ABSTRACT.
Strongyloides stercoralis affects more than half a billion people worldwide, and hyperinfection in immunocompromised patients can be fatal. Elimination of this neglected tropical disease requires field-applicable diagnostic tools. We conducted a laboratory evaluation of a lateral flow rapid dipstick test (SsRapid™) using sera samples from a Strongyloides-endemic area in northeast Thailand. Group 1 was S. stercoralis-positive and larvae- and/or antibody-positive (according to the IgG ELISA) (N = 100). Group 2 had negative fecal examination and IgG ELISA results (N = 25). Group 3 had other parasitic infections and negative IgG ELISA results (N = 25). The results showed good diagnostic sensitivity (82%) and excellent specificity (96%). Suggested improvements in the SsRapid™ test include increased diagnostic sensitivity and conversion to the more robust cassette format. Field studies should be performed as well.
Strongyloides stercoralis is the main causative agent of strongyloidiasis, a neglected tropical disease affecting 613.9 million people worldwide.1 A high prevalence of S. stercoralis infection (as high as 61%) has been reported in northeastern communities in Thailand.2 The availability of a field-applicable rapid test is essential for epidemiological studies to assess its true prevalence and impact and to facilitate an elimination program. It is also beneficial for diagnosing patients in low-resource settings.
To date, there have been only three reports of the development of prototype lateral flow rapid tests for strongyloidiasis.3–5 One of them, SsRapid™, is an IgG4 dipstick test lined with S. stercoralis recombinant proteins.5 The IgG4 level is significantly increased in individuals with chronic strongyloidiasis, and this level is higher in treatment-resistant patients.6,7 IgG4 is also known to be highly specific for the detection of strongyloidiasis and other helminth infections. Our previous study showed that the diagnostic specificity increased 13.3% when IgG4 replaced IgG as the secondary antibody used for an ELISA.8
The initial laboratory-based evaluation of SsRapid™ showed that its diagnostic sensitivity and specificity were 91.3% and 100%, respectively.5 Further studies are needed to evaluate the diagnostic value of the rapid test and to suggest further improvements. Therefore, the present study aimed to evaluate SsRapid™ using serum samples collected from an endemic area in northeast Thailand.
The sample subjects recruited during this study were residents of a suburban area of Khon Kaen province, northeast Thailand. The map of the study area is available elsewhere.9
After the project protocols were explained, written informed consents were requested from the sample subjects. Blood and fecal samples were collected from the volunteers. Finally, 150 matched samples were used for the analysis during this study. Blood samples were collected by venipuncture. Fecal samples were collected in wide-mouth containers, kept at ambient temperature, and transported to the laboratory. The study protocol for collecting and examining clinical samples was approved by the Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (HE621073).
The participants were assigned to three groups based on fecal examination and serum based-IgG ELISA results (Table 1). Group 1 was S. stercoralis-positive and larvae- and/or antibody-positive (IgG ELISA) (N = 100). Group 1 was divided into group 1.1 (positive for larvae and antibody) and group 1.2 (positive for antibody). Group 2 was negative according to both testing methods (N = 25). Group 3 had other parasitic infections and negative IgG ELISA results (N = 25).
Table 1.
