ABSTRACT.
Maternal group B Streptococcus (GBS) colonization is a major risk factor for neonatal GBS infection. However, data on GBS are scarce in low- and middle-income countries. Using sociodemographic data and vaginal swabs collected from an international cohort of mothers and newborns, this study aimed to estimate the prevalence of GBS colonization among pregnant women in Madagascar (n = 1,603) and Senegal (n = 616). The prevalence was 5.0% (95% CI, 3.9–6.1) and 16.1% (95% CI, 13.1–19.0) in Madagascar and Senegal, respectively. No factors among sociodemographic characteristics, living conditions, and obstetric history were found to be associated independently with GBS colonization in both countries. This community-based study provides one of the first estimates of maternal GBS colonization among pregnant women from Madagascar and Senegal.
INTRODUCTION
The burden of group B Streptococcus (GBS) infection among infants younger than 3 months was estimated to be 90,000 deaths worldwide in 2015. In addition, GBS is estimated to have caused up to 3.5 million preterm births and 57,000 stillbirths.1 The burden is predominantly in low- and middle-income countries (LMICs). African countries accounted for 54,000 infant deaths and 42,000 estimated stillbirths resulting from GBS infection.1
GBS is a Gram-positive bacterium frequently present in adult gastrointestinal and genital tracts.2 It is responsible for two different types of severe neonatal infection: early-onset GBS infection, resulting from mother-to-child transmission either in utero or at delivery3; and late-onset GBS infection, with a mode of transmission that appears to involve both vertical and horizontal transmission.4 Maternal GBS colonization in late-stage pregnancy is the most important risk factor for neonatal GBS infection.5 This association has been explored extensively in high-income countries (HICs). A recent review estimated the mean prevalence of maternal GBS colonization to be 17.9% worldwide.6 However, in some regions of the world, primarily low-income and rural settings such as Central and West Africa, there is currently a lack in GBS prevalence data.7
In most HICs, the use of intravenous intrapartum antibiotic prophylaxis (IAP) (penicillin) in culture-positive women was implemented in the 1990s and led to a spectacular reduction in early-onset GBS cases.8 However, the level of late-onset GBS cases remained unmodified.9 In LMICs, the feasibility of IAP is not fully confirmed. Maternal immunization could be a promising cost-effective strategy to reduce the overall burden of GBS infection in newborns.10,11 An effective vaccine could also be an asset for addressing cases of late-onset GBS, preventing GBS-linked preterm births and stillbirths, as well as invasive infections in non-obstetric contexts in both HICs and LMICs.12
In the context of advances in vaccine development13 and uncertainties in implementing IAP programs in LMICs, accurate assessment of the burden of GBS infections in these settings is urgently needed. Investigating maternal GBS colonization in LMICs will provide useful data on the reservoir for GBS transmission to newborns and inform the impact of upcoming vaccines. Thus, using the data from an international cohort of mothers and newborns (Bacterial Infections and anti-microbial Drug Resistant Diseases among Young Children in Low-Income Countries [BIRDY]), we sought to estimate the prevalence of GBS colonization in pregnant women in Madagascar and Senegal and to identify factors potentially associated with such colonization.
MATERIALS AND METHODS
Data source, inclusion criteria, and study setting.
BIRDY is a multicenter cohort study launched to address the lack of epidemiological data concerning drug-resistant neonatal and infantile bacterial infections in LMICs.14 As part of the project, our study included pregnant women in Antananarivo (an urban setting with three districts near Institut Pasteur de Madagascar; 4,100 women of reproductive age according to a local census) and Moramanga (a rural setting with six districts; 3,800 women of reproductive age) in Madagascar, and in Guédiawaye (an urban setting within the Wakhinane Nimzatt district; 4,000 births/year) and Sokone (a rural setting with14,500 inhabitants) in Senegal (Figure 1). At each study site, pregnant women were recruited consecutively via the local primary health-care center—which covers the selected districts, door-to-door home visits by “matrons” (influential women within the local communities who play a role in pregnancy follow-up and delivery), and investigators within the selected locations—during their third trimester of pregnancy or at delivery, at which point a lower vaginal swab was collected to screen for GBS colonization. Health-care workers (nurses and midwives) and collaborating matrons were trained for the project. Recruitment occurred from September 2012 to December 2016 in Madagascar. In Senegal, women were recruited from October 2013 to December 2018. In our study, we only included women from the cohort who underwent an effective GBS screening.
Figure 1.
Study sites maps. This figure appears in color at www.ajtmh.org.
