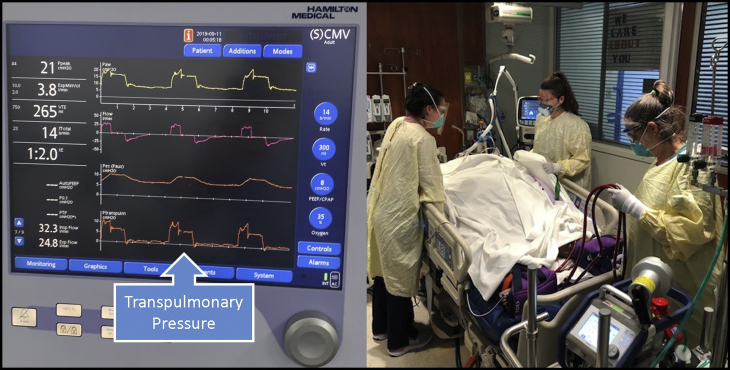
An esophageal probe accurately measures transpulmonary pressure in patients with class 3 obesity.
Central Message.
Venovenous ECMO in obese patients poses unique challenges that may be mitigated through planning and precise execution. Prone positioning, use of esophageal balloons, and a thoughtful ventilator strategy might obviate need for ECMO.
Feature Editor's Note—The use of venovenous (VV) extracorporeal membrane oxygenation (ECMO) has experienced broader adoption over the preceding decade for the management of respiratory failure. As experience with VV-ECMO has grown, VV-ECMO has been applied to more patients afflicted with a greater burden of comorbidities, which has been particularly evident during the global COVID-19 pandemic. One of the more common comorbidities is that of obesity. The use of VV ECMO in obese patients poses significant challenges and requires adoption of different techniques to achieve adequate respiratory support and successful outcomes. In this Invited Expert Technique article, Javidfar and colleagues review respiratory management and VV-ECMO techniques for application in obese patients. The authors discuss optimal respiratory management practices including use of esophageal pressure monitoring to assess lung pressures and special considerations in use of prone positioning. Optimal cannulation techniques are reviewed as well as techniques to obtain optimal ECMO flow, gas exchange and oxygenation, and facilitate airway management and physical therapy. Importantly, the authors review thresholds and situations in which conversion to veno-arterial ECMO might be necessary. Javidar and colleagues should be congratulated on a timely, insightful, and comprehensive review on the topic VV-ECMO for respiratory failure in obese patients and for providing an excellent guide to achieving optimal outcomes.
Francis D. Pagani, MD, PhD
Historically, obesity was not considered a major risk factor for adverse outcomes in patients with acute respiratory distress syndrome (ARDS). Commonly extracorporeal membrane oxygenation (ECMO) survivability prediction scores (ie, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score) do not consider obesity as a risk factor.1,2 Indeed, obesity typically decreased the risk of mortality. However, in patients with COVID-19, obesity (body mass index 30-40) and severe obesity (body mass index >40) are considered risk factors for poor outcomes related to acute hypoxemic respiratory failure (Table 1).3,4
Table 1.
Obesity
| Obesity class∗ | Body mass index |
|---|---|
| Class 1 | 30-35 |
| Class 2 | 35-40 |
| Class 3 (Severe obesity) | >40 |
CDC definition.
Venovenous (VV) ECMO has been used increasingly in the management of ARDS secondary to severe acute respiratory failure coronavirus-2. Safe vascular cannulation and adequate blood flow generation through the ECMO circuit are potentially serious obstacles for health care teams caring for obese COVID-19 patients. We seek to provide an expert review of an approach to the obese patient requiring VV ECMO.
Physiological Challenges of Obesity
Obesity is characterized by anatomical and physiological alterations that negatively affect pulmonary and cardiovascular function in critically ill patients. Obese patients are disproportionately susceptible to hypoxemia from chronic ventilation/perfusion mismatch.5 Increased chest wall mass and intra-abdominal pressure also contribute to a decrease in functional residual capacity.6 This might lead to an increase in pulmonary vascular resistance over time. Furthermore, patients with class 2 and 3 obesity have a higher resting oxygen consumption.7 To meet the increased oxygen demands and to perfuse the excess body mass, obese patients have increased cardiac output and blood volume.8 In normotensive obese patients, elevated cardiac output is associated with low peripheral vascular resistance and increased left ventricular end-diastolic pressure.9 Finally, patients are susceptible to obesity hypoventilation syndrome, obstructive sleep apnea, secondary pulmonary hypertension, and right ventricular failure.10
Special Considerations for Mechanical Ventilation Before ECMO
Mechanical ventilation in patients with class 2 and 3 obesity and ARDS should be approached in a different manner than patients with a normal habitus.11 The higher intra-abdominal pressures of obese patients can be countered with the use of reverse Trendelenburg position, which contributes to a decrease in transpulmonary pressure (Ptp). It is also imperative to use the minimal fraction of inspired oxygen to achieve the required partial pressure of oxygen (PaO2) of 55 to 80 mm Hg. Studies have shown that hyperoxemia is associated with increased mortality in critically ill patients.12,13 Especially for obese patients, hyperoxemia can exacerbate the already existing chronic inflammation associated with poor outcomes.14 Obese patients with ARDS can be optimized with low tidal volume of 4 to 6 cc/kg on the basis of predicted body weight and plateau pressure <30 cm H2O.15 However, it is important to emphasize that lung size does not change with body weight, and tidal volumes should not be on the basis of actual body weight. Lower driving pressures have been linked to improved survival in patients with ARDS.16,17 However, this benefit did not carry over in the obese population likely because of difficulties with accurate pressure measurements.18 In patients with class 2 and 3 obesity, Ptp can be most accurately calculated using esophageal pressure monitoring.
