ABSTRACT.
Influenza is known to cause severe respiratory illness in HIV-infected adults, but there are few data describing the relationship between HIV infection and influenza in West African countries such as Ghana. We conducted a prospective cohort study in the Shai-Osudoku and Ningo Prampram districts of Ghana from 2014 to 2016. Beginning May 2014, 266 HIV-infected and 510 HIV-uninfected participants age 18 to 73 years were enrolled and monitored for 12 months. We observed 4 and 11 laboratory-confirmed influenza cases among HIV-infected and HIV-uninfected persons, respectively. The overall rate of laboratory-confirmed influenza among HIV-infected participants was 15.0 per 1,000 person years (PY) (95% CI, 0.3–29.80 per 1,000 PY), whereas that among HIV-uninfected participants was 21.6 per 1,000 PY (95% CI, 8.8–34.3 per 1,000 PY) (incidence density ratio, 0.70; P = 0.56). Our study found no significant difference in the incidence of laboratory-confirmed influenza-associated illness among HIV-infected and HIV-uninfected individuals in Ghana.
INTRODUCTION
Acute respiratory infections remain a leading cause of morbidity, mortality, and economic loss worldwide.1 According to recent estimates, up to 650,000 annual deaths are associated with respiratory diseases from seasonal influenza.2 A 2017 report published by the WHO estimated approximately 25,000 deaths resulting from influenza and pneumonia in Ghana—about 12% of the total number of deaths that occurred that year.3 Seasonal influenza vaccines are available in some West African countries, but they are not used routinely in Ghana.4 Nevertheless, Ghana has an established influenza surveillance system, and its National Influenza Center conducts routine influenza testing as part of the WHO Global Influenza Surveillance and Response System.
Human immunodeficiency virus (HIV) infection increases a person’s susceptibility to respiratory infection and is a risk factor for severe, hospitalized influenza-associated illness.5–9 In 2014, Ghana had an HIV prevalence of 1.6%, with 260,000 persons living with HIV.10 Although seasonal influenza is a common cause of respiratory illness in HIV-infected adults,11 a better understanding of the association between HIV and influenza is needed to inform influenza prevention and control strategies among HIV-infected persons. Hence, our goal was to measure the incidence of influenza-associated illness among cohorts of HIV-infected and HIV-uninfected adults in two districts in Ghana.
METHODS
We conducted a prospective cohort study in the Shai-Osudoku and Ningo Prampram districts, of the Greater Accra Region, located in southeast Ghana as a part of a larger, population-based surveillance platform for respiratory infections to estimate influenza incidence.12 We recruited 300 HIV-infected and 600 HIV-uninfected participants in a 1:2 ratio matched by age (±5 years), district of residence (either Shai-Osudoku or Ningo Prampram), and gender. HIV-infected participants were recruited from antiretroviral therapy (ART) clinics at Shai-Osudoku District Hospital, Akuse Government Hospital, and Battor Catholic Hospital over a 3-month period. These subjects were identified during regular medical visits and were invited to participate in the study. We recruited HIV-uninfected participants through a Know Your HIV Status campaign organized by the District Health Directorate in conjunction with the Noguchi Memorial Institute for Medical Research and the National AIDS & STI Control Program of the Ghana Health Service. The two cohorts were matched within the same week of recruitment to enable follow-up of the matched pairs during the same period. Residents of districts other than Shai-Osudoku and Ningo Prampram, those younger than 18 years of age, pregnant women, and persons who declined to undergo HIV testing were excluded from the study. Informed consent was obtained prior to enrollment. At enrollment, we interviewed participants using a standardized questionnaire to collect demographic and medical history information. Blood and respiratory samples were also collected at enrollment for HIV and influenza testing, respectively. This study was approved by the University of Ghana Ethics Committee, and the CDC’s internal review board relied on this approval.