Summary of the evaluation results of the SsRapid™ dipstick test using serum samples from a Strongyloides endemic area in northeast Thailand
| Group | Assessed, n | SsRapid™ reactivity | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Group 1 Positive S. stercoralis | 100 | 82 | 18 | ||
| 1.1 Positive S. stercoralis larvae and antibody | 84 | 69 | 15 | 69/84 (82) | |
| 1.2 Positive S. stercoralis antibody | 16 | 13 | 3 | ||
| Group 2 Negative in endemic areas | 25 | 6 | 19 | ||
| Group 3 Other parasites | 25 | 1 | 24 | 24/25 (96) | |
| Echinostoma sp. | 1 | 0 | 1 | ||
| Hookworm | 9 | 1 | 8 | ||
| Minute intestinal fluke | 1 | 0 | 1 | ||
| Opisthorchis viverrini | 7 | 0 | 7 | ||
| Taenia sp. | 1 | 0 | 1 | ||
| Minute intestinal fluke + hookworm | 1 | 0 | 1 | ||
| O. viverrini + hookworm | 1 | 0 | 1 | ||
| O. viverrini + minute intestinal fluke | 1 | 0 | 1 | ||
| O. viverrini + Taenia sp. | 2 | 0 | 2 | ||
| O. viverrini + minute intestinal fluke + Echinostoma sp. | 1 | 0 | 1 | ||
Group 1: Positive for S. larvae and/or antibody (S. ratti ELISA). Cutoff: 132 units.
Group 2: Negative for S. stercoralis antibody and fecal examination. This group resides in endemic areas.
Group 3: Positive for other parasites according to the fecal examination (formalin-ethyl acetate concentration technique) results.
The positive and negative predictive values of the SsRapid are 98.6% and 61.5%, respectively, and the accuracy is 85.3%. The receiver-operating characteristic curve shows an area under the curve of 0.8303 to 0.9631 (P < 0.0001) (Supplemental Figure S5).
Fecal samples were processed to detect S. stercoralis larvae using the agar plate culture technique (APCT) and formalin-ethyl acetate concentration technique (FECT).10–12 When using the FECT, the results of triplicate examinations of each fecal sample were combined. The results of the sample were defined as positive if either the APCT or the FECT found at least one larva. The serum IgG ELISA using the S. ratti antigen was performed according to previously reported prototcols.13,14
The dipsticks were prepared at the Institute for Research in Molecular Medicine, Universiti Sains Malaysia, as previously described.5 Mouse anti-human (Merck Millipore, Darmstadt, Germany) was conjugated to colloidal gold particles (optical density 10 at 530 nm).15 A volume of 25 μL of the conjugate in drying buffer (0.05 M Na2HPO4, 1% w/v bovine serum albumin, 0.1% w/v NaN3, 5% v/v trehalose) was added to the middle well of a triplet-well unit of a flat-bottomed ELISA microplate and dried at 35°C. In a dry room (≈20% humidity), aluminum test pouches were prepared. Each pouch contained a dipstick, a triplet-well unit (wells A, B, C), and a desiccant. All pouches were sealed. One-hundred fifty rapid tests and two microplate well holders were couriered from Universiti Sains Malaysia to Khon Kaen University at room temperature. On arrival at Khon Kaen University, they were kept for approximately 2 months at 4°C before use.
The test was performed as reported previously.5 Briefly, two drops of buffer A were placed into well C, and 25 μL of buffer B was pipetted in well B to reconstitute the dried IgG4–gold. In well A, serum (10 μL) was mixed with an equal volume of buffer B. After the mixture migrated up the dipstick, it was transferred to well B. When the IgG4–gold conjugate was fully absorbed, the dipstick was transferred to well C for a washing step. After approximately 10 minutes, the background cleared and the dipstick result was read: two red lines (control and test lines) on the dipstick indicate a positive result, and one red control line denotes a negative result. Several documents, including a quick procedure guide (flow chart), an instruction sheet, a video of how to perform the test, a dipstick line intensity chart, and images of some dipstick results, were sent via e-mail to Professor Sithithaworn at Khon Kaen University (Supplemental Figures S1, S2, S3, and S4).
Table 1 shows the evaluation results. The positive and negative results were converted into binary data for Receiver Operating Characteristic (ROC) analysis using GraphPad Prism version 8.0.2. The area under the curve (AUC) is 0.8303 to 0.9631 (P < 0.0001) (Supplemental Figure S5). The diagnostic sensitivity of SsRapid™ based on the serum samples (larvae-positive, IgG-positive) of group 1.1 was 82% (69/84), and the diagnostic specificity based on the serum samples (other parasitic infections, IgG-negative) of group 3 was 96% (24/25). The positive and negative predictive values were 98.6% and 61.5%, respectively, and the accuracy was 85.3%.
