare workers previously reported are lack of knowledge about TB transmission, prevention strategies, and consequences of interrupt and incomplete TB treatment, as well as, decreased motivation and lack of commitment or knowledge about Directly Observed Treatment Short-Course (DOTS) strategy from medical and nursing students, doctors, and policy makers.44–48 Healthcare system structural obstacles include administrative barriers that have been reported in health services in Colombia: limited supply of anti-tuberculous therapy by health service,49 scheduling delays, issues related to missing registration of individuals in the health insurance database, electronic system dysfunction due to poor network connectivity, rejection or delay of the service, delay for approval of service, bureaucratic obstacles, treatment center is separate from diagnostic center, and treatment and diagnostic centers are detached from the approval center that authorize additional laboratory or radiographic exams, appointments, or medications that the patient needs and that are not included in the regular health insurance plan, the three centers are distinct entities geographically distant from each other.50
Another factor that may explain the association between diagnosis in secondary and tertiary healthcare institutions and unsuccessful TB treatment outcomes could be the urban violence that affects some regions of Antioquia and Medellin, mainly related to illegal activities and criminal organizations that create “invisible” barriers, limiting the movement between some neighborhoods and reduced mobility due to safety considerations. A qualitative study conducted in Brazil among professionals that worked in the Tuberculosis Control Program in Rio de Janeiro, providing home visits for the purpose of TB treatment and contact tracing, found that obstacles to TB treatment were related to violence due to drug trafficking rules, the restrictions imposed on the mobility of health professionals, curtailing the access of patients and their contacts in seeking to treat or be treated, in addition to restrictions imposed by police.51 Although there are no studies about the impact of urban violence on TB diagnosis and TB treatment outcomes in Colombia, we strongly believe that these obstacles play a critical role, affecting the success of TB treatment and should be explored in future studies.
In our study, living in scattered rural areas compared with urban areas was a protective factor against unsuccessful treatment, an unexpected finding, since those populations may have less access to health services. Better outcomes of TB treatment may be a result of the support of multiple national and international governmental and nongovernmental organizations supporting the population from remote rural areas (the most affected population by the internal war), including healthcare assistance. Another possible reason could be that individuals with severe TB disease may be migrating to urban area to seek health assistance. Again, further studies are necessary to directly assess this association and glean better understanding of the mechanisms behind this observation.
The finding of extrapulmonary TB as a protective factor for unsuccessful treatment was unexpected as it was identified as a risk factor in our literature review.52 We hypothesized that the consideration of extrapulmonary TB as a more severe condition by healthcare providers and patients, could translate to higher adherence to TB treatment and closer follow-up. A recent article reported a similar finding to ours,53 however, their observation was not further explained.
The WHO’s post-2015 End TB established the goal of 90% of successful TB treatment, remains beyond Antioquia’s reach, despite strong efforts of the government at the state and municipality levels. The state of Antioquia, as well as Medellin municipality have invested a tremendous effort to increase the success of TB treatment. In addition to DOTS, technical visits twice a year to health facilities in all 125 municipalities were instituted in 2010 to verify guideline adherence. At the same time, the Advocacy, Communication, and Social mobilization strategy was implemented. This strategy includes legal support for patients who have no access to individualized TB treatment or additional services to restore their rights; psychosocial visits to drug-resistance TB cases, lost to follow up patients and those who did not attend TB treatment of a week or more; and psychological workshops focused on decreasing stigma and discrimination for prioritized patients and their families.
In 2013 “TB treatment without frontiers” was launched, providing TB treatment in the nearest health facility according to patients’ residence. In 2015, the TB program initiated weekly monitoring of individuals diagnosed by the laboratory and linking with data from health facilities to identify whether diagnosed patients are linked to the TB program. If not, the institution had to enroll the patient and notify the healthcare authorities of the outcome. In 2016, by merging the database of mandatory report for public health diseases and the TB program database, the TB program identified 200 TB patients notified but not enrolled in the TB program. Healthcare workers were notified and tasked to identify and linked these individuals to care.