We defined influenza-like illness (ILI) as a respiratory illness with a history of fever or measured axillary temperature of ≥ 37.5°C and cough with illness onset within the past 10 days. Severe acute respiratory infection (SARI) was defined as an ILI requiring hospitalization. We defined acute respiratory infection (ARI) as either an ILI or a SARI episode. Both cohorts were monitored for cough and fever fortnightly via telephone calls for 12 months, and symptomatic patients were instructed to visit a nearby health facility or the ART clinic (HIV-infected participants), where they were interviewed, and respiratory samples were collected for influenza testing.
Blood samples were collected at recruitment, 6 months and 12 months post-enrollment, and were tested to confirm HIV status and assess seroconversion during follow-up (negative or positive), HIV type (HIV 1 or 2), CD4 count, and HIV-1 viral load. Nasopharyngeal or oropharyngeal swabs were collected and tested for influenza by real-time reverse transcription polymerase chain reaction (PCR) whenever a participant sought care for ARI. Real-time reverse transcription PCR was performed according to standardized protocols of the CDC, Atlanta, GA.13
All data analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC) statistical software. Participants who died during the study period or those who did not complete follow-up were excluded from the analysis. We used χ2 tests to assess differences in categorical variables and calculated incidence rates, incidence density ratios (IDRs), and 95% CIs for influenza-associated ILI and/or SARI among HIV-infected and HIV-uninfected per 1,000 person-years (PY).
RESULTS
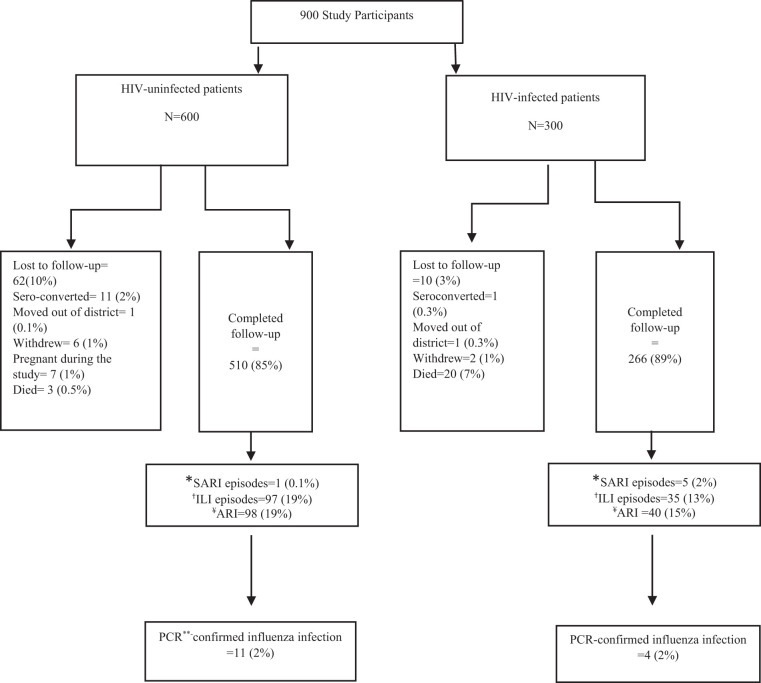
In total, 266 of 300 (89%) HIV-infected and 510 of 600 (85%) HIV-uninfected participants completed the study follow-up (Figure 1). The median age for both HIV-infected and HIV-uninfected participants was 39 years, 80% of the participants were female, and 40% lived in the Ningo-Prampram district. The HIV-infected cohort was more likely to be unemployed (P < 0.01), not know how to read or write (P < 0.01), and to have at least one child younger than 5 years old in the household (P < 0.01) in comparison to the HIV-uninfected cohort (Table 1). Among HIV-infected participants, 35% (92 of 266) had a viral load of ≥ 1,000 copies/mL, 81% (215 of 266) were on ART, and 5% (14 of 266) reported a history of tuberculosis. Almost half (45%) of the HIV-infected persons on ART had CD4 counts less than 500 and 28% had viral loads more than 1,000 copies/mL (Table 1).14
Figure 1.
