ABSTRACT.
This cross-sectional study evaluated epidemiologic characteristics of persons living with HIV (PWH) coinfected with Trypanosoma cruzi in Cochabamba, Bolivia, and estimated T. cruzi parasitemia by real-time quantitative polymerase chain reaction (qPCR) in patients with and without evidence of reactivation by direct microscopy. Thirty-two of the 116 HIV patients evaluated had positive serology for T. cruzi indicative of chronic Chagas disease (27.6%). Sixteen of the 32 (50%) patients with positive serology were positive by quantitative polymerase chain reaction (qPCR), and four of the 32 (12.5%) were positive by direct microscopy. The median parasite load by qPCR in those with CD4+ < 200 was 168 parasites/mL (73-9951) compared with 28.5 parasites/mL (15–1,528) in those with CD4+ ≥ 200 (P = 0.89). There was a significant inverse relationship between the degree of parasitemia estimated by qPCR from blood clot and CD4+ count on the logarithmic scale (rsBC= –0.70, P = 0.007). The correlation between T. cruzi estimated by qPCR+ blood clot and HIV viral load was statistically significant with rsBC = 0.61, P = 0.047. Given the significant mortality of PWH and Chagas reactivation and that 57% of our patients with CD4+ counts < 200 cells/mm3 showed evidence of reactivation, we propose that screening for chronic Chagas disease be considered in PWH in regions endemic for Chagas disease and in the immigrant populations in nonendemic regions. Additionally, our study showed that PWH with advancing immunosuppression have higher levels of estimated parasitemia measured by qPCR and suggests a role for active surveillance for Chagas reactivation with consideration of treatment with antitrypanosomal therapy until immune reconstitution can be achieved.
INTRODUCTION
Chagas disease is caused by infection with the protozoan parasite Trypanosoma cruzi and is endemic to Latin America, particularly Bolivia.1 The estimated global prevalence of infection is 6 million persons, and 70 million remain at risk of vectorborne infection.1 Bolivia has the highest prevalence of T. cruzi infection in the world, with an estimated national prevalence of 6%1 and a prevalence of 30% in adults aged 20 to 60 years in communities near Cochabamba, where the current study was conducted.2 Since the first reports in Bolivia in 1985, infection with HIV increased to an estimated 19,000 persons living with HIV in 2016, with an estimated incidence of 1,100 new cases per year.3 Thus, coinfection with HIV and T. cruzi are likely substantial in Bolivia and have the potential to increase if the HIV epidemic is not controlled.3
Chagas disease is most often transmitted through the feces of the triatomine vector; however, transmission by intravenous drug use, transfusion of contaminated blood products, transplantation of organs from infected donors, and congenital transmission are also possible.4 There are three phases of Chagas disease infection: the acute phase, chronic phase, and reactivation. The acute phase is rarely diagnosed because patients often have mild, nonspecific symptoms that resolve after 4 to 8 weeks; parasitemia can be detectable microscopically during the acute stage. The chronic phase is diagnosed by detection of IgG antibodies to T. cruzi by serology. Although parasites may be detected in the blood by quantitative polymerase chain reaction (qPCR) intermittently and at low levels, the sensitivity of microscopic examination for trypomastigotes is too low for reliable diagnostic use during the chronic phase of illness. The conventional definition of reactivation is the recurrence of visible parasitemia by microscopy. Reactivation occurs only in an immunosuppressed host and has been reported in patients living with HIV, in post–organ transplant and stem cell transplant patients, in cancer patients on chemotherapy, and in patients receiving immunomodulatory agents for systemic lupus erythematosus, rheumatoid arthritis, and similar conditions5–9
Symptomatic reactivation in patients with HIV is usually associated with severe clinical manifestations with mortality ranging from 64% to 79%.10,11 Reactivation most often presents with central nervous system involvement (CNS; 74%), such as meningoencephalitis or CNS chagoma, or myocarditis (17%).12 There are reports of successful treatment with benznidazole, highlighting the need to identify at-risk patients.8,11
Infection with T. cruzi and other intracellular protozoan parasites leads to downregulation of T-cell function and apoptosis, which may contribute to a decrease in CD4+ counts and increasing parasitemia, as well as the increased mortality seen with HIV–T. cruzi coinfection.14 Few studies have directly compared this relationship among parasitemia, CD4+ counts, and viral loads in patients coinfected with HIV and T. cruzi.15,16 Our aim is to further characterize this relationship using real-time qPCR and demonstrate the trends of clinical signs and symptoms of both ambulatory and hospitalized patients at varying levels of CD4+ counts in patients with and without reactivation.
METHODS
Study design.