The Medellin municipality has implemented additional interventions since 2014: permanent healthcare workers in the TB program (healthcare workers hired by the state of Antioquia are intermittent); monthly visits to healthcare institutions for supervision and technical advice; visits to both patients and to those who are nonadherent; an exclusive physician for multi-drug resistant TB; DOTS delivered at home by nurses for selected patients; and institutions to treat homeless population.
Despite these efforts to implement multiple strategies, the average percentage of successful TB treatment was 73.4%, and almost half of patients with TB were diagnosed during a hospitalization. The findings have multiple implications to individuals and communities: 1) prolonged infectivity, 2) higher mortality,54 and 3) high cost for the healthcare system as a recently published article showed that hospitalizations due to TB between 2000 and 2010 in United States cost $6.96 billion USD.55
Literature has reported novel approaches to increase TB treatment success rates that could be evaluated within the state of Antioquia and may complement existing strategies including the following:
-
1.
Use of short message service (SMS) with reminders and educational material.56
-
2.
Offering cash transfer to poor household contacts to defray/reimburse their cost expenses. These are both TB-specific approaches and could be a more affordable and effective strategy going forward. This strategy has shown to improve poor household contact incomes compared with incomes before TB diagnosis (TB-sensitive approach).57 Although this strategy is challenging for the government due to budget constraints (the average total budget for cash transfer needed to prevent catastrophic costs in Colombia was $981,000 USD [95% CI 830,000–1,222,300] for TB-specific approach, and was $4,860,000 USD [95% CI 3,600,000–6,117,000] for TB-sensitive approach),57 it is necessary to evaluate these approaches to prevent catastrophic costs for households. The aforementioned incentives have increased enthusiasm for treatment and results in improvement in adherence among selected patients providing improved access to food, supplements, or personal needs during TB treatment.58
-
3.
The implementation of community-based DOTS59 could lead to improved treatment outcomes as well. A study conducted in Medellin between 2006 and 2009, showed that a special DOTS (follow up to household contacts of TB patients every 6 months, phone calls every 3 months, and trained personnel available by phone on Monday to Friday for free consultation about symptoms) had a successful treatment rate of 85.5% compared with 66.1% of regular DOTS.28
-
4.
The introduction of community health workers to provide TB education, referral, and treatment follow up. In Ethiopia, workers received a year of training in basic health service delivery and were paid by the government.60 They spent 75% working in the community, providing TB education, referral, treatment follow up, and were able to reached one-third of TB patients who initiated TB treatment.60 Based on these findings, Fedaku et al.60 suggested that collection and transport of sputum sample by community health workers to diagnostic centers would improve TB diagnosis compared with patient referral and would decrease the time from symptoms identification to TB diagnosis confirmation, resulting in cost savings.
-
5.
The use of technological tools such as telemedicine, e-health, e-learning to train healthcare workers, patients, and community-members to decrease barriers related to healthcare system or patients.61
-
6.
Lastly, evaluate the current TB program using the methodology of “patient-pathway analysis”62 to identify potential gaps in healthcare screening, diagnosis, treatment, and follow up of patients.
Some of these alternatives will be challenging to the government secondary to budgetary constraints. Nevertheless, it is necessary to evaluate at least some of these approaches to improving TB treatment outcomes within Colombia. Otherwise, it is highly unlikely that the country will reach national and international TB eradication goals, with the population bearing the ongoing burden of this preventable disease.
The main limitation of our study was the inconsistent data that was found in some variables in the routine surveillance databases (SIVIGILA and SITB), such as weight and height required for BMI calculation. In addition, we found duplicate data when we merged the databases, which led to the exclusion of 472 records (6.5%). However, due to the high number of patients that we had in this study we consider that it is representative of the TB situation within the state, and it will allow policy makers to make evidence-based decisions.
Another limitation is that not all TB patients were tested for drug resistance, it is therefore possible that some of drug-resistant TB patients were included in the analyses. In addition, the percentage of cases tested for drug resistance was available only for previously treated patients (50% had a drug-susceptibility test). In Colombia, drug-susceptibility testing is recommended for high TB risk populations, such as prisoners, homeless population, contact of a resistant TB case or of a person who died because of TB, indigenous, HIV, malnutrition, chronic obstructive disease, illicit drug-users, previously treated TB case, extrapulmonary TB, and people who are in TB treatment with persistent sputum-smear positive. This population may have more access to DST. TB cases that presented with documented drug resistance were excluded from our study. Since most of those exposures are associated with unsuccessful TB treatment outcome, we think that the selection bias was very low, and it may lead to an underestimation of the magnitude of association between vulnerable exposures (e.g., nonaccess to health insurance) and unsuccessful TB treatment outcome.