Total laboratory-confirmed influenza infections identified among HIV-infected and HIV-uninfected participants, Ghana, 2014 to 2016. *Severe acute respiratory infection. †Influenza-like illness. ¥Acute respiratory infection (influenza-like illness and severe acute respiratory infection). **Polymerase chain reaction.
Table 1.
Participant characteristics by HIV status, Ghana, 2014 to 2016
| Characteristics | HIV infected, n (%) | HIV-uninfected, n (%) | P value |
|---|---|---|---|
| Total (N) | 266 | 510 | – |
| Age, y; median (range) | 39 (33–46) | 39 (33–46) | 0.5689 |
| Female gender | 214 (80) | 397 (78) | 0.5852 |
| Ningo-Prampram district | 111 (42) | 218 (43) | 0.7858 |
| Employment status* | |||
| Unemployed | 27 (11) | 13 (3) | < 0.001 |
| Employed | 222 (89) | 455 (97) | – |
| Education† | |||
| Received no formal education | 81 (32) | 111 (22) | < 0.001 |
| Received at least some formal education | 169 (68) | 395 (78) | – |
| Smoking status | |||
| Smoker (past and current) | 10 (4) | 12 (2) | 0.2635 |
| Non-smoker | 256 (96) | 498 (98) | – |
| Young children in household, at least 1ne child < 5 years old | 133 (50) | 197 (39) | 0.002 |
| Chronic medical condition‡ | |||
| Yes | 31 (12) | 74 (15) | 0.27 |
| No | 235 (88) | 436 (85) | – |
| TB | 14 (5) | 0 (0) | – |
| ARI episodes | 40 (15) | 98 (19) | 0.5 |
| Antiretroviral therapy | |||
| Yes | 215 (81) | N/A | N/A |
| No | 51 (19) | N/A | N/A |
| Average CD4 count | |||
| < 500 | 124 (47) | N/A | N/A |
| ≥ 500 | 142 (53) | N/A | N/A |
| Viral load | |||
| Undetectable | 78 (29) | N/A | N/A |
| Low viral load (< 1,000 copies/mL) | 96 (36) | N/A | N/A |
| High viral load (≥ 1,000 copies/mL) | 92 (35) | N/A | N/A |
ARI = acute respiratory infection; ART = antiretroviral therapy; N/A = not applicable; TB = tuberculosis.
Missing data: HIV negative = 42, HIV positive = 17.
Missing data: HIV negative = 4, HIV positive = 16.
Includes asthma, chronic obstructive pulmonary disease, congenital heart disease, cystic fibrosis, congestive heart failure, coronary artery disease, sickle cell anemia and diabetes; Missing = 4.
Among HIV-infected participants, 40 ARI episodes (11%) were reported, and four (2%) tested positive for influenza viruses. Among HIV-uninfected participants, 98 episodes (11%) of ARI were observed, and 11 (2%) tested positive for influenza viruses. There was no statistically significant difference in the prevalence of ARI or laboratory-confirmed influenza between HIV-infected and HIV-uninfected participants.
Among the laboratory-confirmed cases of influenza, the average age of HIV-infected participants was 39 years whereas that among HIV-uninfected was 37 years. There were equal numbers of influenza A (H3N2) (n = 2) and influenza B Yamagata infections (n = 2) among HIV-infected participants, whereas influenza B Yamagata (64%) predominated among HIV-uninfected participants, followed by influenza A (H3N2) (27%) and influenza A (H1N1) pdm09 (9%). All four of the HIV-infected participants with laboratory-confirmed influenza were on ART and had CD4 counts less than 500. Two participants had a high HIV viral load of > 90,000 copies/mL, suggesting high viremia.