This was a cross-sectional observational study in which we evaluated each patient and obtained samples for laboratory analysis during one encounter. In hospitalized patients, clinical information was recorded for the duration of the hospitalization. The study took place in the city of Cochabamba, Bolivia, from January 2011 to December 2011. Cochabamba is located on a high altitude plain (8,432') and has a population of 600,000.17 Hospitalized patients were recruited from the infectious diseases ward of Hospital Viedma, the public hospital associated with Universidad de San Simon. Ambulatory patients were recruited from the hospital’s clinic and from Centro Departamental de Vigilancia y Referencia (CDVIR), a public HIV clinic separate from the university.
Inclusion criteria included patients seeking care at a participating facility, known HIV infection confirmed by Western blot, age 18 years or older, and ability to give consent. If the patient was unable to consent, a patient’s family member was able to give informed consent. The patient’s history was provided by the patient or a family member; chart review elicited relevant clinical information; echocardiogram (ECG) and Mini-Mental Exam were performed by the study team.
Institutional review board approval was obtained from Fundación Ciencia y Estudios Aplicados Para el Desarrollo en Salud y Medio Ambiente (CEADES) in Cochabamba, Bolivia, and Asociación Benéfica PRISMA in Lima, Peru.
Sample collection and analysis.
Blood was drawn from each patient and divided into samples of whole blood for direct microscopy and qPCR, blood clot for qPCR, and serum for serological testing.
Immediately after collection, guanidine was added to the sample of whole blood with ethylenediaminetetraacetic acid in a 1:1 ratio. Samples were brought to the CEADES laboratory in Cochabamba for direct observation microscopy and serology tests (recombinant ELISA, conventional ELISA, and hemagglutination). The microhematocrit concentration method was performed before microscopic examination. Six heparinized capillary tubes were filled with peripheral venous blood sample and centrifuged in a microhematocrit centrifuge at 12,000 rpm for 5 minutes. After centrifugation, the buffy coat within the capillary tubes was observed under the light microscope. The samples reported as positive had one to three parasites per high power field observed at magnification 400×. The samples for qPCR were stored at –20°C at a CEADES laboratory in Cochabamba and transported 1 to 12 months after collection to a laboratory at Universidad Peruana Cayetano Heredia in Lima, Peru, for qPCR.
Diagnosis of chronic Chagas disease was made if samples were positive by two of the three serology tests in accordance with WHO criteria for chronic Chagas disease.18 The diagnosis of Chagas reactivation was made if trypomastigotes of T. cruzi were visualized by direct microscopy by the microhematocrit method described earlier.
DNA was extracted following a standard phenol-chloroform protocol.19 Real-time quantitative (q)PCR was performed on the basis of previously published methods,20 with a few modifications (Supplemental File 1). All laboratory workers were blinded from results of clinical evaluations, and technicians who performed the qPCR assays were blinded to the serologic results.
ECG abnormalities included as potentially caused by Chagas disease were bradycardia (< 60 beats/min), first- or second-degree heart block, right bundle branch block, left anterior fascicular block, atrial fibrillation, and atrial flutter. Patients were evaluated for cardiomegaly on chest radiograph defined by cardiothoracic ratio > 50% on PA film. Score of < 25 on the Folstein’s Mini-Mental Status Exam met criteria for mild cognitive deficit, ≤ 20 or less for moderate cognitive deficit, and ≤ 10 or less for severe cognitive deficit.21
Data were entered into Excel, and the analysis was carried out in STATA/IC version 10.1 (Stata Corp LP, College Station, TX). Continuous variables were described as median and 25 to 75 percentiles because they were not normally distributed. Categorical variables were described as absolute or relative frequencies. For continuous variables, medians were compared using Mann-Whitney test. For categorical variables, χ2 test or Z-test was used, and Fisher’s exact test was used for the comparison of two proportions. The correlation between estimated parasitemia by qPCR (number of parasites per milliliter) and CD4+ count (in the logarithm scale), as well as correlation between parasitemia by qPCR and HIV viral load were estimated through the Spearman’s rank correlation coefficient.
RESULTS
One hundred sixteen patients were recruited and had a blood clot sample processed by qPCR. Sixty patients (52%) also had a sample of whole blood processed by qPCR. Thirty-seven percent of patients were female, the median age was 32, and 47% were hospitalized. Median CD4 + count was 139 cells/mm3 with a median HIV viral load of 56,645 copies/mL. 38% of all patients were on antiretroviral therapy (ART) for longer than one week at the time of enrollment. The most common antiretroviral regimens in these patients were either lamivudine, tenofovir disoproxil fumarate, and efavirenz or lamivudine, zidovudine, and efavirenz (Table 1).
Table 1.