Data collection.
Collected variables were as follows: 1) sociodemographic factors (age, marital status, education, and employment), 2) living conditions (access to electricity, type of sanitation [indoor latrines and outdoor pour-flush latrines were considered as improved sanitation], number of people living under the same roof), 3) brachial circumference to determine nutritional status (undernutrition if < 24 cm15), 4) obstetric history (gravidity/parity, history of child death/stillbirth/miscarriage), 5) pregnancy follow-up information (number of antenatal consultations, professional pregnancy follow-up [physician, midwife, or nurse], and 6) medication during the current pregnancy (antibiotic consumption; iron/folate supplementation; intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine [IPTp-SP], defined as an intake of at least one dose during pregnancy; number of doses of IPTp-SP; mosquito net use). The case report form is presented in Supplemental Figure 1. Collected data underwent quality assessment initiated both locally and centralized by the project’s data manager (Institut Pasteur). Paper data entry forms were reviewed, and errors and inconsistencies were corrected by clarifying with the source.
GBS screening.
Trained personnel collected the samples using sterile dry swabs in health-care facilities or at home. All samples were transported without transport medium to Institut Pasteur laboratories then stored in refrigerators before GBS screening was conducted.
Isolates were plated onto a selective growth medium—BD Group B Streptococcus Differential agar (Granada Medium, Becton Dickinson, Franklin Lakes, NJ, a proteose peptone starch agar with 3-[N-morpholino]propanesulfonic acid, which is a buffering agent] and phosphate, and supplemented with methotrexate and antibiotics) in Senegal and BD Colombia colistin and nalidixic acid (CNA) agar (Becton Dickinson, Franklin Lakes, NJ) with 5% sheep blood in Madagascar—and incubated for 24 to 48 hours at 37°C in 5% carbon dioxide (anaerobic incubation for Granada Medium). In Senegal, the colonies were identified by morphological determination and a latex agglutination test (SLIDEX® Strepto Plus, bioMérieux, Marcy-l’Étoile, France). In Madagascar, the colonies were identified by morphological determination, beta hemolysis, and directly by matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI Biotyper®, Bruker Daltonics, Billerica, MA).
Statistical methods.
Because Senegalese and Malagasy study population characteristics—demographics, socioeconomic background, cultural practices, and habits—and prevalence of maternal GBS colonization differed substantially, separate analyses were carried out. Quantitative variables were expressed as median (interquartile range); qualitative variables were expressed as a percentage and a 95% CI for GBS colonization prevalence. The GBS-positive versus GBS-negative groups were compared using the χ2 test or Fisher’s exact test for qualitative variables, and Wilcoxon rank-sum test for quantitative variables. Factors associated potentially with maternal GBS colonization were selected based on prior knowledge.16–18 Because of the important number of missing values concerning antibiotic consumption during pregnancy, this variable was not included in the analysis. The selected variables were first assessed in a univariate analysis and were then included in a logistic regression with backward elimination if the P value was less than 0.25. When two or more variables were correlated, the variables with the smaller P value were retained (age, gravidity, and parity). The significance threshold was fixed at 0.05. Statistical analyses were performed using Stata 14.0 (StataCorp LLC, College Station, TX).
Ethics and data protection.
The BIRDY protocol was approved by the relevant national ethics committees for health research of Madagascar (068-MSANP/CE), Senegal (SEN 14-20) and France (IRB/2016/08/03, Institut Pasteur). Women were included after receiving information about the project, agreeing to biological sampling on themselves and their newborns, and signing an informed consent form. BIRDY provided free-of-charge tests and treatments of infantile infections during the follow-up period. BIRDY data collection has been declared to the Commission nationale de l’informatique et des libertés (a French national data protection authority), in accordance with French law.
RESULTS
Prevalence of maternal GBS colonization.