Finally, not all notified patients enter the TB program because they were either lost to follow up or died before TB treatment initiation. This may have led to underestimation of the magnitude of the association we have found.
CONCLUSION
In this study, we found multiple risk factors associated with unsuccessful TB treatment outcomes in 125 municipalities within Colombia. Based upon those factors, we concluded that it is vitally important to evaluate novel strategies that could improve TB diagnosis in primary healthcare institutions instead of tertiary care hospitals. In addition, it is imperative to do new research about the barriers and obstacles related to patients, healthcare workers and services, and health systems, including the analysis of urban violence, to better understand why the goal of TB treatment success has not been reached despite the efforts and strategies that have been implemented by the state of Antioquia.
Supplemental Material
ACKNOWLEDGMENTS
This research was carried out through the Structured Operational Research and Training Initiative (SORT IT), a global alliance led by the Special Programme for Research and Training in Tropical Diseases (TDR), UNICEF/UNDP/World Bank/WHO. The program SORT IT Colombia where this research was conducted, was developed, and executed jointly by the following committee: the Communicable Diseases Research Program of the Department of Communicable Diseases and Health Analysis (CHA) at the Pan American Health Organization (PAHO), the Universidad Pontificia Bolivariana, Medellín (Colombia), and the International Organization for Migration (Colombia); the initial work plan was prepared and approved by the National Technical Committee of Tuberculosis in Colombia consisting of: Ministerio de Salud y Protección Social (MSPS), Instituto Nacional de Salud of Colombia (INS), Pan American Health Organization (PAHO Colombia), International Organization for Migration (IOM), the Liga Antituberculosa Colombiana y de Enfermedades Respiratorias (LAC), Financial Fund of Development projects (FONADE), and Tuberculosis Group of the Country Coordinating Mechanism for Colombia (MCP). The staff who supported the training and analysis were professors of the Universidad Pontificia Bolivariana, Medellín, the Universidad de Antioquia, and the Universidad del Cauca from Colombia, Ministry of Health of Brazil, Instituto Nacional de Enfermedades Respiratorias (INER) of Argentina. Thanks to our funders: The SORT IT program was funded by The Global Fund to Fight AIDS, Tuberculosis and Malaria through the Tuberculosis project that Tuberculosis Group of the Country Coordinating Mechanism for Colombia (MCP) in the 10th round presented to The Global Fund. Specifically, this activity was approved as a strategy of sustainability and closure of the project in territories that were prioritized because of the highest burden of tuberculosis in Colombia. The funders did not play any role in the design of the study, the compilation and analysis of the data, the decision to publish or the writing of the article. We thank Yoav Keynan, and Jeffrey Edwards for their English review and spelling corrections.
Note: Supplemental information, tables, and figure appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization, 2021 Global Tuberculosis Report 2020 [Internet]. Available at: https://www.who.int/publications-detail-redirect/9789240013131.
- 2. World Health Organization , 2016. The End TB Strategy [Internet]. Available at: http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1.