Patient characteristics of hospitalized and ambulatory patients
| All enrolled patients (N = 116) | Hospitalized patients (n = 55) | Ambulatory patients (n = 61) |
P value | |
|---|---|---|---|---|
| Female (%) | 43 (37.1%) | 15 (27.3%) | 28 (45.9%) | 0.038† |
| Median age, years (IQR) | 32 (25–41.5) | 33 (26–41) | 31 (25–42) | 0.574* |
| Pts on ART for > 1 week (%) | 44 (37.9%) | 12 (21.8%) | 32 (52.5%) | 0.001† |
| Patients diagnosed with HIV within the month before enrollment | 35 (30.1%) | 29 (52.7%) | 6 (9.8%) | < 0.001 |
| Median CD4+ count, cells/mm3 (IQR) | 139 (63–330) | 64 (21–121) | 277 (135–460) | < 0.001* |
| Median HIV viral load (copies/mL) | 56,646 (2,863–277,072) | 118,816 (13,072–436,942) | 9,437 (62–158,497) | 0.005* |
| Lived in infested home | 71/115 (61.7) | 29/54 (53.7%) | 42 (68.9%) | 0.095† |
| Family member with Chagas | 17/115 (14.8%) | 7/54 (13%) | 10 (16.4%) | 0.605† |
| Cardiomegaly on chest X-ray | 6/77 (7.8%) | 3/53 (5.7%) | 3/24 (12.5%) | 0.300† |
| Mild or moderate cognitive deficit | 22/111 (19.8%) | 13/50 (26%) | 9 (14.8%) | 0.139† |
| Positive T. cruzi serology | 32 (27.6%) | 11 (20.0%) | 21 (34.4%) | 0.083† |
| T. cruzi positive by qPCR | 16 (13.8%) | 6 (10.9%) | 10 (16.4%) | 0.710† |
ART = antiretroviral therapy; IQR = interquartile range; qPCR = quantitative polymerase chain reaction; T. cruzi = Trypanosoma cruzi.
Mann-Whitney test.
Chi2 test.
Thirty-two of the 116 HIV patients (27.6%) were positive by T. cruzi serology, which included 11 of the 55 hospitalized patients (20.0%) and 21 of the 61 ambulatory patients (34.4%) (Table 2).
Table 2.
Patient characteristics by Trypanosoma cruzi serology status
|
T. cruzi serology positive patients* (n = 32) |
T. cruzi serology negative patients* (n = 84) |
P value | |
|---|---|---|---|
| Female | 17 (53%) | 26 (31%) | 0.027† |
| Median age, years (IQR) | 34.5 (26.5-48) | 31.5 (25-38) | 0.221§ |
| No. hospitalized patients | 11 (34.4%) | 44 (52.4%) | 0.083† |
| No. ambulatory patients | 21 (65.6%) | 40 (47.6%) | |
| Lived in an infested home | 25 (78.1%) | 46/83 (55.4%) | 0.025† |
| Family member with Chagas | 10 (31.3%) | 7/83 (8.4%) | 0.002† |
| Patients on ART for > 1 week | 15 (46.9%) | 29 (34.5%) | 0.220† |
| Median CD4+ count (cells/mm3) | 210 (48–482) | 130 (64–295) | 0.464§ |
| Median HIV VL (copies/mL) | 7,208 (206–203,750) | 71,113 (5,427–335,556) | 0.170§ |
| Patients with HIV VL < 100 copies/mL | 5/24 (20.8%) | 10/63 (15.9%) | 0.751‡ |
| Patients with HIV VL > 100,000 copies/mL | 8/24 (33.3%) | 28/63 (31.7%) | 0.486† |
| ECG findings that could be attributed to Chagas | 7 (21.8%) | 16/80 (20%) | 0.984† |
| Cardiomegaly on chest X-ray | 3/22 (13.6%) | 3/55 (5.5%) | 0.226‡ |
| Mild or moderate cognitive deficits | 6 (18.8%) | 16/79 (20.3%) | 0.86† |
| T. cruzi positive by qPCR | 16 (50%) | 2/84 (2.4%) | < 0.001† |
ART = antiretroviral therapy; ECG = echocardiogram; IQR = interquartile range; qPCR = quantitative polymerase chain reaction; VL = viral load.
T. cruzi–infected versus –uninfected status was determined by positive result in two of three serology tests (conventional ELISA, recombinant ELISA, and Hemagglutination inhibition test). Data with a denominator that is less than the group n represent data that were not available or not collected.
Chi2 test.
Fisher’s exact test.
Mann-Whitney test.
Four of the 32 T. cruzi serology–positive patients (12.5%) were positive by direct microscopy. Each of these with reactivation had CD4+ counts < 100 cells/mm3, were male, and aged 20 to 30 years. All were diagnosed with HIV at the time of diagnosis of Chagas reactivation. Each had some form of neurologic symptoms, although nonspecific in three of the cases.