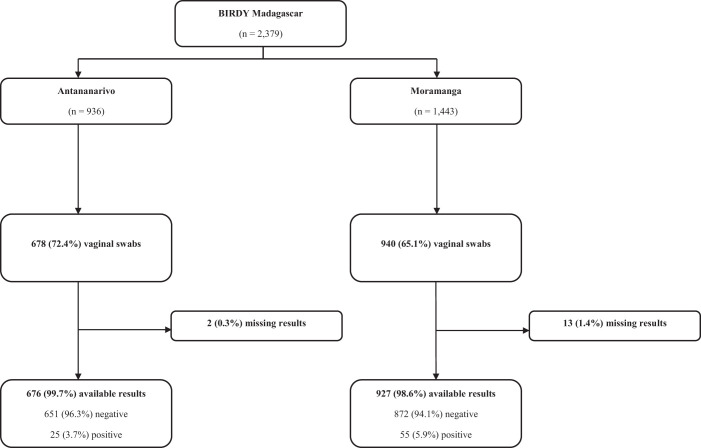
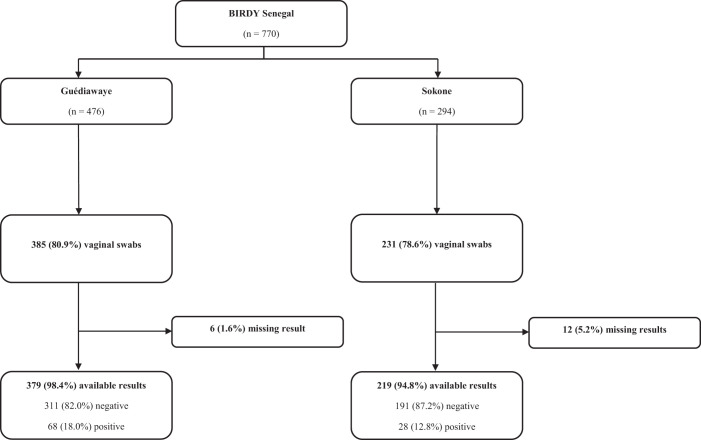
A vaginal swab was performed in 1,618 (68.0%) of the 2,379 women recruited in Madagascar. Samples suitable for analysis were reported for 1,603 women. In Senegal, 616 vaginal swabs out of 770 participants (80.0%) were collected.
Comparison of the main characteristics of the women according to their screening status (effectively screened versus not screened for GBS) showed those with effective GBS screening came more frequently from the urban setting in Madagascar (72.2% of the women from the urban setting were effectively screened versus 64.2% in the rural setting; P < 0.001). Age and gravidity were slightly greater in women who were not screened for GBS. No significant difference between the rural and urban settings was observed in Senegal (79.6% of the women from the urban setting were effectively screened versus 74.5% in the rural setting; P = 0.097). Age and gravidity were also comparable between women according to their screening status.
Sociodemographic characteristics and pregnancy follow-up outcomes of women effectively screened for GBS colonization according to country and setting are available in Tables 1 and 2. Women from Senegal were slightly older and had greater gravidity. Education level was greater in Madagascar. Women from Senegal had more frequent access to electricity at home and improved sanitation compared with women from Madagascar.
Table 1.
Characteristics of pregnant women according to country and study site
| Variable | Madagascar | Senegal | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 1,603) | Urban (n = 676) | Rural (n = 927) | P value* | Overall (n = 598) | Urban (n = 379) | Rural (n = 219) | P value* | |
| Inclusion site, no. (%) | – | – | – | < 0.001 | – | – | – | NS |
| Hospital, private clinic, local health station | 941/1,603 (59) | 361/676 (53) | 580/927 (63) | – | 89/595 (15) | 57/379 (15) | 32/216 (15) | – |
| Home, midwife’s office, private practice, other | 662/1,603 (41) | 315/676 (47) | 347/927 (37) | – | 506/595 (85) | 322/379 (85) | 184/216 (85) | – |
| Sociodemographic characteristics | ||||||||
| Age, y; median (IQR) | 24 (20–30) | 24 (20–29) | 24 (21–30) | 0.0477 | 28 (23–33) | 28 (23–33) | 27 (21–33) | NS |
| Married, n (%) | 1,521/1,603 (95) | 647/676 (96) | 874/927 (94) | NS | 552/598 (92) | 351/379 (93) | 201/219 (92) | NS |
| Education, n (%) | – | – | – | NS | – | – | – | 0.019 |
| Absence or primary | 399/1,603 (25) | 176/676 (26) | 223/927 (24) | – | 440/598 (74) | 291/379 (77) | 149/219 (68) | – |
| Secondary and beyond | 1,204/1,603 (75) | 500/676 (74) | 704/927 (76) | – | 158/598 (26) | 88/379 (23) | 70/219 (32) | – |
| Unemployed, n (%) | 1,128/1,603 (70) | 408/676 (60) | 720/927 (78) | < 0.001 | 412/578 (71) | 252/359 (70) | 160/219 (73) | NS |
| Electricity available at home, n (%) | 1,219/1,603 (76) | 559/676 (83) | 660/927 (71) | < 0.001 | 582/595 (98) | 377/379 (100) | 205/216 (95) | < 0.001 |
| Improved sanitation, n (%) | 168/1,603 (10) | 107/676 (16) | 61/927 (7) | < 0.001 | 337/594 (57) | 126/379 (33) | 211/215 (98) | < 0.001 |
| No. of persons living together, median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.0076 | 10 (7–15) | 10 (6–15) | 10 (7–16) | NS |