Esophageal Balloon for Titration of Positive End-Expiratory Pressure
The compliance of the respiratory system represents the combined compliance of the chest wall and the lungs. In patients with class 2 and 3 obesity, a sizeable fraction of applied positive end-expiratory pressure (PEEP) may be diverted to lifting the heavier chest wall and overlying subcutaneous tissue off of the lungs with little actual effect on Ptp. This might lead to mechanical ventilation-induced lung injury from repetitive opening and closing of alveoli at low lung volumes (atelectrauma). Conversely, the blind application of high levels of PEEP might result in alveolar overdistension at end inspiration (barotrauma), as well as adverse cardiovascular consequences (Table E1).
The use of an esophageal balloon can effectively titrate PEEP to increase lung compliance and improve arterial oxygenation in patients with ARDS19 (Figure 1). However, a definitive survival benefit has not yet been shown using this technique in patients with severe ARDS.20 Two assumptions are inherent. Esophageal pressure equates with pleural pressure, and pleural pressure is uniform throughout the hemithorax.21 After the esophageal balloon is correctly calibrated and positioned, Ptp can easily be displayed on the ventilator monitor. This allows the bedside clinician to visually inspect the true pulmonary distending pressures at any given time. Applied PEEP is then titrated in 2-cm H20 increments to maintain a Ptp PEEP of > 0 cm H2O to mitigate against atelectrauma while ensuring the Ptp plateau remains <20 cm H2O to avoid barotrauma (Table E2). Although the esophageal balloon has its limitations, in patients with class 2 and 3 obesity, it is reasonable to use it to optimize PEEP and PaO2 before committing to VV ECMO.
Figure 1.
The use of an esophageal balloon can effectively titrate positive end-expiratory pressure to increase lung compliance and improve arterial oxygenation in patients with acute respiratory distress syndrome.
Prone Positioning of Obese Patients
Prone positioning improves oxygenation by enhancing ventilation/perfusion matching by providing a more homogenous distribution of Ptp throughout the lungs.22 It either directly enhances lung compliance or indirectly improves it by reducing body wall compliance.23 In patients with severe ARDS, early proning is associated with reduced mortality.24 Prone positioning in obese patients has showed an improved PaO2:fraction of inspired oxygen ratio without higher complication rates25 (Figure E1).
Figure E1.
An algorithm for the management of hypoxia in obese patients with acute respiratory distress syndrome (ARDS) that is refractory to the mechanical ventilator and standard venovenous (VV) extracorporeal membrane oxygenation (ECMO) cannulation. PEEP, Positive end-expiratory pressure; ICU, intensive care unit; LPM, liters per minute; VVV, veno-venovenous.
Before proning, it is important to develop a bedside checklist and to have well-trained staff with clear assignment and roles. Obese patients might be exposed to pressure injuries so adhesive pads should be applied to forehead, chest, iliac crests, and knees. Also, special head support devices can reduce the incidence of skin ulceration of the face. Automated proning beds have the advantage of requiring fewer staff and can facilitate rapid return to supine position (Table E3).
Consideration for Cannulation of Obese Patients
When managing the morbidly obese patient in whom conventional management is failing, ECMO support should be considered. The safe placement of the ECMO cannulas requires specific surgical expertise that can be beyond the capabilities of centers with lower ECMO case volume or experience.26 It might be prohibitive if cannulation is being contemplated remotely at a satellite hospital (Table E4).
There are general principles of cannulation for obese patients, particularly those with class 3 obesity. First, because of habitus, there is an alteration of anatomical landmarks. In these situations it is most beneficial to use 2-view, real-time ultrasound guidance to access the vessels.27,28 Because of the increased amount of soft tissue, the vessels tend to be deeper from the surface and vascular access might require longer access needles and sheaths. One might need to use steeper angles for vascular access and during percutaneous soft tissue dilation (Figure 2). The risk of guide wire kinking during cannulation is increased. One must maintain a low threshold to exchange and use fresh and/or stiffer wires when needed. The latter requires image guidance to minimize risk of vessel injury or rupture, and can be placed using exchange catheters even at the bedside. To further mitigate against kinking of the wire, the proceduralist should keep the needle angle as shallow as possible when initially accessing the vessel. Using longer, more gently tapered silicone dilators might also be useful. To assist with safe cannula placement, a second point of image guidance in the form of radiograph and/or cardiac echo (transthoracic or transesophageal) should be used.
Figure 2.
Because of depth of subcutaneous tissue, shallow needle angle might not reach the vessel.
Last, using 2 cannulas has the theoretical advantage of offering increased ECMO flows while minimizing hemolysis. In the interest of maximizing flows, use as large of a drainage cannula as possible to avoid high venous pressures29 (Table 2). This not only facilitates enough gas exchange initially to support an obese patient, but provides a buffer of excess flow that allows for diuresis later in the patient's ECMO management course (Figure E1). Use of the largest available drainage cannula should be considered during initial cannulation. If additional flows are needed, a third cannula can be placed either in the remaining femoral vein or an upper body vein to allow for a “VVV” configuration with bicaval drainage (Figure 3, A). Alternatively, the lungs can be bypassed with a venoarterial-venous (VAV) ECMO configuration.