The incidence of PCR-positive influenza among the HIV-infected cohort [Incidence rate = 15 (95% CI: 0.3–29.8) per 1000 PY] was similar to that of the HIV-uninfected cohort [Incidence rate = 21.6 (95% CI: 8.8–34.3) per 1000 PY] (IDR, 0.70; 95% CI, 0.19–2.12; P = 0.562). There was also no difference in the rate of ARI episodes among HIV-infected participants (Incidence rate = 150.40; 95% CI, 103.8–1,970 per 1,000 PY) compared with HIV-uninfected participants (Incidence rate = 192.2; 95% CI, 154.1–230.2 per 1,000 PY) (IDR, 0.78; 95% CI, 0.54–1.12; P = 0.189) (Table 2).
Table 2.
Prevalence and Incidence of acute respiratory illness (ARI) and PCR positive influenza by HIV status, 2014–2016
| HIV infected (n = 266) | HIV uninfected (n = 510) | P value | |
|---|---|---|---|
| PCR-positive influenza* n (%) | 4 (2) | 11 (2) | 0.56 |
| Type of influenza, n (%) | |||
| Influenza A (H3N2) | 2 (50) | 3 (27) | 0.64 |
| Influenza B Yamagata | 2 (50) | 7 (64) | |
| Influenza A (H1N1) pdm09 | 0 (0) | 1 (9) | |
| Overall incidence per 1,000 PY (95% CI) | 15.0 (0.3–29.8) | 21.6 (8.8–34.3) | 0.57 |
| Acute respiratory infection (ILI or SARI) n (%) | 40 (15) | 98 (19) | 0.15 |
| Overall incidence per 1,000 PY (95% CI) | 150.4 (103.8–197.0) | 192.2 (154.1–230.2) | 0.19 |
ILI = influenza-like illness; PY = person-years; SARI = severe acute respiratory infection.
DISCUSSION
During the 1-year follow-up period, we found a similar incidence of influenza-associated acute respiratory infection in HIV-infected and HIV-uninfected participants. A study conducted in Malawi concluded that influenza positivity was associated inversely with HIV infection (adjusted odds ratio, 0.53; 95% CI, 0.36–0.76; P < 0.001).15 Our primary hypothesis was that HIV-infected individuals have a higher incidence of influenza and ARI, but consistent with the Malawian study, this was not the case. In contrast, our findings are not consistent with the findings from another Malawian study that reported an influenza-positive ILI incidence of 46 per 1,000 PY among HIV-infected participants and 14.5 per 1,000 PY among HIV-uninfected participants (IDR, 2.75; 95% CI, 1.02–7.44, P = 0.03).7
The overall rate of PCR-positive influenza in the current study was found to be 19.3 per 1,000 PY (95% CI, 11.2–31.2), which is greater than that reported in rural Kenya15 (Incidence rate = 3.8; 95% CI, 2.6–5.7 per 1,000 persons) and Ghana12 (Incidence rate = 844; 95% CI, 501–1,099 per 100,000 persons), possibly a result of varying methods of enrollment, the cohorts enrolled, and the influenza seasons studied. In the two latter studies, patients seeking healthcare for ILI or SARI symptoms were enrolled passively, and symptomatic cases in all age groups were recruited. In our study, participants older than 18 years of age were recruited, and active phone-based follow-up was performed. The PCR-positive influenza rate in our study is slightly less than the estimates from another Kenyan study15,16 (influenza A, 2.6 per 100 PY; influenza B, 0.2 per 100 PY),16 and this could be because the study population in Kenya included children 5 years of age or older who experience greater rates of respiratory illness.
In our study, all four of the HIV-infected participants with laboratory-confirmed influenza virus infection had CD4 counts less than 500, and two had a high viral load (≥ 1,000 copies/mL).17 This finding suggests that participants with low CD4 counts could be more susceptible to influenza viruses. Jambo et al.18 suggested that participants with AIDS have a lower naturally acquired response to influenza-specific CD4(+) T-cell responses, which causes greater susceptibility to influenza virus infection.