| Undernourished | 395/1,496 (26) | 174/604 (29) | 221/892 (25) | < 0.001 | 47/463 (10) | 33/297 (11) | 14/166 (8) | NS |
| Obstetric history | ||||||||
| Gravidity, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.0052 | 3 (2–4) | 3 (2–4) | 3 (2–5) | NS |
| Parity, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.002 | 1 (0–3) | 1 (0–3) | 1.5 (1–3) | NS |
| History of miscarriage, n (%) | 225/1,603 (14) | 112/676 (17) | 113/1,603 (12) | < 0.0001 | 121/597 (20) | 77/379 (20) | 44/218 (20) | NS |
| History of stillbirth, n (%) | 57/1,603 (4) | 21/676 (3) | 36/927 (4) | NS | 38/597 (6) | 26/379 (7) | 12/218 (6) | NS |
| History of child death, n (%) | 100/1,603 (6) | 51/676 (8) | 49/927 (5) | NS | 23/596 (4) | 18/378 (5) | 5/218 (2) | NS |
IQR = interquartile range; NS = not significant. Bold values indicate statistically significant differences.
P values resulting from comparisons of urban and rural populations.
Table 2.
Pregnancy course follow-up according to country and study site
| Variable | Madagascar | Senegal | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 1,603) | Urban (n = 676) | Rural (n = 927) | P value* | Overall (n = 598) | Urban (n = 379) | Rural (n = 219) | P value* | |
| Pregnancy follow-up setting | ||||||||
| No. of antenatal consultations, median (IQR) | 3 (2–4) | 3 (2–4) | 4 (3–4) | < 0.0001 | 2 (1–3) | 2 (2–3) | 2 (1–3) | NS |
| Professional follow-up, n (%) | 1,589/1,602 (99) | 668/676 (99) | 921/926 (99) | NS | 557/563 (99) | 343/345 (99) | 214/218 (98) | NS |
| Antimicrobial consumption during pregnancy, n (%) | 214/1,435 (15) | 95/590 (16) | 119/845 (14) | NS | 39/454 (9) | 28/243 (11) | 12/211 (6) | 0.040 |
| Supplementation | ||||||||
| Iron supplementation, n (%) | 1,319/1,602 (82) | 498/675 (74) | 821/927 (89) | < 0.001 | 573/595 (96) | 366/377 (97) | 207/218 (95) | NS |
| Folate supplementation, n (%) | 1,260/1,594 (79) | 461/671 (69) | 799/923 (87) | < 0.001 | 415/574 (72) | 266/361 (74) | 149/213 (70) | NS |
| Malaria-related information | ||||||||
| IPTp-SP, n (%) | 844/1,603 (53) | 72/676 (11) | 772/927 (83) | < 0.001 | 460/592 (78) | 288/377 (76) | 172/215 (80) | NS |
| No. of IPTp-SP doses, median (IQR) | 1 (0–2) | 0 (0–0) | 2 (1–2) | < 0.0001 | 1 (1–1) | 1 (1–2) | 1 (1–1) | < 0.0001 |
| Mosquito net use, n (%) | 1,045/1,603 (65) | 296/676 (44) | 749/927 (81) | < 0.001 | 364/583 (62) | 173/376 (46) | 191/207 (92) | < 0.001 |
| Reported malaria cases, n (%) | 2 | 0 | 2 | – | 1 | 0 | 1 | – |
IPTp-SP = intermittent preventive treatment of malaria in pregnancy with sulfadoxine–pyrimethamine; IQR = interquartile range; NS = not significant. Bold values indicate statistically significant differences.
P values resulting from comparisons of urban and rural populations.
In Madagascar, the prevalence of maternal GBS colonization was 5.0% (95% CI, 3.9–6.1), with 80 GBS-positive women. The prevalence was greater in the rural setting (5.9% versus 3.7%; P = 0.042). In Senegal, the prevalence of maternal GBS colonization was 16.1% (95% CI, 13.1–19.0), with 96 GBS-positive women. The prevalence was greater in the urban setting compared with rural setting, although the difference was not statistically significant (17.9% versus 12.8%; P = 0.098). Detailed study flowcharts are shown in Figures 2 and 3.