Table 2.
Sample cannulation strategy
| Body mass index <40 | Body mass index >40 | |||
|---|---|---|---|---|
| Cannula size | 20-Fr return 25-Fr drainage | 4-5 LPM | VV 21-Fr return 27-Fr or 29-Fr drainage |
5.5-6 LPM |
| VVV 23-Fr return 20-Fr or 29-Fr drainage |
>6 LPM | |||
Fr, French; LPM, liters per minute; VV, venovenous extracorporeal membrane oxygenation; VVV, veno-venovenous.
Figure 3.
A, Extracorporeal membrane oxygenation (ECMO) flows can be maximized in a veno–venovenous ECMO configuration using the right internal jugular and right femoral veins for bicaval drainage, and the left subclavian vein for oxygenated return. B, With venovenous or veno–venovenous ECMO there is a risk of recirculation if the cannula placement is not image-guided. C, The left subclavian vein can be used for dual lumen cannula placement. Ao, Aorta; PA, pulmonary artery; LA, left atrium; RA, right atrium; RV, right ventricle; LV, left ventricle; IVC, inferior vena cava.
There are specific pros and cons to the various configurations of VV ECMO in the class 2 and 3 obese patient (Table 3). If partial cardiac support is required then VAV ECMO offers the added benefit of partial cardiac support (Table 4). Even with the use of ultrasound, accessing the cervical vessels might be challenging. The right internal jugular (IJ) vein tends to be the easiest site for peripheral cannulation in this population because it is anatomically in line with the superior vena cava. This can be for either a single-site dual lumen cannula or as part of a femoral-IJ configuration. A dual lumen cannula has the benefit of requiring only a single vascular puncture, but should only be placed by experienced cannulators using the requisite image guidance to prevent vascular or cardiac injury. This necessitates the availability of image guidance in the intensive care unit or that the patient is stable enough for transportation to the operating room or catheterization lab. Maximum flows tend to be approximately 4.5 liters per minute (LPM), and this might be insufficient to support the class 2 and 3 obese patient in the acute phase of their ARDS.30 However, a dual-lumen cannula has the advantages of obviating the need for a femoral cannula, which can facilitate patient mobilization, easier rehabilitation, and increase patient comfort.31
Table 3.
Pros and cons of cannula configuration
| Cannulation strategy | Pros | Cons |
|---|---|---|
| Standard configurations | ||
| Venovenous (2 sites) | ||
| Femoral–femoral veins |
|
Femoral vein risks∗
|
|
||
| Femoral–internal jugular veins |
|
LIJ risks†
|
|
||
| Femoral–left subclavian vein |
|
Left subclavian vein risks‡
|
|
||
| Venovenous (dual lumen) |
|
|
| Internal jugular vein |
|
|
| Left subclavian vein |
|
|
| Configurations for extra flow | ||
| Veno-venovenous (VVV) |
|
|
| Femoral–internal jugular veins, left subclavian |
|
Femoral vein risks∗
|
| Femoral–internal jugular vein, femoral vein |
|
LIJ risks†
|
| Femoral–left subclavian veins, femoral vein |
|
|
| Venovenous (2 sites) | ||
| Femoral–internal jugular veins with extra-large cannula |
|
|
| DL–femoral vein (VV-VDL) Upper body DL with additional femoral venous drainage line |
|
|
IVC, Inferior vena cava; RIJ, right internal jugular vein; SVC, superior vena cava; LIJ, left internal jugular vein; ROM, range of motion; ECMO, extracorporeal membrane oxygenation; DL, dual lumen; VV-VDL, venovenous-veno dual lumen.
Femoral vein risks.
LIJ risks.
Left subclavian vein risks.
Table 4.
Pros and cons of ECMO cannula configuration; partial cardiac support
| Cannulation strategy, venovenous (2 sites) | Pros | Cons |
|---|---|---|
Veno-arterial–venous (VAV)
|
|
|
Femoral cannulation, femoral-femoral, or femoral-IJ configuration can be used. Two cannulation sites have the advantage of greater flows due to enhanced drainage versus a single-site, dual-lumen cannula. However, a large pannus and significant amount of subcutaneous tissue can make femoral cannulation challenging. To mitigate against challenges such as kinking of guide wires and difficulties with serial dilation one should take care to meticulously retract the pannus in a secure fashion before prepping and draping. The preferential use of the right groin might be advisable because the right femoral vein is anatomically more in line with the inferior vena cava. However, bifemoral cannulation can be performed safely at the bedside, and, if used with image guidance, offers a low risk of recirculation (Figure 3, B).
If the patient does not require high flows, IJ vein or left subclavian vein cannulation with a dual-lumen cannula might offer the advantage of obviating the need for a femoral cannula, facilitating mobilization and freeing up a peripheral venous access site (Figure 3, C). The left subclavian vein has the added advantage of being further away from the tracheostomy site and less likely to be soiled by its secretions. Because of the anatomy and habitus, radiographic guidance should be routinely used for all dual-lumen cannulations.