Reactivation patient #1 presented to the hospital with worsening headache, right-sided discoordination with inability to walk without assistance, and altered mental status. He was found to have papilledema on exam. Computed tomography (CT) of the brain showed a large, edematous lesion in the right posterior fossa. ECG showed right bundle branch block. Direct microscopy revealed one to three trypomastigotes of Trypanosoma cruzi per high power field. qPCR of whole blood estimated moderate parasitemia at 35 parasites/mL; qPCR by blood clot was negative. Benznidazole and antiretroviral therapy were initiated for treatment of Chagas reactivation and HIV. The patient improved clinically with resolution of his headache after 1 week and was able to walk with a normal gait before leaving the hospital on day 30. Repeat head CT before discharge from the hospital showed resolving brain lesion. Reactivation patient #2 was treated with benznidazole and was doing well at the time of discharge. Reactivation patients #3 and #4 were also coinfected with tuberculosis; patient #3 was started on antitrypanosomal therapy but died before hospital discharge. Patient #4 refused antitrypanosomal treatment and left against medical advice with a likely poor outcome (Table 3).
Table 3.
Patients with Chagas reactivation by direct microscopy
| n | Sex | Age (yrs) | CD4+ (cells/mm3) | VL (copies/mL) | qPCR blood clot (parasites/mL) | qPCR whole blood (parasites/mL) | ECG | Neurologic symptoms | Other diagnoses | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 22 | 52 | 689,568 | 0.00 | 34.94 | RBBB | HA, left-sided weakness; space occupying lesion on CT | Possible toxoplasmosis | Discharged on benznidazole |
| 2 | M | 27 | 15 | 6,705 | 9,951.48 | 21,462.13 | Normal | Syncope, nausea, dizziness | Gastroenteritis, community-acquired pneumonia, esophageal candidiasis | Discharged on benznidazole |
| 3 | M | 26 | 29 | 317,789 | 0.00 | 9.28 | Normal | Fever, nausea, vomiting | Miliary TB | Died in the hospital, secondary to Chagas vs. miliary TB |
| 4 | M | 26 | 3 | 237,684 | 3,264.64 | 1,492.53 | Normal | Headache, fever, confusion, nausea, dizziness | Pulmonary TB, Anemia, gastroenteritis | Refused treatment, left hospital against medical advice |
CT = computed tomography; ECG = echocardiogram; HA = headache; IQR = interquartile range; M = male; qPCR = quantitative polymerase chain reaction; RBBB = right bundle branch block; TB = tuberculosis; VL = viral load.
Sixteen of the 32 (50%) seropositive patients were positive by qPCR by blood clot, whole blood, or both. Patients with positive qPCR were less likely to be on ART than those with negative qPCR (P = 0.013). There was no statistically significant association between positive qPCR with CD4+ count or HIV viral load. However, there were trends toward higher HIV viral loads in patients who were qPCR positive versus qPCR negative. qPCR-positive patients trended toward reporting fever and weight loss and had statistically significantly higher incidences of reported headaches (Table 4).
Table 4.
Patient Characteristics of patients with positive Trypanosoma cruzi serology divided by those with and without detectable T. cruzi by qPCR*
| qPCR+ patients (n = 16) |
qPCR– patients (n = 16) |
P value | |
|---|---|---|---|
| Female | 10 (63%) | 7 (44%) | 0.288† |
| Median age, years (IQR) | 28 (22–42) | 41.5 (30–53) | 0.073† |
| Hospitalized (vs. ambulatory) | 6 (38%) | 5 (31%) | 0.710† |
| Patients on ART for > 1 week | 4 (25%) | 11 (69%) | 0.013† |
| Fever | 8/16 (50%) | 6/16 (38%) | 0.361† |
| Weight loss | 9/16 (56%) | 5/16 (31%) | 0.143† |
| Headache | 11/16 (69%) | 5/16 (31%) | 0.038† |
| Mean body mass index (SD) | 21.6 (19.1–26.6) | 23.8 (22.0–26.0) | 0.176‡ |
| Median CD4+ count, cells/mm3 (IQR) | 231 (29–393) | 176.5 (86.5–505.5) | 0.635‡ |
| CD4+ < 100 cells/mm3 | 6/15 (40.0%) | 4 (25%) | 0.458† |
| CD4+ < 200 cells/mm3 | 7/15 (47%) | 8 (50%) | 0.578† |
| Median HIV VL, copies/mL (IQR) | 7711 (2706–237,684) | 3235 (53–169,815) | 0.385§ |
| Patients with HIV VL < 100 copies/mL | 2/13 (15%) | 3/11 (27%) | 0.630§ |
| Patients with HIV VL > 100,000 copies/mL | 5/13 (38%) | 3/11 (27%) | 0.679§ |
| ECG findings that could be attributed to Chagas disease | 3 (19%) | 2 (13%) | 0.500§ |
| Cardiomegaly on chest X-ray | 3/14 (21%) | 0/8 (0%) | 0.273§ |
| Mild or moderate cognitive deficits | 4 (25%) | 2 (13%) | 0.365§ |
| Reactivation | 4 (20%) | 0 (0%) | 0.051§ |
ART = antiretroviral therapy; ECG = echocardiogram; IQR = interquartile range; qPCR = quantitative polymerase chain reaction; VL = viral load.
Positive qPCR includes those that were positive by either blood clot or whole blood.