Our study has some major limitations. Because patients were contacted by telephone fortnightly, we could have missed mildly symptomatic cases or cases when the individual was not contacted at the prescribed interval. Participants may have been less likely to report symptoms if they were unwilling to seek care and have a specimen collected. We detected few PCR-confirmed influenza cases among the participants, which limited our analysis significantly. Future studies with more than a single season of follow-up, larger sample sizes, and more frequent direct follow-up are required to provide more precise estimates of influenza burden. Alternative methods of follow-up such as home visits at regular time intervals for the duration of study might also be explored to improve respiratory illness detection.
In conclusion, we found no difference in rates of influenza-associated acute respiratory infection in HIV-infected and HIV-uninfected cohorts. However, given the low rate of acute respiratory infection detection, we may have been underpowered to detect a difference.
References
- 1. Ferkol T Schraufnagel D , 2014. The global burden of respiratory disease. Ann Am Thorac Soc 11: 404–406. [DOI] [PubMed] [Google Scholar]
- 2. Iuliano AD. et al. , 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391: 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wor ld Life Expectancy , 2017. Ghana: Influenza and Pneumonia. Available at: http://www.worldlifeexpectancy.com/ghana-influenza-pneumonia. Accessed June 3, 2021.
- 4. Duque J McMorrow ML Cohen AL , 2014. Influenza vaccines and influenza antiviral drugs in Africa: are they available and do guidelines for their use exist? BMC Public Health 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck JM Rosen MJ Peavy HH , 2001. Pulmonary complications of HIV infection: report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med 164: 2120–2126. [DOI] [PubMed] [Google Scholar]
- 6. Cohen C et al. 2015. Epidemiology of severe acute respiratory illness (SARI) among adults and children aged ≥ 5 years in a high HIV-prevalence setting, 2009–2012. PLoS One 10: e0117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho A et al. 2018. Impact of human immunodeficiency virus on the burden and severity of influenza illness in Malawian adults: a prospective cohort and parallel case–control study. Clin Infect Dis 66: 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Safrin S Rush JD Mills J , 1990. Influenza in patients with human immunodeficiency virus infection. Chest 98: 33–37. [DOI] [PubMed] [Google Scholar]
- 9. Cohen C et al. 2013. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 19: 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korte S Pettke A Kossow A Mellmann A Willems S Kipp F , 2016. Norovirus outbreak management: how much cohorting is necessary? J Hosp Infect 92: 259–262. [DOI] [PubMed] [Google Scholar]
- 11. Klein MB Lu Y DelBalso L Cote S Boivin G , 2007. Influenza virus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Nephrol Dial Transplant 45: 234–240. [DOI] [PubMed] [Google Scholar]
- 12. Ntiri MP et al. 2016. Incidence of medically attended influenza among residents of Shai-Osudoku and Ningo-Prampram districts, Ghana, May 2013–April 2015. BMC Infect Dis 16: 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization , 2009. CDC Protocol of Realtime RTPCR for Influenza A(H1N1). Available at: https://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Accessed June 3, 2021.
- 14. Murri R. et al. , 2006. Is moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy: evidence from the Italian cohort of antiretroviral-naive patients study. J Acquir Immune Defic Syndr 41: 23–30. [DOI] [PubMed] [Google Scholar]
- 15. Em ukule GO et al. 2014. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS One 9: e105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feikin DR et al. 2012. Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS One 7: e43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization , 2017. Consolidated Guidelines on Person-Centred HIV Patient Monitoring and Case Surveillance. Available at: https://apps.who.int/iris/bitstream/handle/10665/255702/9789241512633-eng.pdf?sequence=1. Accessed June 3, 2021.
- 18. Jambo KC et al. 2012. Naturally-acquired influenza-specific CD4+ T-cell proliferative responses are impaired in HIV-infected African adults. PLoS One 7: e38628. [DOI] [PMC free article] [PubMed] [Google Scholar]