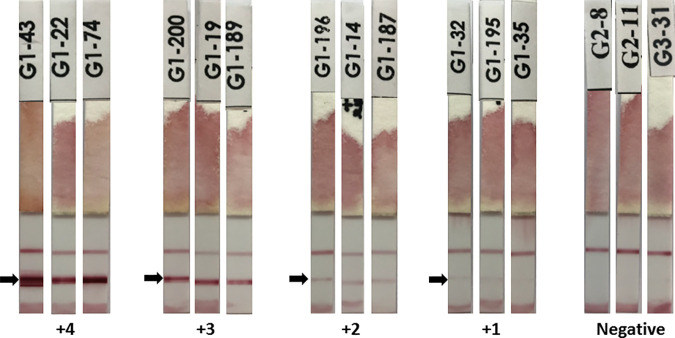
SsRapid™ was positive for 81.3% (13/16) of group 1.2 serum samples (larvae-negative, IgG-positive). The IgG4 rapid test was positive for 24% (6/25) of group 2 sera from apparently healthy people from a Strongyloides-endemic area. The group 2 samples were excluded from the diagnostic specificity determination because some group members may have been infected but asymptomatic and IgG-negative. An analysis of the IgG antibody levels and positive rates found by the IgG4 dipstick revealed a statistically significant positive association (chi-square test, P < 0.05) (Supplemental Table S6). Figure 1 shows the representative images of the dipstick results.
Figure 1.
Representative images of the SsRapid™ dipstick results. The arrow shows the test line. The control line is above the test line. The reddish color at the bottom end of the dipstick, which may appear like a line, is the stain of the IgG4–gold conjugate; therefore, it is ignored when reading the results. This figure appears in color at www.ajtmh.org.
Table 2 shows a comparison of the results and parameters of the present study and the other reported studies using lateral flow rapid tests for strongyloidiasis. The diagnostic specificity of the cassette tests using larvae extract or S. stercoralis immunoreactive (SsIR) recombinant antigen protein was approximately 84% to 85%. The diagnostic sensitivity of the rapid IgG cassette tests using larvae extract and S. stercoralis immunoreactive recombinant antigen protein were similar (91.7% and 93.3%, respectively). The sensitivity rates were higher than that of the IgG4 cassette rapid test (78.3%) using the same recombinant protein.3,4 Strongyloides IgG4 antibody levels are increased during chronic infection, whereas IgG is detected with both acute and chronic strongyloidiasis.7 Therefore, it is not surprising that, generally, the infection prevalence detected by IgG is higher than that detected by IgG4.
Table 2.
Studies of lateral flow rapid tests for the detection of Strongyloides stercoralis infection
| No. | Format | Antigen | Detecting antibody (gold-conjugated) | Sample dilution | Diagnostic sensitivity | Diagnostic specificity | Positive and negative predictive values | Intensity reading chart | Location | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Cassette | Recombinant protein (SsIR) | IgG | 1:100 | 91.7% (55/60) | 83.8% (88/105) | 76.4%, 94.6% | Nine line intensity levels, 8–0.5; cutoff intensity level ≥ 1 | Thailand | Boonroumkaew et al. (2020) |
| 1b | Cassette | Recombinant protein (SsIR) | IgG4 | 1:100 | 78.3% (47/60) | 84.8% (89/105) | 76.4%, 87.3% | Nine line intensity levels, 8–0.5; cutoff intensity level ≥ 0.5 | Thailand | Boonroumkaew et al. (2020) |
| 2 | Cassette | Larvae extract of Strongyloides stercoralis | IgG | 1:50 | 93.3% (56/60) | 83.7% (87/104) | 76.7%, 95.6% | Nine line intensity levels, 8–0.5; cutoff intensity level ≥ 0.5 | Thailand | Sadaow et al. (2020) |
| 3 | Dipstick | Recombinant proteins (rNIE and rSs1a) | IgG4 | 1:2 | 91.3% (21/23) | 100% (82/82) | 100%, 7.6% | Intensity of the test line was quantified using image analysis software | Malaysia | Yunus et al. (2019) |
| 4 | Dipstick | Recombinant proteins (rNIE and rSs1a) | IgG4 | 1:2 | 82% (69/84) | 96% (24/25) | 98.6%, 61.5% | Four intensity scores for positive results: +1 to +4; negative result: no test line | Thailand | Present study |
SsIR = S. stercoralis immunoreactive recombinant antigen. All evaluation studies except study 4 were performed at the location where the tests were developed.