Chi-square test.
Fisher’s exact test.
Mann-Whitney test.
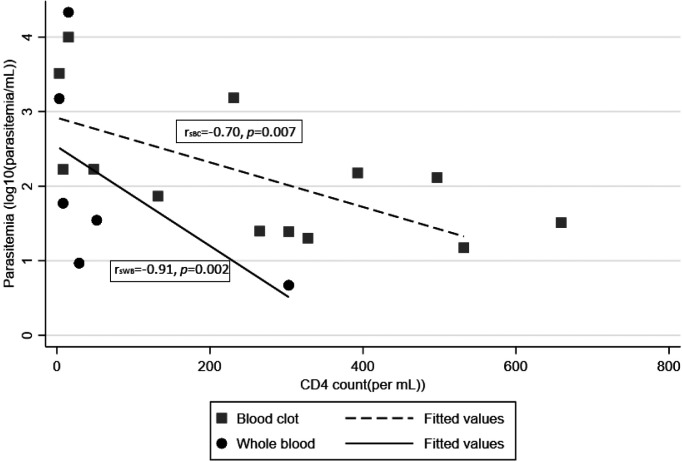
Of those who were positive by qPCR, higher parasite burden estimated by qPCR was associated with lower CD4+ counts and higher HIV viral loads (Table 5). There was a significant negative correlation between CD4+ counts and degree of T. cruzi by qPCR in the logarithmic scale. Of those qPCR positive by blood clot, the correlation of estimated parasitemia to CD4+ count was rsBC= –0.70, P = 0.007; and qPCR+ by whole blood (N = 6) was rsWB= –0.91, P = 0.002 (Figure 1). Those with CD4+ < 200 had median parasite load 168 parasites/mL (range 73–9,951), compared with median parasite load 28.5 (range 15–1,528) in patients with CD4+ > 200 copies/mm3 (P = 0.89).
Table 5.
Clinical characteristics of patients with positive quantitative polymerase chain reaction divided by estimated parasite load
| Parasite load > 100 parasites/mL (n = 7) | Parasite load < 100 parasites/mL (N = 9) | P value | |
|---|---|---|---|
| Median HIV viral load | 63,494 (4,698–237,684) | 7,711 (68–317,789) | 0.668 |
| Median CD4+ count | 48 (8–393) | 284 (92–430) | 0.164 |
| Hospitalized (vs. ambulatory) | 4 (57%) | 2 (22%) | 0.152 |
| Fever | 6 (85.7%) | 2 (22%) | 0.012 |
| Headache | 5 (71%) | 6 (67%) | 0.838 |
| Weight loss | 5 (71%) | 4 (44%) | 0.280 |
| Body mass index < 20 | 4/6 (67%) | 0 (0%) | 0.011 |
Figure 1.
Scatterplot of estimated parasitemia by quantitative polymerase chain reaction (blood clot and whole blood) by CD4+ count.
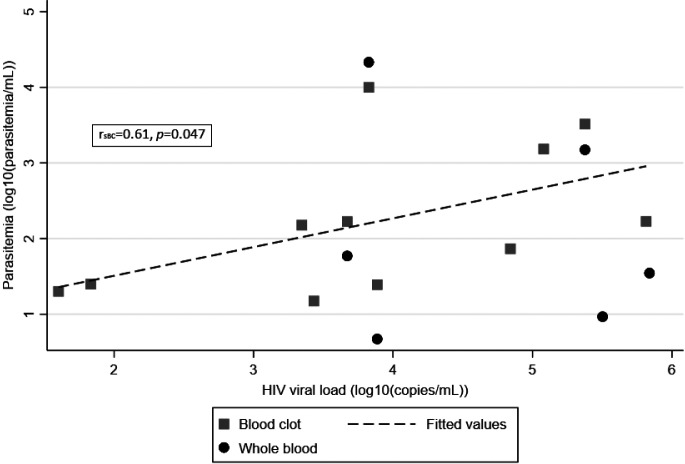
Although not statistically significant, the median HIV viral load was higher in patients with higher parasite loads (Table 5). The correlation between T. cruzi estimated by qPCR positivity in blood clot (N = 11) and HIV viral load was statistically significant with rsBC = 0.61, P = 0.047 (Figure 2).
Figure 2.
Scatterplot of estimated parasitemia by quantitative polymerase chain reaction (blood clot and whole blood) by HIV viral load.
Of the 32 patients with T. cruzi–positive serology, 20 had both a sample of blood clot and whole blood processed by qPCR. Nine of these (45%) were qPCR positive by at least one of the two tests, and 11 (55%) were negative by both tests. Three (15%) were qPCR positive only by blood clot, and two (10%) were qPCR positive only by whole blood. The agreement of qualitative results between qPCR by clot and by whole blood was 75% and the kappa index was 0.432.