Considerations During ECMO Management
The primary goal of VV ECMO is to provide adequate gas exchange and oxygenation while allowing the lungs time to recover. Oxygenation depends on rate of flow, hemoglobin concentration, oxygenator surface area, and the fraction of inspired oxygen of the gas flow. To maximize oxygen delivery, high ECMO flow (6-7 LPM) is needed, which is dependent on cannula size, position, and patient volume status. Elevating serum hemoglobin levels can increase blood oxygen content.32 Finally, increasing oxygenator surface area by using 2 oxygenators in parallel can improve oxygenation and gas exchange.33 The pharmacokinetics in obese patients receiving ECMO has not been well-studied. However, the literature shows that differences in opioid and benzodiazepine requirements were not as great as initially predicted for obese versus nonobese patients receiving VV ECMO.34
Bedside Management Considerations
Obese patients can be difficult to manage in the critical care setting. Prone positioning of obese patients receiving VV ECMO has specific pitfalls. Most concerning, are accidental decannulation, extubation, or removal of invasive lines from the patient. However, in experienced centers many of these complications tend to be avoided.35 Proning patients receiving ECMO is also labor-intensive and can lead to staff injuries. Furthermore, proning obese patients receiving VV ECMO can increase intra-abdominal pressures and impair ECMO flows. Finally, as discussed previously, class 2 and 3 obese patients are at an increased risk of cannula kinking, skin breakdown, and pressure ulcer formation.
Airway Management During ECMO
Tracheostomy in the ECMO population is considered safe.36 After patients are receiving ECMO, each center should continue to follow its established protocols and guidelines for airway management including timing and surgical approach to tracheostomy. When possible, early tracheostomy should be considered. The use of extra-long tracheostomy tubes should be considered to minimize the risk of dislodgement, fistula formation, and tracheal injury.37
Physical Therapy During ECMO
Patients with class 2 and 3 obesity who require VV ECMO are at particular risk for critical illness myopathy and muscle loss. Mobilization also poses a challenge. Practical risks include kinking of lower body cannulas due to the pannus as a result of the angle that the femoral cannula travels through the soft tissue.38,39 In instances in which 2 or more sites of cannulation are required, physical activity might be limited to bed exercises, such as active and passive range of motions activities. It is important to firmly secure the cannula and tubing to the skin with suture and attachment devices. Usually bed-based pedal bikes can be logistically prohibitive because of the habitus and ergonomics. Patients may sit up in a bed converted to the chair position. Tilt table beds specially designed for physical therapy allow for standing and weight-bearing. They can be used to build core strength without flexion at the hips.
It is easier to mobilize patients who can solely be supported using upper body dual-lumen cannula.40,41 As ECMO flow requirements decrease below 4 to 4.5 LPM, a patient can be converted to a dual-lumen cannula, and out of bed exercises can begin. This can include dangling, sit to stand, and then walking on portable treadmills. Patients can also strengthen their core by spending hours a day sitting in a chair. At the peak of their physical therapy training patients can walk laps in the intensive care unit (because they are usually off of isolation precautions by this point). Stationary bikes may also be used.
Anticoagulation During ECMO
Patients with class 2 or 3 obesity tend to be hypercoagulable in part due to the higher inflammatory state. This, when possible, calls fora higher degree of anticoagulation. There is also a trend toward bivalirudin usage in lieu of heparin for daily anticoagulation management in VV ECMO patients. Intravenous bivalirudin has been associated with decreased number of ECMO circuit-related thrombotic events and decreased blood transfusions compared with unfractionated heparin drip.42 In practice, this can still include a heparin bolus of 5000 to 8000 units for cannulation. The initial use of heparin is on the basis of practicality and availability in patients with emergency or remote cannulations. However, it is dosed at a higher level than for nonobese patients. For maintenance, patients can be transitioned to a bivalirudin drip with activated partial thromboplastin time (aPTT) monitoring. The dosing is managed with a nomogram with a starting aPTT goal of 60 to 80 seconds. Alternatively, a heparin drip with a Factor Xa or an aPTT monitoring nomogram can be used. The anticoagulation should be titrated depending on instance and severity of events such as intracranial bleeding, gastrointestinal bleeding, and bleeding from the naso/oro pharynx or tracheostomy site. Additional benefits of bivalirudin include a short half-life, easy monitoring, and avoidance of heparin-induced thrombocytopenia and/or antithrombin III deficiency. All post-ECMO patients with class 2 or 3 obesity would benefit from 4-limb surveillance ultrasound examination, because some might need continued post ECMO anticoagulation.
Management of the Right Heart During VV ECMO
The pre- and peri-ECMO phases are usually when patients with refractory respiratory failure are the most hemodynamically unstable. They usually have significant acid-base disarray, and can require high doses of multiple pressors and/or ionotropes. Maximal ventilator support might fail, which includes high PEEP levels that can diminish venous return. In this situation, patients are vulnerable to varying degrees of right heart failure that can be made worse by the physiologic changes associated with class 2 and 3 obesity. If significant hemodynamic instability is being experienced, additional right heart support can be derived from inhaled pulmonary vasodilators (such as nitric oxide and epoprostenol sodium) and/or ionotropes. In our experience in obese class 2 and 3 patients with isolated respiratory failure severe right heart failure is transient. Significant hemodynamic benefits can be derived from VV ECMO alone without directed mechanical support of the right heart. VV ECMO allows for a rapid correction of pH and improvement in pharmacokinetics because enzymes and medications tend to work optimally at a normal pH. As the ventilator is weaned further toward rest settings, a normalization of the previously very high PEEP improves venous return. Often after a brief period of VV ECMO, pressors, ionotropes, and the ventilator can all be weaned as the patient's hemodynamics stabilize.