- 3. Wu Y-C, Lo H-Y, Yang S-L, Chu D-C, Chou P, 2015. Comparing the factors correlated with tuberculosis-specific and non-tuberculosis-specific deaths in different age groups among tuberculosis-related deaths in Taiwan. PloS One 10: e0118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Djouma FN, Noubom M, Ngomba AV, Hubert D, Kouomboua PSM, Saah MAF, 2015. Determinants of death among tuberculosis patients in a semi urban diagnostic and treatment centre of Bafoussam, West Cameroon: a retrospective case-control study. Pan Afr Med J 22: 253. Available at: http://www.panafrican-med-journal.com/content/article/22/253/full/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin Y-S, Yen Y-F, 2015. Determinants of mortality before start of and during tuberculosis treatment among elderly patients: a population-based retrospective cohort study. Age Ageing 44: 490–496. [DOI] [PubMed] [Google Scholar]
- 6.de Faria Gomes NM, da Mota Bastos MC, Marins RM, Barbosa AA, Soares LC, de Oliveira Wilken de Abreu AM, Souto Filho JT, 2015. Differences between risk factors associated with tuberculosis treatment abandonment and mortality. Pulm Med 2015: 546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alavi-Naini R, Moghtaderi A, Metanat M, Mohammadi M, Zabetian M, 2013. Factors associated with mortality in tuberculosis patients. J Res Med Sci 18: 52–55. [PMC free article] [PubMed] [Google Scholar]
- 8. Gadoev J. et al. , 2015. Factors associated with unfavorable treatment outcomes in new and previously treated TB patients in Uzbekistan: a five year countrywide study. PloS One 10: e0128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y-C, Lo H-Y, Yang S-L, Chou P, 2014. Factors correlated with tuberculosis reported after death. Int J Tuberc Lung Dis 18: 1485–1490. [DOI] [PubMed] [Google Scholar]
- 10. Shuldiner J, Leventhal A, Chemtob D, Mor Z, 2016. Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. Int J Tuberc Lung Dis 20: 43–48. [DOI] [PubMed] [Google Scholar]
- 11. Lackey B, Seas C, Stuyft PV der, Otero L, 2015. Patient characteristics associated with tuberculosis treatment default: a cohort study in a high-incidence area of Lima, Peru. PloS One 10: e0128541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heunis JC, Kigozi NG, Chikobvu P, Botha S, van Rensburg HD, 2017. Risk factors for mortality in TB patients: a 10-year electronic record review in a South African province. BMC Public Health 17: 38. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5217308/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sawadogo B, Tint KS, Tshimanga M, Kuonza L, Ouedraogo L, 2015. Risk factors for tuberculosis treatment failure among pulmonary tuberculosis patients in four health regions of Burkina Faso, 2009: case control study. Pan Afr Med J 21: 152. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4546781/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amante TD, Ahmed TA, 2014. Risk factors for unsuccessful tuberculosis treatment outcome (failure, default and death) in public health institutions, Eastern Ethiopia. Pan Afr Med J 20 20: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gebrezgabiher G, Romha G, Ejeta E, Asebe G, Zemene E, Ameni G, 2016. Treatment outcome of tuberculosis patients under directly observed treatment short course and factors affecting outcome in Southern Ethiopia: a five-year retrospective study. PloS One 11: e0150560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Pérez MP, 2017. Informe de Evento Tuberculosis, Colombia . Available at: https://www.ins.gov.co/buscador-eventos/Informesdeevento/TUBERCULOSIS%202017.pdf.
- 17. Eventos de Interés en Salud Pública por Subregiones y Municipios , 2017. Antioquia 2007–2016 [Internet]. Secretaría Seccional de Salud y Protección Social de Antioquia. Available at: http://www.dssa.gov.co/index.php/estadisticas/eventos-en-salud-publica.
- 18. The World Bank , 2018. Colombia | Data [Internet]. Available at: https://data.worldbank.org/country/colombia.
- 19. Ministerio de Salud y Protección Social , 2018. Cifras del Aseguramiento en Salud con Corte Julio de 2018 [Internet]. Available at: https://www.minsalud.gov.co/proteccionsocial/Paginas/cifras-aseguramiento-salud.aspx.
- 20. Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB, 2010. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 51: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization , 2011. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis-2011 update [Internet] . Available at: http://apps.who.int/iris/bitstream/10665/44597/1/9789241501583_eng.pdf. [PubMed]
- 22. Ministerio de Salud y Protección Social , 2015. Circular Externa 0007 de 2015: Actualización de los Lineamientos para el Manejo Programático de Tuberculosis y lepra en Colombia [Internet]. Available at: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/circular-externa-0007-de-2015.pdf.