Figure 2.
Madagascar flowchart.
Figure 3.
Senegal flowchart.
Factors associated with maternal GBS colonization.
The results of univariate and multivariate analyses are shown in Table 3. In Madagascar, urban setting (odds ratio, 0.61; 95% CI, 0.38–0.99) and history of miscarriage (odds ratio, 0.39; 95% CI, 0.16–0.99]) were associated with a lower risk of maternal GBS colonization in univariate analysis. No factors were found to be associated independently with maternal GBS colonization in the multivariate analysis. In Senegal, none of the factors were associated with a greater risk of maternal GBS colonization in univariate and multivariate analyses.
Table 3.
Crude and adjusted odds ratio for maternal group B Streptococcus colonization
| Madagascar* | GBS– (n = 1,523) | GBS+ (n = 80) | Crude OR | 95% CI | P value | aOR (n = 1,603)† | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|
| Urban setting (vs. rural setting) | 651/1,523 (43) | 25/80 (31) | 0.61 | 0.38–0.99 | 0.0393 | 0.62 | 0.38–1.00 | 0.051 |
| Married (yes vs. no) | 1,446/1,523 (95) | 75/80 (94) | 0.80 | 0.31–2.03 | 0.6468 | – | – | – |
| Education (secondary and more vs. absence or primary) | 1,144/1,523 (75) | 60/80 (75) | 0.99 | 0.59–1.67 | 0.9815 | – | – | – |
| Unemployed (yes vs. no) | 1,070/1,523 (70) | 58/80 (73) | 1.12 | 0.68–1.85 | 0.6662 | – | – | – |
| Electricity available at home (yes vs. no) | 1,157/1,523 (76.0) | 62/80 (78) | 1.09 | 0.64–1.87 | 0.7528 | – | – | – |
| Improved sanitation (yes vs. no) | 160/1,523 (11) | 8/80 (10) | 0.95 | 0.45–2.00 | 0.8848 | – | – | – |
| Gravidity (per 1 year) | 2 (1–3) | 2 (1–3) | 0.90 | 0.76–1.06 | 0.1882 | 0.93 | 0.78–1.11 | 0.430 |
| History of miscarriage (yes vs. no) | 220/1,523 (14) | 5/80 (6) | 0.39 | 0.16–0.99 | 0.0236 | 0.45 | 0.17–1.18 | 0.104 |
| History of stillbirth (yes vs. no) | 54/1,523 (4) | 3/80 (4) | 1.06 | 0.32–3.47 | 0.9240 | – | – | – |
| IPTp-SP (yes vs. no) | 800/1,523 (53) | 44/80 (55) | 1.10 | 0.70–1.74 | 0.6657 | – | – | – |
| Senegal* | GBS– (n = 502) | GBS+ (n = 96) | Crude OR | 95% CI | P value | aOR (n = 594)† | 95% CI | P value |
| Urban setting (vs. rural setting) | 311/502 (62) | 68/96 (71) | 1.49 | 0.93–2.40 | 0.0934 | – | – | – |
| Married (yes vs. no) | 463/502 (92) | 89/96 (93) | 1.07 | 0.46–2.47 | 0.8714 | – | – | – |
| Education (secondary and more vs. absence or primary) | 140/502 (28) | 18/96 (19) | 0.60 | 0.34–1.03 | 0.0553 | 0.62 | 0.36–1.07 | 0.087 |
| Unemployed (yes vs. no) | 349/487 (72) | 63/91 (69) | 0.89 | 0.55–1.45 | 0.6397 | – | – | – |
| Electricity available at home (yes vs. no) | 489/499 (98) | 93/96 (97) | 0.63 | 0.17–2.35 | 0.5123 | – | – | – |
| Improved sanitation (yes vs. no) | 290/498 (58) | 47/96 (49) | 0.69 | 0.44–1.07 | 0.0944 | 0.71 | 0.46–1.10 | 0.122 |
| Age (per 1 year) | 27 (22–32) | 28 (25–34) | 1.02 | 0.99–1.06 | 0.1485 | – | – | – |
| History of miscarriage (yes vs. no) | 97/501 (19) | 24/96 (25) | 1.39 | 0.83–2.32 | 0.2177 | – | – | – |
| History of stillbirth (yes vs. no) | 31/501 (6) | 7/96 (7) | 1.19 | 0.51–2.79 | 0.6899 | – | – | – |
| IPTp-SP (yes vs. no) | 390/496 (79) | 70/96 (73) | 0.73 | 0.44–1.20 | 0.2270 | – | – | – |
aOR = adjusted odds ratio; GBS = group B Streptococcus; GBS– = GBS-negative women; GBS+ = GBS-positive women; IPTp-SP = intermittent preventive treatment of malaria in pregnancy with sulfadoxine–pyrimethamine; OR = odds ratio. Bold values indicate statistically significant differences.