SsRapid™ is an IgG4 test. The first evaluation study was performed in-house (at Universiti Sains Malaysia) using positive samples from Malaysia, and it showed high diagnostic sensitivity (91.3%) and specificity (100%). During the present study, SsRapid™ was evaluated using serum samples from a northeast Thailand endemic area. The results showed reduced diagnostic sensitivity (82%), but the specificity remained high (96%). Whether the reduced sensitivity can be attributed to the fact that the Strongyloides serum samples were from a different country or to technical issues must be investigated. The dipstick format requires two pipetting steps and moving the dipstick from well to well. Therefore, it may be more prone to technical inconsistency than a cassette test format with a much simpler procedure. Nevertheless, the diagnostic sensitivity of SsRapid™ during the present study (82%) was slightly higher than that of the IgG4 cassette test (78.3%) as reported by Boonroumkaew et al.2 There was no cross-reactivity with Opisthorchis viverrini and other parasites (Taenia, minute intestinal flukes, and Echinostome) in northeast Thailand. A cross-reaction with hookworms (1 of 9 cases) was observed. In northeast Thailand, Strongyloides and O. viverrini are the two most common parasites, followed by a very low prevalence of the other helminth infections (Table 1). To date, there has been no report of Schistosoma in the area; therefore, they were not included in the specificity evaluation. During our previous study performed at the Universiti Sains Malaysia, 11 schistosomiasis sera were negative when tested with the rapid dipstick test.5 Other notable results from this study were the significant area under the curve of the receiver-operating characteristic curve, significant correlation between the IgG ELISA units and SsRapid™ positive rates, and good test accuracy.
A simpler and more robust cassette format of SsRapid™ needs to be developed. The diagnostic sensitivity should be at least 90% when tested with samples from larvae-positive individuals, and the diagnostic specificity should remain higher than 95%. Some of the samples with false-negative results during the present evaluation will be used to optimize the SsRapid™ cassette test to help improve its diagnostic sensitivity. Afterwards, it should be evaluated at Khon Kaen University and other places, and during field studies. The test should also work well with whole blood and eluted blood samples to make it field-applicable.
Near the end of a disease elimination program, the infection prevalence is low; therefore, a diagnostic test with high specificity is crucial to support decisions to stop mass drug administration.16 The high specificity of SsRapid™ may indicate its suitability for such use in the future.
Supplemental table and figures
ACKNOWLEDGMENTS
This study was funded by grants from the Malaysian Ministry of Higher Education (MOHE) MyLab grant 1/2018 (no 203.CIPPM.673014 2) and Fluke Free Thailand, National Research Council of Thailand. We also acknowledge support from MOHE’s Institution Centre of Excellence (HICoE) program (no: 311/CIPPM/4401005).
Note: Supplemental table and figures appear at www.ajtmh.org.