- 23. World Health Organization , 2013. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. Geneva, Switzerland: WHO Publications, 1–47. [Google Scholar]
- 24. Castro AF, Arteaga NG, Llinás G, Mora DA, Rueda-Gallardo JA, Villamil MA, 2015. Definición de Categorías de Ruralidad. Departamento Nacional De Planeación. Available at: https://ideas.repec.org/p/col/000118/013652.html. [Google Scholar]
- 25. Honaker J, King G, Blackwell M, 2011. Amelia II: a program for missing data. J Stat Softw 45: 1–47. [Google Scholar]
- 26. Mansournia MA, 2018. Population attributable fraction. BMJ 360: k757. Available at: https://www.bmj.com/content/360/bmj.k757. [DOI] [PubMed] [Google Scholar]
- 27. Hosmer D, Lemeshow S, 1992. Confidence interval estimation of interaction. Epidemiol Camb Mass 3: 452–456. [DOI] [PubMed] [Google Scholar]
- 28. Nieto E. et al. , 2012. Costo-efectividad de un tratamiento antituberculoso alternativo: seguimiento a convivientes residenciales de los pacientes. Rev Panam Salud Pública 32: 178–184. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Sánchez LM et al., 2017. Costos de bolsillo de pacientes con diagnóstico de tuberculosis en Colombia. An Fac Med 78: 37--40. [Google Scholar]
- 30. Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K, 2014. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 43: 1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization , 2018. Tracking Universal Health Coverage: First Global Monitoring Report. Available at: http://www.who.int/healthinfo/universal_health_coverage/report/2015/en/.
- 32. Woldemichael B, Darega J, Dida N, Tesfaye T, 2021. Treatment outcomes of tuberculosis patients and associated factors in Bale Zone, southeast Ethiopia: a retrospective study. J Int Med Res 49: 300060520984916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gadoev J. et al. , 2015. Factors associated with unfavorable treatment outcomes in new and previously treated TB Patients in Uzbekistan: a five year countrywide study. PloS One 10: e0128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ranzani OT, Carvalho CRR, Waldman EA, Rodrigues LC, 2016. The impact of being homeless on the unsuccessful outcome of treatment of pulmonary TB in São Paulo State, Brazil. BMC Med 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dias M. et al. , 2017. Tuberculosis among the homeless: should we change the strategy? Int J Tuberc Lung Dis 21: 327–332. [DOI] [PubMed] [Google Scholar]
- 36. Tesfahuneygn G, Medhin G, Legesse M, 2015. Adherence to anti-tuberculosis treatment and treatment outcomes among tuberculosis patients in Alamata district, northeast Ethiopia. BMC Res Notes 8: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charoensakulchai S. et al. , 2021. Six-year trend and risk factors of unsuccessful pulmonary tuberculosis treatment outcomes in Thai Community Hospital. BMC Res Notes 14: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M, 2014. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis 18: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osei E, Akweongo P, Binka F, 2015. Factors associated with DELAY in diagnosis among tuberculosis patients in Hohoe Municipality, Ghana. BMC Public Health 15: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cremers AL, Laat MM de, Kapata N, Gerrets R, Klipstein-Grobusch K, Grobusch MP, 2015. Assessing the consequences of stigma for tuberculosis patients in urban Zambia. PloS One 10: e0119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodríguez DA. et al. , 2016. Monitoring delays in diagnosis of pulmonary tuberculosis in eight cities in Colombia. Rev Panam Salud Publica Pan Am J Public Health 39: 12–18. [PubMed] [Google Scholar]
- 42. Cai J, Wang X, Ma A, Wang Q, Han X, Li Y, 2015. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PloS One 10: e0120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bogale S, Diro E, Shiferaw AM, Yenit MK, 2017. Factors associated with the length of delay with tuberculosis diagnosis and treatment among adult tuberculosis patients attending at public health facilities in Gondar town, northwest, Ethiopia. BMC Infect Dis 17: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Acharya PR, D’Souza M, Sahoo RC, 2017. Tuberculosis knowledge and attitude in aspiring doctors and nurses – is it time for our TB teaching methods to evolve? Indian J Tuberc 64: 20–25. [DOI] [PubMed] [Google Scholar]