Of the four patients with reactivation by direct microscopy, two had very high levels of T. cruzi (> 1,000 parasites/mL) estimated by qPCR of clot and by whole blood; however, two patients with reactivation had no parasites detected by qPCR of clot and only moderate levels T. cruzi estimated by whole blood (< 40 parasites/mL).
Two patients that had negative serology and negative direct microscopy were positive by qPCR by blood clot (patients A and B). Patient A was qPCR negative from whole blood sample; patient B did not have a sample of whole blood collected.
DISCUSSION
This study is one of few that examines levels of T. cruzi parasitemia by qPCR in HIV patients with and without reactivation, according to the conventional definition of visible parasitemia by direct microscopy.15
The prevalence of reactivation by microscopy among confirmed T. cruzi serology–positive patients in our study was 12.5%. Two other longitudinal studies in Brazil reported a cumulative incidence of reactivation of 15% to 21% over 38 to 65 months.10,15 Our study only examined evidence of reactivation at one point in time and included ambulatory patients with higher CD4+ counts, both of which likely contributed to our lower prevalence of reactivation. In our study, 57% (4/7) of T. cruzi qPCR-positive patients with CD4+ counts < 200 cells/mm3 had evidence of reactivation, and 67% (four of six) of those with CD4+ counts < 100 cells/mm3 had evidence of reactivation. The risk of reactivation seen with lower CD4+ counts and the fact that Chagas reactivation has a mortality rate of 64% to 79%10,11 highlight the risk of morbidity and mortality of patients with uncontrolled HIV coinfected with T. cruzi.
When we look at estimates of parasitemia as detected by qPCR, 50% of the seropositive patients were positive by qPCR, much higher than the 12.5% seen by microscopy. Of those qPCR-positive, higher estimated levels of parasitemia by qPCR were associated with lower CD4+ counts and higher HIV viral loads. There was a statistically significant negative correlation between CD4+ counts and degree of estimated parasitemia by qPCR. qPCR-positive patients had more fever, weight loss, headaches, decreased body mass index, hospitalizations, and increased cognitive deficits compared with qPCR-negative patients. Previous studies have suggested poor sensitivity of direct microscopy for reactivation and demonstrated that quantifiable parasitemia of T. cruzi by qPCR in immunosuppressed patients preceded the syndrome of Chagas reactivation by direct microscopy.15,22–24 A similar finding of increased sensitivity of qPCR compared with direct microscopy has been described in congenital Chagas disease,25 and qPCR has been described as the method of choice for diagnosis of congenital Chagas when resources are available.26 These studies call into question whether direct microscopy should continue to be the criteria for the diagnosis of Chagas reactivation in immunosuppressed patients.
On the basis of our findings here and those previously discussed, it seems plausible that Chagas coinfection in HIV patients is a spectrum, where qPCR-positive patients who are asymptomatic potentially progress until symptomatic and positive by direct microscopy. Once patients are positive by direct microscopy and symptomatic, mortality is high, ranging from 64% to 79%.10,11 In HIV patients, rising levels of T. cruzi estimated by qPCR may be the first sign that a patient will progress to symptomatic reactivation, as has been reported in transplant patients.22–24
Detecting Chagas reactivation earlier by qPCR could potentially allow for patients’ treatment to be optimized, although it is unclear at what level of qPCR positivity warrants antitrypanosomal therapy or if initiation of antiretroviral therapy (ART) would be sufficient. Although an argument could be made for starting antitrypanosomal therapy in all HIV patients found to be coinfected with T. cruzi, we must also keep in mind that benznidazole is not a benign medication, and it is possible that initiation of ART for immune reconstitution would cause the Chagas to subside to a chronic infection. Additionally, it is important to acknowledge that qPCR positivity could be transient and come with little clinical significance at low levels given that De Freitas et al. noted 51% of their asymptomatic HIV-negative patients to have qPCR positivity.13 In a symptomatic patient, however, treating with benznidazole before immune reconstitution is achieved, especially in a patient with CNS lesions, could be lifesaving. Whether benznidazole should be initiated in all HIV patients coinfected with T. cruzi and a certain level of qPCR positivity, a combination of qPCR positivity and signs/symptoms consistent with Chagas reactivation, or only in patients with microscopy results consistent with Chagas reactivation is a question that remains unanswered. We express concern that waiting to start antitrypanosomal therapy until a patient is at the stage of reactivation by direct microscopy comes with a high risk of morbidity and mortality. Additionally, we are concerned that a substantial number of patients living with HIV and Chagas reactivation may be misdiagnosed as having another opportunistic infection with a similar clinical picture, such as toxoplasmosis. We encourage clinical teams to consider the diagnosis of Chagas reactivation in any patient with profound immunosuppression that has lived in a region endemic to Chagas disease and is requiring hospitalization. The suspicion for Chagas reactivation should be increased with neurologic signs or symptoms.