Variables are expressed as frequency (percentage) or median (interquartile range).
Total number of included in final model.
DISCUSSION
To our knowledge, our study is the first to report the prevalence of GBS colonization among pregnant women in Madagascar, and one of the few reported studies in Senegal. A strength of this study was the community-based recruitment, which covered a high proportion of pregnant women, followed in the local primary health-care center in both urban and rural settings. In fact, the majority of maternal GBS colonization prevalence estimates in low-income settings come from tertiary referral hospital-based or laboratory-based sampling, which may tend to overrepresent women from urban areas. All cases of GBS colonization were confirmed by quality laboratory analysis.
Observed prevalence of maternal GBS colonization was greater in Senegal (16.1%) and was consistent with previous studies in Dakar19 and the Gambia.20 In Senegal, GBS were identified using the same techniques as those used by Brochet et al.19 on samples from Dakar and Bangui. The prevalence in Madagascar (5.0%) was unexpectedly lower than available estimates in eastern Africa, which are usually found to be around 20%.7,21 In fact, different microbiology techniques were used in Madagascar and Senegal. More specifically, two different growth media were used in Madagascar and Senegal (sheep blood agar with CNA and Granada medium, respectively). Although both media are considered to be suitable for GBS culture,22,23 the sensitivity of the sheep blood agar with CNA seems to be decreased by 25% to 35% compared with Granada medium, according to available data.22,24 The different microbiology techniques could explain a part of the observed difference in prevalence of maternal GBS colonization between the two countries.21 However, the different techniques do not fully explain the significant difference of prevalence observed between the two countries. The literature shows that maternal GBS colonization prevalence varies across different regions of the world. According to existing systematic reviews and meta-analyses, the prevalence tends to be greatest in sub-Saharan Africa (within which southern Africa presents the highest estimates, at 25–30%; followed by central and eastern Africa, at 20%; and finally western Africa, at 15–20%), lowest in Asia-Pacific regions, and intermediate in Europe and the Americas.6,21,25 Cambodia was also part of BIRDY; however, data from Cambodia were not included in this study as a result of too few cases of maternal GBS colonization (only four women of 819 tested positive). According to our results, Madagascar’s prevalence seems to be closer to the estimates observed in Asia. This suggests the potential role of maternal host factors, including genetic determinants, considering the partially Asian ancestry of Malagasy people. The prevalence of maternal GBS colonization and the incidence of neonatal GBS infection seem to differ according to study location and ethnic group even when assessed within the same country.26,27 This difference may, in part, be a result of maternal immune response to GBS colonization.19
No independent factors associated with GBS colonization were identified in Madagascar and Senegal, probably because of the relatively small number of GBS-positive women and the decision to not pool data from the two countries as a result of substantial differences in population characteristics. Although prevalence of maternal GBS colonization seems to vary across settings, age, ethnicity, personal hygiene practices, sexual practices, and gynecological and obstetrical history, and some particular health conditions such as obesity, are the most frequently described factors associated with GBS colonization in women of reproductive age.28 Although GBS was understood previously to be sexually transmissible, sexual transmission does not seem to be the main mode of contamination.29 Certain foods as well as hygienic and sexual practices may explain GBS colonization, suggesting multiple pathways of transmission.30 Apart from some variables related to living conditions, these data were not collected in our study and further research is needed to clarify the specific modes of transmission of GBS and host-related factors associated with GBS colonization.