However, if patients VV ECMO plus inhaled pulmonary vasodilator and ionotropes fail, then several mechanical options are available. The first would involve converting the patient from VV ECMO to VAV ECMO by adding a peripheral arterial limb. This would provide the mechanical benefit of unloading the right and left heart, but can be technically challenging in the class 2 and 3 obese patient. The arterial limb can be placed percutaneously or open in a femoral artery, but this is subject to all of the associated challenges of femoral venous cannulation including difficult access and kinking. Additionally, the patient would require a distal perfusion cannula placed into the ipsilateral superficial femoral artery. This can be particularly challenging to perform reliably at the bedside in an obese patient. In this situation, we recommend using image guidance such as ultrasound or preferably, an angiogram in a hybrid operating room or catheterization lab. Because of the depth of soft tissue required to be transversed and to prevent inadvertent dislodgement we suggest using an extra-long wire-reinforced catheter. Alternatively, a retrograde reperfusion cannula can be placed in the posterior tibial artery where the soft tissue tends to be less extensive. Other surgical options for an arterial limb include tunneled cannulation of a graft sewn onto the axillary or femoral artery. It would obviate the need for a distal perfusion cannula, but the tunneled graft and cannula can kink in the soft tissue of class 2 or 3 patients. In the axilla, there is also a potential risk of brachial plexus compression from a deep hematoma that was not detected because of the patient's habitus. Additional options include transjugular pulmonary artery cannulation. This can be via either a 2-site technique that includes femoral venous drainage or making use of the ProTek Duo (Liva Nova), which is a dual-lumen cannula commonly placed in the right IJ vein, which bypasses the right ventricle. This cannula can be technically challenging to place, commonly requires fluoroscopy, and only carries with it an indication for 6 hours of use. It would not be ideal for emergency bedside placement. Consideration for transjugular pulmonary artery cannulation should be for rare instances and performed by those with the requisite technical expertise (Figure E2).
Figure E2.
An algorithm for the management of worsening right heart in obese patients with acute respiratory distress syndrome (ARDS) who are already receiving venovenous (VV) extracorporeal membrane oxygenation (ECMO). VAV, Venoarterial-venous; PA, pulmonary artery.
Ventilation Management During ECMO
Patients with class 2 or 3 obesity initially receiving VV ECMO should continue to be ventilated with similar strategies outlined herein for the pre-ECMO phase. Reverse Trendelenburg bed positioning and use of an esophageal probe can optimize respiratory mechanics while accurately recreating rest settings per ARDS Net protocols. As lung recovery occurs, the sequence for weaning of the mechanical ventilator and ECMO should conform to the ECMO center's best practices. Specifically, if a center routinely manages VV ECMO patients without positive pressure ventilation, then the strategy can be applied to select patients with class 2 or 3 obesity. The potential advantages of liberating VV ECMO patients from mechanical ventilation include earlier mobilization, decreased sedation requirements, improved mentation, and improved respiratory muscle usage.43 This would also apply to those with class 2 or 3 obesity. We would, however, caution against using these patients as the first cohort for trialing ECMO without a mechanical ventilator. Mobilizing patients with class 2 or 3 obesity while they are receiving ECMO can be rather challenging. Early tracheostomy could be a comprise by providing a protected airway while allowing for periods free from positive pressure ventilation.
Conclusions
Acute lung injury in the patients with class 2 and 3 obesity can be difficult to manage particularly as a patient progresses to ARDS. Ventilator optimization might involve the use of an esophageal probe to measure Ptp's and where feasible, proning patients should be considered before initiating VV ECMO. However, if respiratory failure is refractory to conventional means, then mechanical circulatory support is a reasonable option in appropriately resourced and experienced centers.
ECMO therapy for the class 2 and 3 obese patient population presents certain unique challenges that require thoughtful planning and meticulous execution of cannulation. Although ECMO cannulation can be a “routine” urgent procedure, it requires experienced cannulators with access to appropriate imaging. After ECMO is started, standard management practices might need to be adjusted to mitigate against mechanical and physiological challenges posed by obesity, including the need for increased flow. Mobilizing this patient population might be difficult, but it is possible and necessary to ensure a meaningful recovery. For patients with severe morbid obesity VV ECMO should be considered an option in experienced hands.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
Illustrations were graciously provided by Andy Mattlock, Department of Surgery, Emory School of Medicine. The authors thank the Washington MedStar Healthsystem ECMO team and the Emory Healthcare ECMO team for their checklists and protocols.
Footnotes
Internal funds were used to support this work.
G.P. and R.A. contributed as co-senior authors.
Appendix E1
Table E1.
Key points
|
|
|
PEEP, Positive end-expiratory pressure; PaO2, partial pressure of oxygen; ECMO, extracorporeal membrane oxygenation.
Table E2.
Definitions
| Transpulmonary pressure: the distending pressure of lung tissue expressed mathematically as: transpulmonary pressure = alveolar pressure − pleural pressure |
| Transpulmonary plateau pressure: transpulmonary pressure measured at end inhalation = plateau pressure − esophageal pressure at end inhalation |
| Transpulmonary PEEP: transpulmonary pressure measured at end exhalation = PEEP − esophageal pressure at end exhalation |
PEEP, Positive end-expiratory pressure.