References
- 1. Buonfrate D. et al. , 2020. The global prevalence of Strongyloides stercoralis infection. Pathogens 9: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jongsuksuntigul P, Intapan PM, Wongsaroj T, Nilpan S, Singthong S, Veerakul S, Maleewong W, 2003. Prevalence of Strongyloides stercoralis infection in northeastern Thailand (agar plate culture detection). J Med Assoc Tha 86: 737–741. [PubMed] [Google Scholar]
- 3. Boonroumkaew P, Sadaow L, Sanpool O, Rodpai R, Thanchomnang T, Phupiewkham W, Intapan PM, Maleewong W, 2020. Effectiveness of Strongyloides recombinant IgG immunoreactive antigen in detecting IgG and IgG4 subclass antibodies for diagnosis of human strongyloidiasis using rapid immunochromatographic tests. Diagnostics (Basel) 10: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadaow L, Sanpool O, Rodpai R, Boonroumkaew P, Maleewong W, Intapan PM, 2020. Development of immunochromatographic device as a point-of-care tool for serodiagnosis of human strongyloidiasis cases. Eur J Clin Microbiol Infect Dis 39: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yunus MH, Arifin N, Balachandra D, Anuar NS, Noordin R, 2019. Lateral flow dipstick test for serodiagnosis of strongyloidiasis. Am J Trop Med Hyg 101: 432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satoh M, Toma H, Sato Y, Kikuchi M, Takara M, Shiroma Y, Kiyuna S, Hirayama K, 1999. Production of a high level of specific IgG4 antibody associated with resistance to albendazole treatment in HLA-DRB1*0901-positive patients with strongyloidiasis. Am J Trop Med Hyg 61: 668–671. [DOI] [PubMed] [Google Scholar]
- 7. Arifin N, Hanafiah KM, Ahmad H, Noordin R, 2019. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect 52: 371–378. [DOI] [PubMed] [Google Scholar]
- 8. Norsyahida A, Riazi M, Sadjjadi SM, Muhammad Hafiznur Y, Low HC, Zeehaida M, Noordin R, 2013. Laboratory detection of strongyloidiasis: IgG-, IgG4 - and IgE-ELISAs and cross-reactivity with lymphatic filariasis. Parasite Immunol 35: 174–179. [DOI] [PubMed] [Google Scholar]
- 9. Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C, 2014. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21: 301–308. [DOI] [PubMed] [Google Scholar]
- 10. Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H, 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg 45: 518–521. [DOI] [PubMed] [Google Scholar]
- 11. Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, Bhudhisawasdi V, Uttaravichien T, 1990. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg 84: 715–719. [DOI] [PubMed] [Google Scholar]
- 12. Sithithaworn P, Srisawangwong T, Tesana S, Daenseekaew W, Sithithaworn J, Fujimaki Y, Ando K, 2003. Epidemiology of Strongyloides stercoralis in north-east Thailand: application of the agar plate culture technique compared with the enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg 97: 398–402. [DOI] [PubMed] [Google Scholar]
- 13. Eamudomkarn C, Sithithaworn P, Sithithaworn J, Kaewkes S, Sripa B, Itoh M, 2015. Comparative evaluation of Strongyloides ratti and S. stercoralis larval antigen for diagnosis of strongyloidiasis in an endemic area of opisthorchiasis. Parasitol Res 114: 2543–2551. [DOI] [PubMed] [Google Scholar]
- 14. Eamudomkarn C. et al. , 2018. Diagnostic performance of urinary IgG antibody detection: A novel approach for population screening of strongyloidiasis. PLoS One 13: e0192598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makhsin SR, Razak KA, Noordin R, Zakaria ND, Chun TS, 2012. The effects of size and synthesis methods of gold nanoparticle-conjugated MαHIgG4 for use in an immunochromatographic strip test to detect brugian filariasis. Nanotechnology 23: 495719. [DOI] [PubMed] [Google Scholar]
- 16. Gass K, 2020. Time for a diagnostic sea-change: Rethinking neglected tropical disease diagnostics to achieve elimination. PLoS Negl Trop Dis 14: e0008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.