Once a clinician arrives at the diagnosis of Chagas disease and the decision is made to treat with benznidazole, another unanswered question is whether ART should be started simultaneously or should be delayed for a certain period of time. There have not been reports of immune reconstitution syndrome clearly described in patients with Chagas disease started on ART, but there is a theoretical risk present. All treatment decisions are complicated by the fact that many of the symptoms of Chagas disease are nonspecific and can be caused by HIV itself or other opportunistic infections. At this time, management decisions regarding HIV patients coinfected with T. cruzi should be made with the complexities of each case considered. The level of immunosuppression of the patient, coinfection with other opportunistic infections, and other medications should be considered. Clinical trials are needed to provide clear guidance for these management decisions, including recommended doses, duration of treatment, and whether secondary prophylaxis should continue until immune reconstitution is achieved or whether the patient should instead be monitored closely for progression of disease instead of administering treatment based on a certain level of asymptomatic parasitemia.
Our study was limited by its cross-sectional, nonrandomized design. Two of our patients positive for reactivation by direct microscopy were not positive by analysis of blood clot by qPCR (R#1 and R#3), although their whole blood samples were moderately positive (< 40 parasites/mL). We suspect that the length of time between sample collection and analysis by qPCR (24 months) affected the results of these two samples because previous studies have shown that whole blood is more stable than blood clot in samples that are saved for an extended period of time.27
An unexpected result from our study is that the median CD4 count of our qPCR-positive patients was higher than qPCR-negative patients, although this did not reach a level of statistical significance (P = 0.635). This may be a result of small sample size or may be due to inadequate volume of blood drawn from each patient, leading to a lower than expected number of patients with detectable parasitemia by qPCR. Or some of our qPCR-positive results may be due to transient, asymptomatic, low-level parasitemia that does not reflect the level of immunosuppression, with questionable clinical significance. Our seropositive patients also had higher CD4 counts than seronegative patients (P = 0.464). It is possible that these patients presented earlier in their HIV course when coinfected secondary to increased symptoms from their Chagas disease. This is supported by the fact that we found a trend toward increased symptoms in patients who were qPCR positive and that among our ambulatory patients, we found a higher percentage of seropositive patients, indicating that in patients with less advanced HIV, symptoms related to coinfection with T. cruzi may drive them to present for care earlier than they would for HIV alone.
Two patients were seronegative for Chagas disease but were qPCR positive by blood clot, which was difficult to interpret. This could be due to early acute Chagas, contamination of qPCR results, or transmission of T. cruzi at a time when the patient was at a stage of profound immunosuppression resulting in inability to mount a sufficient antibody response. This has been described in other studies,9,28,29 which supports the scenario that patients with immunosuppression may be unable to mount an appropriate immunologic response, but we acknowledge the possibility of contamination of qPCR. Given the uncertainty of this clinical scenario, we thought best not to include these two patients with the others who were positive by both serology and qPCR in our data analysis.
CONCLUSION
It is likely that the number of patients coinfected with HIV and T. cruzi will continue to increase in both endemic and nonendemic countries, yet there is a lack of clinical data and an absence of clear recommendations for the management of the interaction of these two diseases. Given that 57% of our patients with CD4 counts < 200 cells/mm3 showed evidence of reactivation, and the significant mortality shown in studies of HIV patients with Chagas reactivation, we propose that screening for chronic Chagas disease be considered in HIV patients in regions endemic for Chagas disease and in the immigrant populations in nonendemic regions. Additionally, in serologically positive coinfected patients, we recommend consideration of surveillance by qPCR once the CD4 count drops below 200 cells/mm3 and consideration of treatment with antitrypanosomal therapy for those with estimated parasitemia by qPCR, with particular attention given to those with neurologic symptoms. We hope that further studies will provide a better understanding of this coinfection and a patient’s risk of progression to symptomatic disease to provide clear guidance for the management of the vulnerable populations that are affected by these two deadly disease processes.
Supplemental file
Note: Supplemental file appears at www.ajtmh.org.
References
- 1. World Health Organization , 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90: 33–44. [PubMed] [Google Scholar]
- 2. Yager JE, Lozano Beltran DF, Torrico F, Gilman RH, Bern C, 2015. Prevalence of Chagas heart disease in a region endemic for Trypanosoma cruzi: evidence from a central Bolivian community. Glob Heart 10: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS, 2019. Bolivia Datasheet. Available at: https://www.unaids.org/en/regionscountries/countries/bolivia. Accessed September 4, 2019.