Table E3.
Ceiling lift proning checklist for ECMO patients
| Adjust ventilator equipment, tubing, leads, and lines so there is enough slack during movement |
| Visualize cannulas and confirm with perfusionist positioning of cannula and securement. While patient is still in supine position, place chux pad, and bariatric (green) Ehob cushion over the chest and hip, and a flat sheet on top of the cushion. Fold flat sheet to expose femoral access |
| Place hand into pocket of ceiling lift and slide into the mattress surface to mitigate skin shear for proper placement |
| Insert 1 ceiling lift so the top is aligned with armpits |
| Place the second ceiling lift near the greater trochanter (depending on location of cannula, placement of ceiling lift may be placed above the pelvis. This might require additional LE support during turn). Insert straps from side onto which patient is being turned leaving short end loops visible. The patient will turn in the direction of the long side of the straps |
| Tuck patient's hand under each hip |
| Wrap the edge of the sheets as closely as possible to the patient's body (like a burrito) and inflate the bed to the maximum |
| Lower sling bar and rotate it parallel to length of the patient. Attach the longer loop from the side onto which the patient is being turned, and the short loop from the short side of the ceiling lift, taking care to ensure the strap is seated properly on sling bar |
| One caregiver raises the sling bar until there is tension in the straps, then the team confirms that all the required loops are secure in the sling bar |
| Perfusionist is responsible for visualizing cannulas throughout the turn |
| Ensure the team member at the head of the bed is responsible for initiating the turn maneuver via a verbal countdown, as well as securing the patients ETT and head throughout the maneuver. At this point, the patient is ready to be lifted |
| On the count of 3, the caregiver on the side from which the patient is being turned away will operate the lift, while the other caregiver(s) monitor the lines and patient's response |
| The caregiver at the head of bed will monitor and support the patient's head and airway. Caregivers at the foot of the bed are responsible for managing the LEs |
| Upon lifting, the lift equipment will begin to rotate the patient to the side. The lift movement is slow and controlled |
| Lift the patient high enough so they easily slide, but do not lift the patient fully off the bed. Slide patient to opposite side from which you are turning so they are centered when proned. The team should continue to manage tubing and lines as patient is being lowered |
| At this point, while patient suspended in lift, place flat sheet and chux pad on bed surface. |
| When all caregivers indicate they are ready, the person at the patients' back lowers the patient and guides them into the prone position |
| Position patient's head on prone pillow, Z-Flo, or air waffle cushion, in a manner that minimizes pressure of holder and tube to face and enables visualization of the ETT. The ceiling lift positioners can remain under the patient for returning the patient to supine position |
| Perfusionist to assess cannula and flow, reposition as needed |
| Position LEs on pillows to prevent pressure on bony prominences |
| Return ICU bed to normal mattress setting |
| Before exiting the room, intensivist/APP to monitor vitals and assess patient's tolerance to position |
| When returning the patient to the supine position, simply reconnect the ceiling lift to the bar in the same manner as when proning and follow the same procedures described above to return to supine position |
| Please note, when returning the patient from prone to supine, ceiling lift used in lower portion of body should be inserted with care to avoid disturbing/dislodging femoral cannula access |
Z-Flo is from Mölnlycke Health Care. LE, Lower extremity; ETT, endotracheal tube; ICU, intensive care unit; APP, advanced practice provider.
Table E4.
ECMO transport supplies (sample packing list)
| CardioHelp console bag | Quantity |
|---|---|
| Maquet CardioHelp with hand crank | 1 |
| Maquet CardioHelp console | 1 |
| Bottle sterile saline | 1 |
| Tumi syringe | 2 |
| Baxter Plasma Lyte-A (priming) | 2 |
| Sterile metal basin | 1 |
| Power extension cord | 1 |
| Transport sterile instrument pan | 1 |
| CardioHelp circuit bag | |
|---|---|
| Maquet HLS pack | 2 |
| Transport heater/water | 1 |
| Black cannula bag (long) | Location | |
|---|---|---|
| Vascular dilator kit | Top - middle pocket | 2 |
| Maquet Avalon access/dilator kit 210 cm | Large compartment - top side | 2 |
| Medtronic Fem Ven cannula Carmeda2 | 2 | |
| Medtronic Fem Ven 21-Fr cannula | Large compartment - top side | 2 |
| Medtronic Fem Ven 25Fr cannula | Large compartment - top side | 2 |
| Maquet HLS Fem Ven 29Fr cannula | Large compartment - bottom side | 2 |
| Maquet HLS Fem Ven 23Fr cannula | Large compartment - bottom side | 2 |
| Maquet HLS Fem Ven 25Fr cannula | Large compartment - bottom side | 2 |
| Fem Flex Fem Art 16-Fr cannula | Mid compartment | 2 |
| Fem Flex Fem Art 18-Fr cannula | Mid compartment | 2 |
| Fem Flex Fem Art 20-Fr cannula | Mid compartment | 2 |
| Medtronic EOPA 22-Fr cannula | Mid compartment | 2 |
| Teleflex/Arrow 6-Fr introducer (lower leg) | Underside of mid compartment - larger pocket | 2 |
| Teleflex/Arrow 8-Fr introducer (lower leg) | Underside of mid compartment - larger pocket | 2 |
| Perfusion clamps | Underside of mid compartment - smaller pocket | 6 |
| Connector 3/8–3/8 | Top - left pocket | 3 |
| Connector 3/8–3/8–3/8 | 3 | |
| Extra 3/8 tubing | Large compartment - top side | 1 |
| 1/4-inch supplemental (yellow) tubing | Large compartment - top side | 1 |
| 1/4-inch male swivel | Top–left pocket | 4 |
| Boston Scientific Amplatz wire | Mid compartment | 2 |
| Micropuncture kit | Underside of mid compartment - larger pocket | 6 |
| Cook Medical Rosen wire | Mid compartment | 2 |
| Black bag (small) | |
|---|---|
| Kerlex gauze | 2 |
| Sterile 4 × 4 | 4 |
| Sterile towels | 4 |
| Sterile gowns | 4 |
| Sterile gloves, multiple sizes | |
| ACT Machine/PowerCord | 1 |
| ACT LR cuvettes | 6 |
| Istat ABG machine | 1 |
| Istat Cartridges/Istat LQC | 5 |
| Syringes/blunt tip needle | 10 |
| 3/4 Sterile drape | 4 |
| Chlora Prep | 6 |
| Sterile intravenous tubing (short) | 2 |
| 0 Silk stitch multipack | 6 |
| Tegaderm | 4 |
| Arterial line transducer Safe Set 2 | |
| Tumi syringe | 2 |
| Bulb syringe | 2 |
Fem, Femoral; Ven, venous; Fr, French; Art, artery; ACT, activated clotting time; LR, lactated ringers; ABG, arterial blood gas; LQC, liquid quality control.