- 45. Tlale L. et al. , 2016. Factors influencing health care workers’ implementation of tuberculosis contact tracing in Kweneng, Botswana. Pan Afr Med J 24: 229. Available at: http://www.panafrican-med-journal.com/content/article/24/229/full/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira RCC. et al. , 2015. Speeches of managers about the policy of the directly observed treatment for tuberculosis. Rev Bras Enferm 68: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 47. Anticona Huaynate CF. et al. , 2015. Diagnostics barriers and innovations in rural areas: insights from junior medical doctors on the frontlines of rural care in Peru. BMC Health Serv Res 15: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Purohit MR, Sharma M, Rosales-Klintz S, Lundborg CS, 2015. ‘Multiple-test’ approach to the laboratory diagnosis of tuberculosis-perception of medical doctors from Ujjain, India. BMC Infect Dis 15: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernández JMR, Rubiano DPR, Barona JCC, 2015. Barreras de acceso administrativo a los servicios de salud en población Colombiana, 2013. Ciênc Saúde Coletiva 20: 1947–1958. [DOI] [PubMed] [Google Scholar]
- 50. Carvajal-Barona R, Tovar-Cuevas LM, Aristizábal-Grisales JC, Varela-Arévalo MT, 2012. Barreras asociadas a la adherencia al tratamiento de tuberculosis en Cali y Buenaventura, Colombia, 2012. Rev Gerenc Políticas Salud 16: 68–84. [Google Scholar]
- 51. Souza FBA de, Villa TCS, Cavalcante SC, Ruffino Netto A, Lopes LB, Conde MB, 2007. Peculiarities of tuberculosis control in a scenario of urban violence in a disadvantaged community in Rio de Janeiro, Brazil. J Bras Pneumol 33: 318–322. [DOI] [PubMed] [Google Scholar]
- 52. Sariem CN, Odumosu P, Dapar MP, Musa J, Ibrahim L, Aguiyi J, 2020. Tuberculosis treatment outcomes: a fifteen-year retrospective study in Jos-North and Mangu, Plateau State, North—Central Nigeria. BMC Public Health 20: 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mundra A, Deshmukh PR, Dawale A, 2017. Magnitude and determinants of adverse treatment outcomes among tuberculosis patients registered under Revised National Tuberculosis Control Program in a Tuberculosis Unit, Wardha, Central India: a record-based cohort study. J Epidemiol Glob Health 7: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bartholomay P. et al. , 2016. Quality of tuberculosis care at different levels of health care in Brazil in 2013. Rev Panam Salud Pública 39: 3–11. [PubMed] [Google Scholar]
- 55. Allareddy V, Rampa S, Allareddy V, Nalliah RP, 2017. Tuberculosis management continues to utilize a large amount of hospital resources in the United States. Clin Respir J 11: 21–27. [DOI] [PubMed] [Google Scholar]
- 56. Fang X-H. et al. , 2017. Effect of short message service on management of pulmonary tuberculosis patients in Anhui Province, China: a prospective, randomized, controlled study. Med Sci Monit 23: 2465–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rudgard WE. et al. , 2017. Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: an economic modelling study. PLOS Med 14: e1002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ukwaja KN, Alobu I, Mustapha G, Onazi O, Oshi DC, 2017. ‘Sustaining the DOTS’: stakeholders’ experience of a social protection intervention for TB in Nigeria. Int Health 9: 112–117. [DOI] [PubMed] [Google Scholar]
- 59. Zhang H, Ehiri J, Yang H, Tang S, Li Y, 2016. Impact of community-based DOT on tuberculosis treatment outcomes: a systematic review and meta-analysis. PloS One 11: e0147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fekadu L, Hanson C, Osberg M, Makayova J, Mingkwan P, Chin D, 2017. Increasing access to tuberculosis services in Ethiopia: findings from a patient-pathway analysis. J Infect Dis 216: S696–S701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huaynate CFA. et al. , 2015. Diagnostics barriers and innovations in rural areas: insights from junior medical doctors on the frontlines of rural care in Peru. BMC Health Serv Res 15: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hanson CL, Osberg M, Brown J, Durham G, Chin DP, 2017. Conducting patient-pathway analysis to inform programming of tuberculosis services: methods. J Infect Dis 216: S679–S685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.