- 4. Bern C, 2015. Chagas’ disease. N Engl J Med 373: 456–466. [DOI] [PubMed] [Google Scholar]
- 5. Rodrigues D. et al. , 2005. Cytokine serum levels in patients infected by human immunodeficiency virus with and without Trypanosoma cruzi coinfection. Rev Soc Bras Med Trop 38: 483–487. [DOI] [PubMed] [Google Scholar]
- 6. Fontes Rezende RE. et al. , 2006. Reactivation of Chagas’ disease in a patient with non-Hodgkin’s lymphoma: gastric, esophageal and laryngeal involvement. Trans R Soc Trop Med Hyg 100: 74–78. [DOI] [PubMed] [Google Scholar]
- 7. Kohl S, Pickerin LK, Frankel LS, Yaeger RG, 1982. Reactivation of Chagas’ disease during therapy of acute lymphocytic leukemia. Cancer 50: 827–828. [DOI] [PubMed] [Google Scholar]
- 8. Chalela CM. et al. , 2020. Reactivation of Chagas disease after autologous hematopoietic stem cell transplantation. Rev Soc Bras Med Trop 54: e20200143. Published 2020 Dec 21. doi: 10.1590/0037-8682-0143-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinazo MJ. et al. , 2013. Immunosuppression and Chagas disease: a management challenge. PLoS Negl Trop Dis 7: e1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sartori AM. et al. , 2007. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann Trop Med Parasitol 101: 31–50. [DOI] [PubMed] [Google Scholar]
- 11. Cordova E, Boschi A, Ambrosioni J, Cudos C, Corti M, 2008. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int J Infect Dis 12: 587–592. [DOI] [PubMed] [Google Scholar]
- 12. de Almeida EA, Ramos Júnior AN, Correia D, Shikanai-Yasuda MA, 2011. Co-infection Trypanosoma cruzi/HIV: systematic review (1980–2010). Rev Soc Bras Med Trop 44: 762–770. [DOI] [PubMed] [Google Scholar]
- 13. Corti M, Yampolsky C, 2006. Prolonged survival and immune reconstitution after chagasic meningoencephalitis in a patient with acquired immunodeficiency syndrome. Rev Soc Bras Med Trop 39: 85–88. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues V. et al. , 2014. Impairment of T cell function in parasitic infections. PLoS Negl Trop Dis 8: e2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Freitas VL. et al. , 2011. Real-time PCR in HIV/Trypanosoma cruzi coinfection with and without Chagas disease reactivation: association with HIV viral load and CD4 level. PLoS Negl Trop Dis 5: e1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sartori AM. et al. , 2002. Trypanosoma cruzi parasitemia in chronic Chagas disease: comparison between human immunodeficiency virus (HIV)-positive and HIV-negative patients. J Infect Dis 186: 872. [DOI] [PubMed] [Google Scholar]
- 17. Encyclopaedia Britannica , 2013. Cochabamba. Available at: https://www.britannica.com/place/Cochabamba. Accessed July 21, 2019.
- 18. World Health Organization , 2002. Control of Chagas Disease. WHO Technical Report Series 25. [PubMed]
- 19. Wincker P, Britto C, Pereira JB, Cardoso MA, Oelemann W, Morel CM, 1994. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg 51: 771. [DOI] [PubMed] [Google Scholar]
- 20. Piron M. et al. , 2007. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 103: 195–200. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22. Diez M. et al. , 2007. Usefulness of PCR strategies for early diagnosis of Chagas’ disease reactivation and treatment follow-up in heart transplantation. Am J Transplant 7: 1633–1640. [DOI] [PubMed] [Google Scholar]
- 23. Sartori AM. et al. , 1998. Reactivation of Chagas’ disease in a human immunodeficiency virus- infected patient leading to severe heart disease with a late positive direct microscopic examination of the blood. Am J Trop Med Hyg 59: 784–786. [DOI] [PubMed] [Google Scholar]
- 24. Gray EB. et al. , 2018. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012–2016. Transpl Infect Dis 20: e12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russomando G, de Tomassone MM, de Guillen I, Acosta N, Vera N, Almiron M, Candia N, Calcena MF, Figueredo A, 1998. Treatment of congenital Chagas’ disease diagnosed and followed up by the polymerase chain reaction. Am J Trop Med Hyg 59: 487–491. [DOI] [PubMed] [Google Scholar]
- 26. López-Vélez R, Norman FF, Bern C, 2020. American trypanosomiasis (Chagas disease). Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th edition. New York, NY: Elsevier, 762–775. [Google Scholar]
- 27. Fitzwater S. et al. , 2008. Polymerase chain reaction for chronic Trypanosoma cruzi infection yields higher sensitivity in blood clot than buffy coat or whole blood specimens. Am J Trop Med Hyg 79: 768–770. [PubMed] [Google Scholar]
- 28. Benchetrit AG, Fernández M, Bava AJ, Corti M, Porteiro N, Martínez Peralta L, 2018. Clinical and epidemiological features of chronic Trypanosoma cruzi infection in patients with HIV/AIDS in Buenos Aires, Argentina. Int J Infect Dis 67: 118–121. [DOI] [PubMed] [Google Scholar]
- 29. Hernandez C, Cucunuba Z, Parra E, Toro G, Zambrano P, Ramirez JD, 2014. Chagas disease (Trypanosoma cruzi) and HIV co-infection in Colombia. Int J Infect Dis 26: 146–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.