References
- 1.Schmidt M., Bailey M., Sheldrake J., Hodgson C., Aubron C., Rycus P.T., et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 2.Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Defining adult overweight & obesity. https://www.cdc.gov/obesity/adult/defining.html Accessed February 2, 2021.
- 4.Centers for Disease Control and Prevention People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed February 10, 2021.
- 5.Holley H.S., Milic-Emili J., Becklake M.R., Bates D.V. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46:475–481. doi: 10.1172/JCI105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones R.L., Nzekwu M.M. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 7.Kress J.P., Pohlman A.S., Alverdy J., Hall J.B. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160:883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 8.Lemmens H.J., Bernstein D.P., Brodsky J.B. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16:773–776. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 9.Alpert M.A., Omran J., Bostick B.P. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep. 2016;5:424–434. doi: 10.1007/s13679-016-0235-6. [DOI] [PubMed] [Google Scholar]
- 10.Kauppert C.A., Dvorak I., Kollert F., Heinemann F., Jorres R.A., Pfeifer M., et al. Pulmonary hypertension in obesity-hypoventilation syndrome. Respir Med. 2013;107:2061–2070. doi: 10.1016/j.rmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 11.De Jong A., Verzilli D., Jaber S. ARDS in obese patients: specificities and management. Crit Care. 2019;23:74. doi: 10.1186/s13054-019-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer E., Post B., Klapaukh R., Marra G., MacCallum N.S., Brealey D., et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients. A multicenter observational cohort study. Am J Respir Crit Care Med. 2019;200:1373–1380. doi: 10.1164/rccm.201904-0849OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You J., Fan X., Bi X., Xian Y., Xie D., Fan M., et al. Association between arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. J Crit Care. 2018;47:260–268. doi: 10.1016/j.jcrc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 14.González-Muniesa P., Garcia-Gerique L., Quintero P., Arriaza S., Lopez-Pascual A., Martinez J.A. Effects of hyperoxia on oxygen-related inflammation with a focus on obesity. Oxid Med Cell Longev. 2015;2015:8957827. doi: 10.1155/2016/8957827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Guérin C., Papazian L., Reignier J., Ayzac L., Loundou A., Forel J.M., et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care. 2016;20:384. doi: 10.1186/s13054-016-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amato M.B.P., Meade M.O., Slutsky A.S., Brochard L., Costa E.L.V., Schoenfeld D.A., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 18.De Jong A., Cossic J., Verzilli D., Monet C., Carr J., Conseil M., et al. Impact of the driving pressure on mortality in obese and non-obese ARDS patients: a retrospective study of 362 cases. Intensive Care Med. 2018;44:1106–1114. doi: 10.1007/s00134-018-5241-6. [DOI] [PubMed] [Google Scholar]
- 19.Talmor D., Sarge T., Malhotra A., O'Donnell C.R., Ritz R., Lisbon A., et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beitler J.R., Sarge T., Banner-Goodspeed V.M., Gong M.N., Cook D., Novack V., et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321:846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahetya S., Brower R.G. The promises and problems of transpulmonary pressure measurements in acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22:7–13. doi: 10.1097/MCC.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard J.C., Bregeon F., Costes N., Bars D.L.E., Tourvieille C., Lavenne F., et al. Effects of prone position and positive end-expiratory pressure on lung perfusion and ventilation. Crit Care Med. 2008;36:2373–2380. doi: 10.1097/CCM.0b013e31818094a9. [DOI] [PubMed] [Google Scholar]
- 23.Mentzelopoulos S.D., Zakynthinos S.G., Roussos C., Tzoufi M.J., Michalopoulos A.S. Prone position improves lung mechanical behavior and enhances gas exchange efficiency in mechanically ventilated chronic obstructive pulmonary disease patients. Anesth Analg. 2003;96:1756–1767. doi: 10.1213/01.ANE.0000064282.79068.1E. [DOI] [PubMed] [Google Scholar]
- 24.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 25.De Jong A., Molinari N., Sebbane M., Prades A., Futier E., Jung B., et al. Feasibility and effectiveness of prone position in morbidly obese patients with ARDS: a case-control clinical study. Chest. 2013;143:1554–1561. doi: 10.1378/chest.12-2115. [DOI] [PubMed] [Google Scholar]