Our study has several limitations. First, coverage of GBS screening was only partially complete. In fact, GBS results were available for 1,603 women (67.1%) in Madagascar and 598 women (77.7%) in Senegal. This resulted from screening material not being available at the beginning of the study. No differences were observed in sociodemographic characteristics among women according to their screening status in Senegal. Consequently, we can assume the prevalence of GBS colonization was not biased by the incomplete screening. In Madagascar, however, women who were not screened for GBS colonization tended to be from the rural setting (prevalence was greater in the rural setting in Madagascar), which could have contributed to underestimating the overall prevalence of maternal GBS colonization. A second limitation is linked to sampling strategy and the material used to collect and transport samples. In fact, the CDC guidelines4 recommend rectovaginal sampling to maximize the probability of detection of GBS. In our study, GBS screening was based solely on vaginal sampling instead of rectovaginal sampling. Moreover, collected samples from rural sites were transported without a transport medium. Using an appropriate transport medium or commercial solutions such as flocked swabs (ESwab™; Copan Diagnostics, Brescia, Italy) could have increased the recovery of GBS from collected samples. Therefore, GBS colonization prevalence is likely to be underestimated in both countries. Transport duration and delay between sample reception and processing at the laboratories possibly have an impact on observed results. However, the exact time of sampling was not collected in our study; consequently, GBS positivity could not be assessed in relation to time from sample collection to its processing. Last, because the cohort was designed primarily to examine drug-resistant neonatal and infantile bacterial infections, we did not have the resources to perform serotype identification on all collected samples. Hence, serotype identification was performed on a small number of randomly selected samples (n =16) from Madagascar, among which three “hypervirulent” serotype III, sequence type 17 (ST-17) strains, known to cause neonatal meningitis and invasive late-onset GBS,31 were identified.
GBS remains one of the priority targets of vaccine development as defined by the WHO.32 Numerous trials implying different types of candidates for maternal immunization are currently underway.33 Because maternal GBS colonization was not the main research question of the BIRDY cohort, it could only be explored in part. Nonetheless, using the available data, our study results provide rare estimates of maternal GBS colonization in Madagascar and Senegal.
Supplemental Material
ACKNOWLEDGMENTS
We are grateful to all the mothers and their newborns for their participation. We thank all physicians, laboratory staff, field interviewers, and community workers for their involvement in this project. We thank all collaborators of the BIRDY program: Laurence Borand, Marguerite Diatta, Pape Samba Dieye, Joseph Faye, Benoît Garin, Amy Gassama Sow (deceased), Alexandra Kerleguer, Elsa Kermorvant-Duchemin, Siyin Lach, Agathe de Lauzanne, Bouya Ndao, Abibatou Ndiaye, Veronique Ngo, Michael Padget, Patrice Piola, Feno Manitra, Jacob Rakotoarimanana, Bodonirina Tanjona Raheliarivao, Frédérique Randrianirina, Vincent Richard, Maud Seguy, Balla Sy, Arnaud Tarantola, Sok Touch, Laurence Watier, and Abdou Armya Youssouf. We also thank Tania Crucitti for her precious advice on the content of the manuscript.
Note: A supplemental figure appears at www.ajtmh.org.
REFERENCES
- 1. Seale AC. et al. , 2017. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis Off Publ Infect Dis Soc Am 65: S200–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berardi A, Tzialla C, Riva M, Cerbo RM, Creti R, 2007. Group B Streptococcus: early- and late-onset infections. J Chemother 19 ( Suppl 2 ): 24–27. [DOI] [PubMed] [Google Scholar]
- 3. Schuchat A, 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11: 497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention , 2007. Perinatal group B streptococcal disease after universal screening recommendations: United States, 2003–2005. MMWR Morb Mortal Wkly Rep 56: 701–705. [PubMed] [Google Scholar]
- 5. Benitz WE, Gould JB, Druzin ML, 1999. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics 103: e77. [DOI] [PubMed] [Google Scholar]
- 6. Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA, 2016. Prevalence of maternal colonisation with group B Streptococcus: a systematic review and meta-analysis. Lancet Infect Dis 16: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 7. Russell NJ. et al. , 2017. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis Off Publ Infect Dis Soc Am 65: S100–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A, 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 342: 15–20. [DOI] [PubMed] [Google Scholar]