- 26.Rupprecht L., Lunz D., Philipp A., Lubnow M., Schmid C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015;7:320–326. [PMC free article] [PubMed] [Google Scholar]
- 27.Ull C., Buchwald D., Strauch J., Schildhauer T.A., Swol J. Extremely obese patients treated with venovenous ECMO–an intensivist's challenge. Am J Emerg Med. 2015;33:1720. doi: 10.1016/j.ajem.2015.03.065. e1723-4. [DOI] [PubMed] [Google Scholar]
- 28.Swol J., Buchwald D., Dudda M., Strauch J., Schildhauer T.A. Veno-venous extracorporeal membrane oxygenation in obese surgical patients with hypercapnic lung failure. Acta Anaesthesiol Scand. 2014;58:534–538. doi: 10.1111/aas.12297. [DOI] [PubMed] [Google Scholar]
- 29.Kon Z.N., Dahi S., Evans C.F., Byrnes K.A., Bittle G.J., Wehman B., et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg. 2015;100:1855–1860. doi: 10.1016/j.athoracsur.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 30.https://www.getinge.com/dam/hospital/documents/english/avalon_elite_brochure-en-non_us.pdf Accessed November 15, 2021.
- 31.Rubino A., Vuylsteke A., Jenkins D.P., Fowles J., Hockings L., Valchanov K. Direct complications of the Avalon bicaval dual-lumen cannula in respiratory extracorporeal membrane oxygenation (ECMO): single-center experience. Int J Artif Organs. 2014;37:741–747. doi: 10.5301/ijao.5000357. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett R.H. Physiology of gas exchange during ECMO for respiratory failure. J Intensive Care Med. 2017;32:243–248. doi: 10.1177/0885066616641383. [DOI] [PubMed] [Google Scholar]
- 33.Malik A., Shears L.L., Zubkus D., Kaczorowski D.J. Parallel circuits for refractory hypoxemia on venovenous extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2017;153:e49–e51. doi: 10.1016/j.jtcvs.2016.10.067. [DOI] [PubMed] [Google Scholar]
- 34.Verkerk B.S., Dzierba A.L., Muir J., Der-Nigoghossian C., Brodie D., Bacchetta M.D., et al. Opioid and benzodiazepine requirements in obese adult patients receiving extracorporeal membrane oxygenation. Ann Pharmacother. 2020;54:144–150. doi: 10.1177/1060028019872940. [DOI] [PubMed] [Google Scholar]
- 35.Culbreth R.E., Goodfellow L.T. Complications of prone positioning during extracorporeal membrane oxygenation for respiratory failure: a systematic review. Respir Care. 2016;61:249–254. doi: 10.4187/respcare.03882. [DOI] [PubMed] [Google Scholar]
- 36.Salna M., Tipograf Y., Liou P., Chicotka S., Biscotti M., Agerstrand C., et al. Tracheostomy is safe during extracorporeal membrane oxygenation support. ASAIO J. 2020;66:652–656. doi: 10.1097/MAT.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 37.Barrera S.C., Sanford E.J., Ammerman S.B., Ferrell J.K., Simpson C.B., Dominguez L.M. Postoperative complications in obese patients after tracheostomy. OTO Open. 2020;4:1–6. doi: 10.1177/2473974X20953090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells C.L., Forrester J., Vogel J., Rector R., Tabatabai A., Herr D. Safety and feasibility of early physical therapy for patients on extracorporeal membrane oxygenator: University of Maryland Medical Center experience. Crit Care Med. 2018;46:53–59. doi: 10.1097/CCM.0000000000002770. [DOI] [PubMed] [Google Scholar]
- 39.Ko Y.J., Cho Y.H., Park Y.H., Lee H., Suh G.Y., Yang J.H., et al. Feasibility and safety of early physical therapy and active mobilization for patients on extracorporeal membrane oxygenation. ASAIO J. 2015;61:564–568. doi: 10.1097/MAT.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 40.Javidfar J. The challenges faced with early mobilization of patients on extracorporeal membrane oxygenation. Crit Care Med. 2018;46:161–163. doi: 10.1097/CCM.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 41.Abrams D., Javidfar J., Farrand E., Mongero L.B., Agerstrand C.L., Ryan P., et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18:R38. doi: 10.1186/cc13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivosecchi R.M., Arakelians A.R., Ryan J., Murray H., Raj Ramanan, Gomez H., et al. Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: heparin versus bivalirudin. Crit Care Med. 2021;49:1129–1136. doi: 10.1097/CCM.0000000000004944. [DOI] [PubMed] [Google Scholar]
- 43.Levin N.M., Ciullo A.L., Overton S., Mitchell N., Skidmore C.R., Tonna J.E. Characteristics of patients managed without positive pressure ventilation while on extracorporeal membrane oxygenation for acute respiratory distress syndrome. J Clin Med. 2021;10:251. doi: 10.3390/jcm10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]