- 9. Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) , 2010. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep 59: 1–36. [PubMed] [Google Scholar]
- 10.Madhi SA, Dangor Z, 2017. Prospects for preventing infant invasive GBS disease through maternal vaccination. Vaccine 35: 4457–4460. [DOI] [PubMed] [Google Scholar]
- 11. Kim S-Y, Russell LB, Park J, Verani JR, Madhi SA, Cutland CL, Schrag SJ, Sinha A, 2014. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine 32: 1954–1963. [DOI] [PubMed] [Google Scholar]
- 12. Schuchat A, 1999. Group B Streptococcus. Lancet 353: 51–56. [DOI] [PubMed] [Google Scholar]
- 13. Heath PT, 2011. An update on vaccination against group B Streptococcus. Expert Rev Vaccines 10: 685–694. [DOI] [PubMed] [Google Scholar]
- 14. Huynh B-T. et al. , 2018. Bacterial infections in neonates, Madagascar, 2012–2014. Emerg Infect Dis 24: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ververs M, Antierens A, Sackl A, Staderini N, Captier V, 2013. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr 5: ecurrents.dis.54a8b618c1bc031ea140e3f2934599c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regan JA, Klebanoff MA, Nugent RP, 1991. The epidemiology of group B streptococcal colonization in pregnancy: vaginal Infections and Prematurity Study Group. Obstet Gynecol 77: 604–610. [PubMed] [Google Scholar]
- 17. Collins TS, Calderon M, Gilman RH, Vivar A, Charache P, 1998. Group B streptococcal colonization in a developing country: its association with sexually transmitted disease and socioeconomic factors. Am J Trop Med Hyg 59: 633–636. [DOI] [PubMed] [Google Scholar]
- 18. Sharmila V, Joseph NM, Arun Babu T, Chaturvedula L, Sistla S, 2011. Genital tract group B streptococcal colonization in pregnant women: a south Indian perspective. J Infect Dev Countries 5: 592–595. [DOI] [PubMed] [Google Scholar]
- 19. Brochet M, Couvé E, Bercion R, Sire J-M, Glaser P, 2009. Population structure of human isolates of Streptococcus agalactiae from Dakar and Bangui. J Clin Microbiol 47: 800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madhi SA, Dangor Z, 2017. Prospects for preventing infant invasive GBS disease through maternal vaccination. Vaccine 35: 4457–4460. [DOI] [PubMed] [Google Scholar]
- 21. Stoll BJ, Schuchat A, 1998. Maternal carriage of group B streptococci in developing countries. Pediatr Infect Dis J 17: 499–503. [DOI] [PubMed] [Google Scholar]
- 22. El Aila NA, Tency I, Claeys G, Saerens B, Cools P, Verstraelen H, Temmerman M, Verhelst R, Vaneechoutte M, 2010. Comparison of different sampling techniques and of different culture methods for detection of group B Streptococcus carriage in pregnant women. BMC Infect Dis 10: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosa-Fraile M, Spellerberg B, 2017. Reliable detection of group B Streptococcus in the clinical laboratory. J Clin Microbiol 55: 2590–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bosch-Mestres J, Martín-Fernández RM, Jiménez de Anta-Losada MT, 2003. [Comparative study of three culture media for detecting group B Streptococcus colonization in pregnant women]. Enferm Infecc Microbiol Clin 21: 346–349. [DOI] [PubMed] [Google Scholar]
- 25. Cools P, Jespers V, Hardy L, Crucitti T, Delany-Moretlwe S, Mwaura M, Ndayisaba GF, van de Wijgert JHHM, Vaneechoutte M, 2016. A multi-country cross-sectional study of vaginal carriage of group B streptococci (GBS) and Escherichia coli in resource-poor settings: prevalences and risk factors. PLoS One 11: e0148052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ, 2000. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol 96: 498–503. [DOI] [PubMed] [Google Scholar]
- 27. Seale AC. et al. , 2016. Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol 1: 16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Doare K, Heath PT, 2013. An overview of global GBS epidemiology. Vaccine 31 (Suppl 4): D7–D12. [DOI] [PubMed] [Google Scholar]
- 29. Honig E, Mouton JW, van der Meijden WI, 2002. The epidemiology of vaginal colonisation with group B streptococci in a sexually transmitted disease clinic. Eur J Obstet Gynecol Reprod Biol 105: 177–180. [DOI] [PubMed] [Google Scholar]
- 30. Manning SD, Neighbors K, Tallman PA, Gillespie B, Marrs CF, Borchardt SM, Baker CJ, Pearlman MD, Foxman B, 2004. Prevalence of group B Streptococcus colonization and potential for transmission by casual contact in healthy young men and women. Clin Infect Dis Off Publ Infect Dis Soc Am 39: 380–388. [DOI] [PubMed] [Google Scholar]
- 31. Manning SD, 2014. Emergence of a hypervirulent neonatal pathogen. Lancet Infect Dis 14: 1028–1030. [DOI] [PubMed] [Google Scholar]
- 32. World Health Organization , 2017. GBS Vaccine Research and Development Technical Roadmap and WHO-Preferred Product Characteristics. Available at: https://apps.who.int/iris/rest/bitstreams/1087677/retrieve. Accessed June 8, 2021.
- 33. Davies HG, Carreras-Abad C, Le Doare K, Heath PT, 2019. Group B Streptococcus: trials and tribulations. Pediatr Infect Dis J 38: S72–S76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.